Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis (original) (raw)
Abstract
AIM: To investigate the relationship between the gut-liver axis and nonalcoholic fatty liver disease (NAFLD), we performed a meta-analysis to evaluate the effects of probiotic therapy in NAFLD.
METHODS: We searched PubMed, Medline, Embase, Web of Science, the Cochrane Library and Chinese Biomedicine Database for all relevant randomized controlled trials on probiotics in patients with NAFLD/nonalcoholic steatohepatitis (NASH). A statistical analysis was performed using RevMan 5.0 software.
RESULTS: Four randomized trials involving 134 NAFLD/NASH patients were included. The results showed that probiotic therapy significantly decreased alanine aminotransferase (ALT), aspartate transaminase (AST), total-cholesterol (T-chol), high density lipoprotein (HDL), tumor necrosis factor (TNF)-α and homeostasis model assessment of insulin resistance (HOMA-IR) [ALT: weighted mean difference (WMD) -23.71, 95%CI: -33.46--13.95, P < 0.00001; AST: WMD -19.77, 95%CI: -32.55--7.00, P = 0.002; T-chol: WMD -0.28, 95%CI: -0.55--0.01, P = 0.04; HDL: WMD -0.09, 95%CI: -0.16-0.01, P = 0.03; TNF-α: WMD -0.32, 95%CI: -0.48--0.17, P < 0.0001; HOMA-IR: WMD -0.46, 95%CI: -0.73--0.19, P = 0.0008]. However, the use of probiotics was not associated with changes in body mass index (BMI), glucose (GLU) and low density lipoprotein (LDL) (BMI: WMD 0.05, 95%CI: -0.18-0.29, P = 0.64; GLU: WMD 0.05, 95%CI: -0.25-0.35, P = 0.76; LDL: WMD -0.38, 95%CI: -0.78-0.02, P = 0.06).
CONCLUSION:Probiotic therapies can reduce liver aminotransferases, total-cholesterol, TNF-α and improve insulin resistance in NAFLD patients. Modulation of the gut microbiota represents a new treatment for NAFLD.
Keywords: Probiotics, Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Liver function, Insulin resistance, Meta-analysis
Core tip: For many decades, researchers have carried out studies on the treatment of nonalcoholic fatty liver disease (NAFLD). However, no firm conclusions have been made regarding the efficacy of various treatments for NAFLD. Here we conducted a meta-analysis of the pooled data from randomized controlled trials to assess the efficacy of probiotic therapies and showed that probiotic therapy significantly decreased alanine aminotransferase, aspartate transaminase, total-cholesterol, high density lipoprotein, tumor necrosis factor-α and homeostasis model assessment of insulin resistance. Thus, probiotics may help to improve liver function, fat metabolism and insulin resistance in NAFLD patients.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is characterized by large vacuoles of triglyceride which accumulate in liver cells via the process of steatosis in non-alcohol users. The condition can progress into more serious liver diseases, such as nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and more rarely, liver carcinoma[1]. It is increasingly recognized as a major cause of liver-related morbidity and mortality. NAFLD is common in Western countries. However, an increase in the prevalence of NAFLD has been observed in China. The underlying mechanisms of disease progression are poorly understood. Diet and lifestyle changes are primary therapies in the management of these patients. Specific pharmacologic treatments for NAFLD/NASH are progressing, such as insulin-sensitizers[2-4], lipid-lowering drugs[5-6], antioxidants[7-8], and anti-tumor necrosis factor (TNF)-α agents[9-11]. However, most of these are not licensed therapies for NAFLD, despite the abundance of clinical trials.
Recently, a new treatment strategy using probiotics was proposed. A probiotic is a live microbial culture or cultured dairy product, which plays a fundamentally important role in health and disease[12-14]. The human intestinal microbiota is composed of 1013-1014 microorganisms whose collective genome contains at least 100 times as many genes as our own genome, representing 500-1000 species in total[15-16]. Miele et al[17] provided the first evidence that NAFLD in humans was associated with increased intestine permeability, and that this abnormality was related to the increased prevalence of small bowel bacterial overgrowth (SIBO) in these patients. The increased permeability appears to be caused by disruption of intercellular tight junctions in the intestine, and it may play an important role in the pathogenesis of NAFLD. Loguercio et al[18] have shown that probiotics may reduce NAFLD liver injury and may improve liver function. Probiotics can inhibit the proliferation of harmful bacteria, reduce SIBO, restore gastrointestinal barrier function and modulate the immune system[19-21], all of which contribute to the improvement of NAFLD.
