Acetaminophen Toxicity and 5-Oxoproline (Pyroglutamic Acid): A Tale of Two Cycles, One an ATP-Depleting Futile Cycle and the Other a Useful Cycle (original) (raw)
Summary
The acquired form of 5-oxoproline (pyroglutamic acid) metabolic acidosis was first described in 1989 and its relationship to chronic acetaminophen ingestion was proposed the next year. Since then, this cause of chronic anion gap metabolic acidosis has been increasingly recognized. Many cases go unrecognized because an assay for 5-oxoproline is not widely available. Most cases occur in malnourished, chronically ill women with a history of chronic acetaminophen ingestion. Acetaminophen levels are very rarely in the toxic range; rather, they are usually therapeutic or low. The disorder generally resolves with cessation of acetaminophen and administration of intravenous fluids. Methionine or _N_-acetyl cysteine may accelerate resolution and methionine is protective in a rodent model. The disorder has been attributed to glutathione depletion and activation of a key enzyme in the _γ_-glutamyl cycle. However, the specific metabolic derangements that cause the 5-oxoproline accumulation remain unclear. An ATP-depleting futile 5-oxoproline cycle can explain the accumulation of 5-oxoproline after chronic acetaminophen ingestion. This cycle is activated by the depletion of both glutathione and cysteine. This explanation contributes to our understanding of acetaminophen-induced 5-oxoproline metabolic acidosis and the beneficial role of _N_-acetyl cysteine therapy. The ATP-depleting futile 5-oxoproline cycle may also play a role in the energy depletions that occur in other acetaminophen-related toxic syndromes.
Introduction
Acetaminophen ingestion can result in several very different toxic syndromes; most are primarily related to alterations of glutathione metabolism and/or depletion of glutathione stores. The best characterized of these disorders is hepatic toxicity produced by an acute acetaminophen overdose (1–3). Acute acetaminophen poisoning can also produce renal injuries that reduce glomerular filtration and cause renal tubule damage characterized by potassium and/or phosphate wasting (4–7). The pathogenic mechanisms responsible for these acute renal disorders may parallel those responsible for hepatic injury but they are less well characterized. More recently, an acquired form of high anion gap metabolic acidosis caused by systemic accumulation of 5-oxoproline was reported in a number of patients who were chronically ingesting acetaminophen (8–37). This disorder most often occurs in chronically ill and malnourished women who have therapeutic acetaminophen drug levels at the time of presentation. It remains unclear why 5-oxoproline levels would increase markedly in serum and urine in association with chronic acetaminophen ingestion in some patients. An ATP-depleting futile 5-oxoproline cycle related to the glutathione synthetic pathway was recently proposed to explain many of the biochemical features of nephropathic cystinosis (38,39). This review describes why this futile metabolic cycle may also become active in patients who chronically ingest acetaminophen and how its activation can explain the 5-oxoproline accumulation. The ATP-depleting futile 5-oxoproline cycle can also contribute to other acute and chronic acetaminophen toxicity syndromes. Furthermore, if this ATP-depleting futile 5-oxoproline cycle is activated by acetaminophen, then it will be aborted by the administration of _N_-acetyl cysteine. Therefore, at least a component of the beneficial effects of _N_-acetyl cysteine in these conditions may be related to interruption of the futile cycle discussed in this article.
Normal Glutathione Metabolism and the _γ_-Glutamyl Cycle
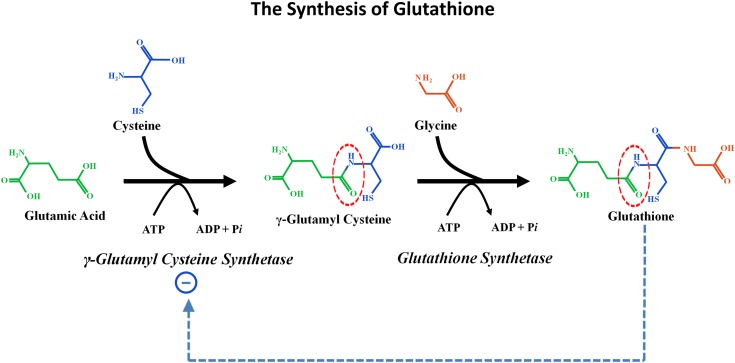
Glutathione, a critically important and very potent antioxidant molecule, is a linear thiol tripeptide that is present at relatively very high (1–10 mmol) concentrations in virtually all mammalian cells. The three amino acids comprising glutathione are glutamate, cysteine, and glycine. The glutamate and cysteine are joined by an unusual chemical bond. In contrast to the typical amide bond (between the _α_-carboxyl group of one amino acid and the _α_-amino group of another) that links virtually all of the amino acids in naturally occurring peptides and proteins (as well as the cysteine and glycine in glutathione), it is the _γ_-carboxyl group of glutamate that is bound to the nitrogen of cysteine (see Figure 1, in which this bond is highlighted by red circles). This _γ_-glutamyl peptide linkage resists splitting by most peptidases; indeed, the only important enzyme that attacks and cleaves this linkage is _γ_-glutamyl transpeptidase (GGT).
Figure 1.
The synthesis of glutathione. Two synthetic reactions combine glutamic acid, cysteine, and glycine to form glutathione. First, the enzyme _γ_-glutamylcysteine synthetase (also called _γ_-glutamylcysteine ligase) catalyzes formation of the unique _γ_-glutamyl bond between glutamic acid and cysteine (shown by the dotted red circle). This reaction requires energy input from the hydrolysis of ATP. Glycine is then linked to _γ_-glutamylcysteine by the enzyme glutathione synthetase. This reaction also requires energy input derived from the hydrolysis of ATP. The enzyme catalyzing the first reaction, _γ_-glutamylcysteine synthetase, is inhibited by normal intracellular glutathione concentrations (interrupted blue arrow shows inhibition).
Glutathione binds to and reduces (i.e., donates electrons) many molecules. These reducing reactions are of great importance for the detoxification of reactive oxygen species, drugs, toxins, and other oxidizing molecules and are also key reducing steps in a variety of normal metabolic synthetic pathways (40). In addition, glutathione has a multitude of other vital metabolic functions: it an important cellular reservoir of the sulfur-containing amino acid cysteine, it is a major cell signaling molecule involved in the regulation of apoptosis, cell cycling, and immunity and it participates in active transcellular amino acid transport (40–42).
