Negative emotionality: monoamine oxidase B gene variants modulate personality traits in healthy humans (original) (raw)
. Author manuscript; available in PMC: 2013 May 14.
Published in final edited form as: J Neural Transm (Vienna). 2009 Aug 6;116(10):1323–1334. doi: 10.1007/s00702-009-0281-2
Abstract
Monoamine oxidase A and B (MAOA and MAOB) appear to be involved in the pathogenesis of Major Depression, and vulnerability of Major Depression is associated with personality traits relating to positive and negative affect. This study aimed to investigate associations between MAOA and MAOB polymorphisms and personality traits of positive and negative emotionality in healthy volunteers, to elucidate mechanisms underlying personality and the risk for depression. Healthy Caucasian volunteers (N = 150) completed the Multiphasic Personality Questionnaire (MPQ), which includes independent superfactors of Positive Emotionality and Negative Emotionality. Participants were genotyped for 8 MAOA and 12 MAOB single nucleotide polymorphisms (SNPs). Association analyses for both SNPs and haplotypes were performed using the permutation approach implemented in PLINK. Negative Emotionality was significantly associated with the two highly linked MAOB polymorphisms rs10521432 and rs6651806 (p < 0.002). Findings were extended in haplotype analyses. For MAOB the 4-SNP haplotype GACG formed from rs1799836, rs10521432, rs6651806 and rs590551 was significantly related to lower Negative Emotionality scores (p < 0.002). MAOA was not related to personality in this study. Our finding provides the first evidence that MAOB polymorphisms influence levels of negative emotionality in healthy human volunteers. If confirmed, these results could lead to a better understanding of personality traits and inter-individual susceptibility developing psychiatric disorders such as major depression.
Keywords: Negative emotionality, Inter-individual differences, Monoamine oxidase B, Human, Polymorphisms, Depression
Introduction
Major Depression (American Psychiatric Association 1994) has a lifetime prevalence of at least 10% and is one of the most common psychiatric disorders (Weissman et al. 1993, 1996).
It has also been established that depression is related to personality traits (Frank et al. 1987; Hirschfeld et al. 1989; Holahan and Moos, 1991; Katon et al. 1994). Specifically, the personality trait dimensions of negative and positive affect have been found to be closely correlated with vulnerability for the development of depression and depressive symptom severity. Negative affect is a stable and highly general trait dimension with aspects ranging from mood to behavior (Watson and Clark 1984). Individuals high on this trait have a higher temperamental sensitivity to negative stimuli (Tellegen 1985) causing negative moods such as anxiety and sadness but also guilt, hostility and self-dissatisfaction (Watson and Clark 1984). In contrast, the stable and highly temperamental dimension positive affect includes personality traits such as positive emotions, energy, and dominance. People who are low in positive affect are less likely to feel joyful, interested, enthusiastic, or energetic (Clark et al. 1994). A growing body of evidence indicates, that personality traits such as anxiety-related traits and harm avoidance (Lesch et al. 1996; Roberts et al. 2004) as well as positive and negative affect are related to genetically controlled patterns of neuro-transmitter metabolism, which are known to be associated, in turn, with psychiatric disorders such as Major Depression (Reif and Lesch 2003).
Genes encoding monoamine oxidase (MAO) are potentially key candidates in studying mechanisms of negative and positive emotionality as well as Major Depression. MAOs are flavin-containing mitochondrial enzymes catalyzing the oxidative deamination of neuro-transmitters and biogenic amides in the brain and peripheral tissues (Shih et al. 1999). Based on substrate selectivity and inhibitor selectivity, two forms of MAO have been designated: MAOA and MAOB (Johnston 1968; Knoll and Magyar 1972), which correspond to two distinct genes. Typically, MAOA catalyses the oxidation of serotonin (5-HT), whereas MAOB acts on 2-phenylethylamine and benzylamine (Shih et al. 1999; Lenders et al. 1996). Dopamine, noradrenaline, adrenaline, tryptamine and tyramine are oxidized by both forms of the enzyme in most species (Youdim and Bakhle 2006), whereas noradrenergic neurotransmitters are preferentially deaminated by MAOA enzymes. Studies have shown that MAO activity is genetically determined and does not change during lifetime under physiological conditions (Murphy et al. 1976; Pedersen et al. 1993). MAOA and B are encoded by separate genes located on theX-chromosome. Each gene comprises 15 exons with different core promoter regions but identical intron-exon organization, indicating that MAOA and B are derived from the duplication of a common ancestral gene (Wong et al. 2002; Zhu et al. 1994).
Studies in animals and humans have shown that MAO activity influences behavior and modulates vulnerability to psychiatric disorders. _MAOA_- and _MAOB_-knockout mice, and mice treated with non-selective MAO inhibitors, are more reactive to stress (Grimsby et al. 1997; Shih et al. 1999). In humans, MAO inhibitors have been used for decades in the treatment of depression (Pletscher 1991; Zisook 1985). Low platelet MAO activity has been linked to vulnerability for depression, suicidality, and substance abuse disorders (Buchsbaum et al. 1977; Fowler et al. 1996; Fowler et al. 2000; Meltzer and Arora 1986; von Knorring et al. 1991). Notably, both enzymes but in particular MAOB activity was found to be associated with characteristics of impulsiveness, and sensation seeking in a sample of male juvenile delinquents (Guerrera 1990; Ruchkin et al. 2005). Genetic variants of MAOA and MAOB have been found to have an influence on personality and behavior. For example, MAOA gene variants were associated with complicated grief in major depression (Kersting et al. 2007), and generalized anxiety in boys with autism (Roohi et al. 2009). Maltreated children with a genotype conferring high levels of MAOA expression were found to be less likely to develop antisocial problems (Caspi et al. 2002). Manuck et al. (2000, 2002) identified a regulatory VNTR in the MAOA promoter region that appears to be associated with variability in impulsivity and aggression in healthy, male subjects. MAOB gene variants may be associated with antidepressant treatment response (Tadic et al. 2007) as well as with vulnerability for attention deficit hyperactivity disorder (ADHD; Li et al. 2008; Ribasés et al. 2009). However, it is still unknown how and which MAOA and MAOB variants affect ‘normal’ variability in emotionality in healthy humans.
