Wall Teichoic Acids of Gram-Positive Bacteria (original) (raw)
. Author manuscript; available in PMC: 2014 Jan 7.
Abstract
The peptidoglycan layers of many gram-positive bacteria are densely functionalized with anionic glycopolymers called wall teichoic acids (WTAs). These polymers play crucial roles in cell shape determination, regulation of cell division, and other fundamental aspects of gram-positive bacterial physiology. Additionally, WTAs are important in pathogenesis and play key roles in antibiotic resistance. We provide an overview of WTA structure and biosynthesis, review recent studies on the biological roles of these polymers, and highlight remaining questions. We also discuss prospects for exploiting WTA biosynthesis as a target for new therapies to overcome resistant infections.
Keywords: peptidoglycan, bacterial cell wall, d-alanylation, MRSA, virulence factor, biofilm
Introduction
Bacteria are surrounded by a complex cell envelope that performs a variety of functions (114). Cell envelopes are varied in structure, but all contain layers of peptidoglycan (PG), a crosslinked matrix of linear carbohydrate (glycan) chains linked to one another via covalent bonds between attached peptides (130). This PG matrix is essential for survival, and in gram-positive organisms it is densely functionalized with other polymers. Wall teichoic acids (WTAs) are the most abundant PG-linked polymers in many gram-positive organisms (91). They are intimately involved in many aspects of cell division and are essential for maintaining cell shape in rod-shaped organisms (120). WTAs are required for β-lactam resistance in methicillin-resistant S. aureus (MRSA), and they modulate susceptibility to cationic antibiotics in several organisms (23; 25; 144). Due to their importance in pathogenesis, WTAs are possible targets for new therapeutics to overcome resistant bacterial infections. Here we review recent work on WTAs and discuss studies implicating the pathway as a therapeutic target.
Teichoic Acid Definition
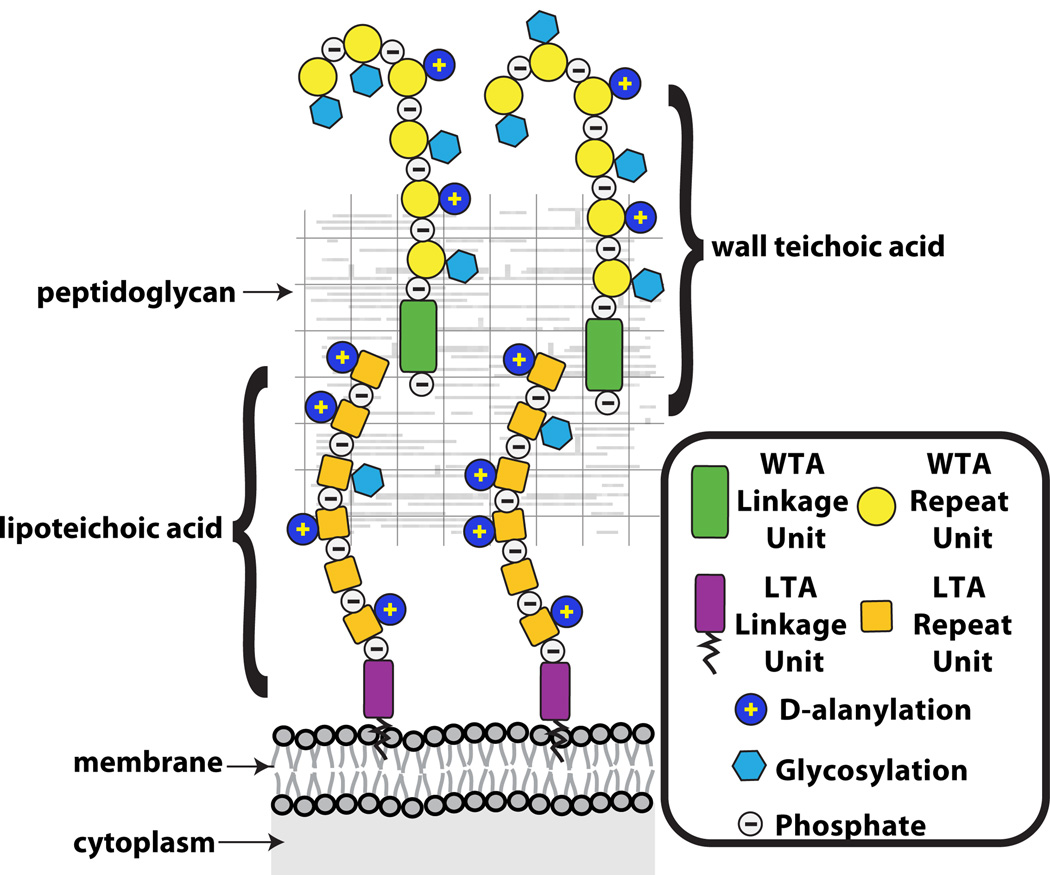
Teichoic acids were discovered in 1958 by Armstrong and co-authors while trying to determine the function of CDP-glycerol and CDP-ribitol in Lactobacillus arabinosus, Bacillus subtilis and several other bacteria (6). The term teichoic acid encompasses a diverse family of cell surface glycopolymers containing phosphodiester-linked polyol repeat units (133). Teichoic acids include both lipoteichoic acids (LTAs), which are anchored in the bacterial membrane via a glycolipid, and WTAs, which are covalently attached to peptidoglycan (Figure 1) (91; 133). This review focuses primarily on WTAs. For information on LTAs the reader is directed to a recent review by Reichmann and Gründling (106).
Figure 1. Teichoic acid polymers are located within the gram-positive cell wall.
Schematic of the gram-positive cell wall showing that wall teichoic acids are covalently anchored to peptidoglycan and lipoteichoic acids are tethered to the membrane. The WTAs extend beyond the PG layer, whereas fully extended LTAs may not be able to reach pass the PG layer.
Wall Teichoic Acid Backbone Structure
Wall teichoic acids are highly abundant modifications of gram-positive cell walls (90). In B. subtilis and S. aureus, it has been estimated that every ninth PG MurNAc residue contains an attached WTA polymer containing 40 to 60 polyol repeats (12; 71). The total mass of WTAs in these and other organisms comprises up to 60% of the cell wall (44; 124). Consistent with their estimated length, cryo-EM images suggest that S. aureus WTAs extend well beyond the PG layer (81; 82; 106).
