Cromolyn Sodium for Insulin-Induced Lipoatrophy: Old Drug, New Use (original) (raw)
Local insulin-induced lipoatrophy, an immune-mediated loss of subcutaneous adipose tissue at insulin administration sites, is now a rare complication of insulin therapy in patients with diabetes. Lipoatrophy incidence, previously noted in 10–55% of patients using animal-derived insulins (1), declined considerably with the advent of and improved purity of modern insulins. Yet, it continues to be reported with insulin analogs (2,3) and poses a clinical challenge owing to erratic insulin absorption at affected areas and distressing cosmetic issues.
We previously demonstrated increased degranulating tryptase/chymase-positive mast cells in biopsies from insulin-induced lipoatrophic sites and reported that topical cromolyn sodium (prepared with 4% cromolyn sodium in petrolatum solvent for topical administration twice daily to affected areas) was efficacious therapy in a small series (4). Since this report, we were contacted by 34 health care providers, caregivers, and patients worldwide, to whom we administered surveys to standardize evaluation of their treatment experiences. The study was approved by the institutional review board of the Joslin Diabetes Center.
Twenty-one responded, providing data on 24 patients with insulin-induced lipoatrophy. Ten patients used cromolyn, while the remaining attempted other therapeutic interventions or observation (Table 1). Ten respondents reported a trial of cromolyn administration. Of the 10 cromolyn users, all had type 1 diabetes and 70% were male, with mean age 16.1 ± 5.0 years, age of diabetes diagnosis 6.1 ± 4.4 years, age when lipoatrophy was first noticed 12.2 ± 6.4 years, duration of insulin use prior to onset of lipoatrophy 6.1 ± 5.2 years, and duration of lipoatrophy 3.9 ± 3.4 years. Three patients had Hashimoto thyroiditis and one other had hyperthyroidism as associated autoimmune diseases.
Table 1.
Therapeutic interventions for insulin-induced lipoatrophy
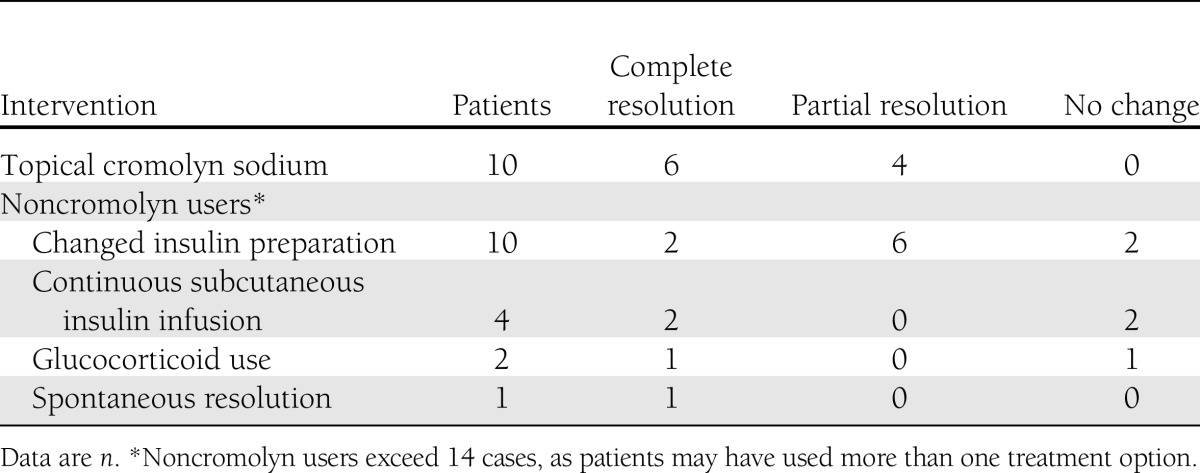
Insulin preparations associated with lipoatrophy included aspart (n = 4), lispro (n = 5), regular human insulin (n = 1), NPH insulin (n = 1), glargine (n = 2), and detemir (n = 1), with three patients reporting use of more than one insulin preparation. Lipoatrophy occurred in regions corresponding to common insulin injection sites: abdomen (n = 6), thighs (n = 5), and buttocks (n = 3), with sizes ranging from 2 × 2 cm to 8 × 10 cm. Eight patients reported multiple lipoatrophic sites.
Among the 10 cromolyn users, 3 initially attempted to treat lipoatrophy by switching insulins, while 2 changed from injections to continuous subcutaneous insulin infusion without improvement. All patients who used cromolyn found it to be at least partially effective; six reported complete resolution of lipoatrophic sites, while four reported partial resolution. These improvements were attributed to cromolyn use by the health care providers or patients. The mean time from initiating cromolyn to noticeable clinical response was 3.1 ± 0.9 months. No side effects or adverse events were reported in association with cromolyn.
Switching insulins and cromolyn were the most frequent therapeutic interventions for lipoatrophy in this series. Cromolyn was reported to be more successful in enabling complete resolution of lipoatrophy than other interventions.
To our knowledge, this is the largest case series to date of patients with insulin-induced lipoatrophy. The retrospective study design, patient/provider qualitative response, and use of multiple interventions for lipoatrophy in noncromolyn users could have restricted the interpretation of results. Nonetheless, topical cromolyn sodium appears to be a well-tolerated and effective option for managing insulin-induced lipoatrophy.
Acknowledgments
This work was supported by grant P30DK036836 from the National Institutes of Health. E.-J.P. received fellowship funding as part of the Health Manpower Development Plan award from Alexandra Health Pte. Ltd. (Singapore).
No potential conflicts of interest relevant to this article were reported.
E.-J.P. researched data, performed statistical analysis, contributed to the discussion, and wrote and edited the manuscript. X.L. and J.R. researched data, contributed to the discussion, and reviewed the manuscript. A.B.G. designed the study, researched data, contributed to the discussion, and reviewed and edited the manuscript. A.B.G. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the health care providers, caregivers, and patients who shared their experience by participating in the study survey.
References
- 1.Reeves WG, Allen BR, Tattersall RB. Insulin-induced lipoatrophy: evidence for an immune pathogenesis. BMJ 1980;280:1500–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: a new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf 2010;9:225–231 [DOI] [PubMed] [Google Scholar]
- 3.Babiker A, Datta V. Lipoatrophy with insulin analogues in type I diabetes. Arch Dis Child 2011;96:101–102 [DOI] [PubMed] [Google Scholar]
- 4.Lopez X, Castells M, Ricker A, Velazquez EF, Mun E, Goldfine AB. Human insulin analog–induced lipoatrophy. Diabetes Care 2008;31:442–444 [DOI] [PubMed] [Google Scholar]