This study aimed to investigate the impact of MAOA and MAOB gene variants on Negative Emotionality (NEM) and Positive Emotionality (PEM) personality traits in humans. The analysis was conducted using data from a pharmacological study assessing associations between responses to acute doses of D-amphetamine and MAOA and MAOB polymorphisms, assuming that subjective drug response is a heritable genetic trait (Comings et al. 1997; Dlugos et al. 2007; Hohoff et al. 2005; Lott et al. 2005; Veenstra-Vanderweele et al. 2006). However, we did not observe an interaction between genotype and response to d-amphet-amine. The results presented here focus only on the influence of MAOA and MAOB gene variants on personality measures of Negative Emotionality (NEM) and Positive Emotionality (PEM), and, in a follow-up analysis, measures of impulsivity and momentary mood states. We performed association analyses with these measures and MAOA and MAOB single nucleotide polymorphisms (SNPs) and haplotypes in a sample of healthy Caucasian subjects. We hypothesized that polymorphisms that alter MAO activity would be associated with PEM and NEM, with increased enzyme activity resulting in higher NEM levels.
Materials and methods
Design
This analysis was conducted in the context of a study investigating the effects of d-amphetamine (0, 10, 20 mg) in healthy volunteers (Dlugos et al. 2007). However, the analyses presented here focus mainly on measures unrelated to the drug. We examine personality measures obtained before the study, and in one instance, mood state measures that were obtained before drugs were administered. We also report briefly that subjects’ acute responses to D-amphetamine were not related to the MAOA or B genotypes.
Participants
72 female and 90 male healthy participants, aged 18–35 years, were recruited. All subjects were Caucasian, which was confirmed using ancestry informative markers as described in the ‘Selection of Polymorphisms and Genotyping’ section, below. Volunteers were excluded if they consumed more than three cups of coffee per day, or smoked more than ten cigarettes per week. All subjects underwent a screening that included a structured clinical psychiatric interview, several screening questionnaires, a psychiatric symptom checklist (SCL-90; Derogatis 1983), the Michigan Alcoholism Screening Test (Selzer 1971) and a health questionnaire. Volunteers were excluded from participation if their BMI was less than 18 or greater than 26 and if they had any medical or psychiatric disorders (American Psychiatric Association, DSM IV). Subjects were also not included if had a history of drug abuse, if they were not fluent in English, if they had less than a high school education, or if they worked a night shift. Demographic characteristics of the subjects, including information about current and lifetime drug use, were obtained during screening.
Procedure
Subjects participated in three laboratory sessions, in which they received placebo or D-amphetamine (10 or 20 mg) under double blind conditions. Before the drug study began, participants gave a blood sample for genotyping and they completed personality questionnaires (see below). On each of the laboratory sessions, they completed mood ratings before and at regular intervals after ingesting a capsule containing drug or placebo. Details of the drug administration test sessions are described elsewhere (Dlugos et al. 2007). Personality traits, pre-drug mood states, and drug responses were examined in relation to genotype groups. This study was approved by the Institutional Review Board of The University of Chicago and was carried out in accordance with the Helsinki Declaration of 1975.
Dependent measures
The primary measure of personality for this analysis was the Multidimensional Personality Questionnaire (MPQ-BF; Patrick et al. 2002). Subjects additionally completed the Barratt Impulsiveness Scale, Version 11 (BIS-11; Barratt 1965).
The MPQ-BF is a tool for investigating the genetic, neurobiological, and psychological substrates of personality (Patrick et al. 2002). It is a self-report questionnaire, on which respondents answer true or false on 155 self-descriptive items (e.g., I am often nervous for no reason). The MPQ gives a comprehensive analysis of personality at both the broader structural levels and the lower order traits. The lower order traits (Well-being, Social Potency, Achievement, Social Closeness, Stress Reaction, Alienation, Aggression, Control, Harm avoidance, Traditionalism, and Absorption) coalesce around the higher order factors (Positive Emotionality, Negative Emotionality, and Constraint), which embody affect and temperament constructs (Patrick et al. 2002). We chose PEM and NEM as primary outcome measures for association analyses between personality traits and MAO polymorphisms based on previous findings that MAOA and MAOB enzymes are associated with psychiatric disorders such as depression, depressed suicide and alcoholism as well as stress response and aggression (Alia-Klein et al. 2008; Biederman et al. 2008; Brummet et al. 2008; Du et al. 2002; Gokturk et al. 2008; Lin et al. 2000; Rivera et al. 2009; Tadic et al. 2007). Previous behavioral-genetic studies have shown that the personality traits measured by the MPQ are heritable and stable, reflecting consistent behavior in the general population (Tellegen et al. 1988). This retrospective analysis was conducted with data from 150 subjects with complete MPQ data. MPQ Response Inconsistency Indices (Patrick et al. 2002) were analyzed in our sample. Response patterns of the individuals showed consistency with respect to item pair content, were consistent and not polarized toward responding either true or false irrespective of item content.
After finding potentially interesting associations between NEM and MAOB we conducted additional post hoc analyses to examine this genotype in relation to two other measures: daily pre-session mood states of Anxiety and Depression from the POMS, and Attentional Impulsiveness from the BIS. We hypothesized that both negative mood states and higher impulsivity would be related to MAOB (Buchsbaum et al. 1977; Cataldo et al. 2005; Meltzer and Arora 1986; Ruchkin et al. 2005; Takahashi et al. 2008).
Anxiety and Depression are two primary scales of the Profile of Mood States (POMS; Johanson and Uhlenhuth 1980), and consists of 8–12 adjectives describing momentary mood states. The subjects’ pre-drug scores of Anxiety and Depression from the three test sessions were averaged for association analyses. 159 individuals completed POMS questionnaires for all 3 sessions. The BIS-11 is a 30-item self-report questionnaire assessing the personality trait of impulsivity on a 4-point Likert scale (Barratt 1994). It consists of six-first-order factors which are used to yield three-second order factors (Attentional, Motor, and Non-planning Impulsiveness) (Patton et al. 1995). The BIS-11 has shown adequate reliability and construct validity in different populations (Patton et al. 1995; Fossati et al. 2001). 145 participants completed the BIS.
Selection of polymorphisms and genotyping
Eight MAOA and 12 MAOB (SNPs) were genotyped using the Addictions Array (Hodgkinson et al. 2008). The Addictions Array was designed to develop a panel of markers able to extract full haplotype information for candidate genes in alcoholism, other addictions, and disorders of mood and anxiety (Hodgkinson et al. 2008). Although the Addictions array evaluates 130 genes, we only performed association analyses between MAOA and MAOB genes and personality traits with a clear hypothesis.