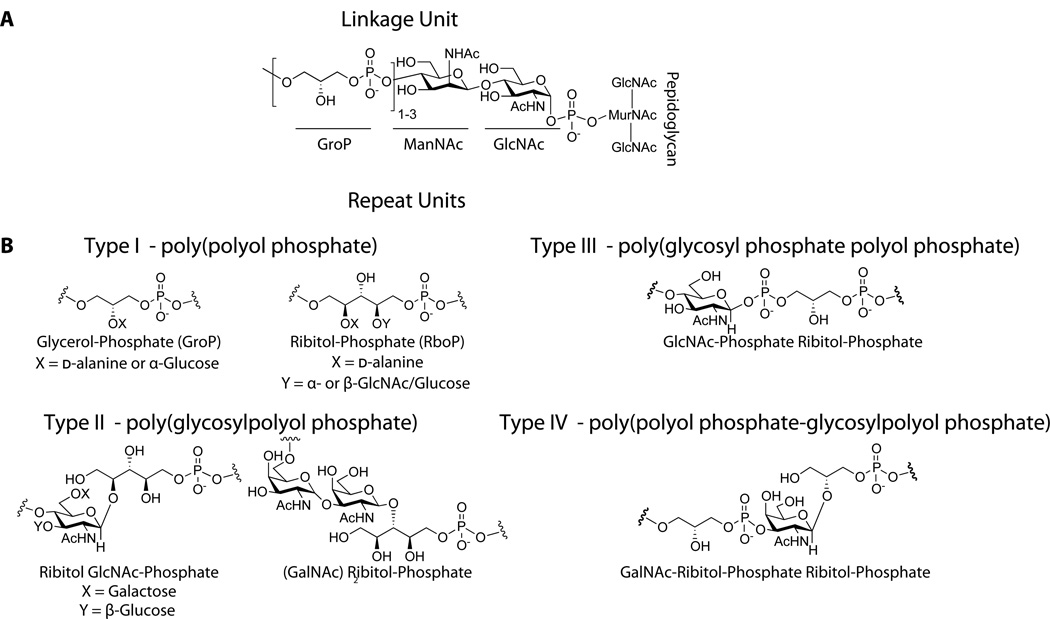
The wall teichoic acid polymer can be divided into two components, a disaccharide linkage unit and a main chain polymer composed of phosphodiester-linked polyol repeat units (Figure 2) (91). The disaccharide linkage unit, which is highly conserved across bacterial species, is comprised of N-acetylmannosamine (β1→4) N-acetylglucosamine-1-phosphate (ManNAc (β1→4) GlcNAc-1P) with one to two glycerol-3-phosphate (GroP) units attached to the C4 oxygen of ManNAc (5; 71; 91). The anomeric phosphate of the linkage unit is covalently attached to peptidoglycan (PG) via a phosphodiester bond to the C6 hydroxyl of N-acetylmuramic acid (MurNAc). The phosphodiester-linked polyol repeats extend from the GroP end of the linkage unit (91; 133).
Figure 2. Wall teichoic acid polymers share a common linkage unit, but exhibit structural diversity in their repeat units.
A. The commonly characterized WTA linkage unit consists of a GroP-ManNAc-GlcNAc-Phosphate that is covalently attached to peptidoglycan. While the stereochemistry of the glycosidic linkage has not been clearly established, it is assumed to be retained as α based on predicted enzymatic catalysis.
B. Four different classes of WTA repeat units found in gram-positive bacteria (90).
The best-characterized WTA structures contain repeat units of 1,5-d-ribitol-phosphate (RboP) or 1,3-l-α-glycerol-phosphate (GroP), but WTA monomer structures can be highly diverse and many published structures are more unusual (45; 46; 90). Other WTA repeat units include variations of glycosyl-polyol phosphate or glycosyl-phosphate polyol-phosphate (Figure 2)(90; 91). WTA structural diversity can exist within the same species, as in B. subtilis, where strains 168 and W23 contain GroP and RboP repeat units, respectively. Lactobacillus plantarum has the ability to switch backbone composition (21). Furthermore, single strains of S. aureus and B. coagulans have been found to contain polymers with distinct repeats expressed simultaneously, but at different levels (102; 128). WTA structural variations may represent adaptations to different environments. Relatedly, bacteria are known to modulate transcription of WTA biosynthetic genes in response to stress conditions (87). Despite their diversity, all WTAs contain a negatively charged anionic backbone and share common functions.
Tailoring Modifications on the Wall Teichoic Acid Polymer
Additional WTA structural diversity arises from the presence or absence of substituents attached to the repeating monomers (Figure 3). The repeat unit hydroxyls can be tailored with cationic d-alanine esters or a variety of mono- or oligosaccharides, commonly glucose or GlcNAc (91). In RboP polymers, d-alanyl residues are installed at position 2 of ribitol while sugars are commonly found at position 4 (91; 128). d-Alanine ester content is variable and depends on several factors, including pH, salt concentration, and temperature (64; 91). In contrast, the WTA sugar substituents do not appear to fluctuate with changes in the cellular environment (32). These tailoring modifications are typically highly abundant. For example, nearly all of the RboP repeats in S. aureus contain O-GlcNAc substituents (64). Depending on the bacterial strain, the anomeric configuration of the glycosidic linkage to the repeat unit can be exclusively α, exclusively β, or a mixture of the two anomers (29; 45; 46; 88). Some S. aureus strains have been found to contain, in the same cell wall, two different poly(RboP) WTAs, one fully α-glycosylated and the other fully β-glycosylated (125). In B. subtilis W23 it was observed that some WTA strands were fully glycosylated, whereas other strands contained no sugar substituents (29).
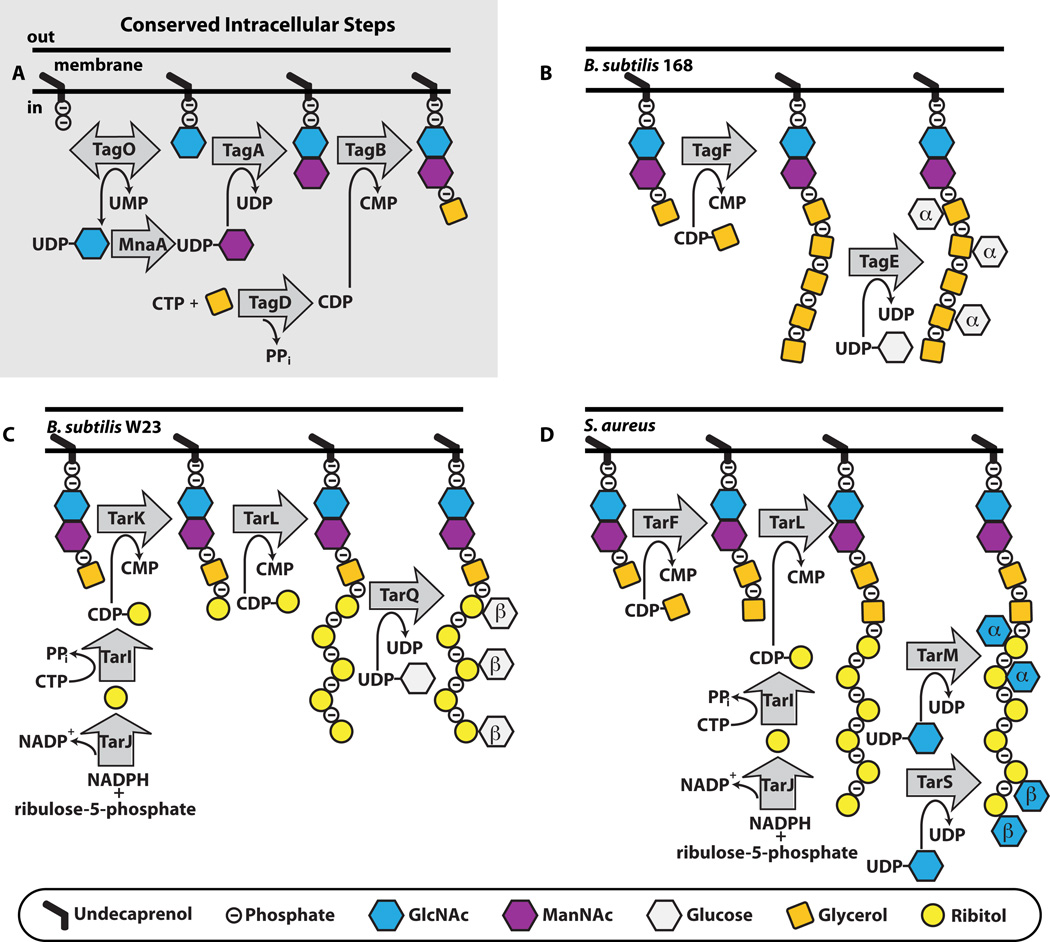
Figure 3. In different organisms wall teichoic acid biosynthesis begins with the same initial steps, but then the pathways diverge.
