Alcoholic Liver Disease and Malnutrition (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 12.
Abstract
Malnutrition, both protein energy malnutrition (PEM) and deficiencies in individual nutrients, is a frequent complication of alcoholic liver disease (ALD). Severity of malnutrition correlates with severity of ALD. Malnutrition also occurs in patients with cirrhosis due to etiologies other than alcohol. The mechanisms for malnutrition are multifactorial, and malnutrition frequently worsens in the hospital due to fasting for procedures and metabolic complications of liver disease, such as hepatic encephalopathy. Aggressive nutritional support is indicated in inpatients with ALD, and patients often need to be fed through an enteral feeding tube to achieve protein and calorie goals. Enteral nutritional support clearly improves nutrition status and may improve clinical outcome. Moreover, late-night snacks in outpatient cirrhotics improve nutritional status and lean body mass. Thus, with no FDA-approved therapy for ALD, careful nutritional intervention should be considered as frontline therapy.
Keywords: Malnutrition, Alcohol, Liver, Micronutrients, Encephalopathy
Malnutrition IS A major complication of alcoholic liver disease (ALD), and this has best been studied in patients with alcoholic hepatitis. Malnutrition worsens clinical outcome in ALD, and nutritional support improves nutritional status and may improve clinical outcome. It is appropriate that an article on malnutrition be included in this symposium honoring Dr. Charles Lieber, a physician scientist who made many of the seminal discoveries concerning critical alcohol:nutrient interactions in the development of ALD. This article will review: (i) methods for diagnosing malnutrition and assessing nutrition status in ALD; (ii) the prevalence of malnutrition in ALD; (iii) potential mechanisms for malnutrition; and (iv) nutritional intervention in ALD.
ASSESSMENT OF NUTRITIONAL STATUS
When evaluating the prevalence of malnutrition in ALD, it is important to use tests that accurately define nutritional status. Unfortunately, assessment of nutritional status in patients with liver disease is often quite difficult. Tests that are most frequently used include: serum visceral protein concentrations, some assessment of immunity (total lymphocyte count or delayed hypersensitivity), anthropometry, percentage of ideal body weight, creatinine-height index, dietary history, muscle strength, subjective global assessment, and, in more sophisticated clinical settings, bioelectric impedance, and body composition determinations (Table 1). Unfortunately, almost all of these tests can be influenced either by underlying liver disease or by factors that may be causing the liver disease, such as chronic alcohol consumption (possibly with superimposed Hepatitis C infection). Visceral proteins are probably the tests most frequently used for evaluating nutritional status, especially protein malnutrition. The visceral proteins such as albumin, prealbumin, and retinol binding protein are all produced in the liver and correlate better with severity of underlying liver disease than with malnutrition status (Merli et al., 1987). Alcohol and viral infection can influence immune function, and edema and ascites can influence anthropometry and bioelectric impedance (Campillo, 2010; Guglielmi et al., 1991; McCullough et al., 1991; O’Keefe, 1980; Shronts, 1998). Impaired renal function frequently occurs in more severe liver disease and influences indicators such as creatinine-height index (Pirlich et al., 1996). Thus, no ideal single indicator of malnutrition in liver disease exists, and often the subjective global assessment in conjunction with a combination of tests is most appropriate for the individual patient and will provide the best possible evaluation (Baker et al., 1982; Campillo, 2010; Detsky, 1987). The subjective global assessment evaluates protein energy malnutrition (PEM) based on clinical findings such as muscle wasting, edema, loss of subcutaneous fat, and glossitis/cheilosis. Malnutrition is clearly evident using subjective global assessment in an alcoholic cirrhotic (Fig. 1_A_) with muscle wasting and ascites, and this markedly improved with 2 years of abstinence (Fig. 1_B_). An evaluation of the various tests of malnutrition in ALD has recently been reviewed in detail by Campillo (2010).
Table 1.
Tests of Nutritional Status/Malnutrition in ALD
- Anthropometry (e.g., triceps skinfold thickness)
- 24-h urinary creatinine height index
- Assessment of muscle strength
- Bioelectrical impedance
- Dual-energy X-ray absorptiometry
- Biological parameters (e.g., visceral proteins)
- Subjective global assessment
- Energy balance
Fig. 1.