Therefore, the aim of this study was to conduct a meta-analysis of the pooled data from RCTs to assess the efficacy of probiotic therapies in modifying liver function, fat metabolism and insulin resistance.
MATERIALS AND METHODS
Search strategy
We searched Medline, Embase, Web of Science, Chinese Biomedicine Database and the China Journal Full Text Database with no language restriction. The search terms were: “(NASH or NAFLD or “nonalcoholic steatohepatitis” or “nonalcoholic fatty liver disease” or “fatty liver”) and (probiotic* or prebiotic* or synbiocit* or bifidobacter* or Lactobacill* or flora)” and “[“Fatty Liver”(Mesh)] AND “Probiotics”(Mesh)”. We also searched the reference lists of each selected study by hand.
Inclusion and exclusion criteria
Inclusion criteria were as follows: randomized controlled trials (RCTs) with participants of any sex or ethnic origin with NAFLD/NASH, diagnosed on the basis of radiological/histological evidence of fatty liver. Exclusion criteria were as follows: other causes of hepatic steatosis or steatofibrosis such as hepatitis B, hepatitis C, autoimmune hepatitis, liver decompensation or malignancy, and genetic liver disease such as Wilson’s disease and hemochromatosis.
The trials should have measured at least one of the following items: BMI, ALT, AST, total-cholesterol, LDL, HDL, GLU, TNF-α and HOMA-IR. Studies must have objective outcome measures, otherwise they were excluded from this review.
Data extraction and methodological quality
Data were abstracted independently by two reviewers and included: author, publication year, study design, population, intervention, duration and outcome. Disagreement was resolved by discussion.
Scored using the Jadad scale, we assessed the quality of the studies by the randomization method, allocation concealment, blinding of outcome assessment and follow-up. All included studies scored ≥ 4.
Statistical analysis
We analyzed the data using Review Manager 5.0. Dichotomous data were presented as odds ratio with 95%CI. Statistical heterogeneity was measured using the χ2 test and the _I_2. A χ2 P value < 0.05 was considered to indicate statistically significant heterogeneity. If there was obvious heterogeneity, the random effects model was chosen; otherwise, the fixed effects model was adopted.
RESULTS
The electronic searches yielded 475 items from Medline, Embase, Web of Science, Chinese Biomedicine Database and the China Journal Full Text Database. Publication dates ranged from 1996 to 2013. After reviewing each publication, we selected 4 original studies (Figure 1).
Figure 1.
Selection of studies.
Table 1 contains specific information on study design, randomization methods, sample size, intervention, duration of treatment and follow-up. Allocation concealment was adequate in three studies. All the studies were double-blind and included a follow-up period. The diagnosis of NAFLD/NASH was confirmed by percutaneous liver biopsy in three studies. All gave detailed baseline information. The main characteristics of the patients included in the two groups were well matched in all RCTs.
Table 1.
Methodological characteristics of the included studies in this meta-analysis
| Ref. | Sample size | Randomization | Blinding | Diagnostic method | Intervention | Duration | Follow-up |
|---|---|---|---|---|---|---|---|
| Aller et al[22] | 28 (14/14) | Table of numbers | Double-blind | Histological | Lactobacillus bulgaricus and Streptococcus thermophilus vs placebo | 3 mo | Yes |
| Vajro et al[23] | 20 (10/10) | Yes | Double-blind | Radiological | Lactobacillus GG vs placebo | 8 wk | Yes |
| Malaguarnera et al[24] | 66 (34/32) | Computer generated | Double-blind | Histological | Bifidobacterium longum + Fos vs placebo | 24 wk | Yes |
| Wong et al[25] | 20 (10/10) | Computer generated | Double-blind | Histological | Lepicol probiotic and prebiotic formula vs nothing | 6 mo | Yes |
All four RCTs[22-25] reported on BMI, but did not show a significant difference in the experimental group compared with the control group [weighted mean difference (WMD) 0.05, 95%CI: -0.18-0.29, P = 0.64]. Significant homogeneity was observed among the studies (_I_2 = 0%, P = 0.77) (Figure 2A).