A. The initial steps to WTA polymer formation that has been found in all thus far characterized pathways. B,C,D. The end stage intracellular steps to form the WTA polymer in B. subtilis 168 (B), B. subtilis W23 (C) and S. aureus (D). TarIJ reactions to form CDP-ribitol exist in both B. subtilis W23 and S. aureus. The α- and β- within the sugar denotes the stereochemistry of attachment. We have drawn α- and β-GlcNAc attachment to S. aureus occurring on the same strand, but it is possible that the TarS and TarM enzymes do not attach GlcNAc to the same polymer.
Wall Teichoic Acid Biosynthesis
Overview
Much of the early work to understand WTA biosynthesis utilized particulate enzyme preparations. Though this work enabled characterization of the enzymatic steps in WTA polymer formation, identification of the enzymes responsible for these steps remained elusive (133). Later, genetic analyses of temperature-sensitive and phage-resistant mutants led to the identification of the poly(GroP) WTA gene cluster in B. subtilis 168 and the poly(RboP) WTA gene cluster in B. subtilis W23 (65; 148). Sequence analysis led to proposals for the enzymatic activity encoded by each gene (75; 91; 133). However, it proved challenging to characterize the pathway fully using genetics because many of the biosynthetic genes are essential (14; 15; 34; 36; 69). Efforts to characterize WTA enzymes biochemically were hampered because the substrates contain 55-carbon lipid chains that make them difficult to handle. Moreover, these lipid-linked substrates are present in very low abundance and cannot be isolated in useful quantities from bacterial cells. The development of chemoenzymatic methods to make WTA substrates has not only allowed elucidation of biosynthetic pathways, but also has permitted mechanistic studies on purified teichoic acid enzymes in the absence of membranes (22; 24; 54; 110; 113; 150).
Linkage Unit Synthesis
In 1978 Archibald and colleagues used electron microscopy to show that WTA synthesis begins in the cytoplasm at the wall-membrane interface and that the WTA polymer subsequently appears on the outer surface of the cell (3; 133). TagO catalyzes the first synthetic step: the transfer of GlcNAc-1-phosphate from UDP-GlcNAc to a membrane-anchored undecaprenyl-phosphate carrier lipid, an intermediate shared with peptidoglycan biosynthesis (107; 116). The TagO product is used in the synthesis of several B. subtilis 168 cell wall polymers, including the major wall teichoic acid (poly(GroP)), the minor teichoic acid (poly(glucosyl N-acetylgalactosamine 1-phosphate)) and teichuronic acid (poly(glucuronyl N-acetylgalactosamine)) (116). Consequently, the first committed step in WTA biosynthesis is the transfer of ManNAc from UDP-ManNAc to the C4 hydroxyl of GlcNAc to form the ManNAc-β1,4-GlcNAc disaccharide, catalyzed by TarA (33; 54; 150). The UDP-ManNAc donor sugar is derived from UDP-GlcNAc via MnaA (yvyH), an epimerase that catalyzes stereochemical inversion at the C2 position (117). TagB, a glycerophosphotransferase, catalyzes the transfer of a glycerol-phosphate unit from CDP-glycerol to the C4 position of ManNAc (16; 54). These first three steps to complete the synthesis of the WTA linkage unit are highly conserved across all thus far characterized strains (Figure 3). After these steps, WTA pathways diverge.
Poly(glycerol-phosphate) Wall Teichoic Acid Synthesis
The genes encoding proteins involved in poly(GroP) WTA synthesis are annotated as tag genes (teichoic acid glycerol) (19). B. subtilis 168 is the most studied of the WTA poly(GroP)-producing organisms (Figure 3). After the action of TagO, TagA, and TagB to complete synthesis of the linkage unit, TagF adds 45 – 60 glycerol-phosphate units to the TagB product to assemble the polymer (78; 98; 109; 110; 113). The GroP moiety originates from the activated precursor CDP-glycerol, which is synthesized by TagD, a cytidylyltransferase that catalyzes the transfer of l-α-glycerol-3-phosphate to CTP, releasing pyrophosphate (94). While the Tag enzymes from B. subtilis 168 remain the most characterized of the poly(GroP) WTA biosynthetic enzymes, homologs have been identified and loosely characterized in other bacteria known to contain poly(GroP), including Enterococcus faecalis, Lactobacillus plantarum, Staphylococcus epidermidis, and several Streptomycetes (37; 39; 50; 86; 89).
In 2010, Strynadka and co-authors published the first and so far only crystal structure of a teichoic acid enzyme involved in polymer synthesis (78). The structure showed that S. epidermidis TagF possesses a GT-B glycosyltransferase fold and an extended open active site that appears capable of accommodating the rebinding of CDP-glycerol without product release. While this observation suggests a processive mechanism, kinetic studies found that polymer length depended on the ratio of CDP-glycerol to lipid acceptor, supporting a distributive mechanism (98; 110; 113). The crystal structure of TagF should allow for further mechanistic studies on this class of phosphotransferases. TagB and TagF share 50% similarity in their catalytic domains, but features governing primase versus polymerase activity await identification.
Poly(ribitol-phosphate) Wall Teichoic Acid Synthesis
Enzymes making poly(RboP) WTAs are designated Tar for teichoic acid ribitol (19), but it should be noted that TarO, TarA, TarB, and TarD have the same biochemical functions as TagO, TagA, TagB, and TagD. In S. aureus, following formation of the lipid-diphospho-ManNAc-GlcNAc-GroP product by TarO, TarA and TarB, poly(RboP) synthesis continues with the TarF-mediated transfer of a (predominantly) single unit of glycerol-phosphate from CDP-glycerol, synthesized by TarD (9; 22; 24; 85; 135). As described above, the synthesis of poly(GroP) WTAs requires a GroP primase (TagB) as well as a GroP polymerase (TagF), and it was proposed that poly(RboP) WTA synthesis in S. aureus requires both a RboP primase and a RboP polymerase to make the main chain polymer (75; 105). Recent work has shown, however, that S. aureus contains a single enzyme, TarL, that primes the linkage unit and then attaches more than 40 ribitol-phosphates to complete the polymer (Figure 3) (24; 85; 97). The CDP-ribitol substrate utilized by TarL is made by the combined action of TarI, a cytidylyl transferase, and TarJ, an alcohol dehydrogenase (96; 151). All S. aureus strains contain two sets of tarIJL genes (the second set is designated tarI’J’K) (105). The significance of these duplications is still unclear (85; 97; 105; 120; 144).