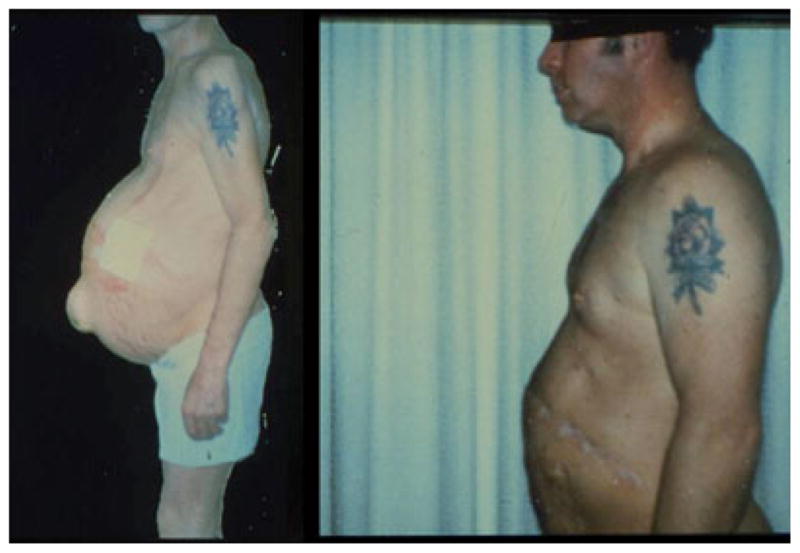
Patient with alcoholic cirrhosis with severe PEM as determined by subjective global assessment with ascites and muscle wasting (left panel). This was corrected with 2 years of abstinence (right panel).
PREVALENCE OF MALNUTRITION IN ALD
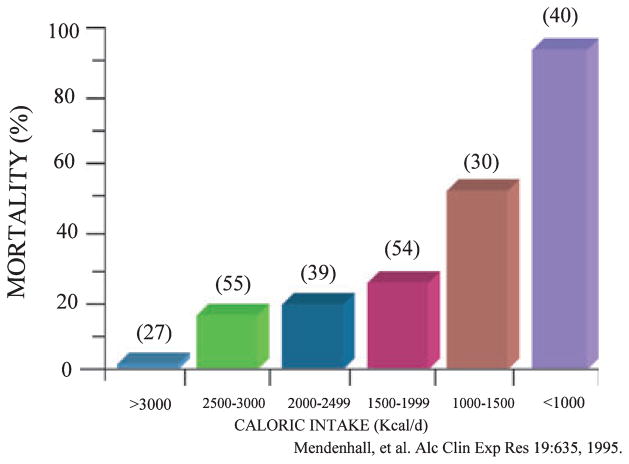
Probably the most extensive studies of nutritional status in patients with liver disease are in patients with ALD. The most detailed reports are two large studies from the Veterans Health Administration (VA) Cooperative Studies Program in patients having alcoholic hepatitis (Mendenhall et al., 1984, 1986, 1995a, 1995b). The first of these studies (Study #119) demonstrated that virtually every patient with alcoholic hepatitis had some degree of malnutrition (Mendenhall et al., 1984). Patients (284 with complete nutritional assessments) were divided into groups with mild, moderate, or severe alcoholic hepatitis based on clinical and biochemical parameters. Patients had a mean alcohol consumption of 228 g/d (with almost 50% of energy intake coming from alcohol). Thus, while calorie intake was frequently not inadequate, there was often deficient intake of protein and critical micronutrients. The severity of liver disease generally correlated with the severity of malnutrition. Similar data were generated in a follow- up VA study on alcoholic hepatitis (Study #275) (Mendenhall et al., 1993). In both of these studies, patients were given a balanced 2500-kcal hospital diet (monitored carefully by a dietitian) and encouraged to consume the diet. In the second study, patients in the therapy arm of the protocol also received an enteral nutritional support product high in branched-chain amino acids (BCAAs) as well as the anabolic steroid oxandrolone (80 mg/d). Patients were not fed by tube if voluntary oral intake was inadequate in either study (probably a study design flaw in retrospect). Voluntary oral food intake correlated in a stepwise fashion with 6-month mortality data. Thus, patients who voluntarily consumed >3000 kcal/d had virtually no mortality whereas those consuming <1000 kcal/d had >80% 6-month mortality (Fig. 2) (Mendenhall et al., 1995a). Moreover, the degree of malnutrition correlated with the development of serious complications such as encephalopathy, ascites, and hepatorenal syndrome (Mendenhall et al., 1995a). In the VA Cooperative Studies the chronic alcohol-consuming control population without liver disease also frequently had some degree of protein-energy malnutrition. This is in contrast with some other studies in which only alcoholics with underlying liver disease demonstrated significant protein-energy malnutrition (Antonow and McClain, 1985).