Figure 2.
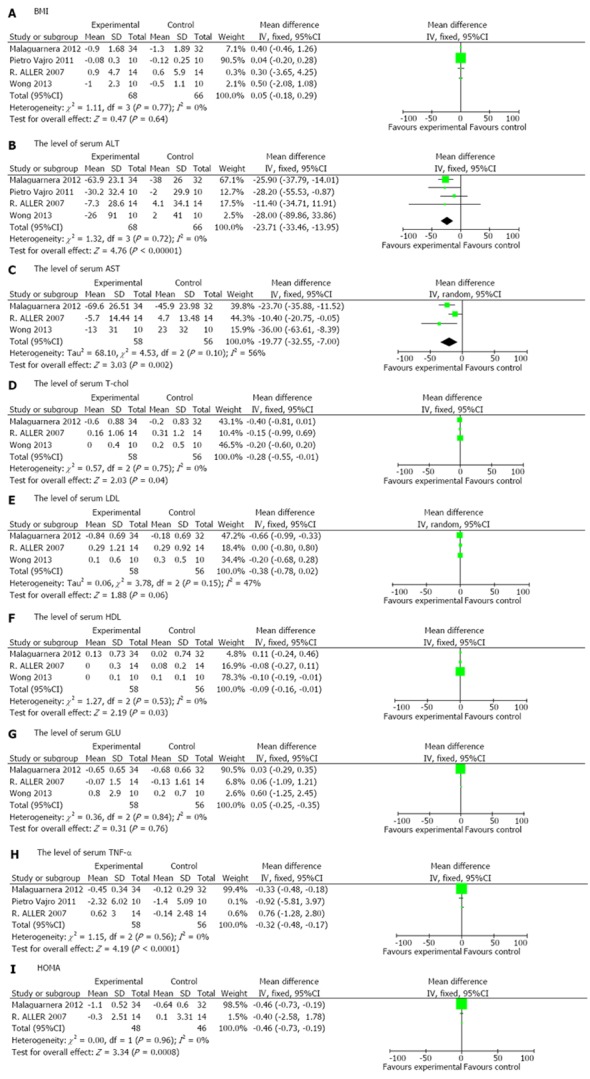
Forest plot of the effects of probiotics in patients with nonalcoholic fatty liver disease. A: BMI; B: The level of serum ALT; C: The level of serum AST; D:The level of serum T-chol; E: The level of serum LDL; F: The level of serum HDL; G: The level of serum GLU; H: The level of serum TNF-α; I: HOMA. BMI: Body mass index; ALT: Alanine aminotransferase; AST: Aspartate transaminase; T-chol: total-cholesterol; LDL: Low density lipoprotein; HDL: High density lipoprotein; GLU: Glucose; HOMA: Homeostasis model assessment.
Four RCTs[22-25] assessed the effect of probiotics on the level of serum ALT and showed a significant difference between patients treated with probiotics compared with those treated with placebo (WMD -23.71, 95%CI: -33.46--13.95, P < 0.00001). The included studies were homogeneous (_I_2 = 0%, P = 0.72) (Figure 2B).
Three RCTs[22,24,25] analyzed the effect of probiotics on AST and T-chol in NAFLD/NASH patients compared with placebo. Probiotics had a significantly better effect on normalizing AST and T-chol (AST: WMD -19.77, 95%CI: -32.55--7.00, P = 0.002; T-chol: WMD -0.28, 95%CI: -0.55--0.01, P = 0.04). The included studies on AST were not homogeneous (_I_2 = 56%, P = 0.1), while the studies on T-chol were significantly homogeneous (_I_2 = 0%, P = 0.75) (Figure 2C, D).