Genotyping was performed blind to the phenotypic data and via an Illumina GoldenGate, 96-well format, Sentrix array as described (Hodgkinson et al. 2008). The array evaluates 130 genes via SNP tagging, and also includes ancestry informative markers as will be described. For this study, only MAOA and MAOB were selected for primary analysis. Arrays were imaged using an Illumina Beadstation GX500 and the data analyzed using GenCall v6.2.0.4 and GTS Reports software v5.1.2.0 (Illumina). Criteria for sample exclusion and classification as genotyping failure were previously described (Hodgkinson et al. 2008). The genotyping error rate was <1% for genotyping results of the addictions array based on concordance between duplicate samples. Participants were assigned to one of three genotype groups: homozygous for the first or second allele and heterozygous. Of 162 original study participants, genotype was undetermined for a single subject at MAOA single nucleotide polymorphism (SNP) rs1465108 and for five subjects at MAOA rs2072744. Because there were only eight subjects with the genotype T/T and seven subjects with the genotype A/T at MAOA rs3027405, the T/T and A/T groups were combined for this locus.
A panel of 186 ancestry informative markers (AIMs) was selected for this array (Hodgkinson et al. 2008). To confirm participants’ self-reported Caucasian ethnicity and rule out ethnic stratification, genotypes of these AIMs were analyzed with Structure 2.1 (Pritchard et al. 2000). Analysis was performed in relation to a worldwide diversity panel consisting of 51 geographically defined reference populations, and totaling 1,051 individuals. Ethnic proportions for each of seven worldwide factors corresponding to the geographic regions of Africa, Europe, Middle East, Central Asia, Far East Asia, America, and Oceania were estimated for each individual.
Statistical analyses
Association analyses between (1) SNP genotype groups and personality measures, (2) between SNP genotype groups and demographic traits as well as (3) between demographic traits and the outcome measures, were performed using PLINK (Purcell et al. 2007; http://pngu.mgh.harvard.edu/purcell/plink/). PLINK can calculate empirical significance levels. This is desirable because it is robust to non-normally distributed data, violations of Hardy–Weinberg equilibrium, small sample sizes and because it provides a means to account for multiple testing. PLINK can also account for X-linked genes (such as MAO A and B); for these genes, men are hemizygous whereas women can be homozygous or heterozygous and can include covariates such as caffeine use. We used the max (T) permutation approach (1,000 permutations) for each SNP. This allowed us to calculate two sets of empirical significance values: point wise estimates of the significance of individual SNPs and also an experiment-wide threshold for significance that accounts for the fact that many SNPs were tested. This is achieved by comparing each observed test statistic against the maximum of all permuted statistics for each of the four replicates. This approach adjusts for multiple testing while preserving the correlational structure between SNPs. In contrast, a Bonferroni correction is over conservative because it implicitly assumes that all tests (SNPs) are independent, whereas we know them to be in LD and thus inter-correlated with one another.
Hardy–Weinberg equilibrium for each marker and linkage disequilibrium between the markers were analyzed using the Haploview software version 4.0 (http://www.broad.mit.edu/mpg/haploview/). Haploview was also used to generate a linkage disequilibrium map of MAOA and MAOB with the available HapMap data (The International HapMap Genome Browser B36). Linkage disequilibrium parameters between SNPs in our sample did not significantly differ from those given in the HapMap project. Haplotype blocks were assessed according to the CEU-Hapmap data. Haplotype pairs were estimated and correlation analyses between haplotypes and personality markers were performed using the max (T) permutation approach implemented in PLINK performing 1,000 permutations for each haplotype to control for multiple testing. A permutated p value of <0.05 was set as a threshold for associations between the outcome measures and MAO polymorphisms and haplotypes.
Results
All subjects were of Caucasian origin. They were aged 18–25 years (mean 22.8, SD ± 0.3), and reported 12–20 years of formal education (mean 15.13, SD ± 1.43). They reported a mean weekly consumption of 4.5 (SD ± 0.3) alcohol-containing drinks, 7.3 (SD ± 0.5) caffeine-containing beverages, and 0.8 (SD ± 0.1) cigarettes. About half the subjects (54.3%) had never consumed any marijuana, and the remainder reported using marijuana about once a month (mean occasion per month 0.88; SD ± 2.26). Less than half (48.1%) reported having never used recreational stimulants, 6.8% reported having ever used sedatives, and 22.2% reported having used opiates recreationally. 29.0% had used hallucinogens, and 9.9% had used inhalants in their lifetime.
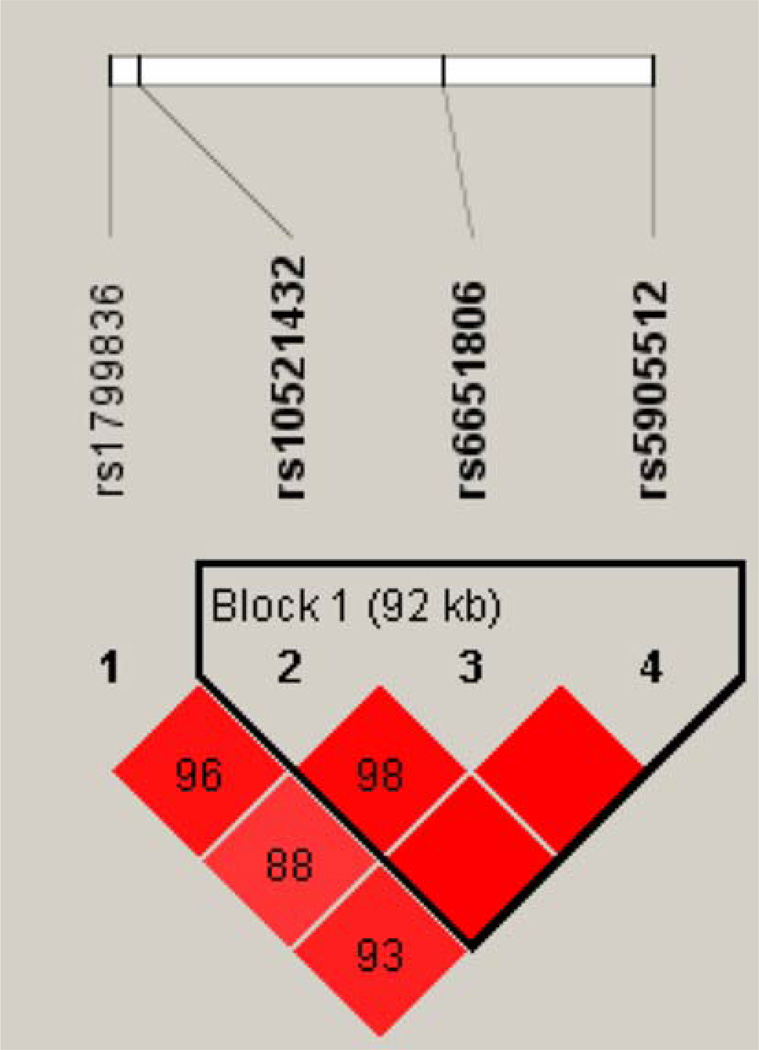
Of 72 female and 90 male study participants, 150 subjects completed the MPQ, 159 subjects the POMS and 145 participants the BIS. Genotype and allele frequencies for the investigated MAOA and B SNPs are shown in Table 1. Eight of the investigated MAOB SNPs (rs6520901, rs7879356, rs3027459, rs12394221, rs7883073, rs2239441, rs9887047 and rs12391346) were not polymorphic in our sample and these were not further considered here. As both MAOA and MAOB are located on the X-chromosome, Hardy–Weinberg-equilibrium was calculated separately for men and women. Genetic variants were in Hardy–Weinberg-Equilibrium in both sexes (p > 0.05) for all polymorphisms. Allele frequency proportions did not significantly differ from those given in the Hapmap project (The International HapMap Genome Browser B36) except for MAOA SNP rs3027405 (Minor allele frequency in study sample: 0.071 and in the HapMap project: 0.28). Polymorphisms within each gene were in high intermarker linkage disequilibrium forming two haplotype blocks in MAOA and one haplotype block in MAOB. Haplotype blocks and D′ values between the genetic variants for MAOB are shown in Fig. 1. The observations were consistent with the HapMap data (The International HapMap Genome Browser B36).