The two synthetic reactions that unite glutamate, cysteine, and glycine to form glutathione are shown in Figure 1. The enzyme _γ_-glutamylcysteine synthetase (also called _γ_-glutamylcysteine ligase) catalyzes the unique _γ_-glutamyl linkage between glutamate and cysteine. Next, glutathione synthetase catalyzes the addition of glycine to the _γ_-glutamylcysteine (via the more usual _α_-amide linkage) to form glutathione. Each of these synthetic reactions requires energy provided by the hydrolysis of ATP. Two aspects of great importance to the pathophysiology discussed in this article are as follows: (1) _γ_-glutamylcysteine synthetase, the enzyme driving the first reaction, is normally inhibited by physiologic concentrations of the downstream product glutathione (this inhibition is shown by the broken blue line in Figure 1); and (2) the usual rate-limiting intracellular reactant in this synthetic sequence is cytosolic cysteine (43,44).
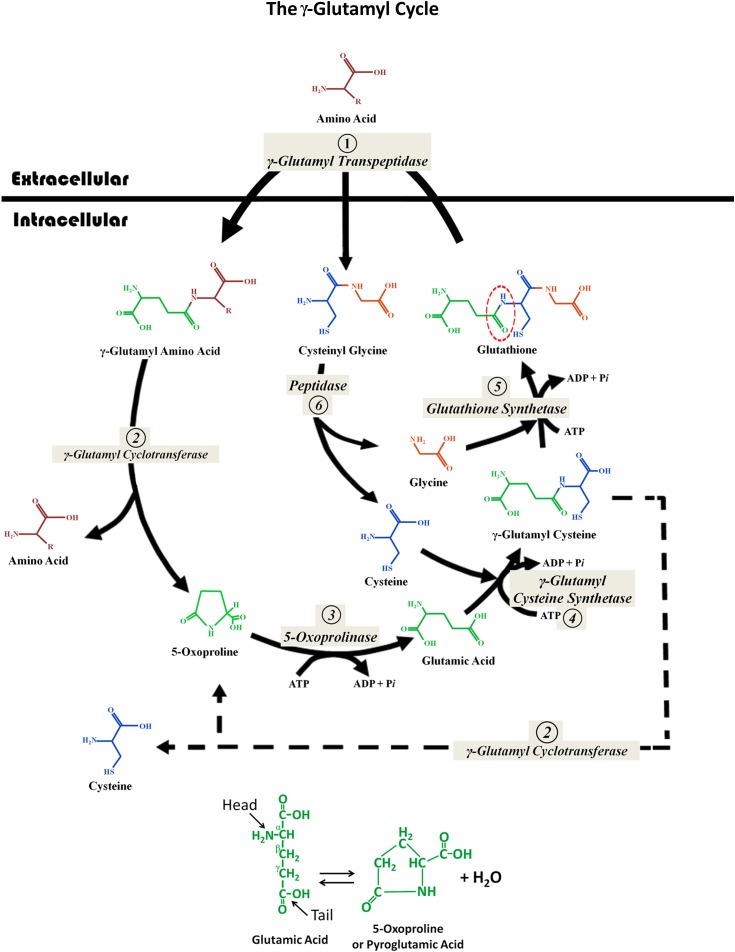
In the early 1970s, Alton Meister and his coworkers proposed that the two reactions shown in Figure 1 were part of a larger metabolic cycle that they named the _γ_-glutamyl cycle (45). This cycle consists of six enzymatic reactions—the pair of glutathione synthesizing reactions shown in Figure 1—and four degradation steps (see Figure 2). Follow this cycle starting at step 1 at the top of the diagram, where glutathione crosses the cell membrane and is attacked by membrane bound GGT. First, the _γ_-glutamyl bond is split and the glutamate is bound, again via the same unusual _γ_-linkage, to another extracellular amino acid. This reaction usually results in two dipeptides: _γ_-glutamyl-amino acid and cysteinylglycine. Both dipeptides enter the cell. (The enzyme GGT can also catalyze a reaction with water or other oligopeptides as the substrate to generate glutamic acid or γ-glutamyloligopeptides and also release cysteinylglycine.) In the cell, _γ_-glutamyl cyclotransferase (step 2) releases the transported amino acid and “cyclizes” the glutamic acid to form 5-oxoproline (also called pyroglutamic acid). This ring molecule is a circular form of glutamic acid created by an internal bond between the _γ_-carboxyl group and the _α_-amino group of this amino acid. Ernest Beutler colorfully described this reaction and the subsequent breaking of the ring as “…the head of glutamic acid biting its tail. With the help of another enzyme, pyroglutamate hydrolase (5-oxoprolinase), and energy from ATP, the head and tail of glutamic acid come undone, making the glutamic acid whole once again” (46) (see bottom of Figure 2). Next, 5-oxoprolinase opens the ring reforming glutamic acid (step 3). In step 4, the glutamic acid combines with cysteine to form _γ_-glutamylcysteine, and then in step 5, glycine is added to resynthesize glutathione. (These last two steps are the same enzymatic reactions shown in Figure 1.)
Figure 2.
The _γ_-glutamyl cycle. This cycle consists of six enzymatic reactions composed of two synthetic reactions and four degradation steps. (Step 1) Glutathione crosses the cell membrane where the enzyme _γ_-glutamyl transpeptidase, which is bound to the outer cell membrane, splits the molecule at the glutamyl _γ_-linkage (dotted circle). The released glutamic acid is then attached to another extracellular amino acid (or a peptide or water), again via the unusual _γ_-linkage. The _γ_-glutamyl-amino acid dipeptide and the cysteinylglycine dipeptide are both transported into the cell. (Step 2) The reaction catalyzed by _γ_-glutamyl cyclotransferase releases the amino acid and also “cyclizes” the glutamic acid to form 5-oxoproline (pyroglutamic acid). (Step 3) The 5-oxoproline ring is broken open by 5-oxoprolinase (pyroglutamate hydrolase) with energy input from ATP hydrolysis. (Step 4) Cysteine is bound to glutamic acid via a _γ_-linkage by the enzyme _γ_-glutamyl cysteine synthetase with energy input from ATP hydrolysis. This forms _γ_-glutamyl cysteine. (Step 5) Glutathione synthetase, with energy input from ATP hydrolysis, adds a glycine to reform glutathione. (Note steps 4 and 5 are the same as shown in Figure 2.) (Step 6) Cysteinylglycine is split by dipeptidase into cysteine and glycine, which are utilized in steps 4 and 5. Each complete rotation of this cycle requires the energy input derived from the hydrolysis of three molecules of ATP (steps 3–5). The net “work” produced by each turn of this cycle is the transport of one amino acid molecule across the cell membrane from the extracellular fluid into the cytoplasm. The interrupted line shows that under certain conditions (e.g., glycine deficiency), _γ_-glutamylcysteine can also be split by the enzyme _γ_-glutamyl cyclotransferase into cysteine and 5-oxoproline. The interconversion of glutamic acid and 5-oxoproline is shown below the cycle.
