A Brief History of Hair Cell Regeneration Research and Speculations on the Future (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 1.
Abstract
Millions of people worldwide suffer from hearing and balance disorders caused by loss of the sensory hair cells that convert sound vibrations and head movements into electrical signals that are conveyed to the brain. In mammals, the great majority of hair cells are produced during embryogenesis. Hair cells that are lost after birth are virtually irreplaceable, leading to permanent disability. Other vertebrates, such as fish and amphibians produce hair cells throughout life. However, hair cell replacement after damage to the mature inner ear was either not investigated or assumed to be impossible until studies in the late 1980s proved this to be false. Adult birds were shown to regenerate lost hair cells in the auditory sensory epithelium after noise- and ototoxic drug-induced damage. Since then, the field of hair cell regeneration has continued to investigate the capacity of the auditory and vestibular epithelia in vertebrates (fishes, birds, reptiles, and mammals) to regenerate hair cells and to recover function, the molecular mechanisms governing these regenerative capabilities, and the prospect of designing biologically-based treatments for hearing loss and balance disorders. Here, we review the major findings of the field during the past 25 years and speculate how future inner ear repair may one day be achieved.
Keywords: hair cell regeneration, historical review, proliferation, transdifferentiation, sensory epithelia, hair cells, supporting cells
Introduction
Damage and loss of hair cells in the inner ear through aging, exposure to noise, environmental chemical toxins, medications, disease and genetic disorders cause hearing and balance disorders in millions of people each year. In the past, clinicians seldom envisioned treatments of the inner ear to prevent hearing or balance disorders, or treatments to restore the cells that are damaged or lost. Instead, prevention of hearing loss is mainly limited to peripheral protection (e.g., ear plugs), and treatment is based on either increasing the stimulation of remaining hair cells (amplification) or bypassing the hair cells entirely (cochlear and brainstem implants). While enormous progress has been made during the past fifty years in those treatment modalities, we believe that during the next two-to-five decades, biologically-based methods will allow prevention of hearing and balance disorders and replacement of lost receptor elements through regeneration or transplantation. This prediction is based on two important discoveries that occurred over the past three decades. The first, with overwhelming importance to all of biology and medicine, is the discovery that most cell death is governed by the activation of a well-conserved and relatively small number of pathways that have been grouped together under the name of “apoptosis”. This important discovery forms the basis for research aimed at developing treatments that will prevent a significant percentage of hearing and balance disorders. The second discovery was mainly of interest to hearing biologists; the discovery that most vertebrates have the capacity to repair and restore function to the damaged inner ear through the regeneration of hair cells. Most of this contribution will review the history of this latter discovery, highlighting some of the most important findings of the early period of the field. In addition, we indicate where some of this germinal research is providing exciting prospects for today and for the future.
The potential for new hair cell production throughout life in some vertebrates has been recognized for over 80 years. For example, Leon Stone studied regeneration of lateral line amputation and regeneration of the entire tail in amphibian embryos in the 1930s (Stone, 1933; 1937). In the early 1980s, a number of groups showed continuous production of hair cells in the inner ear of mature rays and fishes (Corwin; 1981, 1983; Popper and Hoxter, 1984). These studies demonstrated that addition of hair cells to the periphery of vestibular epithelia in many cold-blooded vertebrates occurs throughout life, as the animals continually grow. Until the late 1980's, it was generally accepted that warm-blooded vertebrates could not form new hair cells after development, either normally or in response to damage. This assumption was not challenged for several reasons. First, it appears that earlier investigators had not attempted to examine the possibility that hair cells in the inner ear or lateral line organs of cold-blooded vertebrates would regenerate after localized damage to these organs. Furthermore, it was already known that little growth of inner ear sensory organs occurs in mammals or birds after they reach maturity, and that mammals show little recovery of hearing after hair cell damage (e.g., McGill and Schuknecht, 1976; Blakeslee et al., 1978; Li, 1992; Sliwinska-Kowalska et al., 1992). Finally it was generally assumed that post-embryonic production of sensory or neuronal cells in mammals is limited to a few unique sites, such as the olfactory neuroepithelium and taste buds (e.g., Farbman, 1990) and that specialized undifferentiated precursor cells were limited to these sites. Therefore, few if any researchers had investigated the possibility of hair cell regeneration in vertebrates. In both birds and mammals, all hair cells in the hearing organ (the organ of Corti in mammals and the basilar papilla in birds) are produced during embryogenesis or shortly thereafter (Ruben, 1967; Cotanche and Sulik 1984; Katayama and Corwin, 1989). Although some mitotic activities may continue after embryogenesis, the new cells apparently do not differentiate into hair cells (Oesterle and Rubel, 1993). However, as discussed in detail below, serendipitous findings in two laboratories led to the realization that birds at any age can make new hair cells in their basilar papillae when damage has occurred (Cotanche, 1987a; Cruz et al., 1987; Corwin and Cotanche, 1988; Ryals and Rubel, 1988). Further, the vestibular epithelia of birds and most other non-mammalian vertebrates normally renew their hair cells throughout life (Jørgensen and Mathiesen, 1988), and production of new vestibular hair cells increases after damage (Weisleder and Rubel, 1993). Hair cell replacement in auditory and vestibular epithelia following hair cell loss results in near-complete recovery of hearing and balance function (reviewed in Bermingham-McDonogh and Rubel, 2003 and discussed below). These discoveries initiated a search for methods to stimulate regeneration or replacement of lost hair cells in mammals - a search that, if fruitful, will revolutionize the treatment of sensorineural hearing loss and balance disorders during this century.
In this review, we discuss the origins of some of the major trends in this field, which has now reached its 25th year anniversary and is virtually embryonic in the time-line of science. This is largely meant to be a historical account and therefore deals primarily with the period up to 2000, attempting to provide a historical perspective for the work presented in the remainder of this issue. Due to space limitations, we are unable to include a discussion of many relevant papers. Readers are encouraged to read the following articles or chapters for more comprehensive reviews of the literature: Corwin and Warchol, 1991; Stone et al., 1998; Bermingham-McDonogh and Rubel, 2003; Stone and Cotanche, 2007; Oesterle and Stone, 2008. While this research has yet to result in a new treatment for hearing loss or balance disorders, it has stimulated a field of scientific inquiry that has shed enormous light on the capacity of the inner ear for hair cell regeneration, and it has raised hopes that a biological treatment for the hearing impaired will be available in the near future.
