Pomegranate Juice Augments Memory and fMRI Activity in Middle-Aged and Older Adults with Mild Memory Complaints (original) (raw)
Abstract
Despite increasing emphasis on the potential of dietary antioxidants in preventing memory loss and on diet as a precursor of neurological health, rigorous studies investigating the cognitive effects of foods and their components are rare. Recent animal studies have reported memory and other cognitive benefits of polyphenols, found abundantly in pomegranate juice. We performed a preliminary, placebo-controlled randomized trial of pomegranate juice in older subjects with age-associated memory complaints using memory testing and functional brain activation (fMRI) as outcome measures. Thirty-two subjects (28 completers) were randomly assigned to drink 8 ounces of either pomegranate juice or a flavor-matched placebo drink for 4 weeks. Subjects received memory testing, fMRI scans during cognitive tasks, and blood draws for peripheral biomarkers before and after the intervention. Investigators and subjects were all blind to group membership. After 4 weeks, only the pomegranate group showed a significant improvement in the Buschke selective reminding test of verbal memory and a significant increase in plasma trolox-equivalent antioxidant capacity (TEAC) and urolithin A-glucuronide. Furthermore, compared to the placebo group, the pomegranate group had increased fMRI activity during verbal and visual memory tasks. While preliminary, these results suggest a role for pomegranate juice in augmenting memory function through task-related increases in functional brain activity.
1. Introduction
As people age, their risk for cognitive decline increases. An estimated 40% of people 65 years and older have age-associated memory impairment characterized by self-perception of memory loss and a standardized memory test score demonstrating lower objective memory performance compared with young adults [1–3]. While genetic factors play an important role in age-related memory decline, such nongenetic lifestyle factors as diet and exercise contribute as well [4]. In particular, a growing body of evidence indicates that accumulation of oxidative damage to macromolecules increases progressively during the aging process [5, 6] and contributes to neurodegeneration in Alzheimer's disease [7]. Epidemiological studies have suggested a link between antioxidant consumption and cognitive protection [8–11], and studies using a rodent model of Alzheimer's disease suggest that tau phosphorylation occurs as a compensatory response to oxidative stress [12]. Several clinical trials of antioxidant use in subjects with normal aging or Alzheimer's disease suggest memory benefits [13–15], while others yielded negative results [16–19].
While many fruits are rich in various antioxidants, including ascorbic acid, carotenoids, and phenolics, commonly consumed fruits show large differences in antioxidant capacity, as determined by the ferric reducing/antioxidant power (FRAP) assay [20]. In particular, there is special interest in the pomegranate polyphenols, which have been studied extensively in animal models of Alzheimer's disease [21, 22]. The emerging data on pomegranate fruits and their inherent polyphenols suggest positive benefits ranging from neuroprotective effects [23, 24] to staving off effects of senescent neurodegeneration in animal models of Alzheimer's disease [25–27].
Previously, we found that pomegranate juice has the highest antioxidant capacity among fruit juices using five different in vitro antioxidant assays [28]. In comparison, apple juice, which is low in antioxidant capacity, was less effective than pomegranate juice in affecting antioxidant capacity based on FRAP assays in 26 elderly subjects consuming pomegranate juice or apple juice over a 4-week period [29].
Overexpression of antioxidant enzymes or supplementation of some antioxidants appears effective in extending the life span in several animal models [5, 6], and a few studies have specifically found memory-enhancing effects of polyphenols in mice [30, 31]. However, evidence for memory enhancement from polyphenols in human-controlled trials is limited. One preliminary study [15] found beneficial effects of grape juice on memory in five older adults with age-associated memory impairment compared to seven placebo controls; to our knowledge this small study is the only placebo-controlled human trial of polyphenols that assessed memory change. Therefore, more well-controlled studies are needed to determine potential beneficial effects of antioxidants that occur naturally in various food sources and to elucidate the possible underlying mechanisms.
Moreover, studies that examine possible brain mechanisms of polyphenol treatment in humans are limited. One potential method for identifying mechanisms of recovery in drug trials is functional magnetic resonance imaging (fMRI). While structural MRI studies measure long-term changes in metrics, such as cortical thickness and volume [32–34], fMRI measures blood flow changes in response to memory challenge, which has proven to be effective in determining brain differences among subjects with Alzheimer's disease, mild cognitive impairment, or nondemented subjects at risk for Alzheimer's disease [35–40]. Further, fMRI has proven to be sensitive in measuring changes in neural activation patterns among older subjects in response to memory-enhancing drugs, such as donepezil [41] and memantine [42], with results showing increased activation or restoration of resting-state brain networks following treatment. One investigation used fMRI to examine the effects of antioxidants (flavonoids) in young healthy controls and demonstrated increased fMRI activity to cognitive challenge with flavonoid administration [43]; however, no studies to date have examined fMRI effects of antioxidant therapy in older adults with or without memory complaints. To address this knowledge gap, we explored potential neuroprotective effects derived from polyphenols in pomegranate juice in older volunteers with mild memory complaints in a placebo-controlled, randomized, and double-blind trial and measured three aspects: (1) metabolites of pomegranate juice using blood biomarkers; (2) effects of pomegranate juice on memory performance; and (3) evidence of functional MRI changes during memory activation. We hypothesized that pomegranate juice compared to placebo would increase metabolites, improve memory, and increase task-related brain activation in the left and right hemispheres for verbal and nonverbal memory tasks, respectively. Together these metrics provide preliminary evidence of bioavailability, clinical efficacy, and brain mechanisms of pomegranate juice in older adults with memory complaints.
2. Methods
2.1. Subjects
Thirty-two volunteers were initially enrolled into the study out of 39 who were screened; of these, 28 completed the clinical trial (Table 1). Subjects were recruited via advertisements through local newspapers, flyers, posters, lectures, and word-of-mouth. We specifically recruited nondemented, older right-handed subjects with self-reported age-related memory complaints. The subjects did not carry a diagnosis of mild cognitive impairment (MCI) and were screened using the mini-mental state examination (MMSE). Subjects randomized to placebo versus control groups did not differ in mean age, percentage of women, mean MMSE scores, or baseline memory performance (Table 1). Potential subjects were excluded who had documented neurological, psychiatric, or major medical conditions, including heart disease, diabetes, and gastrointestinal disease, that might affect cognitive function or interfere with study procedures. We also excluded subjects who routinely ate more than three servings per day of fruits and vegetables or were taking vitamin supplements, any medication, or dietary supplement that interferes with the absorption of polyphenols. Potential subjects participating in regular vigorous exercises other than ordinary daily walks or who were unwilling to maintain a sedentary lifestyle during the 28-day study were excluded, as were those for whom MRI was contraindicated (e.g., claustrophobia, dental braces, or implanted ferromagnetic or electronic devices). Seven potential subjects were screened out based on one or more of these exclusion criteria.
Table 1.