Table 1.
Allele and Genotype frequencies of the MAOA and MAOB gene polymorphisms located on the x-Chromosome
| Chr.position | Geneposition | Allele | Genotype | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1/1 | 1/2 | 2/2 | |||
| MAOA | |||||||
| rs1465108A/G | 43423153 | 22800 | 91 | 231 | 31 | 29 | 101 |
| rs5906957A/G | 43432254 | 31901 | 69 | 255 | 20 | 29 | 113 |
| rs909525 A/G | 43438146 | 37793 | 215 | 109 | 91 | 33 | 38 |
| rs2235185C/T | 43480687 | 80334 | 90 | 234 | 31 | 28 | 103 |
| rs3027405A/T | 43481273 | 80920 | 301 | 23 | 147 | 7 | 8 |
| rs2072744A/G | 43484380 | 84027 | 112 | 202 | 41 | 30 | 86 |
| rs979605C/T | 43486307 | 85954 | 134 | 90 | 103 | 28 | 31 |
| rs2239448C/T | 43487623 | 87270 | 233 | 91 | 102 | 29 | 31 |
| MAOB | |||||||
| rs1799836A/G | 43512943 | 2141 | 157 | 167 | 59 | 39 | 64 |
| rs1052143A/G | 43518684 | 7882 | 113 | 211 | 41 | 31 | 90 |
| rs6651806A/C | 43573908 | 63106 | 208 | 116 | 89 | 30 | 43 |
| rs3027459A/G | 43611338 | 100536 | 143 | 181 | 52 | 39 | 71 |
Fig. 1.
Linkage disequilibrium analyses: D′ values of single nucleotide polymorphisms along the MAOB gene, illustrating one haplotype block. D′ values were calculated by Haploview version 4.0
Genotype groups for each SNP were similar on most demographic characteristics. However, subjects with allele A at MAOA rs5906957 reported higher cigarette use per week (mean (+SD): A/A: 1.87 (±2.8), A/G: 0.67 (±1.8), G/G: 0.58 (±1.5), p < 0.01) than subjects with allele G. In a separate analysis, we found no relationship between this demographic trait and the outcome measures.
MAOA and MAOB polymorphisms and personality traits
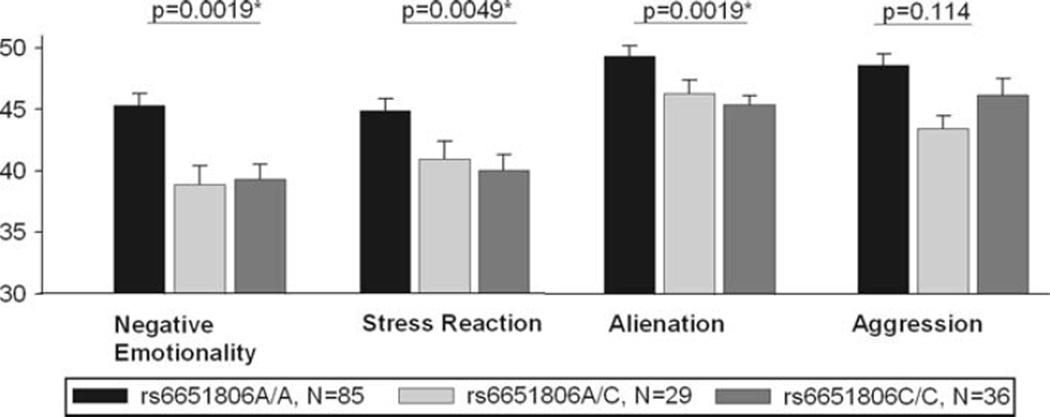
The highly linked MAOB polymorphisms rs10521432 and rs6651806 were significantly associated with the primary outcome measure NEM. Results are shown in Table 2. Subjects with allele G at rs10521432 and allele A at rs6651806 scored significantly higher on NEM compared to subjects with allele G at rs10521432 or allele C at6651806. This observation remained highly significant (p < 0.002) after adjustment for multiple testing using the permutation approach implemented in PLINK (1,000 permutations for each polymorphism). Figure 2 shows Means of NEM between the genotype groups at polymorphism rs6651806.
Table 2.
Associations of MPQ scales with MAOB polymorphisms
| Outcome measures | Betaa | R2b | Tc | Emp. _p_valued | Corrected emp.p valuee |
|---|---|---|---|---|---|
| Primary outc. measures | |||||
| Neg emotionality (NE)—MPQ | |||||
| rs1799836 | 1.748 | 0.028 | 2.087 | 0.0329 | 0.0769 |
| rs10521432 G | −3.085 | 0.082 | −3.638 | 0.0009 | 0.0019** |
| rs6651806 A | −3.288 | 0.095 | −3.959 | 0.0009 | 0.0019** |
| rs5905512 | 1.785 | 0.029 | 2.117 | 0.0439 | 0.0799 |
| Pos emotionality (PE)—MPQ | |||||
| rs1799836 | −0.997 | 0.009 | −1.164 | 0.2360 | 0.4595 |
| rs10521432 | 0.608 | 0.003 | 0.6789 | 0.5110 | 0.8172 |
| rs6651806 | 0.439 | 0.001 | 0.4971 | 0.6190 | 0.8172 |
| rs5905512 | −1.147 | 0.011 | −1.329 | 0.1910 | 0.3487 |
| Post hoc measures | |||||
| Stress reaction—MPQ | |||||
| rs10521432 | −2.560 | 0.059 | −3.065 | 0.0019 | 0.0049** |
| rs6651806 | −2.533 | 0.060 | −3.074 | 0.0019 | 0.0049** |
| Alienation—MPQ | |||||
| rs10521432 | −2.144 | 0.070 | −3.363 | 0.0009 | 0.0009*** |
| rs6651806 | −2.072 | 0.068 | −3.289 | 0.0009 | 0.0019** |
| Aggression—MPQ | |||||
| rs10521432 | −1.44 | 0.024 | −1.912 | 0.0719 | 0.1409 |
| rs6651806 | −1.528 | 0.027 | −2.061 | 0.0499 | 0.1139 |
| Attentional impulsiveness—BIS | |||||
| rs10521432 | −0.670 | 0.031 | −2.143 | 0.0329 | 0.0869 |
| rs6651806 | −0.7829 | 0.043 | −2.551 | 0.0109 | 0.0359* |
Fig. 2.