Initial Experiments: Discovery of Hair Cell Regeneration in Birds
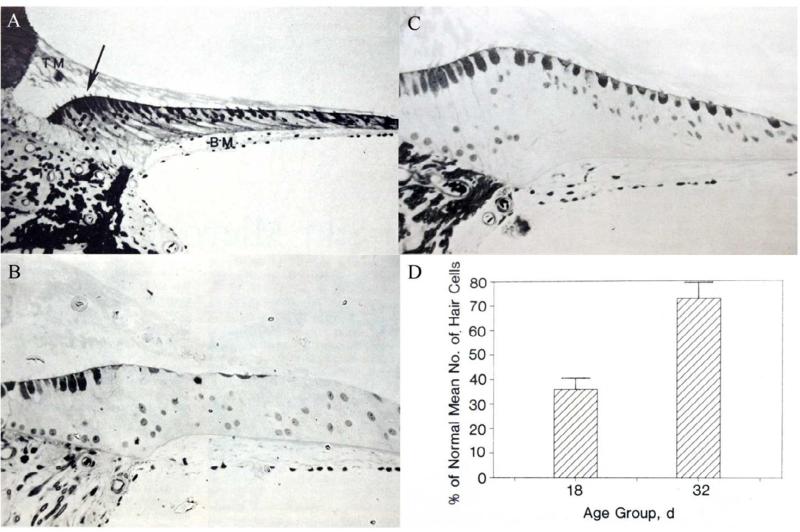
Two serendipitous and nearly simultaneous findings in the mid-1980's suggested that mature birds are able to restore the population of hair cells lost following exposure to ototoxic drugs or intense noise. In 1985 and 1986, Raul Cruz, Paul Lambert, and Edwin Rubel performed an experiment intended to examine the time-course of aminoglycoside-induced hair cell death to the chicken basilar papilla (Figure 1; Cruz et al., 1987). A large group of neonatal chicks was injected daily with 50 mg/kg gentamicin for 10 consecutive days. Experimental and vehicle-injected control animals were allowed to survive varying amounts of time, ranging from 1 day to 3 weeks after the injection period. The goal of this study was simply to determine the onset of hair cell death in order to begin identifying cellular pathways that underlie aminoglycoside-induced hearing loss. Hair cell counts from serial section microscopic analysis revealed that, after the 10-day aminoglycoside treatment, hair cells were nearly totally eliminated in the basal one-third of the basilar papilla. A week later, the damage had spread to eliminate a majority of the hair cells throughout the basal two-thirds of the basilar papilla. Surprisingly however, the number of hair cells had been partially restored at the basal end where the initial hair cell death had occurred. After another 2 weeks, the number of hair cells throughout the basilar papilla appeared almost normal. These unexpected results constituted some of the first evidence that hair cells in warm-blooded vertebrates can regenerate after damage in post-mitotic auditory sensory epithelia.
Figure 1.
Hair cell regeneration occurs in post-hatch (mature) chicken basilar papilla after aminoglycoside-induced hair cell loss. A, Transverse section of mid-portion of normal basilar papilla. Tall and short hair cells are located medially (arrow) and laterally, respectively. TM indicates tectorial membrane, and BM indicates basilar membrane. B, Transverse section of mid-portion of basilar papilla 8 days (age 18 days) after 10 consecutive days of gentamicin injections. C, Transverse section of mid-portion of basilar papilla 22 days (age 32 days) after gentamicin injections. D, Mean hair cell number in gentamicin-treated chickens as a percentage of hair cells counted in undamaged control animals. In the mid-portion of the basilar papilla, hair cell regeneration occurs to restore approximately 70% of lost hair cells by 3 weeks after damage. Bars represent 1 SEM. (From: Cruz RM, Lambert PR, Rubel EW. 1987. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg 113: 1058-1062.)
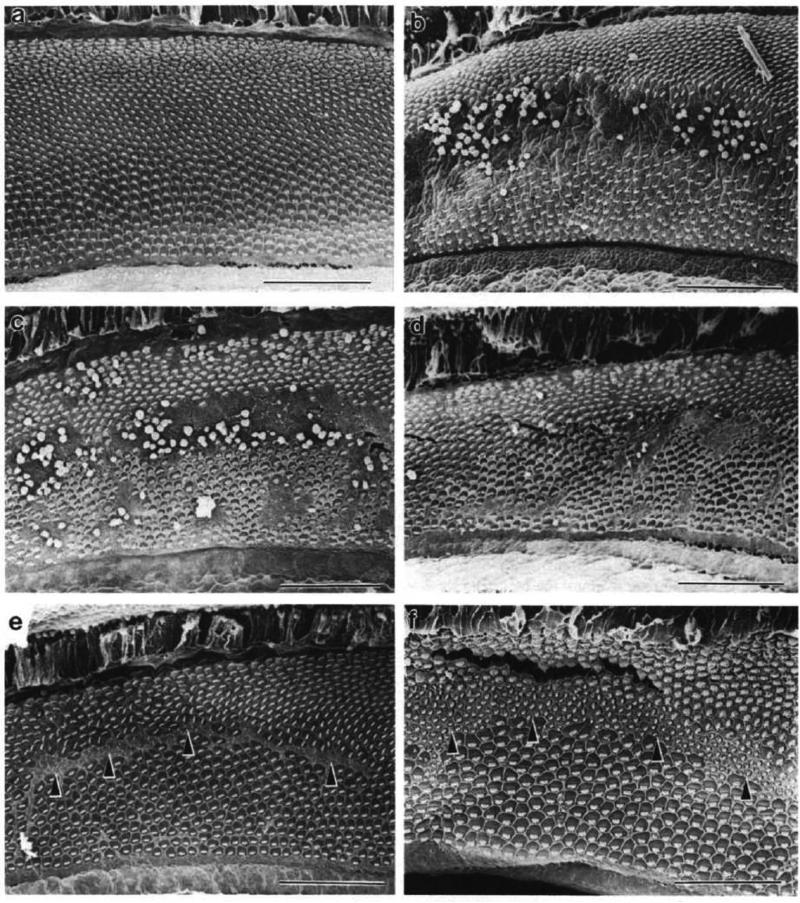
While the Rubel group was counting hair cells following aminoglycoside ototoxicitiy, Douglas Cotanche was examining the neonatal chick basilar papilla following acoustic trauma using scanning electron microscopy (Cotanche, 1987a). The initial purpose of these studies was to examine age differences in the topography of the frequency-place map (see Rubel and Ryals, 1983). Pairs of chickens were subjected to a 1500 Hz pure tone for 48 hrs at 120 dB SPL. One group of chickens was sacrificed immediately, while the others were allowed to recover for 1-10 days. The basilar papillae were processed for scanning and transmission electron microscopy. Immediately following noise exposure, a large area of hair cell loss and damage was found in a tonotopically-stereotyped position. Remarkably however, 2 days later, small stereociliary bundles began populating the area where hair cells had been lost. In addition, apical surfaces of the cells with these small bundles bore a striking resemblance to immature hair cells and the sequence of stereociliary differentiation paralleled that seen in embryogenesis over the course of 10 days of recovery (Figure 2; Cotanche and Sulik, 1984; Cotanche, 1987b).
Figure 2.
Hair cell regeneration occurs in adult chicken basilar papilla after noise-induced hair cell loss. a-f, Scanning electron microscopy analysis conducted on the 1500 Hz region of chicken basilar papillae. a, Control basilar papilla. b-f, Basilar papillae from chickens exposed to 1500 Hz pure tone for 48 hours at 120 dB SPL at 0 hours (b), 24 hours (c), 48 hours (d), 6 days (e), and 10 days (f) after noise exposure. Arrowheads, new stereociliary bundles. Bars represent 100 μm. (From: Cotanche, DA. 1987a. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hearing Research 30: 181-196.)