Demographic and clinical characteristics of subjects at baseline.
| Characteristic | Mean (SD) | |
|---|---|---|
| Pomegranate(n = 15) | Placebo(n = 13) | |
| Mini-mental state examination | 28.0 (1.5) | 27.8 (1.5) |
| Age, y | 63.1 (8.0) | 62.0 (7.8) |
| Female, no. (%) | 11 (73.3) | 10 (76.9) |
| TEAC baseline | 1712 (299) | 1927 (461) |
| Buschke selective reminding test | ||
| Recall | 85.0 (11.7) | 86.5 (12.5) |
| Consistent long-term retrieval | 52.8 (19.9) | 55.8 (25.5) |
Subjects were instructed that they would be removed from the study if they failed to follow the prescribed low polyphenol diet during the one-week run-in period based on self-report in-person interview with a study dietitian. The study was approved by the UCLA Institutional Review Board; all subjects gave informed consent to participate in all procedures and were reconsented for followup evaluations. Funding for this study was provided by the UCLA Center for Human Nutrition from a “various donors” account. The investigators were blind to the contributors to this fund, and there was no communication or input from donors or commercial entities at any stage of the study.
2.2. General Procedures
Following phone screening, volunteers participated in an initial clinical evaluation, which included an explanation of the study procedures, consent, medical screening, MMSE, and routine CBC and chemistry panel. Prior to the intervention phase, each subject met with a registered dietitian and was instructed on a low polyphenol diet, which required that they restrict their intake of several fruits and vegetables, onions, tea, chocolate, and dried beans, for one week prior to the baseline visit and for the duration for the study. At baseline, subjects underwent neuroimaging and neurocognitive testing (described later). We also acquired blood samples to perform the antioxidant assay and to verify compliance with the protocol. Subjects were then randomized to either the pomegranate or placebo groups; investigators and subjects were blind to group membership.
After these procedures, subjects were given a one-week supply of 8-ounce containers of pomegranate or placebo juice with instructions to consume 8 ounces of juice daily. The pomegranate drink was the commercial Pom Wonderful product. The flavor-matched drink contained sugar, citric acid, and food color to match the calorie, taste, and color of the juice. Each week, subjects returned their empty juice containers to ensure compliance, picked up their juice supply for the following week, and met briefly with the dietitian to ensure compliance with the low polyphenol diet. At the end of the study (day 28), subjects again underwent neuroimaging, cognitive assessment, and blood draws.
A placebo drink using sugars and natural colorings and flavoring to imitate the taste and appearance of pomegranate juice was used. The research personnel were blinded as to the assignment of subjects to placebo or pomegranate juice.
2.3. Behavioral Testing Procedures
Each subject underwent fMRI and brief memory testing immediately prior to beginning the trial and following 28 days of pomegranate juice or placebo. Subjects received a memory testing battery and underwent functional MRI scanning on the same day. Memory tests administered were the Buschke-Fuld selective reminding task [44], from which we calculated two performance measures, total items recalled and consistent long-term retrieval; an experimental unrelated word pair associates learning task [36], based on the Wechsler memory scale paired associates learning [45] in which we used identical procedures to that used in the fMRI scan but with different stimuli. We also obtained behavioral data from the MRI tasks administered in the scanner (described below). Of the 28 subjects completing the study, 26 had behavioral data on both occasions (two did not participate in one testing session; their remaining behavioral data were eliminated from analysis). Subjects were administered one of two alternate forms of each task, with the order of forms counterbalanced across subjects, to avoid practice effects. To assess changes in verbal memory, we compared performance on total number of items recalled (TR) on the Buschke-Fuld selective reminding task (sum of all items recalled on all trials) and on the consistent long term retrieval (CLTR) score (sum of items recalled in consecutive trials without item repetition). The TR score emphasizes immediate recall, while CLTR depends upon memory consolidation processes. To determine memory change, we first examine the _t_2 versus _t_1 difference in scores within groups using a paired _t_-test. To determine whether the treatment effects differed between groups, we then tested for differences between the pomegranate juice and placebo groups using a 2-group unpaired _t_-test. Because memory performance in this age range is highly variable, we used time 2 minus time 1 difference scores as our dependent variables. At baseline, there were no differences between groups on either TR performance (two-tailed t = .8; P ≥ .4) or CLTR (two-tailed t = .5; P > .9). For this reason our analysis did not covary baseline performance; instead we performed a _t_-test of group differences on the change scores.
2.4. Plasma Biomarkers
Plasma obtained from all subjects prior to and at the end of the trial was tested for TEAC and urolithin A-glucuronide. The trolox-equivalent antioxidative capacity (TEAC) was determined using 2′, 2′-azinobis(3-thylbenzo thiazoline-6-sulfonic-acid) diammonium (ABTS) radical cations oxidized with by adding solid manganese dioxide in 75 mmol/L sodium/potassium phosphate (Na/K) buffer of pH 7. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water-soluble analog of vitamin E, was used as an antioxidant standard. Plasma was diluted 1 : 20 in Na/K buffer pH 7. Diluted plasma samples were assayed in four replicates. TEAC values were calculated from the trolox standard curve (0–350 _μ_mol/L) and expressed as trolox equivalents (in mmol/L) [46]. Plasma urolithin A-glucuronide was measured using high performance liquid chromatography mass spectrometry (LC-MS) as described previously [47]. Of all biomarker data across the two time points, only three data points were missing (one placebo subject, TEAC Day 0; two PJ subjects, UA-Glu day 28); the calculations were therefore based on the remaining subjects. Plasma TEAC was equivalent between groups at baseline (P > .17; see Table 1), so we calculated the percent change in TEAC for each subject and compared the change scores.
2.5. MRI Procedures
Functional and structural images were collected on a 3T Siemens Trio MRI scanner using a 32-channel head coil. Structural MRI was collected using a _T_1-weighted magnetization prepared rapid gradient echo (MPRAGE) volumetric scan sequence using axial acquisition slicing, TR = 2.3 sec, TE = 2.93 msec, field of view = 256 × 256 mm2, flip angle = 8°, resolution 160 × 192 × 192, voxel size = 1.5 mm3. Scans were reviewed to rule out subjects with brain abnormalities (evidence of stroke, structural lesion, or atrophy indicative of dementia).
Functional MRI was collected using a _T_2*-weighted echoplanar pulse sequence with a TR = 2.5 sec, TE = 35 msec, field of view = 200 × 200 mm, flip angle = 90°, collecting whole brain images with resolution = 64 × 64 × 28, voxel size = 3 mm3. For localization, image registration, and spatial normalization, we collected a coplanar, matched-bandwidth, spin-echo echoplanar scan on each subject (TR = 5000 ms, TE = 33 ms, 128 × 128 matrix size). Experimental tasks (described below) were presented via magnet compatible video and audio system (Resonance Technologies, Inc, Northridge, CA), which included video goggles with lenses for vision correction optimized for each subject. Responses were recorded and made using a button press. Stimuli were presented via a Macintosh computer running MATLAB.
For the functional MRI tasks, subjects received computer training of tasks prior to scan sessions. Two memory tasks reported previously by our group were presented during scanning: a verbal memory task (unrelated word-pair associates learning) [36] and a visual memory task (spatial navigation) [48]. Following 28 days of intervention (_t_2), the fMRI and behavioral testing procedures were repeated and compared to _t_1 using alternate forms.