Fig. 2.
Mortality correlated in a dose-response fashion with voluntary calorie intake in patients with alcoholic hepatitis. Numbers in parentheses indicate the number of patients.
Because both of these VA studies evaluated patients with an acute inflammatory response (hepatitis), it was important to determine nutritional status in patients with stable alcoholic cirrhosis without alcoholic hepatitis. We evaluated patients with stable cirrhosis followed in an ascites clinic who were not actively drinking, were free of alcoholic hepatitis, and had bilirubin levels <51 mmol/l (3mg/dl). They had indicators of malnutrition almost as severe as those in patients with alcoholic hepatitis (e.g., a creatinine-height index of 71% of normal) (Antonow and McClain, 1985).
It is possible that alcohol, rather than the underlying liver pathology, could be the critical variable in malnutrition in liver disease. There have been several major studies evaluating patients having both ALD and nonalcoholic (especially viral) induced liver disease (Caregaro et al., 1996; DiCecco et al., 1989; Lolli et al., 1992; Sarin et al., 1997; Thuluvath and Triger, 1989a, 1989b). These reports from various countries present consistent findings that no difference in malnutrition occurred between alcoholic and non-alcohol-related causes of cirrhosis. For example, Sarin et al. (1997) demonstrated that protein-energy malnutrition was equally severe in alcoholic and non-alcoholic liver disease and that dietary intake decreased equally in both diseases. Caregaro and colleagues (1996) from Italy found that the prevalence, characteristics, and severity of PEM was comparable in alcoholic and viral-induced cirrhosis. Importantly, malnutrition correlated with the severity of the liver disease. Thus, multiple studies suggest that the degree of liver injury rather than the etiology is critical in the development of nutritional disorders. Therefore, important information on nutrition which has been generated in patients with ALD appears to translate to other forms of cirrhosis, especially cirrhosis due to Hepatitis C.
CAUSES OF MALNUTRITION IN ALD
Multiple factors combine to cause malnutrition in patients with liver disease (Table 2). Poor nutritional intake can result from gastrointestinal disturbances, prolonged periods eating nothing during hospitalizations for complications of cirrhosis, and iatrogenic causes. Maldigestion and malabsorption may occur in liver disease and play an important role in causing malnutrition. Typical gastrointestinal disturbances in liver disease include dysgeusia, anorexia, nausea, and early satiety (Madden et al., 1997). Although the exact pathophysiology of how liver dysfunction causes these manifestations is still debated; local and systemic neurohormonal mechanisms are likely involved in causing delayed gastric emptying, small bowel dysmotility and bacterial over-growth, and constipation (Galati, 1994, 1997; Isobe, 1994; Quigley, 1996; Thuluvath and Triger, 1989a, 1989b). Importantly, liver transplantation improves or reverses many of these gastrointestinal manifestations (Madrid, 1997). Concomitant complications typical of liver disease such as upper gastrointestinal bleeding, portal systemic encephalopathy, and sepsis also cause prolonged periods of poor oral intake. Dietary management of fluid retention with salt and water restriction; dietary management of encephalopathy with protein restriction; and carbohydrate and lipid restrictions used in patients with diabetes mellitus, chronic pancreatic insufficiency, and cholestatic liver disease can all affect diet palatability and can severely restrict patients’ food choices.
Table 2.
Causes of Malnutrition
- Anorexia
- Altered taste/smell
- Nausea/vomiting
- Diarrhea/malabsorption
- Poor food availability/quality
- Metabolic disturbances (e.g., hypermetabolism/catabolism)
- Cytokine effects
- Complications of liver disease (PSE, ascites, GI bleeding)
- Unpalatible diets (low Na, protein)
- Fasting for procedures
It is important in hospitalized patients to exert great care to improve nutritional status rather than inadvertently compounding the problem (with long periods of fasting for procedures, unpalatable low-sodium diets, etc.). This concern is highlighted by the fact that 67% of patients in a VA Cooperative Study on alcoholic hepatitis did not consume the recommended 2500 kcal/d even though these patients received expert care by nutritionists and hepatologists who knew that nutrition was a major outcome variable of the study (Mendenhall et al., 1995b). Thus, hospitalized patients with ALD often have their nutritional status worsen due to inadequate food intake.