Taken together, data from these two labs strongly suggested that new hair cells were being produced in the mature basilar papilla to replace those destroyed by aminoglycoside treatment or noise trauma. However, other interpretations were possible; aminoglycosides and noise may cause deterioration or dedifferentiation of hair cells to the extent that they were unrecognizable in the microscopic sections or that the stereocilia deteriorated, and then recovery ensued. Furthermore, even if hair cells were now taking the place of cells that had been destroyed by the treatments, investigators could not conclude that this involved the generation of new cells. It was possible that the damage induced supporting cells in the sensory epithelium (or other cells outside of the sensory epithelium) to transform into hair cells. This is not uncommon in tissues that show continuous cell turnover, as exemplified by olfactory receptor epithelium and taste buds (Beites et al., 2005; Miura et al., 2006).
Proof that hair cells were being regenerated was rapidly provided by two studies that used 3H-thymidine to label mitotically active cells in the inner ear of postnatal birds after noise exposure. This method makes use of the fact that the nucleic acid, thymidine, is incorporated into DNA during S-phase of the cell cycle. Thymidine then remains in the nucleus of the daughter cells for their lifespan, which enables investigators to identify cells born of mitosis. Using this method, two groups, Error! Reference source not found. and Edwin Rubel, and Jeffrey Corwin and Douglas Cotanche, demonstrated that damage to the avian basilar papilla causes a population of supporting cells to enter the cell cycle and to produce new cells, which subsequently differentiate into replacement hair cells and supporting cells (Corwin and Cotanche, 1988; Ryals and Rubel, 1988). An important and complimentary difference between these two studies was that, while Corwin and Cotanche studied neonatal chicks in which hearing is well developed, Ryals and Rubel's experiment was carried out on fully mature quail, removing any assertion that the ability of hair cells to regenerate is only a property of young animals. These studies also indicated that production of new hair cells did not occur in the neonatal chicken or adult quail basilar papilla unless there was hair cell damage. In the same year, however, Jørgensen and Mathiesen (1988) discovered that cell division and production of new hair cells in the budgerigar vestibular epithelium occurs spontaneously throughout life, without intentional hair cell damage. This was subsequently confirmed (Roberson et al., 1992) and extended to include damage-induced regeneration of hair cells in chicken vestibular sensory epithelia (Weisleder and Rubel, 1992; 1993).
In summary, during the period between 1985 and 1993, it became clear that the assumption that warm-blooded vertebrates cannot restore hair cells after injury is wrong. Both young and adult birds can regenerate new hair cells to repopulate areas damaged by aminoglycosides or noise, and they continually replace vestibular hair cells throughout life. In addition, it soon became apparent that the ability to replace damaged hair cells was widespread across vertebrates and hair cell organ systems (Balak et al., 1990; Jones and Corwin, 1993; Baird et al., 1993; Lombarte, et al, 1993).
The discovery of hair cell regeneration had two critical ramifications for the field of hearing research. First, it stimulated a new vibrant area of investigation with the goal of identifying treatments to actually cure hearing loss (and balance disorders). Second, it provided the impetus for renewed interest in the study of inner ear development. While it was assumed that hair cell regeneration would recapitulate hair cell development in many ways, this discovery also made it clear that new hair cells could form in an environment that was quite different from the normal embryonic environment. Thus, it became clear that understanding the cellular and molecular processes underlying development would be essential for stimulating regeneration in the mammalian ear. Accordingly, one could consider this early work on regeneration as a stimulus for some of the developmental investigations seen in the reports that follow our introductory manuscript.
The Source of Hair Cell Regeneration: Supporting Cells and Repair
The early studies that suggested new hair cells arise from mitotically dividing supporting cells (Ryals and Rubel, 1988; Corwin and Cotanche, 1988) were soon followed up by studies that confirmed this assumption. Further evidence for supporting cells as the source of regenerated hair cells after damage came from experiments performed in the lateral line neuromasts of live salamanders. These organs, found in amphibians and fish, contain both hair cells and supporting cells that are analogous in morphology and function to those in avian inner ear sensory epithelia. Balak et al. (1990) killed all hair cells residing in individual neuromasts using laser ablation, leaving only supporting cells in the epithelia. In vivo imaging allowed Balak and colleagues to follow supporting cells in the same neuromast over time, and they clearly observed supporting cells increase rates of cell division compared to control neuromasts, and daughter cells differentiate into both supporting and hair cells.
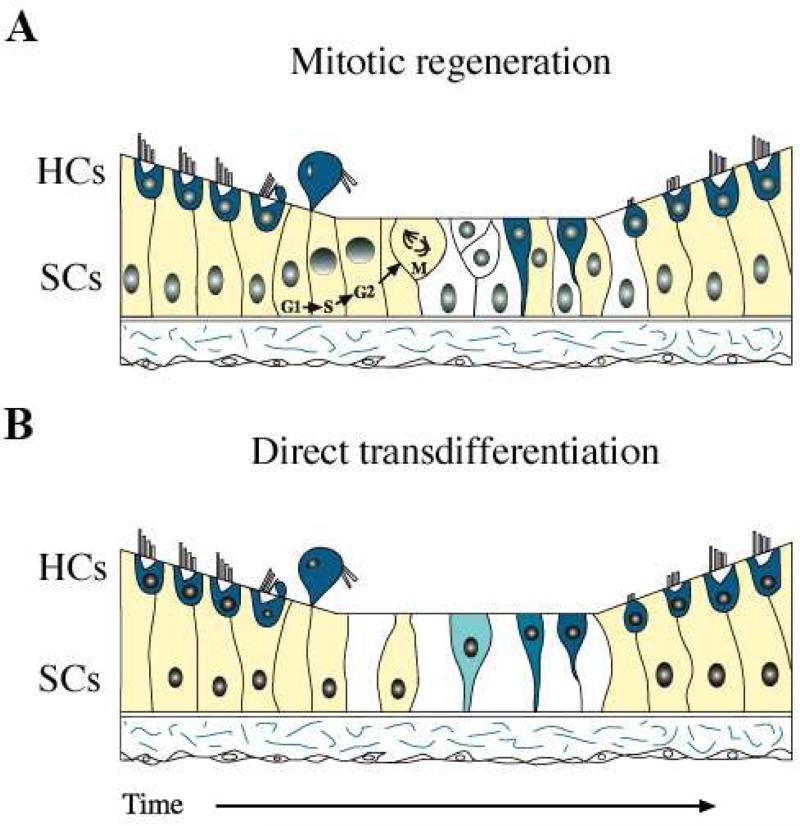
During the same period, studies carried out in avian auditory sensory epithelium demonstrated supporting cells become mitotically active as early as 18-24 hours after the onset of noise exposure and 12 hours after onset of hair cell loss (Girod et al., 1989; Raphael, 1992; Hashino and Salvi, 1993; Stone and Cotanche, 1994). Girod et al. (1989) began 3H-thymidine exposure during the last 6 hrs of an 18 hr noise exposure paradigm and continued it for 6-24 hrs. They reported extensive proliferative activity in the damaged regions of the sensory epithelium 12-27 hrs after the beginning of the noise exposure. As to the source of the new hair cells, Girod et al. state, “At this time, none of the labeled cells had structural similarities with either hair cells or supporting cells in our Toluidine Blue stained material. Instead, they were cytologically indistinguishable from hyaline cells and cuboidal cells.” This study concluded that, in the inferior region of the avian basilar papilla, supporting cells and hyaline/cuboidal cells are “potential precursor populations”, based on the fact that they are the earliest cell types showing 3H-thymidine incorporation. In more superior regions, it was clear that resident supporting cells were the precursors. Other authors have concluded that hyaline/cuboidal cells do not serve as progenitors to new hair cells after noise damage (Cotanche et al., 1995); rather, only supporting cells do, since they divide and give rise to both hair cells and supporting cells (Raphael, 1992; Hashino and Salvi, 1993; Stone and Cotanche, 1994). Additionally, mitotic activity of supporting cells followed the spatial pattern of hair cell loss, suggesting the absence of hair cells signals supporting cells to re-enter the cell cycle (Figure 3a).