Means (±SEM) between genotype groups at rs6651806 for Negative Emotionality, Aggression, Alienation, and Stress Reaction (MPQ). 150 subjects completed the MPQ data
Genotype groups of MAOA and MAOB polymorphisms did not significantly differ on the PEM scale. However, participants with allele G at rs10521432 and allele A at rs6651806 scored lower on PEM compared to subjects with the other respective alleles. No significant associations were found between any of the MAOA polymorphisms and the investigated primary outcome measures.
Post hoc analyses
We conducted several follow-up analyses to investigate the finding that the MPQ higher-order personality dimension NEM was associated with two highly linked MOAB gene polymorphisms. First, we conducted analyses with the three primary MPQ scales that most strongly influence the NEM dimension: Stress Reaction, Alienation, and Aggression (Church and Burke; 1994). Second, we examined subjects’ daily mood ratings of Anxiety and Depression (POMS) from their three sessions, as well as their scores on Attentional Impulsiveness (BIS).
Results of these post hoc association analyses are shown in Tables 2 and 3. Figure 2 shows Mean (±SEM) between genotype groups at rs6651806 for Aggression, Alienation and Stress Reaction (MPQ). Again, participants with allele A of rs1799836 and allele G of rs6651806 scored significantly higher on Stress Reaction and Alienation (MPQ) as well as on Attentional Impulsiveness (BIS) and Anxiety (POMS). These associations remained significant after adjustment for multiple testing using the permutation approach (1,000 permutation performed for each SNP) implemented in PLINK: The investigated outcome measures Aggression (MPQ) and Depression (POMS) showed the same trend but were not significantly correlated with the MAOB polymorphisms.
Table 3.
Associations of POMS scales with MAOB polymorphisms
| POMS outcomemeasures | Betaa | _R_2b | Tc | Emp. p valued | Corrected emp.p valuee |
|---|---|---|---|---|---|
| Post hoc measures | |||||
| Anxiety | |||||
| rs10521432 | −0.055 | 0.045 | −2.747 | 0.0089 | 0.0179* |
| rs6651806 | −0.053 | 0.042 | −2.658 | 0.0119 | 0.0239* |
| Depression | |||||
| rs10521432 | −0.031 | 0.022 | −1.892 | 0.0549 | 0.1299 |
| rs6651806 | −0.030 | 0.020 | −1.816 | 0.0639 | 0.1618 |
Haplotypes and personality traits
D’ -values between the four polymorphisms in our sample are shown in Fig. 1 (Haploview software version: 4.0). Linkage disequilibrium criteria were not significantly different from those given in the HapMap Project. According to the CEU-HapMap data four-SNP haplotype pairs from rs1799836, rs10521432, rs6651806 and rs590551 were estimated for each individual using PLINK allowing for uncertainty of haplotype phases. Six reconstructed haplotypes AGAA [Frequency (F): 0.426], GGAA (F: 0.014), GACG (F: 0.339), AGCG (F: 0.013), GAG (F: 0.037) and GGAG (F: 0.160) were assessed for primary association analyses and post hoc analyses with the outcome measures. Results of association analyses between both, MPQ, BIS and POMS outcome measures and MAOB haplotypes are shown in Table 4.
Table 4.
Association of MPQ and POMS scales and MAOB haplotypes from rs1799836, rs10521432, rs6651806, rs5905512
| Haplotypes | Betaa | R2b | STATc | Emp.p valued | Corrected emp.p valuee |
|---|---|---|---|---|---|
| MPQ | |||||
| Primary outc. measures | |||||
| Neg emotionality (NE)—MPQ | |||||
| AGAA (F: 0.426) | 0.468 | 0.001 | 0.3984 | 0.1179 | 0.5095 |
| GGAA (F: 0.014) | 7.898 | 0.015 | 1.504 | 0.0509 | 0.2178 |
| GACG (F: 0.339) | −4.537 | 0.100 | −4.068 | 0.0019 | 0.0019** |
| AGCG (F: 0.013) | −1.626 | 0.000 | −0.307 | 0.2647 | 0.7902 |
| AGAG (F: 0.037) | 3.995 | 0.009 | 1.218 | 0.2647 | 0.7832 |
| GGAG (F: 0.160) | 2.089 | 0.010 | 1.243 | 0.1279 | 0.5754 |
| Pos emotionality (PE)—MPQ | |||||
| AGAA (F: 0.426)e | −1.096 | 0.005 | −0.9206 | 0.1479 | 0.5544 |
| GGAA (F: 0.014) | −2.524 | 0.001 | −0.471 | 0.959 | 1 |
| GACG (F: 0.339) | 1.002 | 0.004 | 0.843 | 0.5734 | 0.983 |
| AGCG (F: 0.013) | 0.877 | 0.000 | 0.163 | 0.8981 | 1 |
| AGAG (F: 0.037) | 4.077 | 0.010 | 1.227 | 0.4136 | 0.9281 |
| GGAG (F: 0.160) | 1.564 | 0.005 | 0.916 | 0.4895 | 0.976 |
| Post hoc measures | |||||
| Stress reaction—MPQ | |||||
| GACG | −3.104 | 0.049 | −2.782 | 0.0009 | 0.0019** |
| Alienation—MPQ | |||||
| GACG | −2.575 | 0.058 | −3.019 | 0.0029 | 0.0189* |
| Aggression—MPQ | |||||
| GACG | −3.027 | 0.060 | −3.084 | 0.0379 | 0.1728 |
| Attentional imp.—BIS | |||||
| GACG | −0.729 | 0.036 | −2.325 | 0.0189 | 0.1119 |
| POMS | |||||
| Post hoc measures | |||||
| Anxiety | |||||
| GACG (F: 0.346) | −0.076 | 0.046 | −2.777 | 0.0029 | 0.0389* |
| Depression | |||||
| GACG (F: 0.346) | −0.032 | 0.023 | −1.959 | 0.0559 | 0.2218 |
Haplotype CACG from rs1799836, rs10521432, rs6651806 and rs590551 was significantly associated with levels of NEM. Post hoc analyses revealed that haplotype CACG was significantly correlated with scores of Stress Reaction and Alienation and Anxiety (POMS) after adjustment for multiple testing. No significant correlations were found between haplotype CACG and PEM or Aggression, Attentional Impulsiveness (BIS) and POMS Depression. Further, neither PEM nor NEM was associated with any of five other haplotypes.