Impaired lipid metabolism is multifactorial in liver disease. Decreased intraluminal bile salts, small bowel bacterial overgrowth, coexistent pancreatic insufficiency or intestinal disease (inflammatory bowel disease, sprue), and mucosal vascular hypertension and edema can worsen maldigestion and malabsorption. Cholestatic liver disorders are associated with decreased intraluminal concentration of bile salts resulting in lipid and lipid-soluble vitamin malabsorption (Vlahcevic, 1971).
Glycogen stores are depleted in cirrhotic livers. This can result in peripheral muscle proteolysis to provide amino acids for gluconeogenesis, thus contributing to protein malnutrition. Thus, patients with alcoholic cirrhosis go into an early starvation mode after only about 12 h of fasting, while this takes about 2 days in normal subjects (Plank et al., 2008; Swart et al., 1989). Liver disease patients with portal hypertension and ascites and those with acute alcoholic hepatitis are at increased risk of developing a hypermetabolic state (resting energy expenditure >110% of its expected value), which contributes to overall malnutrition (Dolz, 1991; John et al., 1989; Muller, 1992).
Low-grade endotoxemia facilitated by portal hypertension and gut bacterial translocation leads to a low-grade increase in proinflammatory cytokines that further affects nutrient management and overall metabolism (McClain et al., 1999). Dysregulated cytokine metabolism [with elevated proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 8 (IL-8)] is well documented in many forms of liver disease, with ALD having been studied in the greatest detail (Hill et al., 1992, 1993; McClain and Cohen, 1989; McClain et al., 1999, 2004). Increased levels of cytokines have been postulated to cause many of the metabolic and nutritional abnormalities observed in liver disease, especially in acute alcoholic hepatitis and in more decompensated liver disease (Hill et al., 1992, 1993; McClain and Cohen, 1989; McClain et al., 1999, 2004). Thus, abnormalities such as fever, anorexia, muscle breakdown and wasting, and altered mineral metabolism are likely to be at least partially cytokine mediated.
NUTRITION SUPPORT
Initial interest in nutrition therapy was stimulated by Patek and colleagues (1948) who demonstrated that a “nutritious diet” improved the 5-year outcome of patients with alcoholic cirrhosis compared with historical controls. Subsequent studies further supported nutritional support in hospitalized patients with ALD. In one trial, nutritional supplementation through a feeding tube significantly improved liver function in inpatients with ALD as assessed by serum bilirubin levels and antipyrine clearance, compared to inpatients who ate a hospital diet (Kearns et al., 1992). Probably the most compelling data in favor of nutrition therapy come from a multicenter study by Cabré and colleagues (2000) who randomized severe alcoholic hepatitis patients to receive either Prednisone, 40 mg daily or a liver-specific formula containing 2000 calories per day through a feeding tube. The one-month mortality was the same in both groups but the one-year mortality was significantly lower in the enteral nutrition group compared to the glucocorticoid group, mainly due to fewer infectious complications. This study demonstrates the importance of enteral nutrition in severe alcoholic hepatitis. We do not hesitate to place a nasogastric feeding tube as soon as alcoholic hepatitis patients are admitted to the hospital if it is necessary to ensure adequate enteral nutrition.
Limited studies of nutritional support in liver patients are available in the outpatient setting. Hirsch and colleagues demonstrated that outpatients from a liver clinic taking an enteral nutrition supplement (1000 Kcal and 34 gm of protein) had significantly improved protein intake and fewer hospitalizations in comparison with those not receiving the supplements (Hirsch et al., 1993, 1999). The same group later showed that enteral supplements improved nutritional status and immune function in outpatients with alcoholic cirrhosis (Hirsch et al., 1993, 1999). Patients with liver cirrhosis exhibit early onset of gluconeogenesis after short-term fasting. This accelerated metabolic reaction to starvation may result in their increased protein requirements and muscle depletion. A recent randomized controlled trial tested the hypothesis that provision of a lateevening nutritional supplement over a 12-month period would improve body protein stores in patients with cirrhosis. Total body protein (TBP) was measured by neutron activation analysis at baseline, 3, 6, and 12 months. Provision of a nighttime snack to patients with cirrhosis resulted in body protein accrual equivalent to about 2 kg of lean tissue sustained over 12 months and this benefit was not observed with daytime snacks. Thus, nighttime snacks are valuable nutritional interventions in outpatient cirrhotics (Plank et al., 2008).