Figure 3.
Schematic representation of hair cell regeneration from supporting cells after damage. After damage, hair cells are extruded from the epithelium which signals nearby supporting cells to re-enter the cell cycle and produce mitotically-derived daughter cells (A), or convert directly into hair cells (B). Dividing supporting cells can give rise to both hair cells and supporting cells (A). Blue designates hair cells (HCs) and white designates supporting cells (SCs). (From: Oesterle EC and Stone JS. 2008. Hair cell regeneration: mechanisms guiding cellular proliferation and differentiation. Springer Handbook of Auditory Research Chapter 5: 141-196.)
Studies on birds and amphibians also suggested a variety of other previously unknown reparative processes occur in the injured inner ear. These include replacement of lost hair cells through an unusual process called direct transdifferentiation (Beresford, 1990), during which supporting cells convert into hair cells without dividing (Figure 3b). The first evidence for direct transdifferentiation came from several studies showing that blockade of mitosis after injury does not prevent new hair cell production in either the amphibian saccule (Baird et al., 1996; Baird et al., 2000) or the avian basilar papilla (Adler and Raphael, 1996). The same conclusion was reached by Roberson et al. (1996; 2004), who showed that many new hair cells were not labeled for 3H-thymidine or bromodeoxyuridine following infusion of either nucleotide into the chicken inner ear using an osmotic pump during the entire period of regeneration. Finally, cells with morphological or molecular properties intermediate between supporting cells and hair cells were noted in regenerating sensory epithelia (Adler et al., 1997; Steyger et al., 1997).
In addition to direct transdifferentiation, repair of injured hair cells was shown to be another response to hair cell damage. Both noise and drug exposure can cause injury to stereociliary bundles, giving the appearance that the hair cell itself has been lost from the epithelium. Subsequently, bundles were rebuilt (Sobkowicz et al., 1992; 1996; Zheng et al., 1999a; Gale et al., 2002). These observations have important clinical implications, and they remind investigators that morphological recovery cannot be assumed to be due exclusively to regeneration, and modifications including repair should also be examined.
Recovery of Synaptic Contacts, Organ Structure, and Ultrastructure
Other important early experiments defined additional features of hair cell regeneration in the avian inner ear. For example, researchers investigated the time-course of the regenerative response, the dynamics of nuclear migration and cell shape changes during regeneration, and the innervation of the restored sensory epithelium. Of particular interest was the demonstration, first made by Yehoash Raphael (1992), of a stereotyped pattern of nuclear migration of the hair cell precursors during their transitions through the cell cycle (Figure 3a). This pattern reproduced the sequence of events seen by Corwin and Katayama during development of the avian inner ear (Katayama and Corwin, 1989; 1993), further suggesting that many of the regenerative processes stimulated by injury had marked similarities to the processes that occur during normal development, a theme that exists throughout this research area. During this same period, a series of studies from the Rubel laboratory confirmed that supporting cells are the source of regenerated hair cells in damaged vestibular epithelia and showed that their nuclei migrate from near the basement membrane to the lumenal surface during mitosis. Weisleder and Rubel (1992; 1993; 1995) carefully studied the development of 3H-thymidine-labeled cells after aminoglycoside-induced hair cell death, and Tsue et al. (1994) used markers of G1, S, G2, and M phases of the cell cycle to track mitotic activity in the avian vestibular epithelium damaged with ototoxic drugs.
Though supporting cells can produce new hair cells after noise trauma or aminoglycoside treatment, questions remained as to whether new cells would develop the mature stereotyped organization of stereocilia, enabling restoration of normal mechanotransduction, and form synapses with eighth-nerve afferents, enabling transmission of information from the periphery to the brain. Duckert and Rubel (1990) and Cotanche and Corwin (1991) investigated the time-course of stereociliary regeneration and maturation in the chicken basilar papilla after noise trauma or ototoxic drug treatment. Initially, the bundles on regenerated hair cells were disoriented, but around 4 days after noise exposure, they became realigned to one another and to surviving hair cells outside of the lesion (Cotanche and Corwin, 1991). However, after gentamicin-induced hair cell death, there was a longer time-course of restoring stereociliary orientation (Duckert and Rubel, 1990). By 10-12 weeks after gentamicin treatment, most regenerated stereocilia in the mid portion of the basilar papilla were oriented appropriately, but the entire basal end did not look normal for another 10 weeks. Likewise, after 20-25 weeks post-gentamicin treatment, stereotyped patterns of stereocilia height and number of stereocilia in each bundle were also restored (Duckert and Rubel, 1990). This remarkable restoration of the positional gradients of stereociliary length and number, suggests a “memory” for these parameters resides in supporting cells or the basement membrane in the area of damage, or amongst surviving hair cells in nearby regions.
Afferent synaptic terminals formed on regenerating hair cells as early as one day after a 10-day gentamicin treatment protocol (Duckert and Rubel, 1990) and within 10 days of noise exposure (Ryals and Westbrook, 1994). These initially immature-appearing terminals were found quite early, on immature hair cells, some of which had not yet reached the lumen nor formed stereocilia. Vesiculated terminals (presumably from efferent projections) were found most often on short hair cells (either erupted or not), and were evident around one week after gentamicin treatment.
Functional Recovery
While restoration of synaptic contacts, stereociliary structure, and sensory organ organization were being examined, several groups investigated whether hair cell regeneration resulted in restored auditory and vestibular function. Studies using evoked brainstem potentials (ABRs) in chickens were the first to test the recovery of hearing after hair cell destruction. McFadden and Saunders (1989) examined recovery of ABR thresholds after acoustic trauma. In this situation, recovery appeared very fast and appeared to be largely independent of hair cell regeneration. They attributed this rapid hearing recovery to recovery of the tectorial membrane (Cotanche, 1987c). However, when ABR thresholds were followed after aminoglycoside-induced hearing loss Tucci and Rubel (1990) showed that recovery of neural responses very closely follows a time-course similar to the production and differentiation of new hair cells (see also Girod et al., 1991). Soon, behavioral and electrophysiological studies on quail, chickens, starlings, pigeons, and other avian species provided convincing evidence that after severe deficits due to a variety of ototoxic treatments, hearing returns to near normal levels of sensitivity following hair cell regeneration (fully reviewed in Bermingham-McDonogh and Rubel, 2003). Psychoacoustic analyses on starlings (Marean et al., 1998) took these analyses a step further by studying the reemergence of spectral and temporal acuity during regeneration, and subequent studies showed that even complex song recognition and production are restored after severe disturbances due to deafening (Woolley and Rubel, 2002; Dooling et al., 2006). Similarly, several studies showed that regeneration of hair cells in the vestibular epithelium restores vestibular reflexes and eighth-nerve responses to normality (Carey et al., 1996; Goode et al., 1999; Boyle et al, 2002; Dickman and Lim, 2004). Perhaps even more interesting is a later study showing that the central projections underlying the vestibulo-ocular reflex (VOR) do not recover fully when animals are maintained in strobe illumination (depriving them of retinal slip information), but rapidly assumed normal function when normal visual stimulation is resumed (Goode et al., 2001). This study provides an important demonstration that the specificity of central organization does not remain perfectly intact in the absence of vestibular activation, but it can be rapidly restored through an unknown process when each part of the circuit is available.