2.6. Verbal Memory Task
The unrelated word-pair task was identical to that previously reported by Bookheimer et al. [36]. Briefly, subjects were presented seven pairs of unrelated words (e.g., table flower) in six learning blocks, interspersed with baseline fixation and retrieval blocks. During each learning block, subjects heard each word pair presented over two seconds with a two-second interval before the next pair (total block length = 28 sec). Following a 20-second fixation baseline, they performed the retrieval task in which they were presented the first word in each pair and asked to generate the matching word. The word lists were matched for word frequency, imageability, and form. Behavioral performance was collected at the end of each scan and in an alternate form administered outside the scanner to obtain a learning curve. The total scan length was 9 minutes.
2.7. Visual Memory Task
The spatial navigation task involved learning and recalling routes on a virtual map, while subjects play the role of a “taxi driver.” During the encoding phase, subjects watched and rewatched two sets of video clips. In each video, subjects first saw the name of the imaginary town, such as “Johnsberg,” then for 15 second blocks they saw a first-person view of “driving” to a virtual town laid out in 5 × 5 block grids, to a storefront destination with unique characteristics for specific storefronts, stopping for 3 seconds before proceeding to a new store within the same town starting from a different location. After each city, subjects performed a 30-second distractor task where they pressed a left or right button in response to a probe. This was repeated while subjects learned 6 cities in total.
The fMRI data were then collected during memory retrieval in three conditions. Subjects were asked to remember one of three characteristics about each storefront for the separate tasks. Two conditions focused on the physical features of the environment (specific stores-item task and physical placement-location task). In condition 1, participants were asked to identify an appearance change of the store (identification), such as signs, colors, and doors. In condition 2, they were asked to identify the spatial location change of the store within the town, relative to other buildings (location identification), respectively. Condition 3 required subjects to identify the store (source) for a specific item that might be purchase there, requiring the participant to encode the association between an item and a spatial location. For this source task, subjects had to recall whether the store was in the first or second town (memory for object-location associations). Only the latter condition tapped into the associative memory processes. The order of the three conditions was counterbalanced across subjects.
2.8. fMRI Analysis
All functional data were analyzed using FSL version 4.1.4 (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian kernel FWHM = 5 mm), and high-pass temporal filtering (t > 0.01 Hz). Subjects with head motion exceeding an average of 2 voxels were removed from analysis. Twenty-three (13 pomegranate juice, 10 placebo) subjects were able to complete the visual memory fMRI task without significant head motion, while for the verbal memory task, eight pomegranate juice and nine placebo subjects were able to complete both scanning sessions with high quality images. Each functional scan was registered to its corresponding coplanar high-resolution image with rigid body transformations and to the MNI152 standard brain using nonlinear transformation (12 degrees of freedom). Additionally, a high-pass temporal filtering of 100 sec was applied to the functional images. Individual preprocessing and statistical analysis of each subject's functional scan was run using FSL FMRI Expert Analysis Tool (FEAT); FEAT was then used to analyze data at the group level. Without strong a priori hypotheses about region-specific effects of polyphenols (other than presuming left versus right hemispheric changes for verbal and visual memory tasks, resp.), we corrected for multiple comparisons across the entire brain using FSL's FEAT, correcting at the cluster level. FEAT uses an FWE (family-wise error) cluster-based thresholding using random field theory to correct for multiple comparisons across space. Thresholding defines contiguous clusters; each cluster's estimated significance level from Gaussian random field-theory is compared with the cluster probability threshold.
First, for both the verbal and visual memory tasks, we contrasted task versus baseline for each subject at each time point (before and after treatment). To minimize the number of comparisons, we restricted our analysis to learn versus baseline and recall versus baseline for the verbal memory task and task versus baseline for each of the three visual memory conditions, (item, source, and spatial). Next, for each task, the individual subject data were entered into a second-level analysis of within-group means for each contrast. To test the hypothesis that pomegranate juice and placebo groups differed in _t_2 versus _t_1 activation patterns, between-group, between time point contrasts were generated using an ANOVA model in FSL, cluster-corrected at Z > 2.0, P = .05, and corrected for multiple comparisons. Finally, for each task, the individual subject data were entered into additional analyses of within-group between time point, and between-group within time point.
3. Results
3.1. Peripheral Biomarkers
At baseline, the groups did not differ in antioxidant levels (TEAC; t = 1.37, P > .17). The pomegranate juice group had significantly higher TEAC values after day 28 compared with baseline than the placebo group (unpaired t = 2.8, df = 25, P < .05; Figure 1). Similarly, plasma urolithin A-glucuronide increased significantly in the pomegranate juice, but not in the placebo group (unpaired t = 3.75; df = 24, P < .001). To evaluate compliance, we identified the number of participants whose urolithin A-glucuronide values increased versus remained the same or less after day 28 across the groups. Two of 13 placebo subjects had increased urolithin A-glucuronide values after 28 days versus 11 of 14 pomegranate juice subjects, a difference that was significant (chi-square = 4.8; P < .03).
Figure 1.
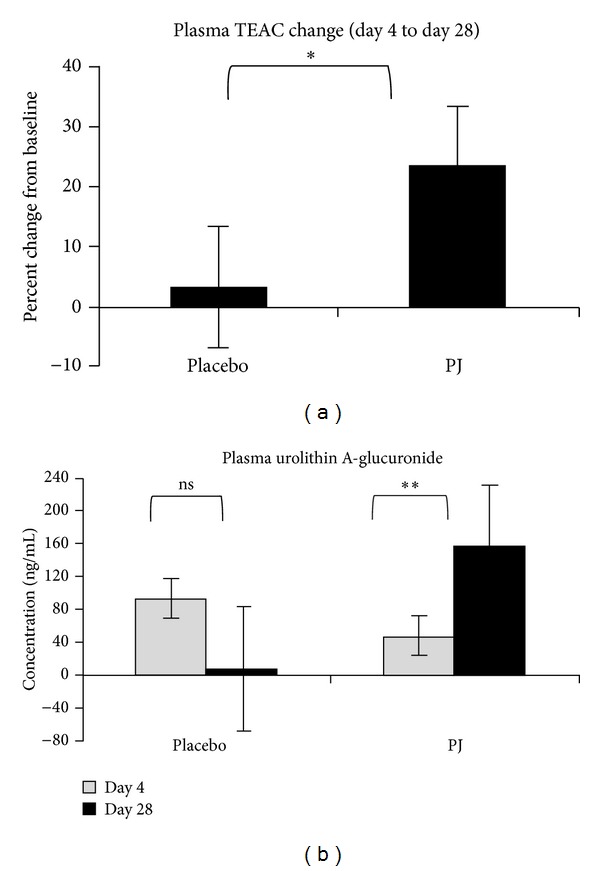
Metabolite results. (a) The pomegranate juice group had significantly higher change in TEAC after day 28 compared with baseline than the placebo group (*unpaired t = 2.8, df = 25, p < .05). (b) Plasma urolithin A-glucuronide significantly increased in the pomegranate juice group (**paired _t_ = 3.93, df = 13, _p_ < .00064), but not in the placebo group (paired _t_ = 1.7, df = 11, _p_ > .09).