A defined approach is necessary to achieve appropriate nutritional support in patients with liver disease (Campillo et al., 2003; Marsano and McClain, 1991, 1992; McCullough, 2000). For the patient who has been actively drinking alcohol, it is useful first to correct electrolyte imbalances and to treat and control withdrawal symptoms when present. This will facilitate control of electrolyte disorders and decrease the risk of having a feeding tube or parenteral nutrition line pulled out. During this period (2 to 3 days), if the mental status of the patient is adequate, the patient can be offered a nutritious diet and energy intake can be measured. If the patient is able to ingest adequate amounts of energy and protein, this diet should be continued. If the patient develops portal-systemic encephalopathy (PSE) and there is no evidence of other precipitating disorders (gastrointestinal bleeding, sedative use, hypoxia, electrolyte or acid-base disturbances, volume depletion, infection, etc.), then protein can be restricted to as low as 20 g/d for only 1 to 2 days. However, protein restriction is utilized too often and for too long in most clinical situations. If employed, it should be only short-term to prevent further muscle catabolism. Indeed, most research casts doubt on the use of protein restriction even during clinical encephalopathy (Cordoba et al., 2004; Mullen and Dasarathy, 2004). Most PSE episodes have a precipitating factor, and long-term protein intolerance is not a frequent problem. As soon as mental status improves, protein intake must be increased to at least 60 g/d and perhaps to 1 to 1.5 g/kg per day while lactulose and neomycin are given as needed. If, despite maximal medical therapy with lactulose and neomycin, an adequate protein intake cannot be obtained, the protein intake should be kept at the highest tolerated amount, and a BCAA-enriched formula can be administered to supplement nitrogen intake (Charlton, 2003; Marchesini et al., 2003; Marsano and McClain, 1993) (Table 3).
Table 3.
Treatment for PSE—Nutritional Emphasis
| Correct precipitating factors—electrolyte abnormalities, constipation, etc. |
|---|
| Treat with non-absorbable disaccharide (Lactulose®) or non-absorbable antibiotic (Neomycin®, Rifaximin®) |
| Consider enteral tube feeding for inpatients; nighttime snacks for outpatients |
| Do not reduce protein intake for more than 2 days for acute PSE; we do not reduce protein intake |
| Consider BCAA enriched products for chronic PSE or acute PSE not responding to therapy |
| Consider other nutritional agents such as zinc, prebiotics, carnitine |
If the patient cannot take in adequate kcals and has a functioning gastrointestinal tract, then a feeding tube should be used and a standard enteral formula should be given following the guidelines already mentioned above. If the patient develops PSE without other precipitating factors, the amount of protein may be decreased until lactulose and neomycin control the PSE, and then protein must be increased to satisfy nitrogen requirements (Marsano and McClain, 1993). If, despite medical therapy, the standard enteral formula leads to the development of PSE, then this can be decreased until well tolerated and a BCAA-enriched formula can be given as a supplement to meet nitrogen needs.
Deficiencies in individual nutrients such as zinc, magnesium, Vitamin A, and so on also are frequent in ALD. A discussion concerning micronutrient supplementation is beyond the scope of this article, but has been reviewed by us previously (Hanje et al., 2006; Zhou et al., 2010) (Table 4).
Table 4.
Possible Nutrient Deficiencies in ALD
| Nutrient deficiency | Possible manifestations |
|---|---|
| Vitamin A | Night blindness, dry skin |
| Thiamine | Neurologic problems, Wernike’s encephalopathy |
| Folate | Anemia, possible increased susceptibility to ALD, altered methionine metabolism |
| Vitamin D | Bone disease altered immune function |
| Vitamin E | Possible increased susceptibility to liver injury, oxidative stress |
| Niacin | Pellegra dermatitis, neurologic alterations, hallucinations |
| Pyridoxine | Hypochromic anemia |
| Zinc | Skin lesions, anorexia, depressed wound healing, hypogonadism, altered immune function, impaired night vision, depressed mental function, diarrhea, increased susceptibility to liver injury |
| Magnesium | Muscle cramps, glucose intolerance |
| Selenium | Myopathy, cardiomyopathy, oxidative stress |
Enteral nutrition is desired over parenteral nutrition because of cost, risk of sepsis of the parenteral nutrition line, preservation of the integrity of the gut mucosa, and prevention of bacterial translocation and multiple organ failure. Moreover, total parenteral nutrition (TPN) can, in some instances, cause liver disease as one of its complications.