What about Hair Cell Regeneration in Mature Mammals?
Following the discovery of hair cell regeneration in the inner ear of birds, several investigators turned to rodents to investigate the possibility that this capability is present but greatly attenuated in the mammalian inner ear. They quickly established that supporting cells in the mature mammalian auditory sensory epithelium (organ of Corti) do not form new hair cells but instead maintain mitotic quiescence, even after hair cell damage (Roberson and Rubel, 1994; Sobkowicz et al., 1997). In contrast, two important investigations by Forge et al. (1993) and Warchol et al. (1993) showed that the mammalian vestibular epithelium contains mechanisms for more plasticity. Careful ultrastructural examination of utricles of gentamicin-treated adult guinea pigs using both SEM and TEM revealed the re-emergence of some cells with immature-appearing stereocilia bundles and undifferentiated cytoplasm, characteristic of developing hair cells (Forge et al., 1993; 1998). Warchol et al. (1993) demonstrated that a small number of supporting cells in adult guinea pig and human utricles re-entered the cell cycle and divided when in culture. However, rates of supporting cell division in adult rodent utricles after in vivo damage were very low, and few if any of the newly formed post-mitotic cells differentiated into bona fide hair cells (Warchol et al., 1993; Rubel et al., 1995; Li and Forge, 1997; Kuntz and Oesterle, 1998; Ogata et al., 1999; Oesterle et al., 2003). The conclusion from these studies was that some degree of hair cell replacement can occur naturally in mature mammalian vestibular epithelia, but neither proliferative nor non-mitotic mechanisms restore significant numbers of hair cells in the mature organ of Corti.
Approaches Toward Identifying Key Regulators of Hair Cell Regeneration
Armed with the understanding that non-mammals mount a robust regenerative response to hair cell loss while mammals do not, it was clear early on that the focus needed to shift toward identifying molecular mechanisms that could robustly promote or inhibit supporting cells to form new hair cells in animals that DO regenerate hair cells (non-mammalian vertebrates) and those that do not mount a robust regenerative response (mammals). To address this problem, it was first necessary to develop in vitro models that enabled investigators to efficiently test specific hypotheses about potential regulators of hair cell regeneration. Two groups performed the initial experiments to develop in vitro preparations of inner ear sensory organs from mature birds or rodents (see Warchol et al., 1993; Oesterle et al., 1993; Warchol and Corwin, 1993; Lambert, 1994). Shortly thereafter, other investigators developed cell culture methods for purified avian and mammalian supporting cells (e.g., Warchol, 1995; Stone et al., 1996; Zheng et al., 1997). Most early investigations focused on developing methods that enabled supporting cell division to proceed. Later, it became evident that more long-term cultures were required to observe hair cell differentiation (e.g., Shang et al., 2010; Slattery and Warchol, 2010; Lin et al., 2011).
At this point, investigators selected candidate molecules to test for their ability to promote or inhibit regeneration using in vitro preparations. Some researchers reasoned that molecules identified as key regulators of hair cell regeneration in non-mammals might be manipulated in damaged mammalian tissue in order to coax regeneration. Others postulated that signals regulating hair cell development could be re-activated in mature damaged tissue to promote regeneration. Still others chose candidate drugs or growth factors to test, based on their roles in other systems. In all cases, investigators’ primary goal was to drive mature supporting cells into the cell cycle or to identify signals that prevented this process. These approaches led to the identification of several molecules with the capacity to stimulate or block hair cell regeneration in birds and mammals (for reviews, see Oesterle and Hume, 1999; Oesterle and Stone, 2008). For example, Oesterle et al. (1997; 2000) showed that insulin-like growth factor 1 (IGF-1) and insulin can stimulate proliferation in cultures of mature avian utricles and basic fibroblast growth factor (FGF-2) inhibits cell proliferation in cultured avian inner ear sensory epithelia. Attempts to perform comprehensive screens for growth factors that induce proliferation in cultured developing mammalian organ of Corti did not yield many strong candidates (Zine and de Ribaupierre, 1998; Zheng et al., 1999b), and technologies remain to be developed to examine cultured adult organ of Corti. However, transforming growth factor- α (TGF-α) did increase cell proliferation in cultures of mature mouse vestibular sensory epithelium (Yamashita and Oesterle, 1995; Lambert, 1994; Zheng et al., 1997; 1999b), and infusion of TGF-α with insulin into the ears of rats caused a modest amount of supporting cell division in the mature vestibular system (Kuntz and Oesterle 1998; Oesterle et al., 2003).
Investigators also took their quest for regulators of hair cell regeneration inside the cell, to look at molecules that more directly control cell cycle entry, progression, and exit. Some of the earliest studies addressed the role of the cyclin-dependent kinase inhibitor, p27kip1, in limiting the period of mitotic activity in the developing organ of Corti (Chen and Segil, 1999; Löwenheim et al., 1999). These studies demonstrated that constitutive deletion of p27kip1 significantly extended the normal developmental period of cell division in the organ of Corti and caused overproduction of hair cells and supporting cells. Unfortunately, supporting cells still established mitotic quiescence by the end of the second postnatal week. Nevertheless, subsequent studies of potential roles for manipulations of p27kip1 and other cell cycle regulatory proteins continue to yield important results defining the role of several cell cycle regulators in supporting cell quiescence (White et al., 2006; Minoda et al., 2007; Laine et al., 2010; Oesterle et al., 2011; White et al., 2012).
Efforts to promote supporting cell division in mature mammals were accompanied by the search for molecules that control hair cell specification and differentiation. It is hoped that such molecules could be used to either promote new post-mitotic cells to differentiate as hair cells or to push supporting cells or other cell types to convert directly into hair cells (via direct transdifferentiation). Early studies focused on molecules that control the differentiation of hair cells during normal development. Huda Zoghbi and collaborators showed that the basic helix-loop-helix transcription factor, Atoh1, is necessary for hair cell differentiation in both auditory and vestibular epithelia (Bermingham et al., 1999). Shortly thereafter, Wei-Qiang Gao and colleagues discovered that misexpression of Atoh1 in a non-sensory region of the cultured developing organ of Corti drove those cells to transdifferentiate into hair cells (Zheng and Gao, 2000). More recent investigations in mature mammalian epithelia showed that Atoh1 can drive mammalian supporting cells to acquire hair cell features in cultured utricles and in vivo (Shou et al., 2003; Staecker et al., 2007). The results of manipulating Atoh1 in vivo in the mature mammalian organ of Corti appear mixed. Some authors report robust regeneration and functional recovery, while others find less convincing evidence (Kawamoto et al., 2003; Izumikawa et al., 2005; Staecker et al., 2007; Schlecker et al., 2011; Liu et al., 2012).