MAOA and MAOB and amphetamine response
Associations between subjective response (POMS and other measures) to amphetamine (10 and 20 mg) and MAOA and MAOB polymorphisms were analyzed in separate analyses (data not shown). Amphetamine response was not significantly modulated by any of the MAOA or MAOB polymorphisms. Further details on the amphetamine study describing the subjective measures, investigating different genes are described in Dlugos et al. (2007), Lott et al. (2006) and Veenstra-VanderWeele et al. (2006).
Discussion
The main finding of the study is that the intronic MAOB polymorphisms rs10521432 and rs6651806 were associated with personality trait of negative emotionality (NEM) in healthy humans. These findings remained significant after adjusting for multiple testing using the permutation approach implemented in PLINK (p < 0.01). Post hoc analyses revealed that subjects with allele G at rs10521432 and allele A at rs6651806 scored significantly higher not only on NEM but also on MPQ primary scales of Stress Reaction and Alienation, Attentional Impulsiveness (BIS) and mood ratings of Anxiety (POMS), but not Depression (POMS) or Aggression (MPQ). The findings were extended in haplotype analyses. The four-SNP haplotype CACG formed with rs1799836, rs10521432, rs6651806 and rs590551 was associated with lower scores on the SNP associated MPQ scales and the POMS scale Anxiety.
MAOA genotype groups at rs5906957 differed in cigarette use. However, it is unlikely that cigarette use influenced any of our findings as the investigated outcome measures were not related to smoking. Moreover, subjects included in this study were light smokers who consumed fewer than 10 cigarettes per week.
There are several reasons to believe that our findings reflect a real association and were not due to chance. MAOB activity is known to be genetically determined (Pedersen et al. 1993; Murphy et al. 1976). Most of the frequent MAOB polymorphisms are in relatively high intermarker disequilibrium, even between haplotype blocks. Thus, it is likely that the associated polymorphisms are in high LD with a functional variant, even if they do not influence MAOB activity themselves. It is also possible that the associated polymorphisms will influence transcription rates as well as mRNA processing, stability or splicing. The associated polymorphisms are located in transcription-factor binding sites, according to the TRANSFAC program (Matinspector; Genomatix Software, http://www.genomatix.de/index.html). Compared to subjects with the A allele at locus rs6651806, subjects with the C allele have the three additional transcription factor– binding sites Ubiquitous GLI-Krueppel like zinc finger involved in cell cycle regulation (V$E4FF), Heat shock factors (V$HEAT) and the Signal transducer and activator of transcription (V$STAT) and they have lost the Fork head domain factors (F$KHD), Hepatic Nuclear Factor 1 (V$HNF1) and OVO homolog-like transcription factors (V$OVOL). Subjects with the A allele at locus rs10521432 have two additional transcription factor binding sites [Onecut homeodomain factor HNF6 (V$HNF6) and RXR heterodimer binding sites (V$RXRF)]. The differences may alter transcription rates of the MAOB gene. This could consequently lead to higher MAOB protein levels, which would result in lower monoamine concentrations in the CNS. These various in silico observations, require confirmation by in vitro MAOB gene-expression studies. In previous studies, the MAOB variant rs1799836 included in this study was found to be associated with the risk of developing Parkinson Disease and ADHD (Gao et al. 2008; Li et al. 2008). Although we did not find rs1799836 to be significantly associated with PEM or NEM, the patterns of response were in the expected direction (Table 2). Furthermore, rs1799836 was in high LD with the associated loci rs10521432 and rs6651806, which did show highly significant associations with these traits.
The present findings are also consistent with previous studies showing that MAOB activity is associated with depression, suicidality, impulsiveness, and stress reaction (Tadic et al. 2007; Roggenbach et al. 2007; Grimsby et al. 1997). Notably, in a study of MAOB activity in juvenile delinquents, Ruchkin et al. (2005) found that MAOB activity was not directly related to psychopathology but rather to specific personality traits such as sensation seeking or impulsiveness, which may predispose individuals to psychopathologic behavior. In the healthy volunteers tested in our study, we found that the associations between NEM and MAOB polymorphisms were mostly related to Alienation and Stress Response scales, as well as Attentional Impulsiveness (BIS) and mood ratings of Anxiety (POMS). Thus, our findings are consistent with the observation of previous studies. Investigations of the identified MAOB variants involving additional outcome measures (e.g. suicidality) in different samples such as patients with Borderline personality disorder would be interesting.
None of the eight investigated MAOA variants was associated with NEM or PEM in our sample, although the MAOA enzyme is more closely related to depressive symptoms than MAOB. Polymorphisms could be correlated with other, not investigated outcome measures such as Aggression. In addition, it is possible that MAOA variants other those investigated here are important. For example, there is a well-characterized functional variable number tandem repeat (VNTR) polymorphism in the promotor region of the MAOA gene in which the longer allele affecting the transcription of the gene 3–4 times more efficiently than the shorter allele (Deckert et al. 1999). Caspi et al. (2002) investigated this VNTR and found the low-activity MAOA genotype to be associated with the development of antisocial problems in maltreated children.
The investigated polymorphisms were also not associated with acute response to amphetamine, suggesting that the investigated polymorphisms might not modulate acute response to the 10 and 20 mg doses of the drug. Other MAOA and MAOB variants than the investigated polymorphisms could influence drug response that requires confirmation in future studies. It is also possible that MAOA and B polymorphisms have an influence on chronic and not acute drug response because their proteins are involved in transmitter catabolism and not receptor function, as for example, the norepinephrine transporter or the dopamine transporter whose genetic variants are known to alter acute amphetamine effects (Dlugos et al. 2007; Lott et al. 2005).