3.2. Memory Performance
The within-group analysis of changes following treatment found that, on average, the pomegranate juice group performed significantly better at _t_2 compared with _t_1 (using alternate test forms; two-tailed t = 2.8, P = .017). For the placebo group, there was no significant difference between _t_2 and _t_1 performance scores (two-tailed t = 1.1, P > .3).
Between groups, the _t_2 versus _t_1 improvement in memory scores was significantly greater in the pomegranate juice group compared to the placebo group on the total recall measure (two-tailed t = 2.3; P = .029; Figure 2). Similarly on consistent long-term retrieval the pomegranate juice group recalled more items compared to the placebo group (two-tailed t = 2.4; P = .022; Figure 2).
Figure 2.
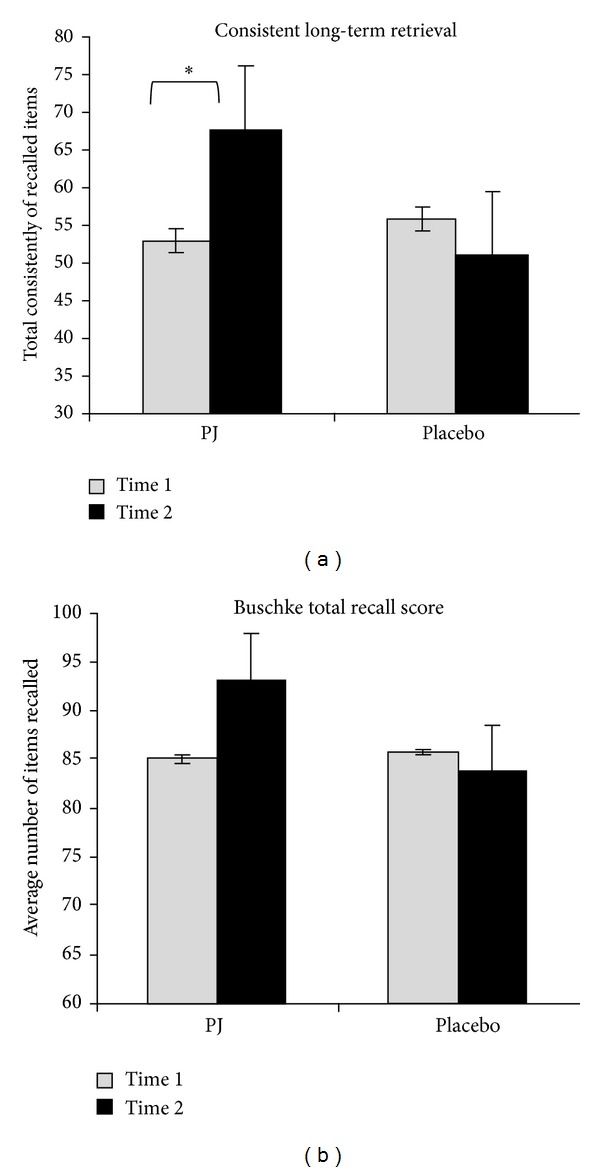
Buschke selective reminding test results. (a) On the total recall measure, the _t_2 versus _t_1 change in memory scores was significantly greater in the pomegranate juice group compared to the placebo group (mean change, number of items recalled: PJ = 7.7, placebo = −2.77; t = 2.3; P = .029). (b) Similarly, on the consistent long-term retrieval score the pomegranate juice group recalled more items compared to the placebo group (mean change, number of items consistently recalled: PJ = 15.2, placebo = −9.7; t = 2.4; P = .022).
3.3. Imaging Results
Performance on the visual and verbal memory tasks during scanning did not differ between groups at either timepoint (all P values > .1); thus subsequent analyses of activation differences could not be attributed to differential performance.
3.3.1. Visual Memory fMRI Task
Looking at within-group means for both groups and at both time points, we found significant activation in visual pathways including bilateral occipital cortex extending into temporal fusiform and parahippocampal gyrus, as well as activation in subcortical region across the three task conditions, consistent with visual memory processing and spatial navigation [48].
To test the hypothesis that brain activity during a visual memory task increased for the treatment group exclusively, we first performed a whole brain, voxel-wise ANOVA comparing the PJ and control groups on the _t_2 versus _t_1 contrast, corrected for multiple comparisons and thresholded at Z > 2.0. Results of the ANOVA are shown in Figure 3; coordinates of active brain regions are listed in Table 2. The pomegranate group showed greater fMRI activation than the placebo group in the _t_2 versus _t_1 contrast, located bilaterally in regions of the basal ganglia and thalamus. At a lower threshold of Z = 1.7 (P < .05), additional regions in the left inferior frontal gyrus and left middle frontal gyrus were recruited. In contrast and as predicted, no brain regions were more active in _t_2 versus _t_1 for the placebo group compared to the pomegranate group (no voxels exceeded threshold).
Figure 3.
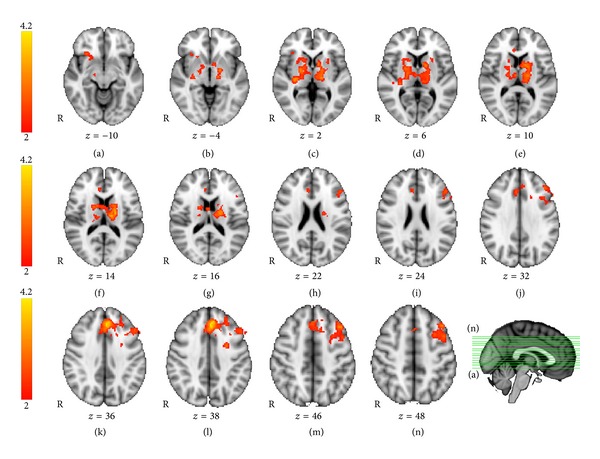
Visual memory fMRI task: between-groups _T_2 versus _T_1 ANOVA. Regions showing greater activation for _t_2 > _t_1, for the pomegranate juice group > placebo group (group by time interaction), were found bilaterally in the basal ganglia and thalamus, including caudate, putamen, and pallidum (ANOVA, Z > 2.0, P = .05, corrected for multiple comparisons). Additional regions significant at an uncorrected threshold (Z > 1.7) are listed in Table 2.
Table 2.