Three RCTs[22,24,25] reported the effects of probiotics on LDL, HDL and GLU in patients with NAFLD/NASH compared with placebo. Probiotics had a significantly better effect in reducing HDL (WMD -0.09, 95%CI: -0.16-0.01, P = 0.03), but no significant difference in reducing LDL and GLU (LDL: WMD -0.38, 95%CI: -0.78-0.02, P =0.06; GLU: WMD 0.05, 95%CI: -0.25-0.35, P = 0.76). The included studies were homogeneous (LDL: _I_2 = 47%, P = 0.15; HDL: _I_2 = 0%, P = 0.53; GLU: _I_2 = 0%, P = 0.84) (Figure 2E, F, G).
Three RCTs[22-24] provided sufficient data to compare the effects of probiotics with those of placebo and showed a statistically significant effect for TNF-α in NAFLD/NASH patients (WMD -0.32, 95%CI: -0.48--0.17, P < 0.0001). Significant homogeneity was observed among the studies (_I_2 = 0%, P = 0.56) (Figure 2H).
Only two RCTs[22,24] reported the effects of probiotics on HOMA-IR in NAFLD/NASH patients. There was a significant reduction in HOMA-IR in NAFLD/NASH patients in the experimental group compared with the control group (WMD -0.46, 95%CI: -0.73--0.19, P = 0.0008). Significant homogeneity was observed among the studies (_I_2 = 0%, P = 0.96) (Figure 2I).
DISCUSSION
NAFLD is a relevant issue in public health due to its epidemiologic burden. The prevalence of NAFLD has apparently increased in proportion to the increasing incidence of obesity in both adults and children[26]. NAFLD is closely associated with obesity and insulin resistance, and is now recognized to represent the hepatic manifestation of the metabolic syndrome. At present, there is no registered drug for the treatment of NAFLD. Although lifestyle intervention is often advocated[27-28], it is difficult to maintain. In 2009, Socha et al[29] performed a meta-analysis of the pharmacological interventions for NAFLD in adults and children, including pioglitazone, vitamin E, ursodeoxycholic acid, probucol, N-acetylcysteine, and low-dose carnitine. However, he was unable to draw firm conclusions on the efficacy of the various treatments for NAFLD. In 2011, Musso et al[30] found that weight loss improved liver histology and the cardio-metabolic profile, as did pioglitazone. It is also important to explore new treatment strategies.
It is well known that liver and intestine have the same origin in embryology the foregut. In addition, the liver continuously receives blood from the gut through the portal system. Therefore, there is a close relationship between the intestine and liver. Evidence has shown that SIBO is present in 50% of patients with non-alcoholic steatosis[17,31]. High-fat diet-induced obesity is associated with changes in the composition of intestinal bacteria in rats[32-33] and in humans[34]. Therefore, changes in the composition of the intestinal bacterial content may be associated with NAFLD or obesity.
Intestinal bacteria may be involved in the etiology of NAFLD by enhancing intestinal permeability[35], direct activation of inflammatory cytokines via release of lipopolysaccharide (LPS) and favoring absorption of endotoxins[36]. Endotoxins activate Kupffer cells in the liver and increase the production of TNF-α and IL-6, which contributes to the onset of liver fibrosis[31,37-38]. Furthermore, a complex mechanism involving extensive lipid accumulation, systemic inflammation, oxidative stress, and insulin resistance causes cytotoxicity and exacerbated hepatopathy[39-40].
Serum ALT and AST levels are well-recognized clinical markers of liver damage and may be involved in the pathogenesis of NAFLD. Cholesterol is also a risk factor for NAFLD. Liver damage can lead to elevated cholesterol or reduced HDL in the blood. TNF-α is secreted directly by hepatocytes and Kupffer cells in the liver[41]. Many studies have shown a relationship between TNF-α expression and NAFLD[42-43]. Assessment of insulin resistance by HOMA-IR has been widely utilized in clinical studies of NAFLD[44-45]. In four RCTs, ALLER, Wong et al[25] reported that probiotics improved liver aminotransferase levels in patients with NAFLD, while Malaguarnera concluded that probiotics reduced TNF-α, serum AST levels and HOMA-IR. Our meta-analysis showed that probiotics significantly reduced ALT, AST, T-chol, TNF-α and HOMA-IR, which are all related to the process, development and consequences of NAFLD. However, the level of HDL was significantly increased in the placebo treatment compared with probiotic treatment, which was contrary to expectation. It is possible that the elevation in HDL requires long-term treatment or there are other mechanisms which have not been explored.