Whereas poly(GroP) WTA biosynthesis has only been fully characterized in one organism, B. subtilis 168, poly(RboP) WTA biosynthesis has been characterized in both B. subtilis W23 and S. aureus (Figure 3) (22; 24). These two organisms produce very similar RboP-WTAs, which differ only in the number of GroP repeats in the linkage unit (one versus two) and in the sugar tailoring modifications (the addition of Glc versus GlcNAc) (91; 147). Both organisms appeared to contain the same tar genes (75; 105), but their WTA biosynthetic pathways were found to differ. Although S. aureus utilizes a single enzyme to prime the linkage unit and build the RboP polymer chain, B. subtilis W23 requires a RboP primase, TarK (22; 85). Furthermore, TarK acts directly on the TagB product. Thus, B. subtilis W23 does not require the enzymatic activity of TarF even though it contains a tarF gene. In S. aureus tarF is essential, but genetic analysis has established that B. subtilis tarF is neither essential nor transcribed under laboratory culture conditions (22). The WTA polymer in B. subtilis W23 is completed by the RboP polymerase TarL, which can attach upwards of 40 RboP units (147). Thus, B. subtilis W23 TarL is not a bifunctional primase-polymerase like its S. aureus homolog, but can only utilize WTA lipid-linked substrates that are primed with RboP by TarK (22; 85).
The biochemical and genetic studies of wall teichoic acid biosynthesis described above have substantially expanded our understanding of how WTA polymers are made, but also highlight challenges in predicting enzymatic pathways from genome sequences. Other pathways will most likely begin with the same initial steps, but may then diverge (Figure 2). In principle, the presence of tagD and tarIJ genes, required to make CDP-glycerol (tagD) and CDP-ribitol (tarIJ) substrates, should facilitate prediction of WTA polymer composition (124) (11; 39). It should be noted, however, that strains containing both types of genes may produce only one type of polymer. For example, some strains of L. plantarum that make only poly(GroP) polymers contain both tarIJKL and tagDF homologs, but the tarIJ gene homologs are not transcribed (21; 124). More perplexing is a recent report of an E. faecalis strain making a WTA ribitol-phosphate polymer that does not have a tarJ homolog (122).
Attachment of sugars to the polyol chain
In the past few years several WTA glycosyltransferases have been identified. In B. subtilis 168, TagE modifies WTAs with α-glucose using UDP-glucose as a donor substrate (1; 10). In B. subtilis W23, TarQ attaches β-glucose units to poly(RboP)-WTAs (23). In S. aureus, RboP-WTA α-O-GlcNAc modifications are installed by TarM (145) and β-O-GlcNAc modifications are installed by TarS (23). As mentioned above, WTAs extracted from cells appear to be heavily modified with sugars of the same stereochemistry or not modified at all (29; 45; 46; 125), suggesting that the glycosyltransferases are processive enzymes that fully glycosylate WTAs. Processivity may ensure that individual polymers are densely modified and, in organisms that contain more than one glycosyltransferase, may also prevent the attachment of sugars of different stereochemistries to the same polymer. The presence or absence of sugars and the glycosidic linkage conformation affects polymer structure and likely influences interactions with other components in the cell envelope or at the cell interface (13). Strains lacking modifications have altered phenotypes with regard to antibiotic susceptibility and virulence (23; 144). Although progress has been made with regard to the roles of the WTA sugar substituents, numerous questions remain.
Polymer Export
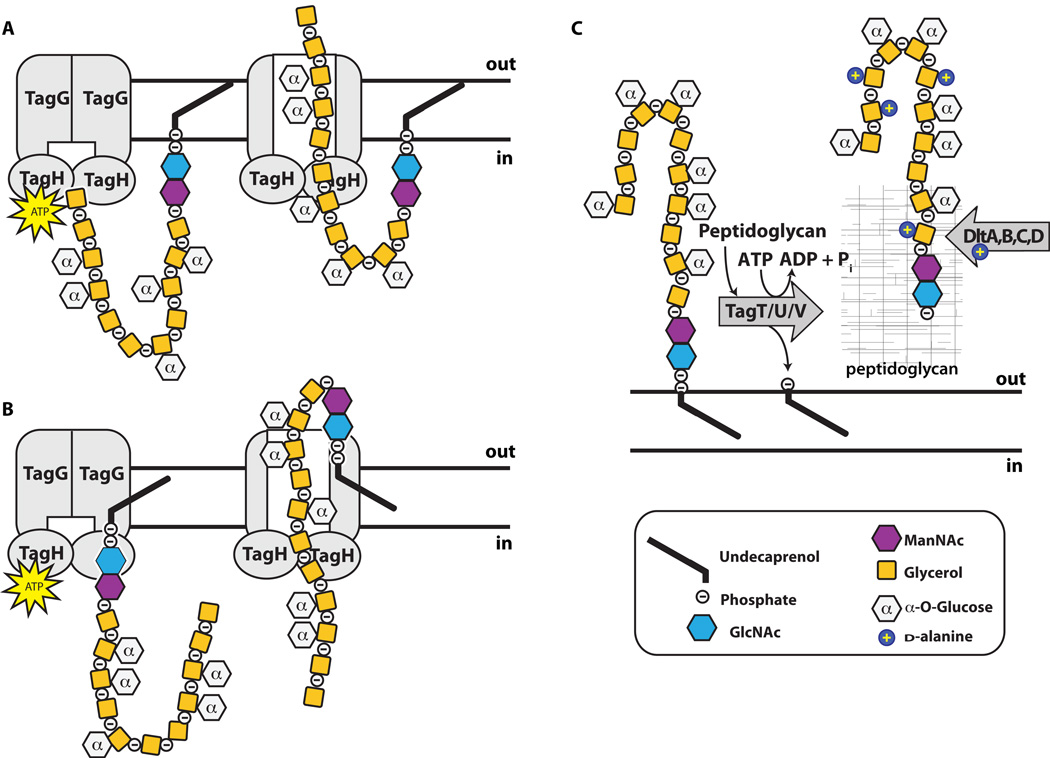
The final steps in the WTA biosynthetic pathway, those following polymer formation and sugar tailoring, are not as clearly defined as earlier steps. For both poly(GroP) and poly(RboP) WTA polymers, the polymer is translocated through the membrane by a two-component ABC (ATP-binding cassette) transporter, TagGH (or TarGH) (76). TagH, containing an ATPase domain, provides energy to drive a conformational change in the transmembrane component, TagG, which somehow facilitates translocation across the membrane (76; 111). The WTA transporter exports polymers containing main chains that can be more than ten times longer than the width of the lipid bilayer. In principle, there are two models for how export may occur: one involves a threading mechanism in which the polymer is fed through the transporter from the non-reducing end, eventually pulling the linkage unit across; the other involves a flipping mechanism in which the lipid-pp-GlcNAc-ManNAc linkage unit is somehow flipped across the membrane, with the polymer chain following (Figure 4). While the former model is conceptually simpler, experimental studies support the latter model, as they show that the linkage unit, and not the main chain, is recognized by TagGH (21; 111). It is not known whether translocation occurs concomitantly with or following polymerization. Reconstitution of the translocation process in proteoliposomes would provide valuable mechanistic insight and may be possible now that all previous steps have been characterized and enzymes to make the precursors are available.