Given Atoh1's remarkable ability to drive hair cell differentiation, investigators became interested in finding ways to promote Atoh1 expression in supporting cells using exogenous agents, which could be more therapeutically wieldy than gene misexpression. Studies in fruit flies and other animals had shown that Atoh1 transcription is strongly antagonized by signaling via the Notch receptor (reviewed in Lewis, 1996). Early investigation of Notch signaling in the developing organ of Corti showed that it is critical for establishing correct cell types and patterning (Lanford et al., 1999). In particular, deletion of the gene encoding Jagged2, a Notch ligand, resulted in hair cell overproduction at the expense of supporting cells. This finding was consistent with Notch's known role in maintaining sensory progenitors during development. The first study to implicate Notch signaling in hair cell regeneration in mature epithelia was Stone and Rubel (1999), which demonstrated upregulation of Notch ligand expression was correlated temporally and spatially with renewed hair cell and supporting cell production. In essence, a developmental program of gene expression had been recapitulated. More recent studies have confirmed the inhibitory role of Notch in hair cell regeneration in neonatal and mature sensory epithelia from non-mammals (Ma et al., 2008; Daudet et al., 2009) and mammals (Doetzlhofer et al., 2009; Collado et al., 2011; Lin et al., 2011; Zhao et al., 2011). To date, all studies that have blocked Notch activity to promote hair cell regeneration in mature animals have been performed in vitro; in vivo effects remain to be examined.
The Future
Scientific efforts in numerous laboratories over the past 25 years have made major contributions to our understanding of hair cell regeneration as a mechanism for restoring hearing or balance function after injury in mature animals. They have demonstrated that most or all non-mammalian vertebrates readily regenerate hair cells after damage, and regenerated hair cells develop normal features, become innervated, and communicate effectively with the brain. Second, investigators showed that adult mammalian supporting cells in auditory and vestibular organs are rigorously blocked from re-entering the cell cycle after hair cell damage, and simple manipulations are only mildly effective in driving their re-entry into the cell cycle. Third, it is now well established that adult mammalian vestibular epithelia contain some supporting cells with the capacity to directly convert into hair cells, but such ability remains to be demonstrated in the mature organ of Corti. Finally, the most recent studies demonstrated that some genetic manipulations can drive mammalian supporting cells to convert into hair cell-like cells, but so far, these manipulations seem to be most effective in the neonatal ear and are insufficient to restore inner ear anatomy and function to normal levels in mature animals.
The next phase of hair cell regeneration research has many challenges. Below, we define some of the major approaches that are currently being taken to promote higher degrees of hair cell replacement in mature mammals.
Identification of methods to induce hair cell regeneration in mature mammals
A major push is underway to define manipulations sufficient to drive adult supporting cells to form adequate numbers and types of new hair cells. To do this, investigators are taking advantage of exciting technological advancements in molecular biology, including gene expression profiling and in vivo manipulation of gene activity.
Gene expression (or transcriptional) profiling is one strategy being used to unravel the molecular cascades involved in supporting cell quiescence and proliferation in auditory and vestibular sensory epithelia. Utilizing this approach, researchers are identifying genes that are up-regulated, down-regulated, and unchanged during hair cell regeneration in non-mammals (e.g., Hawkins et al., 2007; Alvarado et al., 2011; Schuck et al., 2011). They are also using genetic profiling in mammals to identify signals that may restrict supporting cells to quiescence. Comparison of supporting cell gene expression patterns in non-mammals and mammals after hair cell damage will provide important clues as to why adult mammalian supporting cells fail to mount a robust regenerative response, particularly in the organ of Corti. One strength of this approach compared to past approaches is it will identify genes that have not yet been associated with sensory cell fate determination and differentiation in the inner ear or in other tissues.
As the field becomes inundated with results from genetic profiling experiments, the next step will be to test the specific function of genes, gene combinations, and signaling pathways identified as potential regulators of hair cell regeneration. The zebrafish might be an excellent model for relatively quick, high-throughput screening of hair cell regeneration capacity in the presence of modulators of signaling cascades. Lateral line neuromasts are composed of both supporting and hair cells that are analogous in morphology and function to other species, but they reside on the outside of the fish allowing easy access to small molecules (reviewed in Brignull et al., 2009). Small molecules that disrupt various signaling pathways can be applied to zebrafish in vivo to assess their direct involvement in the regenerative processes. Likewise, direct testing of candidate molecules can be accomplished in mammalian systems in vitro and in vivo. Transgenic mouse lines utilizing Cre-loxP and tetracycline-responsive element technology are used to conditionally control gene expression in specific cellular populations before, during, and after development (e.g., Sage et al., 2005; 2006). In such mice, the activity of individual genes can be manipulated in normal and damaged sensory epithelia in vitro and in vivo to test their role in supporting cell proliferation or hair cell differentiation. Use of adenovirally expressed vectors to force overexpression of specific genes or to deliver RNA sequences to knockdown gene expression also provides a solid method for testing hypotheses (e.g., Loponen et al., 2011; Zhao et al., 2011). Not only can adenovirus technology prove useful in manipulating gene expression in cells to provide understanding of molecular mechanisms at work, but it also may serve as a route for genetic therapy in patients one day. A review of these approaches is provided in provided in “Gene therapy for the inner ear” by Fukui and Raphael, in this issue.
Stem cells as a source for new hair cells
Several research groups are investigating ways to promote supporting cells or other cells inside the inner ear to divide or convert into hair cells. Another viable approach toward restoring hair cells is to introduce into the inner ear, cells from other sources that have the capacity to form hair cells. To accomplish this, investigators are studying different types of stem or progenitor cells, defining their capacities to form new hair cells, and characterizing ways to introduce such cells into the inner ear. A review of these efforts is provided in Okano and Kelley, 2012.
Conclusion
In the early 1980s, it was impossible for most auditory scientists to imagine clinicians could someday repair the damaged cochlea by inducing the production of new sensory cells to transduce sound into electrical signals conveyed to the brain. Today, thanks to work pioneered in the 1980s and 1990s, laboratories around the world are eagerly seeking ways to use the recent findings of modern biology to protect and repair the inner ear. The momentum driving this research will only continue to grow and propel the field to develop therapies to treat, and perhaps to cure, hearing and balance impairments.
Highlights.
- Discovery of hair cell regeneration was serendipitous.
- Not known or generally believed possible before 1986-87.
- The future is bright - “we've come a long way, baby”!