This metabolic cycle requires the input of energy, derived from the hydrolysis of three ATP molecules (Figure 2; steps 3–5). The net “work” produced by each turn of this cycle is the active transport of one amino acid molecule into the cell. Also note that each spin of this cycle does not generate any net new glutathione. Instead, a glutathione molecule is split and then later reformed in the process of transporting an amino acid into the cell. To generate new glutathione, its component amino acids must be combined as shown in Figure 1, utilizing other stores of the required three amino acids. The enzymes that comprise the _γ_-glutamyl cycle are widely distributed in the body, with the highest enzyme activities in organs with very active amino acid transport such as the kidney, liver, and small bowel (47).
Inherited Disorders of the _γ_-Glutamyl Cycle
At least two genetic enzyme defects lead to the overproduction of 5-oxoproline. Inherited abnormalities of 5-oxoprolinase (Figure 2, reaction 3), which are autosomal recessive traits, cause the accumulation of this enzyme’s substrate (5-oxoproline) and generate 5-oxoprolinemia and 5-oxoprolinuria. Clinical manifestations include neonatal hypoglycemia, microcytic anemia, and intellectual deficits (48,49). In addition, genetic glutathione synthetase defects (Figure 2, reaction 5), also an autosomal recessive trait, reduce glutathione levels and generate overproduction of 5-oxoproline. The clinical severity of inherited glutathione synthetase defects varies widely. Some patients develop hemolytic anemia and severely affected patients often develop major neurologic disorders and seizures. This enzymatic defect reduces synthesis of glutathione and the resulting low intracellular glutathione levels remove the normal tonic inhibition of the enzyme _γ_-glutamylcysteine synthetase (Figure 1 and Figure 2, reaction 4). Released inhibition accelerates synthesis of _γ_-glutamylcysteine. However, the inherited defective glutathione synthetase blocks or slows the conversion of _γ_-glutamylcysteine to glutathione. Consequently, γ-glutamylcysteine accumulates and is then converted to 5-oxoproline and cysteine by the enzyme _γ_-glutamylcyclotransferase (see dotted line in Figure 2).
Acetaminophen and Acquired 5-Oxoprolinemia/5-Oxoprolinuria
In 1989, Creer et al. reported a 52-year-old woman with anion gap metabolic acidosis caused by systemic accumulation of 5-oxoproline (a strong organic acid; p_K_a=3.6) and 5-oxoprolinuria (8). She had none of the other characteristic clinical features of the above-described two known inherited forms of 5-oxoprolinuria. They hypothesized that she had developed an acquired form of 5-oxoprolinemia/5-oxoprolinuria. A urine drug screen was positive for acetaminophen but the authors did not comment on the potential relationship of this medication to her disorder. The patient’s biochemical abnormalities gradually resolved with intravenous fluids, sodium bicarbonate, and discontinuation of acetaminophen.
One year later, Pitt et al. (9) described a similar case and noted that this patient was chronically ingesting acetaminophen. Glutathione synthetase and 5-oxoprolinase activity measured in skin fibroblasts were normal. The authors proposed that chronic acetaminophen use may have generated glutathione deficiency and that this was in some way responsible for overproduction of 5-oxoproline. Pitt et al. subsequently reported 11 cases of acquired 5-oxoprolinemia metabolic acidosis in patients ingesting acetaminophen (10). Dempsey et al. (12) and Fenves et al. (17) each described four additional cases and a number of individual case reports have been published (8–37).
Acetaminophen-related 5-oxoprolinemia/5-oxoprolinuria is usually associated with long-term outpatient use of the drug, although it has also been reported after inpatient therapeutic dosing (14,16,18,20,21,26). Acetaminophen blood levels are usually therapeutic. This is a distinctly different condition from acute acetaminophen poisoning, which can cause severe and potentially irreversible hepatic and sometimes renal damage. High 5-oxoproline levels have also occasionally been reported with toxic acetaminophen levels (with or without acute liver toxicity) (10,19,24,29).
Although acetaminophen-related anion gap metabolic acidosis due to 5-oxoprolinemia is thought to be a rare disorder, the assay for urine or serum 5-oxoproline is not readily available and many cases are undoubtably missed. Therefore, its true frequency is unknown. A large majority (40 of 49) of reported patients are women with various chronic medical diseases, malnutrition, and renal insufficiency in addition to long-term acetaminophen use. The condition generally resolves with discontinuation of acetaminophen and general supportive measures. Although many reports suggest that administration of _N_-acetyl cysteine may accelerate recovery, this has not yet been proven (10,19,23,31,32,36).
An animal model of acquired 5-oxoprolinemia/5-oxoprolinuria can be produced in rodents by adding acetaminophen to their drinking water (50). Of importance, the addition of methionine to the drinking water together with the acetaminophen prevents development of the disorder. The protective mechanism of methionine is likely due to its conversion to sulfur-containing amino acids, including cysteine, which are depleted by the renal excretion of sulfated acetaminophen metabolites (50).