Figure 4. Wall teichoic acid polymer flipping and attachment to peptidoglycan in B. subtilis 168 proceeds through a series of enzymes.
Graphical representation of the poly(GroP) WTA polymer in B. subtilis 168 transported through the cell membrane by TagGH. Homology suggests energy generated by TagH ATP hydrolysis drives a conformational change that allows for polymer transport. TagGH can presumably either thread the polymer through the membrane by the non-reducing end (A) or recognize the disaccharide linkage unit and flip the polymer across (B). Following transport (C), the polymer is covalently attached to PG by TagTUV. It is unknown whether the TagTUV enzymes work independently or together to ligate WTA to PG. The presumed energy source for this ligation reaction, which we designated as ATP hydrolysis, is undetermined. It remains unknown whether extracellular d-alanylation by DltABCD occurs before or after WTA is covalently attached to PG.
Wall Teichoic Acid Attachment to PG
Once the polymer is outside the cell, the final stage of wall teichoic acid biosynthesis is formation of a phosphodiester linkage between the WTA polymer and the C6 hydroxyl of the PG MurNAc unit. Based on genetic evidence, a set of redundant enzymes, TagTUV, was recently proposed to be responsible for catalyzing this coupling reaction in B. subtilis 168 (66), and similar evidence supports a related function for three homologous enzymes in S. aureus (Figure 4) (40; 62; 92). TagT was found to have geranyl pyrophosphatase activity (42; 66). This activity was presumed to be a surrogate for attack of the WTA phosphodiester bond by the C6 MurNAc hydroxyl of PG, but reconstitution of an authentic coupling reaction must be done to confirm the proposed WTA ligase activity.
When and where WTAs are synthesized and attached to peptidoglycan is still debated. Decades ago, some studies suggested the WTA polymer is attached to nascent (new) peptidoglycan, while others indicated that it may be attached to mature (old) PG. Still others suggested it can be attached to concurrently synthesized as well as pre-existing PG (20; 83; 123; 133; 141). Recently, the timing and location of WTA synthesis has been revisited. In cryo-EM images, Matias and Beveridge identified densely-staining material at the septa of B. subtilis and S. aureus as WTA, and proposed that WTAs are attached to new PG during cell division (80–82). Consistent with this hypothesis, this densely-staining material disappears in a time-dependent fashion when the first step in WTA synthesis is inhibited (25). Furthermore, studies using fluorescent protein fusions show that WTA biosynthetic proteins localize predominantly to the septum in B. subtilis and S. aureus (7; 16; 51), suggesting that WTAs are attached to nascent peptidoglycan during cell division (septum formation). However, other studies suggest that WTAs are attached to older PG (4; 112). Fluorescently-labeled concanavalin A (ConA), a lectin reported to bind to α-GlcNAcylated WTAs, was used to probe the location of WTAs in S. aureus. Fluorescent ConA bound to the half of the cell containing old PG, but not to the half containing PG produced during cross wall formation (cell division) (4; 112). Additional studies using alternative detection methods are required to resolve the location of WTA attachment.
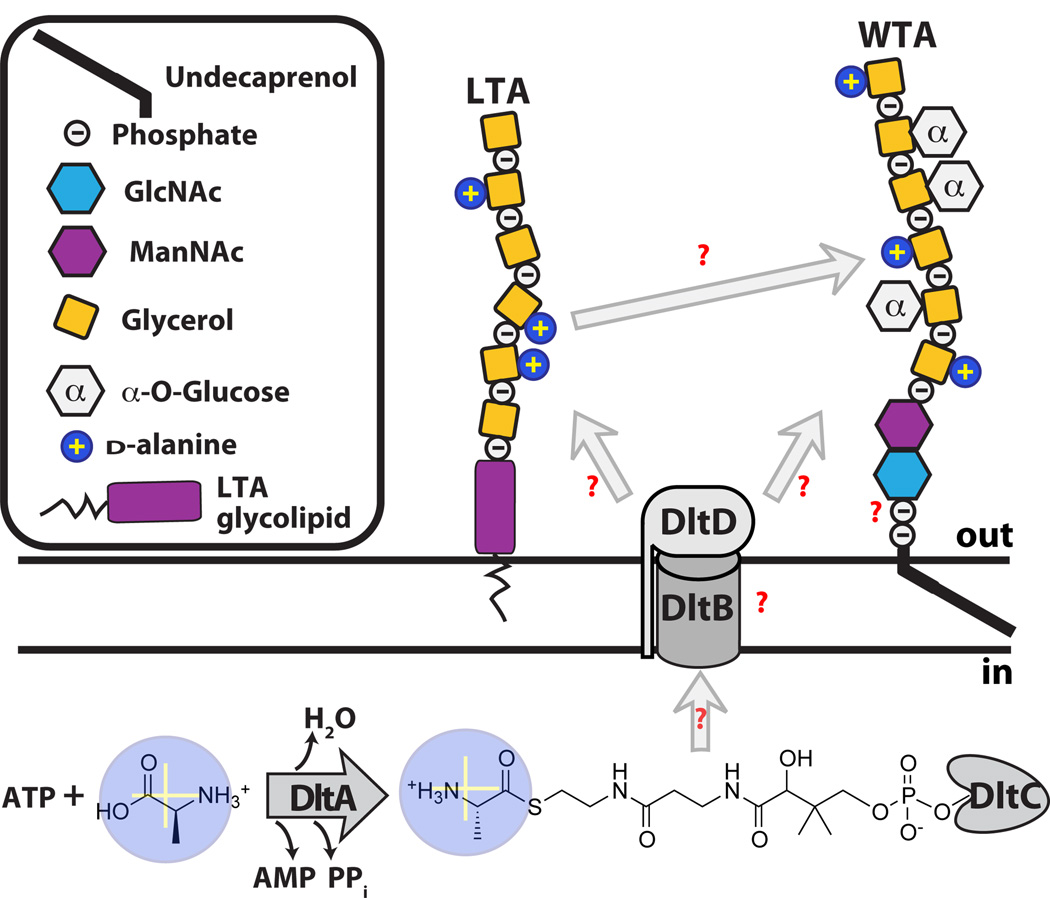
d-Alanylation of the Wall Teichoic Acid repeat unit
Attachment of d-alanine esters to WTAs is an important mechanism by which bacteria modulate surface charge (32). Unlike glycosylation, which occurs inside the cell, d-alanylation occurs following export of WTAs to the cell surface. Four enzymes, encoded by the dltABCD operon, attach d-alanines to both LTAs and WTAs (Figure 5) (72; 84; 95). DltA resembles the adenylation domains of nonribosomal peptide synthetases. It is responsible for activating d-alanine as an AMP ester (58), and then transferring the aminoacyl adenylate to DltC. DltC possesses a pantothenate cofactor that forms a thioester with d-alanine through nucleophilic attack of the sulfhydryl on the mixed anhydride (59; 129). What happens after DltC is charged with d-alanine is unclear, but involves both DltB and DltD (Figure 5). DltB, which contains several predicted membrane-spanning helices, is an uncharacterized membrane protein of the membrane-bound O-acetyltransferase (MBOAT) family. DltD is a membrane-anchored protein predicted to have a short intracellular N-terminus and a large extracellular C-terminal domain with predicted esterase/thioesterase activity. DltD was shown to hydrolyze d-alanine from acyl carrier protein (ACP), which is normally involved in fatty acid biosynthesis; on this basis DltD was suggested to have an editing function and to remove d-alanine from mischarged ACP (38). It was also shown to modestly accelerate the rate of transfer of d-alanine to DltC, suggesting an accessory role in the transfer reaction (38). These proposals for DltD require it to function in the cytoplasm, which is inconsistent with its predicted topology. At this point, the specific function of DltD is unclear.