The change in cholesterol level in our study should be emphasized, as Gilliland et al[46] in the early 1990s found that regular consumption of probiotics reduced cholesterol levels. Over several decades, more and more researchers confirmed that probiotics can lead to a decrease in serum cholesterol in animals and humans[47-50]. However, these RCTs did not report the positive effects of probiotics on reducing cholesterol in NAFLD/NASH patients, while the findings of the present meta-analysis supported the reduction of cholesterol in NAFLD/NASH patients. From this meta-analysis, we can conclude that probiotics have positive effects in patients with NAFLD/NASH.
Of the four RCTs included in this meta-analysis, the studied probiotics included lactobacillus, bifidobacterium and streptococcus. Two studies also determined the effect of probiotics combined with fructo-oligosaccharides in NAFLD[24,25]. Bifidobacteria colonize the intestinal tract soon after birth and are the major components of the microbial barrier in healthy humans. Bifidobacteria produce a range of beneficial effects on host health[51-52]. Lactobacilli and streptococcus are also beneficial, although they are present at much lower levels in the human colon[52]. Probiotics have been shown to enhance the barrier function of epithelial cells[53] and decrease intestinal permeability and endotoxemia in patients with liver disease[54]. At the same time, probiotics can also influence host metabolism in several other ways, such as regulation of energy extraction from nutrients and modulation of genes involved in substrate metabolism[55]. A prebiotic is a nondigestible food ingredient. Due to the general properties of prebiotics, they can influence the growth, activity and metabolites of probiotics[56]. Fructo-oligosaccharides are now becoming increasingly popular due to their prebiotic effects. They can be fermented by bifidobacteria and lactobacilli[57]. Fructo-oligosaccharides can lead to bifidobacteria becoming the dominant species in the large bowel[58] and may help to control or reduce the growth of harmful bacteria[59]. In animal models, treatment with oligofructose reduced adipose tissue inflammation, oxidative stress and led to an improvement in glucose tolerance and to a reduction in body weight, which were beneficial in patients with NAFLD[60]. In conclusion, probiotics and prebiotics are important mediators of diet-induced metabolic disturbances in NAFLD.
There are several limitations to this review. It is well known that liver histology is the gold standard for NAFLD/NASH. Although ultrasonography is reasonably accurate, it cannot identify fatty infiltration of the liver below a threshold of 30%. In our review, three RCTs used liver histological response as an outcome index evaluating the effectiveness of probiotics in the treatment of NAFLD. Regretfully, only one RCT had post-treatment histology results. The diagnostic criteria for NAFLD in another trial included increased ultrasonographic bright liver. Three trials included patients aged 18-70 years, while one trial included children. The researchers ignored the dietary restrictions, exercise and physical activities as in almost all studies they were not described. The sample sizes in some trials, as well as the number of trials for some comparisons, were small. Existing data are difficult to reconcile, given the use of different strains, dosages and duration of treatment.
COMMENTS
Background
The prevalence of and mortality due to nonalcoholic fatty liver disease (NAFLD) are increasing worldwide. Diet and lifestyle changes are primary therapies in the management of NAFLD patients. However, most drug therapies are not licensed for NAFLD. Recent evidence suggests that malfunction of the gut-liver axis contributes to hepatic damage in rats and humans with NAFLD, and probiotics play a fundamentally important role in health and disease. Thus, it was necessary to conduct a meta-analysis to assess the effects of probiotics on liver function, fat metabolism and insulin resistance in NAFLD patients.
Research frontiers
A probiotic is a live microbial culture or cultured dairy product and the human intestinal microbiota is composed of 1013-1014 microorganisms. The gut-liver axis indicates that changes in the composition of the intestinal bacterial content are associated with NAFLD. Most therapies are not licensed for the prevention of NAFLD. Therefore, a research hotspot is whether treatment with probiotics is effective in patients with NAFLD.