Acetaminophen Metabolism, Glutathione, and 5-Oxoproline
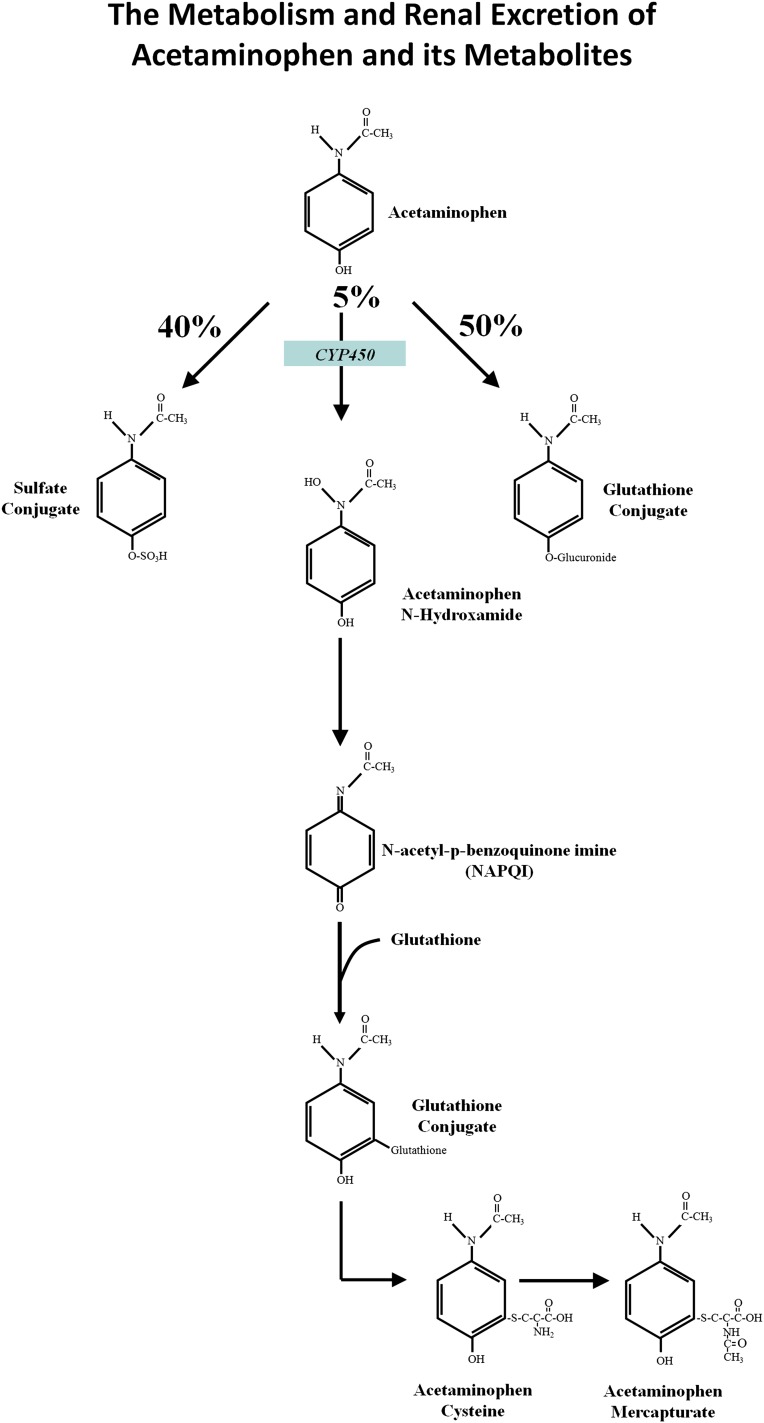
The absorbed complement of a therapeutic dose of acetaminophen is almost entirely metabolized and excreted into the urine. Approximately 50% is excreted as glucuronide acetaminophen conjugates, 40% as sulfated acetaminophen conjugates, and 5% unchanged. The remaining 5% of absorbed acetaminophen is oxidized by cytochrome enzymes, mainly CYP2E1, to produce _N_-acetyl-p-benzoquinoneimine (NAPQI), a highly reactive and short-lived oxidation product or electrophile (Figure 3) (1–3,51). Optimally, this dangerous metabolite is conjugated, reduced, and thereby detoxified by glutathione. The glutathione-NAPQI conjugate is then cleaved to other chemically stable, nontoxic thiol metabolites that are also mainly excreted in the urine.
Figure 3.
The metabolism and renal excretion of acetaminophen and its metabolites. Approximately 50% of a therapeutic dose of acetaminophen is glucuronidated and excreted in the urine, approximately 40% is sulfated and excreted in the urine, and approximately 5% is excreted in the urine unchanged. The remaining 5% of ingested acetaminophen is oxidized by cytochrome enzymes (mainly CYP2E1) to produce acetaminophen _N_-hydroxamide, which is then converted to _N_-acetyl-p-benzoquinoneimine (NAPQI), a highly reactive and short-lived oxidation product or electrophile. Optimally, this dangerous metabolite is conjugated, and thereby detoxified, by glutathione. The glutathione-NAPQI conjugate is then cleaved to chemically stable, nontoxic thiol metabolites, including acetaminophen cysteine and acetaminophen mercapturate, which are largely excreted in the urine.
Acute ingestion of toxic amounts of acetaminophen probably depletes glutathione stores, especially in malnourished individuals and persons with chronic alcoholism (51). If glutathione levels fall, the reactive NAPQI metabolite can combine with various structural proteins and other vital molecules and thereby cause toxicity. This is believed to be a major mechanism of hepatic damage generated by acute acetaminophen poisoning.
Chronic acetaminophen ingestion likely also consumes glutathione, as well as stores of sulfated amino acids. This is especially likely in malnourished, chronically ill patients. Depletion of glutathione and sulfated amino acids is almost certainly an important component of the mechanism responsible for the development of acetaminophen-related 5-oxoprolinemia and 5-oxoprolinuria.
Virtually every report of acetaminophen-related 5-oxoprolinemia/oxoprolinuria includes a diagram of the _γ_-glutamyl cycle similar to that shown in Figure 2 and attributes the overproduction of 5-oxoproline to the metabolic pathway represented by the dotted arrow leading from _γ_-glutamyl cysteine to 5-oxoproline and cysteine. As noted above, the enzyme _γ_-glutamyl cysteine synthetase (Figure 2, reaction 4) is the main rate-limiting step in the _γ_-glutamyl cycle and this reaction is nonallosterically inhibited by physiologic concentrations of glutathione (42,43). Reduced intracellular glutathione levels removes the inhibition of _γ_-glutamyl cysteine synthetase and synthesis of _γ_-glutamyl cysteine increases (Figure 2, reaction 4). Therefore, to the extent that chronic acetaminophen ingestion reduces glutathione levels, _γ_-glutamyl cysteine synthesis should increase. However, if this occurred with chronic acetaminophen ingestion, why would the _γ_-glutamyl cysteine enter the dotted line pathway, be catalyzed by _γ_-glutamylcyclotransferase, and release cysteine and 5-oxoproline? It would seem that the _γ_-glutamyl cysteine should instead add a glycine molecule (glutathione synthetase reaction) (Figure 2, reaction 5) and thereby replenish glutathione. Conceivably, a state of glycine deficiency could slow glutathione synthesis via reaction 5 and this may occur in some malnourished children and pregnant women. Indeed, 5-oxoprolinruia has been used as a nutritional index of glycine sufficiency in these populations (52,53). However, the magnitude of 5-oxoprolinuria that occurs under these conditions is modest and overt metabolic acidosis does not develop (52,53). It is unlikely that deficiency of this nonessential amino acid can account for the degree of 5-oxoprolinemia that develops in patients with acquired 5-oxoproline metabolic acidosis (10). Furthermore, if glycine deficiency was the cause of acetaminophen-related overproduction of 5-oxoproline, then why would provision of methionine or cysteine prevent or reverse the pathology?