Acknowledgments
We are grateful to Elizabeth Oesterle for helpful comments on the manuscript, to Kevin Whitham and Rebecca Lewis for help with figures, and to Dr. Douglas Cotanche for providing Figure 2 and helpful comments on the manuscript. We dedicate this manuscript to Dr. Douglas Cotanche, co-discoverer of hair cell regeneration, friend, mentor and colleague who has unselfishly shared his enthusiasm, ideas and scientific acumen throughout our long association and continuing to the present.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Adler HJ, Komeda M, Raphael Y. Further evidence for supporting cell conversion in the damaged avian basilar papilla. Int J Dev Neurosci. 1997;15:375–85. doi: 10.1016/s0736-5748(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Alvarado DM, Hawkins RD, Bashiardes S, Veile RA, Ku YC, Powder KE, Spriggs MK, Speck JD, Warchol ME, Lovett M. An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci. 2011;31:4535–43. doi: 10.1523/JNEUROSCI.5456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Torres MA, Schuff NR. Hair cell regeneration in the bullfrog vestibular otolith organs following aminoglycoside toxicity. Hear Res. 1993;65:164–74. doi: 10.1016/0378-5955(93)90211-i. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann N Y Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci U S A. 2000;97:11722–9. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–12. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–16. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Direct transdifferentiation: can cells change their phenotype without dividing? Cell Differ Dev. 1990;29:81–93. doi: 10.1016/0922-3371(90)90026-s. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–26. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Blakeslee EA, Hynson K, Hamernik RP, Henderson D. Asymptotic threshold shift in chinchillas exposed to impulse noise. J Acoust Soc Am. 1978;63:876–82. doi: 10.1121/1.381767. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM, Carey JP, Xu J. Functional recovery of anterior semicircular canal afferents following hair cell regeneration in birds. J Assoc Res Otolaryngol. 2002;3:149–66. doi: 10.1007/s101620020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JP, Fuchs AF, Rubel EW. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J Neurophysiol. 1996;76:3301–12. doi: 10.1152/jn.1996.76.5.3301. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Collado MS, Thiede BR, Baker W, Askew C, Igbani LM, Corwin JT. The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs. J Neurosci. 2011;31:11855–66. doi: 10.1523/JNEUROSCI.2525-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic production and aging in inner ear hair cells in sharks. J Comp Neurol. 1981;201:541–53. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic growth of the macula neglecta auditory detector in the ray, Raja clavata: continual increases in hair cell number, neural convergence, and physiological sensitivity. J Comp Neurol. 1983;217:345–56. doi: 10.1002/cne.902170309. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Warchol ME. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–33. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987a;30:181–95. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Development of hair cell stereocilia in the avian cochlea. Hear Res. 1987b;28:35–44. doi: 10.1016/0378-5955(87)90151-1. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of the tectorial membrane in the chick cochlea following severe acoustic trauma. Hear Res. 1987c;30:197–206. doi: 10.1016/0378-5955(87)90136-5. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Sulik KK. The development of stereociliary bundles in the cochlear duct of chick embryos. Brain Res. 1984;318:181–93. doi: 10.1016/0165-3806(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Corwin JT. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear Res. 1991;52:379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Messana EP, Ofsie MS. Migration of hyaline cells into the chick basilar papilla during severe noise damage. Hear Res. 1995;91:148–59. doi: 10.1016/0378-5955(95)00185-9. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–62. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman JD, Lim I. Posture, head stability, and orientation recovery during vestibular regeneration in pigeons. J Assoc Res Otolaryngol. 2004;5:323–36. doi: 10.1007/s10162-004-4047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Dent ML, Reid TL. Perception of complex sounds in budgerigars (Melopsittacus undulatus) with temporary hearing loss. J Acoust Soc Am. 2006;119:2524–32. doi: 10.1121/1.2171839. [DOI] [PubMed] [Google Scholar]
- Duckert LG, Rubel EW. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear Res. 1990;48:161–82. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci 13. 1990;9:362–5. doi: 10.1016/0166-2236(90)90017-5. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog's saccule. J Neurobiol. 2002;50:81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- Girod DA, Duckert LG, Rubel EW. Possible precursors of regenerated hair cells in the avian cochlea following acoustic trauma. Hear Res. 1989;42:175–94. doi: 10.1016/0378-5955(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Girod DA, Tucci DL, Rubel EW. Anatomical correlates of functional recovery in the avian inner ear following aminoglycoside ototoxicity. Laryngoscope. 1991;101:1139–49. doi: 10.1288/00005537-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Goode CT, Carey JP, Fuchs AF, Rubel EW. Recovery of the vestibulocolic reflex after aminoglycoside ototoxicity in domestic chickens. J Neurophysiol. 1999;81:1025–35. doi: 10.1152/jn.1999.81.3.1025. [DOI] [PubMed] [Google Scholar]
- Goode CT, Maney DL, Rubel EW, Fuchs AF. Visual influences on the development and recovery of the vestibuloocular reflex in the chicken. J Neurophysiol. 2001;85:1119–28. doi: 10.1152/jn.2001.85.3.1119. [DOI] [PubMed] [Google Scholar]
- Hashino E, Salvi RJ. Changing spatial patterns of DNA replication in the noise-damaged chick cochlea. J Cell Sci. 1993;105(Pt 1):23–31. doi: 10.1242/jcs.105.1.23. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One. 2007;2:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13:1022–34. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988;75:319–20. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- Katayama A, Corwin JT. Cell production in the chicken cochlea. J Comp Neurol. 1989;281:129–35. doi: 10.1002/cne.902810110. [DOI] [PubMed] [Google Scholar]
- Katayama A, Corwin JT. Cochlear cytogenesis visualized through pulse labeling of chick embryos in culture. J Comp Neurol. 1993;333:28–40. doi: 10.1002/cne.903330103. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz AL, Oesterle EC. Transforming growth factor alpha with insulin stimulates cell proliferation in vivo in adult rat vestibular sensory epithelium. J Comp Neurol. 1998;399:413–23. [PubMed] [Google Scholar]
- Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol. 2010;337:134–46. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Lambert PR. Inner ear hair cell regeneration in a mammal: identification of a triggering factor. Laryngoscope. 1994;104:701–18. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–92. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Li HS. Influence of genotype and age on acute acoustic trauma and recovery in CBA/Ca and C57BL/6J mice. Acta Otolaryngol. 1992;112:956–67. doi: 10.3109/00016489209137496. [DOI] [PubMed] [Google Scholar]
- Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–46. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–39. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32:6600–10. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64:166–74. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Loponen H, Ylikoski J, Albrecht JH, Pirvola U. Restrictions in cell cycle progression of adult vestibular supporting cells in response to ectopic cyclin D1 expression. PLoS One. 2011;6:e27360. doi: 10.1371/journal.pone.0027360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–8. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW. Auditory perception following hair cell regeneration in European starling (Sturnus vulgaris): frequency and temporal resolution. J Acoust Soc Am. 1998;103:3567–80. doi: 10.1121/1.423085. [DOI] [PubMed] [Google Scholar]
- McFadden EA, Saunders JC. Recovery of auditory function following intense sound exposure in the neonatal chick. Hear Res. 1989;41:205–15. doi: 10.1016/0378-5955(89)90012-9. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Schuknecht HF. Human cochlear changes in noise induced hearing loss. Laryngoscope. 1976;86:1293–1302. doi: 10.1288/00005537-197609000-00001. [DOI] [PubMed] [Google Scholar]