Our study has several limitations. One limitation is the relatively small number of subjects that did not allow us to assess gene–gene or gene–environment interactions. It is axiomatic that large sample sizes are needed for genotypic studies of personality. Thus, we plan to confirm findings in a replication sample with at least the double number of subjects. Nevertheless, our sample is very homogenous because we used stringent inclusion and exclusion criteria (see ‘‘Materials and Methods’’). Another limitation is that the outcome measures are based on self-report questionnaires and are vulnerable to reporting biases associated with such ratings. Replication studies involving different subjective outcome measures such as the Tridimensional Personality Questionnaire (TPQ; Cloninger 1987) and the revised version of the Neuroticism Extraversion Personality Inventory (NEO-PI-R; Costa and McCrae 1992) to assess in particular associations with Neuroticism (TPQ) and Harm Avoidance (TPQ) that are closely related to NEM would be of interest. In addition, objective outcome measures, such as functional imaging studies would nicely complement these observed associations between MAOB polymorphisms, MAOB activity, and personality traits. Studies involving objective outcome measures have not been carried out yet related to MAOB variants. The measures that we used are standardized, and have been widely used to study the neurobiological basis of personality. The significant findings reported here are consistent with, and extend, previous reports on the role of MAOB.
In summary, our study emphasizes the importance of MAOB activity and provides the first evidence that MAOB variants are associated with personality traits in healthy volunteers. If they are replicated in future studies, the findings improve understanding of biological underlying mechanisms of personality characteristics and inter individual risks to develop a psychiatric disorder.
Acknowledgments
We gratefully thank Dr. Judith Badner, Dr. Andrew Skol, Shen Pei-Hong, Dr. David Goldman, and Dr. Colin Hodgkinson for their invaluable input and technical support. We also thank Ms. Margo Meverden and Ms. Patricia Kriegel for their skillful technical assistance. This work was supported by DA021336, DA02812 and MO RR00055.
Footnotes
Conflict of interest statement The authors declare that they have no conflict of interest.
Contributor Information
Andrea M. Dlugos, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago, 5841 S. Maryland Ave, Chicago, IL MC3077, USA, andrea.dlugos@ukmuenster.de
Abraham A. Palmer, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago, 5841 S. Maryland Ave, Chicago, IL MC3077, USA Department of Human Genetics, The University of Chicago, Chicago, IL, USA.
Harriet de Wit, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago, 5841 S. Maryland Ave, Chicago, IL MC3077, USA, hdew@uchicago.edu.
References
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: 1994. [Google Scholar]
- Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychol Rep. 1965;16:547–554. doi: 10.2466/pr0.1965.16.2.547. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness and aggression. In: Monahan J, Steadman HJ, editors. Violence and mental disorder: developments in risk assessment. University of Chicago: 1994. pp. 61–79. [Google Scholar]
- Biederman J, Kim JW, Doyle AE, Mick E, Fagerness J, Smoller JW, Faraone SV. Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1511–1518. doi: 10.1002/ajmg.b.30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummet BH, Boyle SH, Siegler IC, Kuhn CM, Surwitt RS, Garrett ME, Collins A, Ashley-Koch A, Williams RB. HPA axis function in male caregivers: effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR) Biol Psychol. 2008;79:250–255. doi: 10.1016/j.biopsycho.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, Murphy DL. Suicide attempts, platelet monoamine oxidase and average evoked response. Acta Psychiatr Scand. 1977;56:57–68. doi: 10.1111/j.1600-0447.1977.tb06665.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M. Impulsivity in depressed children and adolescents: a comparison between behavioral and neuropsychological data. Psychiatry Res. 2005;136:123–133. doi: 10.1016/j.psychres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Church AT, Burke PJ. Exploratory and confirmatory tests of the big five and Tellegen’s three- and four-dimensional models. J Pers Soc Psychol. 1994;66:93–114. doi: 10.1037//0022-3514.66.1.93. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray G. Cannabinoid receptor gene (CNR1) association with i.v. drug use. Mol Psychiatry. 1997;2:161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) an NEO Five Factor Inventory (NEOFFI) professional manual. Odessa: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, No¨then MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Derogatis L. SCL-90-R Manual II. Clinical Psychometric Research; Towson: 1983. [Google Scholar]
- Dlugos A, Freitag C, Hohoff C, McDonald J, Cook EH, Deckert J, de Wit H. Norepinephrine transporter gene variation modulates acute response to D-amphetamine. Biol Psychiatry. 2007;61:1296–1305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Pakovits M, Sotonyi P, Bakish D, Hrdina PD. High activity-related allele of MAO-A gene associated with depressed suicide in males. NeuroReport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- Fossati A, DiCeglie A, Acquarini E, Barratt ES. Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol. 2001;57:815–828. doi: 10.1002/jclp.1051. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Franceschi D, Logan J, Pappas N, Shea C, MacGregor RR, Garza V. Maintenance of brain monoamine oxidase B inhibition in smokers after overnight cigarette abstinence. Am J Psychiatry. 2000;157:1864–1866. doi: 10.1176/appi.ajp.157.11.1864. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Jacob M, Jarrett D. Personality features and response to acute treatment in recurrent depression. J Pers Disord. 1987;1:14–26. [Google Scholar]
- Gao X, Scott WK, Wang G, Mayhew G, Li YJ, Vance JM, Martin ER. Gene-gene interaction between FGF20 and MAOB in Parkinson disease. Ann Hum Genet. 2008;72:157–162. doi: 10.1111/j.1469-1809.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- Gokturk C, Schultze S, Nilsson KW, von Knorring L, Oreland L, Hallman J. Serotonin transporter (5-HTTLPR) and monoamine oxidase (MAOA) promoter polymorphisms in women with severe alcoholism. Arch Womens Ment Health. 2008;11:347–355. doi: 10.1007/s00737-008-0033-6. [DOI] [PubMed] [Google Scholar]