An ATP-Depleting Futile Cycle Can Explain Acetaminophen-Related 5-Oxoproline Accumulation
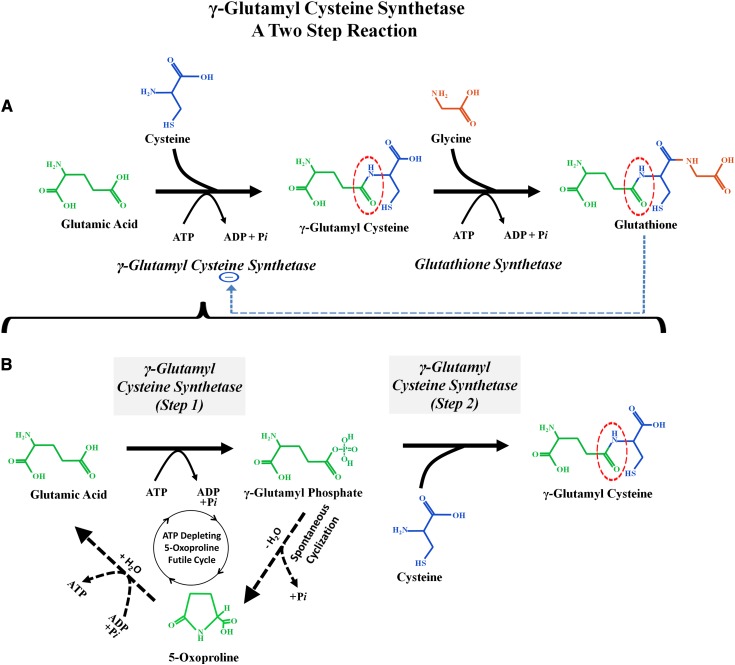
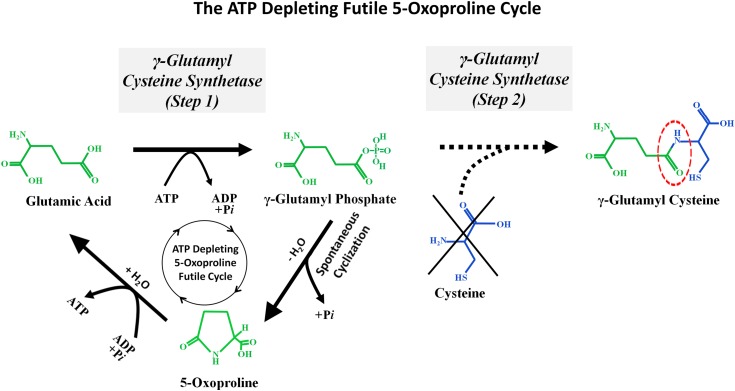
The critical “missing step” that can explain why chronic acetaminophen generates 5-oxoprolinemia/5-oxoprolinuria is shown in Figure 4. Orlowski and Meister found that the ATP-driven reaction that combines glutamate and cysteine to generate _γ_-glutamyl cysteine (catalyzed by _γ_-glutamyl cysteine synthetase) (Figure 4A) can actually be subdivided into two distinct reaction steps (Figure 4B) (38,54). In the first step, ATP hydrolysis phosphorylates glutamate to form the high energy intermediate _γ_-glutamyl phosphate. This intermediate remains within the active site of the enzyme _γ_-glutamyl cysteine synthetase until it combines with cysteine in step 2 to form _γ_-glutamyl cysteine that is then released. However, if cysteine is deficient, then _γ_-glutamyl cysteine cannot be formed and instead the _γ_-glutamyl phosphate will autocyclize to become 5-oxoproline (Figures 4B and 5). The ring of 5-oxoproline can be broken open by the enzyme 5-oxoprolinase to reform glutamate. This reaction consumes ATP. These reactions create an ATP-depleting futile 5-oxoproline cycle that consumes two ATP molecules for each circuit. When ATP levels fall, the 5-oxoprolinase reaction slows and 5-oxoproline accumulates (55). Activation of this ATP-depleting futile 5-oxoproline cycle (glutamate → glutamyl phosphate → 5-oxoproline → glutamate) requires two triggers: reduced cellular glutathione levels, which activates step 1 of the _γ_-glutamyl cysteine synthetase reaction, and reduced cytosol cysteine levels, which blocks step 2 of the reaction sequence and instead causes the accumulating glutamyl phosphate to autocyclize and form 5-oxoproline. It is likely that this milieu of combined glutathione and cysteine depletion develops in some patients who chronically ingest acetaminophen, especially if their intake of cysteine precursors, such as methionine, is deficient.
Figure 4.
_γ_-Glutamyl cysteine synthetase: a two-step reaction sequence. (A) Two synthetic reactions first combine glutamic acid and cysteine to form _γ_-glutamylcysteine and then add glycine to form glutathione. (These two reactions were also shown in Figure 1.) Note the interrupted blue arrow, which represents inhibition of the enzyme _γ_-glutamylcysteine synthetase (also called _γ_-glutamylcysteine ligase) by physiologic concentrations of glutathione. (B) The _γ_-glutamylcysteine synthetase reaction, which combines glutamate and cysteine, occurs in two discrete steps. (Step 1) Glutamate enters the active site of the enzyme and is then “activated” by the addition of phosphate from ATP to form _γ_-glutamyl phosphate. This high energy intermediate molecule remains within the active site of _γ_-glutamyl cysteine synthetase until step 2, when it combines with cysteine to form _γ_-glutamyl cysteine. The _γ_-glutamyl cysteine is then released from the active site of the enzyme. However, if cysteine is deficient, then _γ_-glutamyl phosphate will autocyclize to form 5-oxoproline. The black dotted lines are part of an ATP-depleting 5-oxoproline futile cycle.
Figure 5.