- Minoda R, Izumikawa M, Kawamoto K, Zhang H, Raphael Y. Manipulating cell cycle regulation in the mature cochlea. Hear Res. 2007;232:44–51. doi: 10.1016/j.heares.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–25. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Rubel EW. Postnatal production of supporting cells in the chick cochlea. Hear Res. 1993;66:213–24. doi: 10.1016/0378-5955(93)90141-m. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Reh TA, Rubel EW. Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hear Res. 1993;70:85–108. doi: 10.1016/0378-5955(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor-I and insulin. J Comp Neurol. 1997;380:262–74. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Hume CR. Growth factor regulation of the cell cycle in developing and mature inner ear sensory epithelia. J Neurocytol. 1999;28:877–87. doi: 10.1023/a:1007074222659. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Stone JS. Hair cell regeneration: mechanisms guiding cellular proliferation and differentiation. In Springer Handbook of Auditory Research. 2008;33:141–196. [Google Scholar]
- Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J Comp Neurol. 2000;424:307–26. doi: 10.1002/1096-9861(20000821)424:2<307::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Cunningham DE, Westrum LE, Rubel EW. Ultrastructural analysis of [3H]thymidine-labeled cells in the rat utricular macula. J Comp Neurol. 2003;463:177–95. doi: 10.1002/cne.10756. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10:1237–48. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y, Slepecky NB, Takahashi M. Study of the gerbil utricular macula following treatment with gentamicin, by use of bromodeoxyuridine and calmodulin immunohistochemical labelling. Hear Res. 1999;133:53–60. doi: 10.1016/s0378-5955(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Okano T, Kelley MW. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif. 2012;16:4–18. doi: 10.1177/1084713812440336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper AN, Hoxter B. Growth of a fish ear: 1. Quantitative analysis of hair cell and ganglion cell proliferation. Hear Res. 1984;15:133–42. doi: 10.1016/0378-5955(84)90044-3. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol. 1992;21:663–71. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud Neurosci. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–71. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res. 1992;57:166–74. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Ryals BM. Development of the place principle: acoustic trauma. Science. 1983;219:512–4. doi: 10.1126/science.6823549. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Dew LA, Roberson DW. Mammalian vestibular hair cell regeneration. Science. 1995;267:701–7. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol, Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW. TEM analysis of neural terminals on autoradiographically identified regenerated hair cells. Hear Res. 1994;72:81–8. doi: 10.1016/0378-5955(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, Vetter DE, Chen ZY. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103:7345–50. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–8. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Praetorius M, Brough DE, Presler RG, Jr., Hsu C, Plinkert PK, Staecker H. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 2011;18:884–90. doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck JB, Sun H, Penberthy WT, Cooper NG, Li X, Smith ME. Transcriptomic analysis of the zebrafish inner ear points to growth hormone mediated regeneration following acoustic trauma. BMC Neurosci. 2011;12:88. doi: 10.1186/1471-2202-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J. Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J Assoc Res Otolaryngol. 2010;11:203–22. doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J Neurosci. 2010;30:3473–81. doi: 10.1523/JNEUROSCI.4316-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska-Kowalska M, Sulkowski W, Chrzescijanek M, Kamedula T. Auditory brainstem responses (ABR) in guinea pigs to loud tones noise: a preliminary study. Otolaryngol Pol. 1992;46:409–14. [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Epithelial repair following mechanical injury of the developing organ of Corti in culture: an electron microscopic and autoradiographic study. Exp Neurol. 1992;115:44–9. doi: 10.1016/0014-4886(92)90219-g. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Post-traumatic survival and recovery of the auditory sensory cells in culture. Acta Otolaryngol. 1996;116:257–62. doi: 10.3109/00016489609137836. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Cellular interactions as a response to injury in the organ of Corti in culture. Int J Dev Neurosci. 1997;15:463–85. doi: 10.1016/s0736-5748(96)00104-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Burton M, Hawkins JR, Schuff NR, Baird RA. Calbindin and parvalbumin are early markers of non-mitotically regenerating hair cells in the bullfrog vestibular otolith organs. Int J Dev Neurosci. 1997;15:417–32. doi: 10.1016/s0736-5748(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Stone L. The development of lateral line sense organs in amphibians observed in living and vital-stained preparations. J Comp Neurol. 1933;57:507–540. [Google Scholar]
- Stone L. Further experimental studies of the development of lateral-line sense organs in the amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115. [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–73. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–47. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Oesterle EC, Rubel EW. Recent insights into regeneration of auditory and vestibular hair cells. Curr Opin Neurol. 1998;11:17–24. doi: 10.1097/00019052-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Stone JS, Leano SG, Baker LP, Rubel EW. Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations. J Neurosci. 1996;16:6157–74. doi: 10.1523/JNEUROSCI.16-19-06157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsue TT, Watling DL, Weisleder P, Coltrera MD, Rubel EW. Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J Neurosci. 1994;14:140–52. doi: 10.1523/JNEUROSCI.14-01-00140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci DL, Rubel EW. Physiologic status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990;103:443–50. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Supporting cells in isolated sensory epithelia of avian utricles proliferate in serum-free culture. Neuroreport. 1995;6:981–4. doi: 10.1097/00001756-199505090-00008. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Supporting cells in avian vestibular organs proliferate in serum-free culture. Hear Res. 1993;71:28–36. doi: 10.1016/0378-5955(93)90018-v. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–22. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair Cell Regeneration in the Avian Vestibular Epithelium. Experimental Neurology. 1992;115:2–6. doi: 10.1016/0014-4886(92)90211-8. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Tsue TT, Rubel EW. Hair Cell Replacement in Avian Vestibular Epithelium: Supporting Cell to Type I Hair Cell. Hearing Research. 1995;82:125–133. doi: 10.1016/0378-5955(94)00169-q. [DOI] [PubMed] [Google Scholar]
- White PM, Stone JS, Groves AK, Segil N. EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals. Dev Biol. 2012;363:191–200. doi: 10.1016/j.ydbio.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Rubel EW. Vocal memory and learning in adult Bengalese Finches with regenerated hair cells. J Neurosci. 2002;22:7774–87. doi: 10.1523/JNEUROSCI.22-17-07774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Oesterle EC. Induction of cell proliferation in mammalian inner-ear sensory epithelia by transforming growth factor alpha and epidermal growth factor. Proc Natl Acad Sci U S A. 1995;92:3152–5. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, Gao WQ, Yang SM. Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PLoS One. 2011;6:e23729. doi: 10.1371/journal.pone.0023729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Helbig C, Gao WQ. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cell cultures. J Neurosci. 1997;17:216–26. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999a;19:2161–70. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999b;28:901–12. doi: 10.1023/a:1007078307638. [DOI] [PubMed] [Google Scholar]
- Zine A, de Ribaupierre F. Replacement of mammalian auditory hair cells. Neuroreport. 1998;9:263–8. doi: 10.1097/00001756-199801260-00016. [DOI] [PubMed] [Google Scholar]