Figure 5.
d-alanylation of teichoic acids. The DltABCD machinery is responsible for the activation and attachment of d-alanines to extracellular TAs. Many questions remain regarding the roles of DltB and DltD, the nature of their substrates, and whether d-alanine transesterification is enzymatically catalyzed.
It has been proposed based on pulse-chase experiments that d-alanines are transferred to LTAs by DltC and then to WTAs in an isoenthalpic transesterification reaction (56). Supporting such a mechanism, d-alanyl esters could be transferred between LTAs of differing lengths in nonnative micelles without an enzyme catalyst, though the reaction was slow (28). Alternatively, it has been suggested that d-alanyl esters are transferred back from LTAs to DltC and then to WTAs (68). Since this transfer reaction required bacterial membranes, other factors may also play a role in the process. DltB is also required for d-alanylation, and it has been suggested to facilitate membrane translocation of d-alanine-charged DltC so that it can serve as the direct donor to LTA (91). DltC has not been identified in any proteomic analyses of secreted proteins, and the evidence that it serves as the direct d-alanylation donor is limited (41; 68; 91). While it is clear that d-alanylation of LTAs and WTAs requires the same biosynthetic machinery, many questions remain regarding the enzymology of the process.
Nonessential & Conditionally Essential Wall Teichoic Acid genes
It is possible to delete tagO or tagA, the first two genes in the WTA biosynthetic pathway, and produce bacteria lacking WTA polymers. These bacteria display a range of defects, but are viable in vitro. With the exception of the tailoring enzymes, however, most of the downstream WTA genes are essential and their deletion results in a lethal phenotype unless flux into the WTA pathway is blocked at an early stage (34; 36). The lethality of a late stage block may be due to accumulation of toxic intermediates in the cell or to depletion of cellular pools of undecaprenyl phosphate, which is also required for the synthesis of PG (34–36; 120; 144). Conditionally essential genes are found in pathways for other cell wall polysaccharides that are synthesized on the undecaprenyl phosphate carrier lipid. For example, it was found that deletion of late-stage genes involved in synthesis of the capsular polysaccharide of S. pneumoniae generated bacteria with suppressor mutations in the initiating enzyme that turned off capsule production (63; 142).
Roles of Wall Teichoic Acids
Wall teichoic acid polymers play numerous, varied roles in the cell wall owing to their location, abundance, and polyanionic nature. The molecular mechanisms underlying their many critical functions are not well understood. Genetic and small molecule tools are enabling more detailed studies to elucidate these mechanisms.
Regulation of Cell Morphology and Division
Bacteria lacking WTAs grow slower than wildtype and clump in solution. These strains exhibit numerous morphological abnormalities, including a non-uniform thickening of the peptidoglycan cell wall, increased cell size, and defects in septal positioning and number (4; 25; 31; 34; 93; 112). Furthermore, rod-shaped bacteria such as B. subtilis and L. monocytogenes lose their shape, becoming spherical (14; 19; 43; 47; 116; 117). These phenotypes imply that wall teichoic acids are necessary for proper localization, assembly, and/or activation of cell wall elongation and division machinery. Consistent with this idea, many WTA and PG biosynthetic enzymes have been shown to colocalize and physically interact in B. subtilis (51; 66).
Evidence for functional interactions between WTAs and PG machinery comes from S. aureus, where it was observed that the putative PG cross-linking enzymes PBP4 and FmtA lose septal localization in the absence of WTAs(7; 104). This mislocalization correlates with decreased PG cross-linking found in Δ_tarO_ mutants (112). In addition, Campbell et al. showed that TarO inhibitors potentiate β-lactams in MRSA strains, indicating a functional connection between the PG and WTA biosynthetic pathways (25). Farha et al. used a panel of β-lactams to argue that this synthetic lethality was specifically due to combined disruption of the PG cross-linking enzymes PBP2 (via β-lactams) and PBP4 (via WTA biosynthesis inhibition) (48). Although many details remain to be clarified, there is considerable evidence that WTAs regulate the function of PG biosynthetic machinery, and one mechanism by which they do so is through the recruitment of cell wall assembly proteins.
Regulation of autolytic activity
The failure of mutants to effectively septate and separate during cell division implicates WTAs in coordinating the localization, stability, and/or activity of PG lytic proteins. Strains lacking WTAs have an altered endogenous autolysin expression profile and increased autolysis rates (18; 79; 112; 134). In vitro studies performed with purified LTAs, enzymes, and cell wall substrates explain these observations using a recruitment model, in which polyanionic phosphate backbones bind and sequester positively-charged autolysins (17; 49; 91). Positively-charged d-alanyl esters help mask this charge and free autolysins to degrade PG. This model requires WTAs to be either absent from the septum, or present in a highly d-alanylated state, as autolytic activity must be regulated to ensure separation specifically at the cross wall during cell division (146). Recent studies support an exclusion model (52; 112). Finally, others have proposed that WTAs could indirectly modulate enzyme activity through ion chelation (8; 18; 60). Whether directly or indirectly, or by recruitment or exclusion, it is clear WTAs play a role in the localization of autolytic enzymes (52; 104; 112).
Regulation of Ion Homeostasis
It is thought that WTAs can bind extracellular metal cations by extending beyond the PG layer (67; 140). WTAs have a high affinity for metals and their biosynthesis is upregulated under metal limitation (8; 45). Wall teichoic acids also bind protons, and cells lacking WTAs having a 23% decrease in their proton binding capacity (18). While WTAs do not influence the pH gradient across the cell wall, it has been suggested that they can create localized changes in pH, indirectly modulating the function of some enzymes, (e.g. autolysins) (18). WTA ion binding is also thought to minimize repulsion between nearby phosphate groups, which can affect polymer structure and therefore cell wall integrity (67; 140). d-alanylation masks negatively charged sites on the polymer, reducing WTAs’ binding capacity for cations (60). Some have also speculated ion binding to WTAs could help prevent fluctuations in osmotic pressure between the inside and the outside of the cell (4; 60; 120).