The ATP-depleting futile 5-oxoproline cycle. Glutathione deficiency increases the activity of _γ_-glutamyl cysteine synthase, an ATP utilizing enzyme. However, if cysteine deficiency exists, then step 2 of this reaction sequence is blocked and _γ_-glutamyl cysteine cannot be generated. Instead, the _γ_-glutamyl phosphate synthesized by step 1 will autocyclize to form 5-oxoproline. The 5-oxoproline ring can then be broken open by 5-oxoprolinase to regenerate glutamate. This reaction also requires energy from ATP hydrolysis. This series of reactions creates a futile cycle that consumes two ATP molecules for each circuit. When ATP levels fall, the 5-oxoprolinase reaction is inhibited and this leads to the accumulation of 5-oxoproline.
As discussed and shown in Figure 3, approximately 50% of absorbed acetaminophen is excreted into the urine as sulfated metabolites: acetaminophen sulfate, acetaminophen cysteine, and acetaminophen mercapturate. This can deplete cysteine stores (56,57). In addition, conjugation of acetaminophen to glutathione reduces its levels. The combination of cysteine and glutathione depletion activates the above-described ATP-depleting futile 5-oxoproline cycle.
This ATP-depleting futile 5-oxoproline cycle was first proposed by Kumar and Bachhawat to explain the development of Fanconi syndrome, ATP depletion, and 5-oxoprolinuria in children with nephropathic cystinosis (38,39). The genetic mutation responsible for that disorder causes cystine to accumulate within cellular lysosomes (58–60). Kumar and Bachhawat hypothesized that this leads to the combination of cytosolic cysteine and glutathione depletion, which activates the ATP-depleting futile 5-oxoproline cycle.
ATP-Depleting 5-Oxoproline Futile Cycle May Explain Other Features of Acetaminophen-Related Toxicity
Activation of an ATP-depleting futile 5-oxoproline cycle may also contribute to the development of hepatic and kidney injury after acute acetaminophen poisoning. The pathogenesis of these disorders is generally attributed to the direct toxicity of acetaminophen-derived reactive oxidation products. As described above, the small fraction (approximately 5%) of ingested acetaminophen that is oxidized to NAPQI is usually detoxified by glutathione conjugation. When large acute doses of acetaminophen consume glutathione stores, the reactive and very toxic NAPQI is free to interact with multiple cell macromolecules. This damages mitochondrial and other cell membranes and structures, leading to cell injury and death (3). These toxic reactions are especially pronounced within the hepatocytes, but also proceed in other organs such as the kidney. It is also well established that hepatic ATP depletion occurs with acetaminophen poisoning and disruption of mitochondrial membranes is thought to be the major cause of impaired ATP generation. However, if the ATP-depleting futile 5-oxoproline cycle becomes activated, this may also contribute to the hepatic and renal ATP and energy depletion.
The protective and therapeutic effect of cysteine precursors such as methionine and _N_-acetyl cysteine in patients and animal models of acquired 5-oxoprolinemia and 5-oxoprolinuria is probably related to restoration of both cellular cysteine stores and glutathione. This allows step 2 of the _γ_-glutamyl cysteine synthetase reaction to incorporate cysteine so that substrates will flow toward glutathione synthesis instead of 5-oxoproline. The very dramatic beneficial effect of _N_-acetyl cysteine in cases of acute acetaminophen toxicity is also well documented (51). Its proposed mechanism of action in these patients is attributed to glutathione repletion and subsequent detoxification of acetaminophen reactive oxidation products, especially NAPQI. However, repletion of cellular cysteine stores may also be an important and underemphasized beneficial effect of this drug. To the extent that depleted cysteine stores are restored, the component of ATP depletion caused by the ATP-depleting futile 5-oxoproline cycle will be ameliorated. Renal tubular toxicity of acetaminophen with the development of potassium and phosphate wasting and acute tissue injury may also be linked to energy (ATP) depletion partially generated by the ATP-depleting 5-oxoproline cycle.
The role of dietary glycine deficiency in the development of 5-oxoprolinuria has been mentioned (52,53). If that occurred, then _γ_-glutamyl cysteine, the final substrate of the glutathione synthase reaction (Figure 2, reaction 5), would be shunted into the dotted line pathway shown in Figure 2 instead of synthesis of glutathione. Therefore, adding glycine deficiency to reduced levels of cysteine and glutathione would likely have an additive effect on 5-oxoproline generation and may develop in malnourished, chronically ill patients.
The marked female predominance of the acquired acetaminophen-related form of 5-oxoprolinemia/5-oxoprolinuria syndrome may be due to sex-related differences in the acetaminophen detoxification pathways. Women preferentially metabolize acetaminophen to sulfated derivatives (61). Therefore, women may be more susceptible to depletion of sulfated amino acids when they ingest acetaminophen. However, it should be noted that opposite findings have been reported in rodents, and the animal model of this disorder discussed above utilized male rats (50,62).
Occasional reports of acquired 5-oxoprolinemia and 5-oxoprolinuria have attributed the disorder to the administration of several drugs other than acetaminophen. They include the antibiotics flucloxacillin and netilmicin and the anticonvulsant vigabatrin (16,63,64). The patients were also ingesting acetaminophen in most, but not all, cases. Reports have suggested that these drugs may inhibit various _γ_-glutamyl cycle enzymes, including 5-oxoprolinase. However, there is no evidence that this actually occurs. Furthermore, vigabatrin is likely directly metabolized to the dextro isomer of 5-oxoproline and this may explain that particular association (65).
In conclusion, an ATP-depleting futile 5-oxoproline cycle that can lead to the accumulation of 5-oxoproline is described and shown in Figure 5. This cycle is activated by the combination of glutathione depletion, which increases the activity of _γ_-glutamyl cysteine synthetase, and cysteine deficiency, which prevents the high energy intermediate product _γ_-glutamyl phosphate (step 1) from being converted to _γ_-glutamyl cysteine (step 2). Instead, the _γ_-glutamyl phosphate is autocyclized to form 5-oxoproline. The 5-oxoproline is then converted back to glutamic acid by the enzyme 5-oxoprolinase. When this cycle spins, ATP is utilized at two steps and is depleted. ATP depletion slows the 5-oxoprolinase reaction and causes 5-oxoproline accumulation. This ATP-depleting futile 5-oxoproline cycle can explain the development of 5-oxoprolinemia and 5-oxoprolinuria in patients who chronically ingest acetaminophen. Glycine deficiency can further exacerbate this syndrome. The ATP-depleting futile 5-oxoproline cycle may also play an important role in other acetaminophen toxicity syndromes related to ATP depletion.