Spatial memory fMRI activation clusters. ANOVA results showing the coordinates of maximally activated voxels of clusters for pomegranate juice group > placebo group for _t_2 greater than _t_1 (Z > 2.0, corrected P = 0.05). No regions were found for placebo > pomegranate for _t_2 > _t_1.
| Spatial memory task | MNI coordinates (mm) | |||
|---|---|---|---|---|
| x | y | z | Z stat | |
| Z > 2.0 | ||||
| Subcortical regions | ||||
| Right thalamus | 18 | −14 | 8 | 2.60 |
| Right pallidum | 22 | −4 | 2 | 2.98 |
| Right putamen | 30 | −20 | 0 | 3.51 |
| Left thalamus | −12 | −14 | 10 | 3.44 |
| Left pallidum | −18 | −4 | −2 | 3.34 |
| Left caudate | −12 | 4 | 10 | 3.06 |
| Z > 1.7 | ||||
| Frontal Lobe | ||||
| Left paracingulate gyrus, superior frontal gyrus | −4 | 36 | 38 | 4.23 |
| Left middle frontal gyrus | −38 | 24 | 44 | 3.19 |
| Left middle frontal gyrus | −26 | 2 | 40 | 3.1 |
| Left precentral gyrus | −44 | 0 | 52 | 3.06 |
| Left inferior frontal gyrus, pars opercularis | −42 | 12 | 28 | 2.78 |
| Left middle frontal gyrus, frontal pole | −46 | 32 | 28 | 2.75 |
| Subcortical | ||||
| Right putamen | 30 | −20 | 0 | 3.49 |
| Right pallidum | 26 | −20 | 0 | 3.25 |
| Left pallidum | −18 | −6 | 0 | 3.15 |
| Left Thalamus | −16 | −14 | 12 | 3.67 |
| Left Thalamus | −14 | −18 | 0 | 3.39 |
To further examine the effects of the PJ intervention on visual memory, we performed a secondary analysis comparing the PJ versus placebo groups for the visual memory versus control contrast at _t_2 only. This analysis directly compares visual memory processing between groups after treatment. The results of this analysis are shown in Figure 4. Using unpaired _t_-tests for comparing patterns of activation during the posttreatment scan between groups, the pomegranate group showed significantly greater activation than the placebo group in right occipital and right fusiform regions, extending into parahippocampal cortex (Figure 4). These regions are typically engaged during visual navigation memory tasks, indicating greater recruitment of visual memory regions in the treatment group compared with the placebo subjects. In contrast, and as expected, the placebo group did not activate any brain regions significantly more than the pomegranate group (no voxels exceeding threshold).
Figure 4.
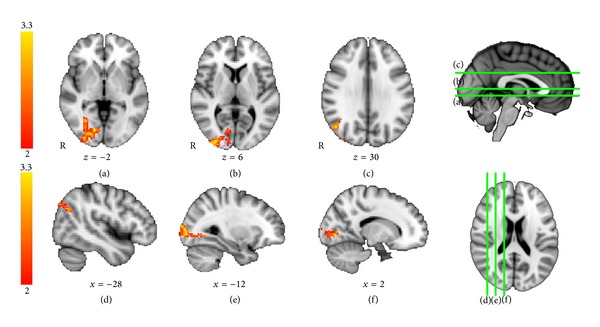
Visual memory fMRI task: between-groups _T_2. Activations for time point, _t_2, for pomegranate > placebo. For the visual memory task, at _t_2, between groups, pomegranate > placebo, there were significant activations in right lateral occipital cortex and fusiform gyrus (unpaired _t_-test, Z > 2.0, P = .05, corrected for multiple comparisons).
3.3.2. Verbal fMRI Memory Task
We first identified brain regions activated during the verbal memory task compared to the baseline condition across all subjects and time intervals. Consistent with prior studies, the task verbal memory activated a wide network of brain regions, including bilateral occipital cortex, frontal lobe, and medial temporal lobe and subcortical regions including thalamus, putamen, and the hippocampus. Of note, for the verbal task, the pomegranate group activated basal ganglia, thalamus, and hippocampus in the posttreatment session and the placebo group did not (Figure 5).
Figure 5.
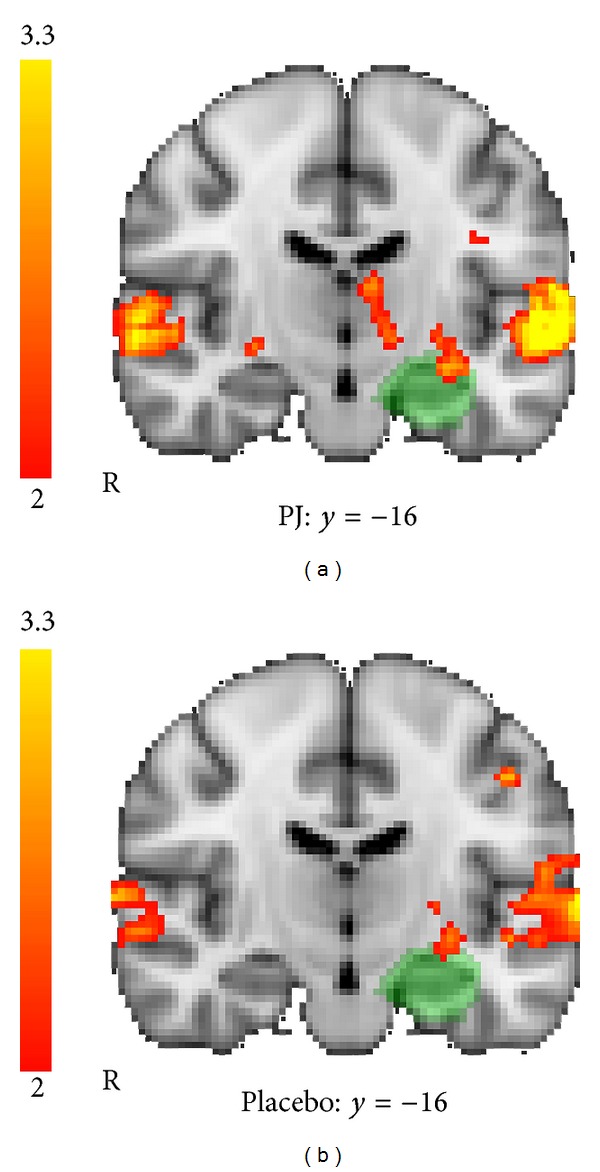
Verbal memory fMRI task: within-group activations at _t_2. Comparing the within-group results for time point _t_2, there were activation similarities and differences in the placebo group ((a): top) and the pomegranate group ((b): bottom). Only the pomegranate group recruited the hippocampus, bilaterally (group mean: placebo, Z > 2.0, P = .05, corrected for multiple comparisons, pomegranate, Z > 2.0, P = .05, corrected for multiple comparisons).
We then compared the group by time interaction of fMRI activations for the PJ versus control group differences using ANOVA, identifying brain regions that showed greater fMRI activation signal after treatment, for _t_2 versus _t_1. For the verbal learning versus control contrast, we found that the PJ group showed significantly greater increases in activation compared to the placebo group for _t_2 versus _t_1. Regions showing differences were found in the left hemisphere, including regions of the left occipital lobe and left fusiform gyrus (Z > 2.0, P = .05, corrected for multiple comparisons), shown in Figure 6 and listed in Table 3. By contrast, but as expected, there were no regions in which the placebo group showed greater activation than the pomegranate juice group for _t_2 > _t_1.
Figure 6.