Protection from Host Defenses and Antibiotics
Wall teichoic acids and their attached substituents contribute to bacterial cell surface charge and hydrophobicity, which in turn affects binding of extracellular molecules. This plays a role in protecting bacteria from various threats and adverse conditions. In the absence of WTAs, bacteria are sensitive to high temperatures and unable to grow in high salt media, indicating WTAs are involved in temperature tolerance and osmotic stress (7; 61; 117; 127). WTA deficient cells are more susceptible to human antibacterial fatty acids (AFAs), presumably because hydrophobic AFAs penetrate the less hydrophilic mutant cell wall more easily, and more efficiently bind to the cell membrane where they can cause damage (70).
Preventing d-alanylation through gene deletion removes d-alanine esters from both LTAs and WTAs, consequently, researchers have not yet been able to identify roles specific to each polymer. Nevertheless, it has been established that TA d-alanylation protects against host-defense mechanisms (73; 74). S. aureus lacking d-alanine have an increased susceptibility to phagocytes, to neutrophil killing (32), and to lysostaphin and lysozyme (100). A reduction in the d-alanyl content of the cell wall results in increased susceptibility to glycopeptide antibiotics and to certain cationic antimicrobials peptides (CAMPs) (32; 99; 100; 126; 131). It was reasoned that the absence of d-alanyl esters increases the overall negative cell surface charge (126), attracting cationic molecules and thus increasing susceptibility to CAMPs. Accordingly, B. subtilis and S. aureus lacking TA d-alanine modifications bind more positively-charged cytochrome c as compared to wildtype (99; 134). In Group B Streptococci, however, d-alanylation showed little influence on the surface-association of fluorescently-labeled CAMPs (108). These data suggest that charge effects are not solely responsible for the observed changes in CAMP susceptibility. Saar-Dover et al. suggest the increased cell wall density observed in strains with d-alanylation prevents penetration of CAMPs, thereby conferring protection (108).
Blocking WTA synthesis renders B. subtilis and MRSA sensitive to β-lactam antibiotics (23; 25; 48; 79; 99; 119; 134). Recent data has replicated this sensitivity by the sole removal of WTA β-GlcNAc modifications (23). It has been speculated that the WTA β-GlcNAc moieties scaffold proteins involved in resistance. Accordingly, recent work has shown that S. aureus WTAs specifically interact with PBP2A (104), the resistant transpeptidase found in MRSA. Since wall teichoic acid glycosyl substituents are also receptors for phage (10; 143), there is precedence for WTA sugar residues mediating protein binding. It is possible that glycosylated WTAs function as ligands for cell surface proteins with carbohydrate recognition sites.
Effects on Adhesion and Colonization
The presence of WTAs and their d-alanyl esters influences bacterial interactions with various surfaces (91). WTA deficient cells have a reduced capability to form biofilms (55; 61; 127; 131). Removal of d-alanine esters also decreases biofilm formation and bacterial adhesion to plastic and glass surfaces, an effect potentially linked to the resultant increase in negative cell surface charge creating increased repulsive forces between bacteria and a stratum (55; 61).
In 1980, Aly et al. showed a 71% reduction of S. aureus binding to nasal epithelial cells that were preincubated with teichoic acid (2; 73; 131; 135; 136; 139). Since that time, several animal studies have established that bacteria lacking TA d-alanine esters or WTAs are attenuated in host colonization and infection (32; 48; 118; 119; 132; 136). Lactobacilli lacking TA d-alanylation colonized mice gastrointestinal tracts at 1% of wildtype levels (131). Similarly, WTA-free S. aureus cells colonized rat nares at levels 90% lower than wildtype and in rabbits these mutant cells were unable to proliferate to adjacent organs (135; 139). TA d-alanine esters are considered virulence factors, since their deletion attenuates pathogenicity but does not cause major cellular defects in vitro (4; 14; 25; 27; 31). WTAs have also been categorized as virulence factors, although they exhibit such profound defects in vitro that they may be better described as “in vivo essential” (30; 138). The decreased ability of WTA null mutants to survive in challenging host environments is due to the combined effects of an inability to adhere to host tissue, an increased susceptibility to host defenses, and cell division defects (74; 91; 131; 137).
The wall teichoic acid pathway as an antibiotic target
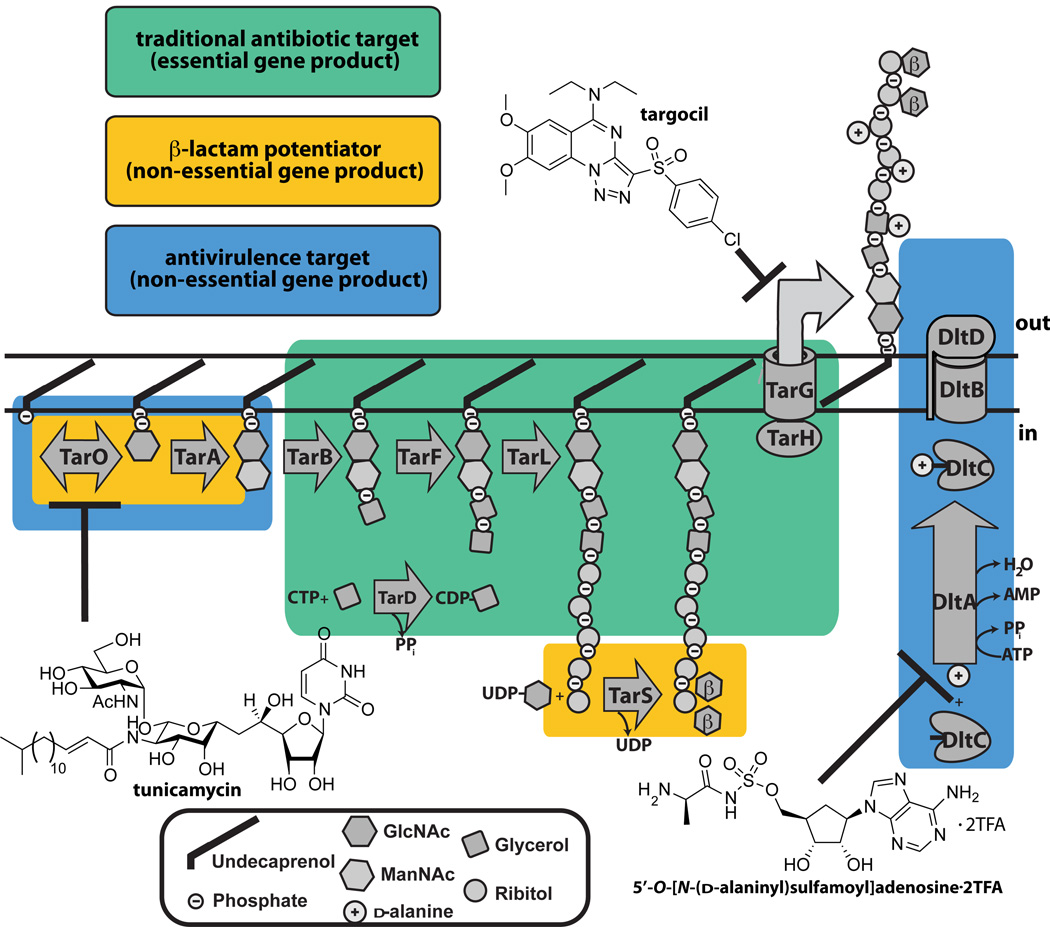
The vital roles of WTAs in physiology and pathogenesis promote this pathway as a target for antibacterial drugs and vaccines (120; 137). The WTA pathway contains three distinct target categories: antivirulence targets, β-lactam potentiator targets, and essential targets (Figure 6).