If enteral nutrition is not possible, then TPN can be used with the knowledge that it is important to return to the enteral route as soon as the small bowel shows evidence of recovered function. TPN can be started with a standard amino acid formula in amounts that are increased until nitrogen needs are met. If the patients develop PSE, then standard therapy with lactulose and neomycin must be given. If the patient is still unable to tolerate the amount of amino acids needed to satisfy nitrogen requirements, then the standard amino acids can be replaced by a BCAA-enriched solution specifically designed for liver disease (Marchesini et al., 2003). It is unusual to require either TPN or BCAA formulas, and our goal is always aggressive enteral support.
CONCLUSIONS
Malnutrition is common in ALD and correlates with the severity of liver disease as well as outcome. The mechanisms for malnutrition are multifactorial, and malnutrition may actually worsen in the hospital unless great care is given to nutrition support. Hospitalized patients often require enteral feeding to achieve nutritional goals. Outpatients should be given late-night snacks in order to prevent muscle wasting. Great attention should be paid to nutrition in both inpatients and outpatients with ALD, and this should be considered initial therapeutic intervention.
Acknowledgments
This work was supported by NIH grants P01AA017103, P30AA019360, R01AA015970, R01AA018016, R01AA 018869, R37AA010762, RC2AA019385, R01DK071765, R01AA014371, the Veterans Administration BX000350, and the University of Louisville’s Center for Environmental Genomics and Integrative Biology (CEGIB) P30ES014443.
References
- Antonow DR, McClain CJ. Nutrition and alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and The Brain. Plenum Publishing; New York: 1985. pp. 81–120. [Google Scholar]
- Baker JP, Detsky AS, Wesson DE, Wolman SL, Stewart S, Whitewell J, Langer B, Jeejeebhoy KN. Nutritional assessment: a comparison of clinical judgment and objective measurements. N Engl J Med. 1982;306:969–972. doi: 10.1056/NEJM198204223061606. [DOI] [PubMed] [Google Scholar]
- Cabré E, Rodríguez-Iglesias P, Caballería J, Quer JC, Sánchez-Lombraña JL, Parés A, Papo M, Planas R, Gassull MA on behalf of the Spanish Group for the Study of Alcoholic Hepatitis. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- Campillo B. Assessment of nutritional status and diagnosis of malnutrition in patients with liver disease. In: Preedy VR, Lakshman R, Srirajaskanthan R, Watson RR, editors. Nutrition, Diet Therapy and the Liver. CRC Press; Boca Raton, FL: 2010. pp. 22–46. [Google Scholar]
- Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19(6):515–521. doi: 10.1016/s0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- Caregaro L, Alberino F, Amodio P, Merkel C, Bolognesi M, Angeli P, Gatta A. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996;63:602–609. doi: 10.1093/ajcn/63.4.602. [DOI] [PubMed] [Google Scholar]
- Charlton M. Branched-chain amino acid-enriched supplements as therapy for liver disease: rasputin lives. Gastroenterology. 2003;124(7):1980–1982. doi: 10.1016/s0016-5085(03)00550-x. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Lopez-Hellin J, Planas M, Sabin P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatology. 2004;41(1):38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Detsky AS. What is subjective global assessment of nutritional status? JPEN J Parenter Enter Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- DiCecco SR, Wieners EJ, Wiesner RH, Southorn PA, Plevak DJ, Krom RA. Assessment of nutritional status of patients with end stage liver disease undergoing liver transplantation. Mayo Clin Proc. 1989;64:95–102. doi: 10.1016/s0025-6196(12)65308-7. [DOI] [PubMed] [Google Scholar]
- Dolz C. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology. 1991;100:738–744. doi: 10.1016/0016-5085(91)80019-6. [DOI] [PubMed] [Google Scholar]