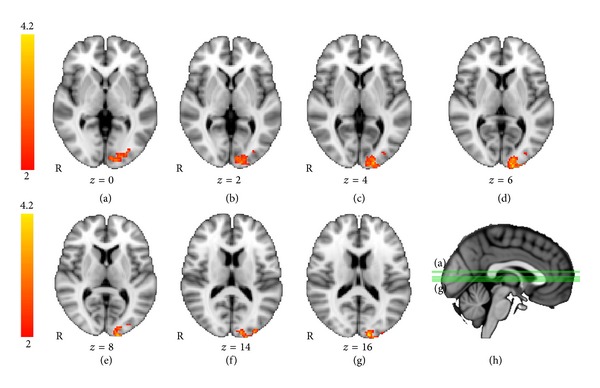
Verbal memory fMRI task: between-groups _T_2 versus _T_1 ANOVA. Regions showing greater activation for _t_2 > _t_1, for pomegranate group > placebo group, were found in the left hemisphere in occipital polar regions (ANOVA, Z > 2.0, P = .05, corrected for multiple comparisons).
Table 3.
Verbal memory fMRI activation CLusters. ANOVA results showing the coordinates of maximally activated voxels of clusters for pomegranate juice group > placebo group for _t_2 greater than _t_1 (Z > 2.0, corrected P = 0.05). No regions were found for placebo > pomegranate for _t_2 > _t_1.
| Verbal memory task | MNI coordinates (mm) | |||
|---|---|---|---|---|
| x | y | z | Z stat | |
| Brain region | ||||
| Occipital lobe | ||||
| Left occipital pole | −14 | −98 | 16 | 3.97 |
| Left fusiform gyrus | −18 | −90 | −4 | 3.55 |
| Left lingual gyrus | −16 | −76 | −6 | 2.82 |
| Left lateral occipital | −32 | −80 | −2 | 2.54 |
To further examine treatment-related effects, we then examined regions of brain activity greater for _t_2 versus _t_1 within groups using paired _t_-tests. Only the pomegranate group showed significant differences in activation in the posttreatment session compared to pretreatment for any brain regions for the learn versus rest condition. Before treatment, the PJ group showed significantly greater fMRI activation in the left inferior temporal gyrus and right occipital lobe compared to the first session (Figure 7). As predicted, the placebo group did not show any regions with significantly greater activation in the second session compared to the first. It is important to note that between groups differences in change over time could be due either to increases in the PJ group or decreases in the placebo group at _t_2 relative to time 1. Within the PJ group, there was greater activation in _t_2 compared to _t_1 and no regions showed the reverse effect; thus the PJ group clearly demonstrated an increase in MRI activation following treatment. However, by examining the reverse contrast (time 1 > time 2), we found that the placebo group only also showed a decrease in fMRI activation at _t_2. Therefore, the ANOVA results appear to reflect both increases in the PJ group and decreases in the placebo group following intervention.
Figure 7.
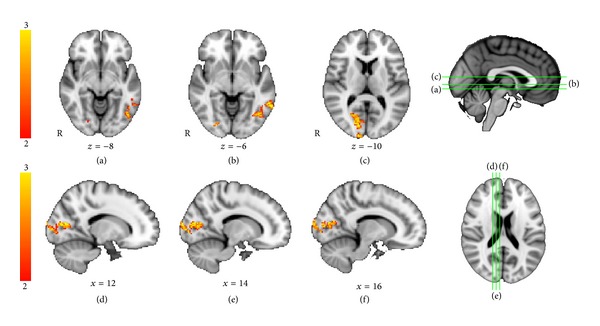
Verbal memory fMRI task: activations for the pomegranate group for _t_2 > _t_1. For the pomegranate group in the verbal memory task, there were significant activations between sessions, in the left inferior temporal gyrus and right occipital lobe (paired _t_-test between time points, Z > 2.0, P = .05, corrected for multiple comparisons). No regions were more active for _t_2 versus _t_1 in the placebo group.
4. Discussion
Our results support the hypothesis that polyphenols derived from pomegranate juice may improve memory in older persons with age-related memory decline. Figure 8 summarizes our major findings integrating the hypothesized mechanism for effects of pomegranate juice on polyphenol metabolites, task-related cerebral blood flow, and memory performance. Peripheral measures of TEAC and urolithin A-glucuronide indicated that our subjects were compliant with their regimen and that the dose administered was sufficient to increase polyphenol metabolites. Moreover, memory assessments indicated that subjects who drank 8 ounces of pomegranate juice for a month experienced significant improvements on a sensitive measure of memory performance. As in fMRI studies of pharmacological interventions in memory-impaired subjects, the pomegranate juice intervention activated only memory-related brain regions, with no increases in the control group and no decreases in activation following intervention in the pomegranate juice group. Taken together, these findings support the hypothesis that drinking pomegranate juice may increase task-related brain activation and improve memory ability in older adults with preexisting mild memory complaints.
Figure 8.
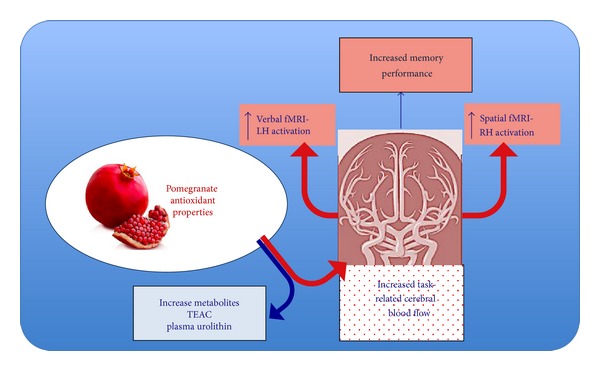
Summary of study results. Our data suggest that the antioxidant properties of pomegranate produce increased task related cerebral blood flow, with lateralized effects for verbal versus visual memory challenge, which in turn increased cerebral blood flow facilitates memory performance. Metabolic measures confirm the increase in polyphenols among the experimental group.
These findings are also consistent with animal studies showing positive effects of polyphenols on memory laboratory animals; previous studies have shown that combating oxidative stress through berry extract supplementation attenuates age-related cognitive deficits in aged rodents [26, 27, 49, 50]. Joseph and coworkers found that such supplementation reversed age-related deficits in working memory performance in the Morris water maze [49]. Other studies have shown that blueberry supplementation was effective in reversing cognitive declines in object recognition [51] Possible mechanisms for this effect of polyphenol supplementation include antioxidant, direct effects on signaling to enhance neuronal communication [50], and the ability to buffer against excess calcium [50]. These animal studies showing putative polyphenol antioxidant effects on cognitive led to our choice of pomegranate juice as an intervention, given the high polyphenol antioxidant properties of pomegranates [52].