Innovations and breakthroughs
In 2009, Socha et al performed a meta-analysis of pharmacological interventions for NAFLD in adults and children, including pioglitazone, vitamin E, ursodeoxycholic acid, probucol, N-acetylcysteine, and low-dose carnitine. However, he was unable to draw firm conclusions on the efficacy of various treatments for NAFLD and he did not study the effect of probiotics. Research on probiotics in rats is popular. However, there have only been a few large randomized controlled trials (RCTs), and the results were inconsistent. Therefore, the aim of this study was to conduct a meta-analysis of the pooled data from RCTs to assess the efficacy of probiotic therapies in modifying liver function, fat metabolism and insulin resistance in patients with NAFLD/nonalcoholic steatohepatitis.
Applications
Probiotic therapies can reduce liver aminotransferase levels, serum cholesterol and tumor necrosis factor-α and improve insulin resistance in patients with NAFLD. Thus, modulation of the gut microbiota using probiotics may represent a new method of treating or preventing NAFLD.
Terminology
NAFLD is characterized by large vacuoles of triglyceride which accumulate in liver cells via the process of steatosis in non-alcohol users. The condition can progress into more serious liver diseases, such as nonalcoholic steatohepatitis, liver fibrosis, cirrhosis, and more rarely, liver carcinoma. A probiotic is a live microbial culture or cultured dairy product, which plays a fundamentally important role in health and disease. The human intestinal microbiota is composed of 1013-1014 microorganisms whose collective genome contains at least 100 times as many genes as our own genome, representing 500-1000 species in total.
Peer review
This meta-analysis is an interesting revision on the present knowledge on probiotics and NAFLD but again introduces the difficulty in obtaining unequivocal correlations between biochemical changes and pharmacological treatment or lifestyle modifications, including the inclusion of probiotics in diet. This meta-analysis suggests that liver inflammatory markers become significantly reduced with the use of probiotics and this has been interpreted as indirect evidence of the effect on inflammation and liver damage by the intervention. Nevertheless, the meta-analysis concludes that many of the data analyzed remain without modification on pooled analysis with the inclusion of probiotics. All of these data make it difficult to assess the real effect of probiotics on NAFLD. Nevertheless, the data obtained by this meta-analysis are interesting since they demonstrate the complexity of the factors which may influence the development of NAFLD.
Footnotes
Supported by National Natural Science Foundation of China, No. 81300303; Zhejiang Provincial Laboratory Animal Science Technology Program of China, No. 2011C37088; Natural Science Foundation of Zhejiang Province, China, No. Y13H030004
P- Reviewers Bordas JM, Herrerias-Gutierrez JM, Petr H S- Editor Qi Y L- Editor A E- Editor Liu XM
References
- 1.Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–1133. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 3.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 4.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 5.Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. doi: 10.1016/s0168-8278(99)80243-8. [DOI] [PubMed] [Google Scholar]
- 6.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193–196. doi: 10.1016/j.atherosclerosis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 8.Pamuk GE, Sonsuz A. N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2003;18:1220–1221. doi: 10.1046/j.1440-1746.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- 9.Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16:39–46. [PubMed] [Google Scholar]
- 10.Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L, Cheng CL, Wee A, Lim SG. A randomized controlled pilot study of Pentoxifylline in patients with non-alcoholic steatohepatitis (NASH) Hepatol Int. 2008;2:196–201. doi: 10.1007/s12072-008-9058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinella ME, Koppe S, Brunt EM, Elias M, Gottstein J, Green RM. Pentoxifylline improves ALT and histology in patients with NASH: a double-blind placebo controlled trial. Gastroenterology. 2009;136 Suppl 1:88–89. [Google Scholar]
- 12.Salminen S. Uniqueness of probiotic strains. IDF Nutr Newsletter. 1996;5:16–18. [Google Scholar]