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- Guerrera RJ. Some biological and behavioural features with clinical personality types. J Nerv Ment Dis. 1990;178:556–566. doi: 10.1097/00005053-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, Klerman GL, Lavori P, Keller M, Griffith P, Coryell W. Premorbid personality assessments of first onset of major depression. Arch Gen Psychiatry. 1989;46:345–350. doi: 10.1001/archpsyc.1989.01810040051008. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohoff C, McDonald JM, Baune BT, Cook EH, Deckert J, de Wit H. Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:42–444. doi: 10.1002/ajmg.b.30228. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH. Life stressors, personal and social resources, and depression. A 4-year structural model. J Abnorm Psychol. 1991;100:31–38. doi: 10.1037//0021-843x.100.1.31. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Katon W, Lin E, Von Korff M, Bush T, Walker E, Simon G, Robinson P. The predictors of persistence of depression in primary care. J Affect Disord. 1994;31:81–90. doi: 10.1016/0165-0327(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Kersting A, Kroker K, Horstmann J, Baune BT, Hohoff C, Mortensen LS, Neumann LC, Arolt V, Domschke K. Association of MAO-A variant with complicated grief in major depression. Neuropsychobiol. 2007;56:191–196. doi: 10.1159/000120624. [DOI] [PubMed] [Google Scholar]
- Knoll J, Magyar K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol. 1972;5:393–408. [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, Kopin IJ, Karoum F, van Gennip AH, Brunner HG. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Invest. 1996;97:1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KR, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Mueller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotoin transporter-gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Hu S, Zhou R, Yu X, Wang B, Guan L, Yang L, Zhang F, Faraone SV. The monoamine oxidase B gene exhibits significant association to ADHD. Am J Med Genet B Neuro-psychiatr Genet. 2008;147:370–374. doi: 10.1002/ajmg.b.30606. [DOI] [PubMed] [Google Scholar]
- Lin S, Jiang S, Wu X, Qian Y, Wang D, Tang G, Gu N. Association analysis between mood disorder and monoamine oxidase gene. Am J Med Genet. 2000;96:12–14. doi: 10.1002/(sici)1096-8628(20000207)96:1<12::aid-ajmg4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim S, Cook EH, Jr, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, de Wit H. Serotonin transporter genotype and acute subjective response to amphetamine. Am J Addict. 2006;15:327–335. doi: 10.1080/10550490600859868. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Muldoon MF, Ferrell RE. Central nervous system serotonergic responsivity and aggressive disposition in men. Physiol Behav. 2002;77:705–709. doi: 10.1016/s0031-9384(02)00922-8. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Arora RC. Platelet markers of suicidality. Ann N Y Acad Sci. 1986;487:271–280. doi: 10.1111/j.1749-6632.1986.tb27906.x. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Wright C, Buchsbaum MS, Nichols A, Costa JL, Wyatt RJ. Platelet and plasma amine oxidase activity in 680 normals:sex and age differences and stability over time. Biochem Med. 1976;16:254–265. [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–1563. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Oreland L, Reynolds C, McClearn GE. Importance of genetic effects for monoamine oxidase activity in thrombocytes in twins reared apart and twins reared together. Psychiatry Res. 1993;46:239–251. doi: 10.1016/0165-1781(93)90092-u. [DOI] [PubMed] [Google Scholar]
- Pletscher A. The discovery of antidepressants: a winding path. Experientia. 1991;47:4–8. doi: 10.1007/BF02041242. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81 doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Lesch KP. Toward a molecular architecture of personality. Behav Brain Res. 2003;139:1–20. doi: 10.1016/s0166-4328(02)00267-x. [DOI] [PubMed] [Google Scholar]
- Ribasés M, Ramos-Quiroga JA, Hervás A, Bosch R, Bielsa A, Gastaminza X, Artigas J, Rodriguez-Ben S, Estivill X, Casas M, Cormand B, Bayés M. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/ hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry. 2009;14:71–85. doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- Rivera M, Gutiérrez B, Molina E, Torres-González F, Bellón JA, Moreno-Küstner B, King M, Nazareth I, Martínez-González LJ, Martínez-Espín E, Muñoz-García MM, Motrico E, Martínez-Cañavate T, Lorente JA, Luna JD, Cervilla JA. High-activity variants of the uMAOA polymorphism increase the risk for depression in a large primary care sample. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:395–402. doi: 10.1002/ajmg.b.30829. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Luty SE, Mulder RT, Joyce PR, Kennedy MA. Association between cytochrome P450 2D6 genotype and harm avoidance. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:90–93. doi: 10.1002/ajmg.b.20163. [DOI] [PubMed] [Google Scholar]
- Roggenbach J, Müller-Oerlinghausen B, Franke L, Uebelhack R, Blank S, Ahrens B. Peripheral serotonergic markers in acutely suicidal patients. 1. Comparison of serotonergic platelet measures between suicidal individuals, nonsuicidal patients with major depression and healthy subjects. J Neural Transm. 2007;114:479–487. doi: 10.1007/s00702-006-0555-x. [DOI] [PubMed] [Google Scholar]
- Roohi J, Devincent CJ, Hatchwell E, Gadow KD. Association of a monoamine oxidase-A gene promoter polymorphism with ADHD and anxiety in boys with autism spectrum disorder. J Autism Dev Disord. 2009;39:67–74. doi: 10.1007/s10803-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchkin VV, Koposov RA, af Klinteberg B, Oreland L, Grigorenko EL. Platelet MAO-B, personality, and psychopathology. J Abnorm Psychol. 2005;114:477–482. doi: 10.1037/0021-843X.114.3.477. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Riddl MJ. Role of MAO A and B in neurotransmitter metabolism and behavior. Pol J Pharmacol. 1999;51:25–29. [PubMed] [Google Scholar]
- Tadić A, Rujescu D, Müller MJ, Kohnen R, Stassen HH, Dahmen N, Szegedi A. A monoamine oxidase B gene variant and short-term antidepressant treatment response. Prog Neuropsy-chopharmacol Biol Psychiatry. 2007;31:1370–1377. doi: 10.1016/j.pnpbp.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects-an analysis based on Tsallis’ statistics. Neuro Endocrinol Lett. 2008;29:351–358. [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale: New Jersey; 1985. pp. 681–706. [Google Scholar]
- Tellegen A, Lykken DT, Bouchard TJ, Jr, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, Jr, de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to D-amphetamine. Neuropsy-chopharmacology. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- von Knorring AL, Hallmann J, von Knoring L, Oreland L. Platelet monoamine oxidase activity in type 1 and type 2 alcoholism. Alcohol Alcohol. 1991;26:409–416. doi: 10.1093/oxfordjournals.alcalc.a045132. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lépine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Wong WK, Ou XM, Chen K, Shih JC. Activation of human monoamine oxidase B gene expression by a protein kinase C MAPK signal transduction pathway involves c-Jun and Egr-1. J Biol Chem. 2002;277:22222–22230. doi: 10.1074/jbc.M202844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. J Pharmacol. 2006;147(Suppl 1):287–296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Chen K, Shih JC. Bidirectional promoter of human monoamine oxidase A (MAO A) controlled by transcription factor Sp1. J Neurosci. 1994;14:7393–7403. doi: 10.1523/JNEUROSCI.14-12-07393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S. A clinical overview of monoamine oxidase inhibitors. Psychosomatics. 1985;26:240–246. doi: 10.1016/S0033-3182(85)72877-0. [DOI] [PubMed] [Google Scholar]