The changes in fMRI activity in the pomegranate group are consistent with results seen on pharmacological trials in patients with mild cognitive impairment and Alzheimer's dementia. It is striking that both the verbal and nonverbal memory tasks showed virtually exclusive increases in activity following intervention (there were some parietal increases for placebo). Saykin and colleagues [41] found that short-term treatment with the cholinesterase inhibitor donepezil enhanced frontal circuitry activity in patients with amnestic mild cognitive impairment, and this increase in activation was related to improved cognition. Such increases may occur because the subjects can better focus on the cognitive task (increased cognitive effort) or alternatively may indicate greater task-related increases in cerebral blood flow. This fMRI method cannot distinguish between these possibilities; however, changes in task strategy tend to produce both task-related increases and decreases in fMRI signal [53, 54], whereas an hypothesis of increased blood flow responses would tend to predict only fMRI increases. Our data show evidence that the PJ group showed increases in fMRI activation at _t_2 that were greater than those for placebo, with no regions greater for the placebo group at time 2. However we also found some regions in which the placebo group showed reduced activation at _t_2 compared to _t_1. Thus the group by time interaction appears to reflect both increases in the experimental and decreases in the control group. Reduction in activation with repeated testing in fMRI is a common finding (e.g., [55]); however we can only conclude that there is relatively greater fMRI activation among the PJ group as we do not have absolute measures of cerebral blood flow.
Although the duration of our study was short, there is evidence in the animal literature suggesting that antioxidant use can affect cerebral blood flow, including task-related CBF, acutely and over short time intervals as in the present study. For example, Huang et al. [56] measured CBF, BOLD response to forepaw stimulation, and oxygen metabolic rate (CMRO2) in rats treated with the antioxidant methylene blue USP; they found increases in task-related BOLD response and CBF under normal breathing conditions; Bitner et al. [57] found that antioxidant carbon clusters restored CBF in an experimental traumatic brain injury model in rate under hypotension. The antioxidant black cumin oil administered over 10 weeks improved spatial cognition in a water maze task in rats, a task reminiscent of our spatial navigation task [58]. One recent study in humans found an increase in BOLD activation during a simple attention-switching task in subjects after 5 days of treatment with flavanol-rich cocoa [43]. In addition, they performed a second, preliminary study (N = 4) of acute flavanol administration during arterial spin labeling (ASL) MRI, which provides a quantitative measure of cerebral blood flow, finding increased CBF following flavanol administration. Together, these studies suggest that antioxidants administered either acutely or chronically increase cerebral blood flow and in one animal study also improved spatial memory [55].
It is also notable that the regional distribution of increased activations was largely specific to each task (i.e., left hemisphere for verbal memory, right hemisphere for visual memory). Of the three conditions in the visual spatial navigation task, the intervention significantly affected bold activation pattern in the source memory task. The source task placed particular emphasis on episodic memory, requiring subjects to learn and retrieve novel associations between specific items and the context in which they were encoded (i.e., episodic memory). Similarly, the verbal memory task was designed to tap associative encoding in the verbal domain. In the verbal task, there were increases in left hemisphere regions after treatment, and the pomegranate group showed activation in the temporal lobe in the posttreatment session that was not apparent in the placebo group. Associative, episodic memory tasks rely particularly on temporal lobe systems, which are critically involved in both age-related memory decline and Alzheimer's disease.
The two measures of the Buschke selective reminding task are thought to assay different memory processes. In this test, subjects hear a long list of words and repeat back as many as they can recall; on subsequent trials they are reminded only of those words they did not recall on the last trial (hence, “selective” reminding). The total score reflects all recalls across up to 10 trials until all words are recalled consistently. By contrast, the consistent long-term retrieval score only counts items once they are consistently recalled for all subsequent trials (without reminders) and thus appears to depend upon consolidation processes mediated by the hippocampus. While the scores are not entirely independent (high scores on total recall require consistent retrieval), they are also not fully redundant, as it is possible to have recall scores of up to 80 (approximately the mean of our subjects' performance) while never retrieving a memory item consistently. The subjects in this study improved on both measures suggesting that the intervention may affect cognition broadly. Because our cognitive battery was limited in this preliminary study, however, we cannot tell if other aspects of cognition are affected by pomegranate juice. Thus, future studies should examine the effects of pomegranate juice or extract using a more comprehensive cognitive battery of tests.
Based on this work and the work on animals, we hypothesize that polyphenols (and other antioxidants) enhance resting and task-related cerebral blood flow. Increased metabolic activity should improve cognitive processing efficiency, in this case during memory tasks, increasing memory performance. If correct, we would predict a correlation between the magnitude of increased task-related activation in these brain regions and the degree of memory improvement after treatment. In our study, the number of subjects completing the fMRI procedures without head motion was smaller than that of those completing the memory tests; with the reduced sample size we were unable to make these direct correlations. Further studies with larger samples will allow us to test this model more directly.
Other limitations of this study include the small sample size and brief treatment duration. Thus our results should be considered preliminary and should be validated in a larger scale, multicenter study. Because the functional MRI measure is a relative one and not a direct measure of cerebral blood flow, reduced base-line blood flow could provide an alternative explanation for the results. Differences between groups in the _T_2 versus _T_1 comparison could be due to either increases in task-related blood flow relative to _T_1 in the pomegranate group or to decreases in blood flow at _T_2 in the placebo group. Future studies could measure cerebral blood flow quantitatively using either H2 15O positron emission tomography or arterial spin-labeled MRI. In this study our regional hypotheses were limited to hemispheric specificity for verbal versus visual tasks, given the lack of prior studies in humans. Accordingly we corrected our results for multiple comparisons across the whole brain, which may have been overly conservative. Future studies with larger N's could target specific regions of interest in relation to treatment-related memory changes.
In summary, this preliminary randomized placebo-controlled, double-blind trial of pomegranate juice in older adults with mild memory complaints suggests that 8 ounces of pomegranate juice taken daily over one month improve a sensitive measure of verbal memory and alter neural activity during a visual source memory task. The presence of polyphenol metabolites validated compliance with the experimental regimen and efficacy of pomegranate juice in releasing polyphenols. fMRI results suggest that the mechanisms of change may involve increases in task-related cerebral blood flow, particularly during associative encoding tasks. Future studies are needed to validate these results within larger samples and determine the long-term effects of pomegranate juice on a range of comprehensive cognitive functions.
Acknowledgment
Dr. Bookheimer, Dr. Renner, Dr. Ekstrom, Dr. Li, Dr. Henning, Dr. Brown, Mr. Jones, and Dr. Moody have no relevant financial conflict of interests. Dr. Gary Small has received an unrestricted educational grant from Pom Wonderful, Inc.; this funding was not used to fund the present study and was received after study completion; he has no other relevant financial conflict of interests. Funding for this study was provided by the UCLA Nutrition Department research funds. POM Wonderful or any other corporate entity or representative had no role in design or analysis of this study.