Disclosures
None.
Acknowledgments
The author would like to thank Dr. John S. Fordtran for his thoughtful input and suggestions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bessems JGM, Vermeulen NPE: Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31: 55–138, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Hinson JA, Roberts DW, James LP: Mechanisms of acetaminophen-induced liver necrosis. Handbook Exp Pharmacol 196: 369–405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H: The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122: 1574–1583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waring WS, Jamie H, Leggett GE: Delayed onset of acute renal failure after significant paracetamol overdose: A case series. Hum Exp Toxicol 29: 63–68, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Mazer M, Perrone J: Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol 4: 2–6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pakravan N, Bateman DN, Goddard J: Effect of acute paracetamol overdose on changes in serum and urine electrolytes. Br J Clin Pharmacol 64: 824–832, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AF, Harvey JM, Vale JA: Hypophosphataemia and phosphaturia in paracetamol poisoning. Lancet 2: 608–609, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Creer MH, Lau BWC, Jones JD, Chan K-M: Pyroglutamic acidemia in an adult patient. Clin Chem 35: 684–686, 1989 [PubMed] [Google Scholar]
- 9.Pitt JJ, Brown GK, Clift V, Christodoulou J: Atypical pyroglutamic aciduria: Possible role of paracetamol. J Inherit Metab Dis 13: 755–756, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Pitt JJ, Hauser S: Transient 5-oxoprolinuria and high anion gap metabolic acidosis: Clinical and biochemical findings in eleven subjects. Clin Chem 44: 1497–1503, 1998 [PubMed] [Google Scholar]
- 11.Mayatepek E: 5-Oxoprolinuria in patients with and without defects in the γ-glutamyl cycle. Eur J Pediatr 158: 221–225, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Dempsey GA, Lyall HJ, Corke CF, Scheinkestel CD: Pyroglutamic acidemia: A cause of high anion gap metabolic acidosis. Crit Care Med 28: 1803–1807, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Tailor P, Raman T, Garganta CL, Njalsson R, Carlsson K, Ristoff E, Carey HB: Recurrent high anion gap metabolic acidosis secondary to 5-oxoproline (pyroglutamic acid). Am J Kidney Dis 46: e4–e10, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Humphreys BD, Forman JP, Zandi-Nejad K, Bazari H, Seifter J, Magee CC: Acetaminophen-induced anion gap metabolic acidosis and 5-oxoprolinuria (pyroglutamic aciduria) acquired in hospital. Am J Kidney Dis 46: 143–146, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Foot CL, Fraser JF, Mullany DV: Pyroglutamic acidosis in a renal transplant patient. Nephrol Dial Transplant 20: 2836–2838, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Peter JV, Rogers N, Murty S, Gerace R, Mackay R, Peake SL: An unusual cause of severe metabolic acidosis. Med J Aust 185: 223–225, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fenves AZ, Kirkpatrick HM, 3rd, Patel VV, Sweetman L, Emmett M: Increased anion gap metabolic acidosis as a result of 5-oxoproline (pyroglutamic acid): A role for acetaminophen. Clin J Am Soc Nephrol 1: 441–447, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Alados Arboledas FJ, de la Oliva Senovilla P, García Muñoz MJ, Alonso Melgar A, Ruza Tarrío F: Acidosis piroglutámica asociada a paracetamol. An Pediatr (Barc) 67: 582–584, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hodgman MJ, Horn JF, Stork CM, Marraffa JM, Holland MG, Cantor R, Carmel PM: Profound metabolic acidosis and oxoprolinuria in an adult. J Med Toxicol 3: 119–124, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooker G, Jeffery J, Nataraj T, Sair M, Ayling R: High anion gap metabolic acidosis secondary to pyroglutamic aciduria (5-oxoprolinuria): Association with prescription drugs and malnutrition. Ann Clin Biochem 44: 406–409, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rolleman EJ, Hoorn EJ, Didden P, Zietse R: Guilty as charged: Unmeasured urinary anions in a case of pyroglutamic acidosis. Neth J Med 66: 351–353, 2008 [PubMed] [Google Scholar]
- 22.Kortmann W, van Agtmael MA, van Diessen J, Kanen BLJ, Jakobs C, Nanayakkara PWB: 5-Oxoproline as a cause of high anion gap metabolic acidosis: An uncommon cause with common risk factors. Neth J Med 66: 354–357, 2008 [PubMed] [Google Scholar]
- 23.Green TJ, Bijlsma JJ, Sweet DD: Profound metabolic acidosis from pyroglutamic acidemia: An underappreciated cause of high anion gap metabolic acidosis. CJEM 12: 449–452, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence DT, Bechtel LK, Charlton NP, Holstege CP: 5-oxoproline-induced anion gap metabolic acidosis after an acute acetaminophen overdose. J Am Osteopath Assoc 110: 545–551, 2010 [PubMed] [Google Scholar]
- 25.Duewall JL, Fenves AZ, Richey DS, Tran LD, Emmett M: 5-Oxoproline (pyroglutamic) acidosis associated with chronic acetaminophen use. Proc (Bayl Univ Med Cent) 23: 19–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong YL, Koh J: Case report: Pyroglutamic acidaemia as a likely cause of high anion gap metabolic acidosis. Respirology 16[Suppl 2]: 254, 2011 [Google Scholar]
- 27.Reddi AS, Kunadi AR: Recurrent anion gap metabolic acidosis in a woman with vertebral disc disease. Am J Emerg Med 29: 962.e3–962.e8, 2011 [DOI] [PubMed]
- 28.Chestnutt J, Heyburn G, Roberts B: Pyroglutamic aciduria: A cause of high anion-gap metabolic acidosis associated with common drugs. Ir Med J 104: 312–313, 2011 [PubMed] [Google Scholar]
- 29.Romero JE, Htyte N: An unusual cause of high anion gap metabolic acidosis: Pyroglutamic acidemia. A case report. Am J Ther 20: 581–584, 2011 [DOI] [PubMed]
- 30.Verma R, Polsani KR, Wilt J, Loehrke ME: 5-Oxoprolinuria as a cause of high anion gap metabolic acidosis. Br J Clin Pharmacol 73: 489–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myall K, Sidney J, Marsh A: Mind the gap! An unusual metabolic acidosis. Lancet 377: 526, 2011 [DOI] [PubMed] [Google Scholar]