- 13.Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004;80:516–526. doi: 10.1136/pgmj.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilly DM, Stillwell RH. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 15.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 18.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane GT, Cummings JH. Probiotics, infection and immunity. Curr Opin Infect Dis. 2002;15:501–506. doi: 10.1097/00001432-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 21.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 22.Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 23.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 24.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 25.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–262. [PubMed] [Google Scholar]
- 26.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. A nn Hepatol. 2011;10:486–492. [PubMed] [Google Scholar]
- 28.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S39–S43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 29.Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587–596. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- 30.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 31.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 33.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 36.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681–687. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 38.Kaplowitz N. Mechanisms of liver cell injury. J Hepatol. 2000;32:39–47. doi: 10.1016/s0168-8278(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 39.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 41.Montecucco F, Mach F. Does non-alcoholic fatty liver disease (NAFLD) increase cardiovascular risk? Endocr Metab Immune Disord Drug Targets. 2008;8:301–307. doi: 10.2174/187153008786848268. [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto Lde F, Compri CM, Fornari JV, Bartchewsky W, Cintra DE, Trevisan M, Carvalho Pde O, Ribeiro ML, Velloso LA, Saad MJ, et al. The immunosuppressant drug, thalidomide, improves hepatic alterations induced by a high-fat diet in mice. Liver Int. 2010;30:603–610. doi: 10.1111/j.1478-3231.2009.02200.x. [DOI] [PubMed] [Google Scholar]
- 44.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 45.Aguilera E, Recasens M, Flores L, Ricart MJ, Casamitjana R, Fernández-Cruz L, Esmatjes E. HOMA test in diabetic patients with simultaneous pancreas and kidney transplantation. Transplant Proc. 2002;34:206–208. doi: 10.1016/s0041-1345(01)02831-7. [DOI] [PubMed] [Google Scholar]
- 46.Gilliland SE, Walker DK. Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J Dairy Sci. 1990;73:905–911. doi: 10.3168/jds.S0022-0302(90)78747-4. [DOI] [PubMed] [Google Scholar]
- 47.De Rodas BZ, Gilliland SE, Maxwell CV. Hypocholesterolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. J Dairy Sci. 1996;79:2121–2128. doi: 10.3168/jds.S0022-0302(96)76586-4. [DOI] [PubMed] [Google Scholar]
- 48.Usman A. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J Dairy Sci. 2000;83:1705–1711. doi: 10.3168/jds.S0022-0302(00)75039-9. [DOI] [PubMed] [Google Scholar]
- 49.Pereira DI, Gibson GR. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol. 2002;37:259–281. doi: 10.1080/10409230290771519. [DOI] [PubMed] [Google Scholar]
- 50.Ibnou-Zekri N, Blum S, Schiffrin EJ, von der Weid T. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect Immun. 2003;71:428–436. doi: 10.1128/IAI.71.1.428-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 52.McCartney AL, Wenzhi W, Tannock GW. Molecular analysis of the composition of the bifidobacterial and lactobacillus microflora of humans. Appl Environ Microbiol. 1996;62:4608–4613. doi: 10.1128/aem.62.12.4608-4613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malasanos TH, Stacpoole PW. Biological effects of omega-3 fatty acids in diabetes mellitus. Diabetes Care. 1991;14:1160–1179. doi: 10.2337/diacare.14.12.1160. [DOI] [PubMed] [Google Scholar]
- 54.Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010;22:199–206. doi: 10.1097/MEG.0b013e328330a8d3. [DOI] [PubMed] [Google Scholar]
- 55.Vanni E, Bugianesi E. The gut-liver axis in nonalcoholic fatty liver disease: Another pathway to insulin resistance? Hepatology. 2009;49:1790–1792. doi: 10.1002/hep.23036. [DOI] [PubMed] [Google Scholar]
- 56.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 57.Tannock GW, Wilson CM, Loach D, Cook GM, Eason J, O’Toole PW, Holtrop G, Lawley B. Resource partitioning in relation to cohabitation of Lactobacillus species in the mouse forestomach. ISME J. 2012;6:927–938. doi: 10.1038/ismej.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 59.Hofacre CL, Mathis GF, Quiroz MA. Natural alternatives to prevent necrotic enteritis. Int Poult Prod. 2005;13:7–9. [Google Scholar]
- 60.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]