References
- 1.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimer’s and Dementia. 2012;8(1):14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Crook T, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change-report of a National Institute of Mental Health Work Group. Developmental Neuropsychology. 1986;2:261–276. [Google Scholar]
- 3.Larrabee GJ, Crook TH., III Estimated prevalence of age-associated memory impairment derived from standardized tests of memory function. International Psychogeriatrics. 1994;6(1):95–104. doi: 10.1017/s1041610294001663. [DOI] [PubMed] [Google Scholar]
- 4.Seeman T, Chen X. Risk and protective factors for physical functioning in older adults with and without chronic conditions: MacArthur studies of successful aging. Journals of Gerontology B. 2002;57(3):S135–S144. doi: 10.1093/geronb/57.3.s135. [DOI] [PubMed] [Google Scholar]
- 5.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radical Biology and Medicine. 2002;33(5):575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 6.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mechanisms of Ageing and Development. 2004;125(10-11):811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochimica et Biophysica Acta. 2010;1801(8):924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins AJ, Hendrie HC, Callahan CM, et al. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination survey. American Journal of Epidemiology. 1999;150(1):37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- 9.Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54(6):1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 10.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. Journal of the American Medical Association. 2002;287(24):3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. The American Journal of Clinical Nutrition. 2003;77(4):975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 12.Koudinov A, Kezlya E, Koudinova N, Berezov T. Amyloid-β, Tau protein, and oxidative changes as a physiological compensatory mechanism to maintain CNS plasticity under Alzheimer’s disease and other neurodegenerative conditions. Journal of Alzheimer’s Disease. 2009;18(2):381–400. doi: 10.3233/JAD-2009-1202. [DOI] [PubMed] [Google Scholar]
- 13.Summers WK, Martin RL, Cunningham M, Deboynton VL, Marsh GM. Complex antioxidant blend improves memory in community-dwelling seniors. Journal of Alzheimer’s Disease. 2010;19(2):429–439. doi: 10.3233/JAD-2010-1229. [DOI] [PubMed] [Google Scholar]
- 14.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. British Journal of Nutrition. 2010;103(5):730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 15.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidants vitamin supplements: the Cache County study. Archives of Neurology. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. American Journal of Epidemiology. 2004;159(10):959–967. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 17.Jae HK, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Archives of Internal Medicine. 2006;166(22):2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 18.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, Vitamin C, Beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the women’s antioxidant and cardiovascular study. Circulation. 2009;119(21):2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Archives of Neurology. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutrition Research. 2003;23(12):1719–1726. [Google Scholar]
- 21.Wang J, Santa-Maria I, Ho L, et al. Grape derived polyphenols attenuate Tau neuropathology in a mouse model of alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;22(2):653–661. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Yang SG, Du XT, et al. Ellagic acid promotes Aβ42 fibrillization and inhibits Aβ42-induced neurotoxicity. Biochemical and Biophysical Research Communications. 2009;390(4):1250–1254. doi: 10.1016/j.bbrc.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 23.Joseph JA, Denisova NA, Arendash G, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutritional Neuroscience. 2003;6(3):153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 24.West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Developmental Neuroscience. 2007;29(4-5):363–372. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos-Esparza MDR, Torres-Ramos MA. Neuroprotection by natural polyphenols: molecular mechanisms. Central Nervous System Agents in Medicinal Chemistry. 2010;10(4):269–277. doi: 10.2174/187152410793429728. [DOI] [PubMed] [Google Scholar]
- 26.Hartman RE, Shah A, Fagan AM, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiology of Disease. 2006;24(3):506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Choi SJ, Lee J-H, Heo HJ, et al. Punica granatum protects against oxidative stress in PC12 cells and oxidative stress-induced alzheimer’s symptoms in mice. Journal of Medicinal Food. 2011;14(7-8):695–701. doi: 10.1089/jmf.2010.1452. [DOI] [PubMed] [Google Scholar]
- 28.Seeram NP, Aviram M, Zhang Y, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. Journal of Agricultural and Food Chemistry. 2008;56(4):1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 29.Guo C, Wei J, Yang J, Xu J, Pang W, Jiang Y. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutrition Research. 2008;28(2):72–77. doi: 10.1016/j.nutres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Rubio J, Yucra S, Gasco M, Gonzales GF. Doseresponse effect of black maca (Lepidium meyenii) in mice with memory impairment induced by ethanol. Toxicology Mechanisms and Methods. 2011;21(8):628–634. doi: 10.3109/15376516.2011.583294. [DOI] [PubMed] [Google Scholar]
- 31.Papandreou MA, Dimakopoulou A, Linardaki ZI, et al. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behavioural Brain Research. 2009;198(2):352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Weiner MW, Sadowsky C, Saxton J, et al. Magnetic resonance imaging and neuropsychological results from a trial of memantine in Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(4):425–435. doi: 10.1016/j.jalz.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Thompson PM, Hayashi KM, Dutton RA, et al. Tracking Alzheimer’s disease. Annals of the New York Academy of Sciences. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burggren AC, Renner B, Jones M, et al. Thickness in entorhinal and subicular cortex predicts episodic memory decline in mild cognitive impairment. International Journal of Alzheimer’s Disease. 2011;2011:9 pages. doi: 10.4061/2011/956053.956053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saykin AJ, Flashman LA, Frutiger SA, et al. Neuroanatomic substrates of semantic memory impairment in Alzheimer’s disease: patterns of functional MRI activation. Journal of the International Neuropsychological Society. 1999;5(5):377–392. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Archives of General Psychiatry. 2011;68(8):845–852. doi: 10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- 41.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127(7):1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzi M, Beltramello A, Mercuri NB, et al. Effect of memantine on resting state default mode network activity in Alzheimer’s disease. Drugs and Aging. 2011;28(3):205–217. doi: 10.2165/11586440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Francis ST, Head K, Morris PG, Macdonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. Journal of Cardiovascular Pharmacology. 2006;47(supplement 2):S215–S220. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 44.Buschke H, Altman Fuld P. Evaluating storage, retention and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Memory Scale. 3rd edition. San Antonio, Tex, USA: The Psychological Corporation; 1997. [Google Scholar]
- 46.Henning SM, Zhang Y, Seeram NP, et al. Antioxidant capacity and phytochemical content of herbs and spices in dry, fresh and blended herb paste form. International Journal of Food Sciences and Nutrition. 2011;62(3):219–225. doi: 10.3109/09637486.2010.530595. [DOI] [PubMed] [Google Scholar]
- 47.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. Journal of Nutrition. 2006;136(10):2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 48.Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning and Memory. 2007;14(10):645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. Journal of Neuroscience. 1999;19(18):8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph JA, Denisova NA, Arendash G, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutritional Neuroscience. 2003;6(3):153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 51.Goyarzu P, Malin DH, Lau FC, et al. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutritional Neuroscience. 2004;7(2):75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- 52.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. International Journal of Cancer. 2005;113(3):423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 53.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 54.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cerebral Cortex. 2005;15(3):303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- 55.Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. NeuroImage. 2004;23(4):1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 56.Huang S, Du F, Shih YY, Shen Q, Gonzalez-Lima F, Duong TQ. Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. Neuroimage. 2013;72:237–242. doi: 10.1016/j.neuroimage.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bitner BR, Marcano DC, Berlin JM, et al. Antioxidant carbon particles improve cerebrovascular dysfunction following traumatic brain injury. ACS Nano. 2012;6(9):8007–8014. doi: 10.1021/nn302615f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azzubaidi MS, Saxena AK, Talib NA, Ahmed QU, Dogarai BB. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiologiae Experimentalis. 2012;72(2):154–165. doi: 10.55782/ane-2012-1888. [DOI] [PubMed] [Google Scholar]
- 59.Francis ST, Head K, Morris PG, Macdonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. Journal of Cardiovascular Pharmacology. 2006;47(supplement 2):S215–S220. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]