- 32.O’Brien LM, Hooper M, Flemmer M, Marik PE: Chronic acetaminophen ingestion resulting in severe anion gap metabolic acidosis secondary to 5-oxoproline accumulation: An under diagnosed phenomenon. Br Med J Case Rep 2012: pii:bcrbcr0320126020, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhuijzen N, Kamphuis S, van den Bergh F, Spronk P, Braber A: Madam, why are you so sour? Cause, diagnosis and complication of 5-oxoprolinemia. Eur J Anaesthesiol 29: 398–400, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Zand L, Muriithi A, Nelsen E, Franco PM, Greene EL, Qian Q, El-Zoghby ZM: Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 344: 501–504, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Prasad S: A case of high anion gap metabolic acidosis: What’s the anion? Crit Care Med 40: U317, 2012 [Google Scholar]
- 36.Armenian P, Gerona RR, Blanc PD, Wu AHB, Mookherjee S: 5-oxoprolinemia causing elevated anion gap metabolic acidosis in the setting of acetaminophen use. J Emerg Med 43: 54–57, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Milosevic S, Tran K, O’Brien B: A rare cause of high anion gap metabolic acidosis. Intern Med J 43: 100–101, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Bachhawat AK: A futile cycle, formed between two ATP-dependant γ-glutamyl cycle enzymes, γ-glutamyl cysteine synthetase and 5-oxoprolinase: The cause of cellular ATP depletion in nephrotic cystinosis? J Biosci 35: 21–25, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Bachhawat AK: Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr Sci 102: 288–297, 2012 [Google Scholar]
- 40.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI: The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem 113: 234–258, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL: Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390: 191–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forman HJ, Zhang H, Rinna A: Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu SC: Regulation of glutathione synthesis. Mol Aspects Med 30: 42–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stipanuk MH, Dominy JE, Jr, Lee JI, Coloso RM: Mammalian cysteine metabolism: New insights into regulation of cysteine metabolism. J Nutr 136[Suppl]: 1652S–1659S, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Orlowski M, Meister A: The γ-glutamyl cycle: A possible transport system for amino acids. Proc Natl Acad Sci U S A 67: 1248–1255, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beutler E: Editorial: Glutathione deficiency, pyroglutamic acidemia and amino acid transport. N Engl J Med 295: 441–443, 1976 [DOI] [PubMed] [Google Scholar]
- 47.Meister A, Griffith OW, Novogrodsky A, Tate SS: New aspects of glutathione metabolism and translocation in mammals. Ciba Found Symp 72: 135–161, 1979 [DOI] [PubMed] [Google Scholar]
- 48.Larsson A, Ristoff E, Anderson ME: Glutathione synthetase deficiency and other disorders of the γ-glutamyl cycle. In: Beaudet, Vogelstein, Kinzler, Antonarakis, Ballabio, eds. Scriver’s Online Metabolic & Molecular Bases of Inherited Disease Available at: http://www.ommbid.com/ Accessed October 25, 2013
- 49.Ristoff E, Larsson A: Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis 2: 16, 2007 [DOI] [PMC free article] [PubMed]
- 50.Ghauri FY, McLean AEM, Beales D, Wilson ID, Nicholson JK: Induction of 5-oxoprolinuria in the rat following chronic feeding with N-acetyl 4-aminophenol (paracetamol). Biochem Pharmacol 46: 953–957, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Heard KJ: Acetylcysteine for acetaminophen poisoning. N Engl J Med 359: 285–292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persaud C, McDermott J, De Benoist B, Jackson AA: The excretion of 5-oxoproline in urine, as an index of glycine status, during normal pregnancy. Br J Obstet Gynaecol 96: 440–444, 1989 [DOI] [PubMed] [Google Scholar]
- 53.Lenton C, Ali Z, Persaud C, Jackson AA: Infants in Trinidad excrete more 5-L-oxoproline (L-pyroglutamic acid) in urine than infants in England: An environmental not ethnic difference. Br J Nutr 80: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Orlowski M, Meister A: Partial reactions catalyzed by gamma-glutamylcysteine synthetase and evidence for an activated glutamate intermediate. J Biol Chem 246: 7095–7105, 1971 [PubMed] [Google Scholar]
- 55.Van Der Werf P, Griffith OW, Meister A: 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Purification and catalytic properties. J Biol Chem 250: 6686–6692, 1975 [PubMed] [Google Scholar]
- 56.Poulsen HE, Thomsen P: Long-term administration of toxic doses of paracetamol (acetaminophen) to rats. Liver 8: 151–156, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Mannery YO, Ziegler TR, Park Y, Jones DP: Oxidation of plasma cysteine/cystine and GSH/GSSG redox potentials by acetaminophen and sulfur amino acid insufficiency in humans. J Pharmacol Exp Ther 333: 939–947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mannucci L, Pastore A, Rizzo C, Piemonte F, Rizzoni G, Emma F: Impaired activity of the gamma-glutamyl cycle in nephropathic cystinosis fibroblasts. Pediatr Res 59: 332–335, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Coor C, Salmon RF, Quigley R, Marver D, Baum M: Role of adenosine triphosphate (ATP) and NaK ATPase in the inhibition of proximal tubule transport with intracellular cystine loading. J Clin Invest 87: 955–961, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilmer MJ, Emma F, Levtchenko EN: The pathogenesis of cystinosis: Mechanisms beyond cystine accumulation. Am J Physiol Renal Physiol 299: F905–F916, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Critchley JA, Nimmo GR, Gregson CA, Woolhouse NM, Prescott LF: Inter-subject and ethnic differences in paracetamol metabolism. Br J Clin Pharmaol 22: 649–657, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kane RE, Tector J, Brems JJ, Li A, Kaminski D: Sulfation and glucuronidation of acetaminophen by cultured hepatocytes reproducing in vivo sex-differences in conjugation on Matrigel and type 1 collagen. In Vitro Cell Dev Biol 27A: 953–960, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Croal BL, Glen AC, Kelly CJ, Logan RW: Transient 5-oxoprolinuria (pyroglutamic aciduria) with systemic acidosis in an adult receiving antibiotic therapy. Clin Chem 44: 336–340, 1998 [PubMed] [Google Scholar]
- 64.Bonham JR, Rattenbury JM, Meeks A, Pollitt RJ: Pyroglutamicaciduria from vigabatrin. Lancet 1: 1452–1453, 1989 [DOI] [PubMed] [Google Scholar]
- 65.Meister A: Vigabatrin and urinary 5-oxoproline. Lancet 2: 1216, 1989 [DOI] [PubMed] [Google Scholar]