- Galati JS. Delayed gastric emptying of both the liquid and solid components of a meal in chronic liver disease. Am J Gastroenterol. 1994;89:708–711. [PubMed] [Google Scholar]
- Galati JS. Gastric emptying and orocecal transit in portal hypertension and end-stage chronic liver disease. Liver Transpl Surg. 1997;3:34–38. doi: 10.1002/lt.500030105. [DOI] [PubMed] [Google Scholar]
- Guglielmi FW, Contento F, Laddaga L, Panella C, Francavilla A. Bioelectric impedance analysis: experience with male patients with cirrhosis. Hepatology. 1991;13:892–895. [PubMed] [Google Scholar]
- Hanje AJ, Fortune B, Song M, Hill DB, McClain CJ. The use of selected nutritional supplements and complementary and alternative medicine in liver disease. Nutr Clin Pract. 2006;21:255–272. doi: 10.1177/0115426506021003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 activity in alcoholic hepatitis. J Lab Clin Med. 1992;119:547–552. [PubMed] [Google Scholar]
- Hill DB, Marsano L, McClain CJ. Increased plasma Interleukin-8 concentrations in alcoholic hepatitis. Hepatology. 1993;18:576–580. [PubMed] [Google Scholar]
- Hirsch S, Bunout D, de la Maza P, Iturriaga H, Petermann M, Icazar G, Gattas V, Ugarte G. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN J Parenter Enter Nutr. 1993;17:119–124. doi: 10.1177/0148607193017002119. [DOI] [PubMed] [Google Scholar]
- Hirsch S, de laMaza MP, Gattas V, Barrera G, Petermann M, Gotteland M, Muñoz C, Lopez M, Bunout D. Nutritional support in alcoholic cirrhotic patients improves host defenses. J Am Coll Nutr. 1999;18:434–441. doi: 10.1080/07315724.1999.10718881. [DOI] [PubMed] [Google Scholar]
- Isobe H. Delayed gastric emptying in patients with liver cirrhosis. Dig Dis Sci. 1994;39:983–987. doi: 10.1007/BF02087548. [DOI] [PubMed] [Google Scholar]
- John WJ, Phillips R, Ott L, Adams LJ, McClain CJ. Resting energy expenditure in patients with alcoholic hepatitis. J Parenter Enter Nutr. 1989;13:124–127. doi: 10.1177/0148607189013002124. [DOI] [PubMed] [Google Scholar]
- Kearns PJ, Young H, Garcia G, Blaschke T, O’Hanlon G, Rinki M, Sucher K, Gregory P. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992;102:200–205. doi: 10.1016/0016-5085(92)91801-a. [DOI] [PubMed] [Google Scholar]
- Lolli R, Marchesini G, Bianchi G, Fabbri A, Bugianesi E, Zoli M, Pisi E. Anthropometric assessment of the nutritional status of patients with liver cirrhosis in the Italian population. Ital J Gastroenterol. 1992;24:429–435. [PubMed] [Google Scholar]
- Madden AM, Bradbury W, Morgan MY. Taste perception in cirrhosis: its relationship to circulation micronutrients and food preferences. Hepatology. 1997;26:40–48. doi: 10.1002/hep.510260106. [DOI] [PubMed] [Google Scholar]
- Madrid AM. Orthotopic liver transplantation improves small bowel motility disorders in cirrhotic patients. Am J Gastroenterol. 1997;92:1044– 1045. [PubMed] [Google Scholar]
- Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124(7):1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Marsano L, McClain CJ. Nutrition and alcoholic liver disease. JPEN J Parenter Enter Nutr. 1991;15:337–344. doi: 10.1177/0148607191015003337. [DOI] [PubMed] [Google Scholar]
- Marsano L, McClain CJ. Nutritional support in alcoholic liver disease. In: Watson RR, Watzl B, editors. Nutrition and Alcohol. CRC Press; Boca Raton, FL: 1992. pp. 385–402. [Google Scholar]
- Marsano L, McClain CJ. How to manage both acute and chronic hepatic encephalopathy. J Crit Illness. 1993;8:579–600. [Google Scholar]
- McClain CJ, Barve S, Deaciuc IV, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G497–502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. Malnutrition in liver disease. Liver Transpl. 2000;6:S85–S96. doi: 10.1002/lt.500060516. [DOI] [PubMed] [Google Scholar]
- McCullough AJ, Mullen KD, Kalhan SC. Measurements of total body and extracellular water in patients with and without ascities. Hepatology. 1991;14:1102–1111. [PubMed] [Google Scholar]
- Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Am J Med. 1984;76:211–222. doi: 10.1016/0002-9343(84)90776-9. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI, Samanta A, Weesner RE, Henderson W, Chen TS, French SW, Chedid A the VA Cooperative Study Group #275. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs Cooperative Study. Hepatology. 1993;17:564–576. doi: 10.1002/hep.1840170407. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI, Samanta A, Weesner MD, Henderson WG, Chen TS, French SA, Chedid A the VA Cooperative Study Group #275. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. J Parent Enter Nutr. 1995b;19:258–265. doi: 10.1177/0148607195019004258. [DOI] [PubMed] [Google Scholar]
- Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration cooperative studies. Alcohol Clin Exp Res. 1995a;19:635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Tosch T, Weesner RE, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorell M, Tamburro C, Zetterman R, Chedid A, Chen T, Rabin L. VA cooperative study on alcoholic hepatitis II: prognostic significance of protein-calorie malnutrition. Am J Clin Nutr. 1986;43:213–218. doi: 10.1093/ajcn/43.2.213. [DOI] [PubMed] [Google Scholar]
- Merli M, Romiti A, Riggio O, Capocaccia L. Optimal nutritional indexes in chronic liver disease. JPEN J Parenter Enteral Nutr. 1987;11(suppl):130s–4s. doi: 10.1177/014860718701100521. [DOI] [PubMed] [Google Scholar]
- Mullen KD, Dasarathy S. Protein restriction in hepatic encephalopathy: necessary evil or illogical dogma? J Hepatol. 2004;41(1):147–148. doi: 10.1016/j.jhep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Muller MJ. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782–794. doi: 10.1002/hep.1840150507. [DOI] [PubMed] [Google Scholar]
- O’Keefe SJ. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 1980:615–617. doi: 10.1016/s0140-6736(80)90284-6. [DOI] [PubMed] [Google Scholar]
- Patek AJ, Jr, Post J, Ratnoff OD, Mankin H, Hillman RW. Dietary treatment of cirrhosis of the liver: results in 124 patients observed during a 10 year period. JAMA. 1948;139:543–549. doi: 10.1001/jama.1948.02900080001001. [DOI] [PubMed] [Google Scholar]
- Pirlich M, Selberg O, Boker K, Schwarze M, Müller MJ. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology. 1996;24:1422–1427. doi: 10.1002/hep.510240620. [DOI] [PubMed] [Google Scholar]
- Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, McIlroy K, Donaghy AJ, McCall JL. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- Quigley EMM. Gastrointestinal dysfunction in liver disease and portal hypertension. Gut-liver interaction revisited. Dig Dis Sci. 1996;41:557–561. doi: 10.1007/BF02282341. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Dhingra N, Bansal A, Malhotra S, Guptan RC. Dietary and nutritional abnormalities in alcoholic liver disease: a comparison with chronic alcoholics without liver disease. Am J Gastroenterol. 1997;92:777–783. [PubMed] [Google Scholar]
- Shronts EP. Nutritional assessment of adults with end stage hepatic failure. Nutr Clin Part. 1998;3:113–119. doi: 10.1177/0115426588003003113. [DOI] [PubMed] [Google Scholar]
- Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BrMed J. 1989;299:1202–1203. doi: 10.1136/bmj.299.6709.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuluvath PJ, Triger DR. Autonomic neuropathy in chronic liver disease. QJM. 1989a;72:737–747. [PubMed] [Google Scholar]
- Thuluvath PJ, Triger DR. Evaluation of nutritional status by using anthropometry in adults with alcoholic and non-alcoholic liver disease. Am J Clin Nutr. 1989b;60:269–273. doi: 10.1093/ajcn/60.2.269. [DOI] [PubMed] [Google Scholar]
- Vlahcevic ZR. Bile acid metabolism in patients with cirrhosis. Gastroenterology. 1971;60:491–498. [PubMed] [Google Scholar]
- Zhou Z, Song Z, Pigneri D, McClain M, Mendenhall CL, Marsano LS, McClain CJ. Longterm management of alcoholic liver disease. In: Preedy VR, Lakshman R, Srirajaskanthan R, Watson RR, editors. Nutrition, Diet Therapy, and the Liver. CRC Press; Boca Raton, FL: 2010. pp. 159–182. [Google Scholar]