Figure 6. Wall teichoic acid biosynthetic enzymes are potential antibiotic targets.
Schematic showing the three types of antibacterial targets present in the S. aureus WTA pathway. Enzymes that are traditional, β-lactam potentiators, or antivirulence antimicrobial targets are boxed. The three chemical structures illustrated are small molecules that are known to inhibit the WTA enzymes, TarO, TarG, and DltA.
Antivirulence Targets
Proponents of virulence factors as antibacterial targets argue that resistance is less likely to emerge to virulence factor inhibitors since there is no selection pressure for survival (30). Despite arguments against these inhibitors, such as their inability to cure established infections or unforeseen in vivo selection pressures, it is worth investigating virulence factor targets, particularly for resistant pathogens. Enzymes of the dlt operon are targets for antivirulence agents since strains lacking TA d-alanine esters are strongly attenuated in vivo but show minimal growth defects in vitro (137). A DltA inhibitor was reported in 2005, but was not further optimized and is likely not specific (84). The enzymes involved in initiating WTA polymer synthesis, TarO and TarA, have also been described as virulence factor targets but, as noted above, these deletion strains are more than attenuated in vivo; they are non-viable (135). Hence, it is possible that inhibitors of these initiating enzymes would behave more like traditional antibiotics in vivo, although this remains to be established. The natural product tunicamycin inhibits TarO at very low concentrations (25; 57; 101), but at higher concentrations it also blocks MraY, an essential UDP-MurNAc-pentapeptide transferase involved in peptidoglycan biosynthesis (103). Tunicamycin has been used to probe the in vitro effects of inhibiting WTA biosynthesis, unfortunately however, it cannot be used in animals because it inhibits GPT, an essential phosphotransferase involved in eukaryotic N-linked glycan biosynthesis (103). Hence, there is still a need for specific nontoxic inhibitors that target TarO, TarA, and Dlt enzymes for use in vivo.
β-lactam potentiator targets
β-lactams are one of the safest and most widely used classes of antibiotics (53), and there is considerable interest in compounds that restore β-lactam sensitivity to resistant microorganisms. β-lactamase inhibitors, for example, have been highly successful as components of compound combinations to treat many β-lactam resistant infections (53). β-lactam resistance in MRSA is not mediated by β-lactamases, but by the resistant transpeptidase PBP2A (149). Since the activity of this transpeptidase depends on the function of various host factors, compound combinations are possible as therapeutic modalities (149). TarO, TarA and TarS are β-lactam potentiator targets. Unlike Δ_tarO_ and Δ_tarA_ strains, Δ_tarS_ mutants divide normally and do not show appreciable growth defects (23). Furthermore, they are not strongly attenuated in virulence. Hence, TarS inhibitors should act exclusively as potentiators, whereas, inhibitors of TarO and TarA would serve as both β-lactam potentiators and antivirulence agents. A cell-based high-throughput β-lactam potentiation screen recently identified the clinically used antiplatelet drug ticlopidine as a TarO inhibitor, although target selectivity was not demonstrated (48). It should be possible to also identify TarS inhibitors from β-lactam potentiation screens, as they can be readily discriminated from TarO or TarA inhibitors based on their cellular effects (23).
Traditional Antibiotic Targets
Inhibitors of the essential late stage WTA biosynthetic enzymes TarB through TarH should have lethal effects on bacterial cells, and thus would be akin to traditional antibiotics. Late stage WTA pathway inhibitors (e.g. targocil) were discovered by screening a Δ_tarO_ mutant and a wildtype strain against the same library to identify compounds that selectively killed the wildtype strain. All identified compounds were found to inhibit TarG (77; 121; 132). It has been suggested that the identification of several different inhibitors against TarG indicates that this is a druggable target. It is likely that WTA precursor export is the rate-limiting step in the pathway, and that TarG may also be more accessible to inhibitors than most other WTA targets since it spans the membrane. All identified TarG inhibitors suffer from a relatively high frequency of resistance. Consequently, the TarG inhibitors were shown to have efficacy in an MRSA infection model only in combination with β-lactams (26; 132). It would be desirable to identify compounds with a lower frequency of resistance to validate late stage enzymes as targets independent of β-lactams.
Conclusion
Wall teichoic acids comprise a huge percentage of the gram-positive cell wall and are thus extremely important for cell wall integrity. They are important for pathogenesis and play fundamental roles in bacterial physiology. Furthermore, their tailoring modifications modulate their structures and interactions with other molecules and cells in complex ways. The variety of enzyme classes involved in WTA biosynthesis (glycosyltransferases, polymerases, ABC transporters) and the diverse functions of WTAs make the ongoing study of this pathway challenging. Small molecule inhibitors of WTA enzymes can be used to probe enzyme mechanism and WTA function in cells, and to explore the potential of the different target types.
Summary Points List.
- WTAs are covalently attached to PG and comprise up to 60% of the cell wall mass in gram-positive bacteria.
- The first three steps in WTA biosynthesis, catalyzed by TagO, TagA, and TagB, lead to the formation of a lipid-anchored disaccharide-glycerol-phosphate and are conserved across gram-positive bacteria.
- Cells lacking WTAs have gross defects in cell division and cell morphology. This and other evidence indicate that the WTA and PG biosynthetic pathways are connected.
- How WTAs function to regulate normal cell physiology is still unclear. They are thought to scaffold some cell wall assembly proteins, exclude other such proteins, and influence protein activity through ion chelation.
- WTAs are required for survival in a host.
- WTA tailoring modifications are involved in biofilm formation, virulence, and antimicrobial resistance.
- The WTA pathway is a possible target for β-lactam potentiators, antivirulence agents, and novel antibiotics.
Future Issues List.
- While most of the steps in WTA biosynthesis have been elucidated, major questions remain regarding the mechanisms of polymer export and attachment to PG.
- The pathway for d-alanylation of TAs is poorly understood. In particular, the enzymatic functions of DltB and DltD are unknown.
- There is a long standing controversy regarding whether WTA precursors are attached to old or new PG. This issue has a direct bearing on how WTAs may regulate PG biosynthesis and cell division.
- The roles of distinct tailoring modifications in modulating WTA function remain unclear. Glycosylated WTAs are required to maintain β-lactam resistance in MRSA, but a molecular mechanism to explain this observation is lacking.
- While small molecules that inhibit WTA synthesis have been identified and some have been shown to have efficacy in animal models, the WTA pathway as a therapeutic target has yet to be validated.
Acronym List
WTA
wall teichoic acid
PG
peptidoglycan
TA
teichoic acid
LTA
lipoteichoic acid
poly(GroP)
poly(glycerol-3-phosphate)
poly(RboP)
poly(ribitol-5-phosphate)
GlcNAc
N-acetyl-D-glucosamine
MRSA
methicillin resistant Staphylococcus aureus
tag (Tag)
teichoic acid glycerol
tar (Tar)
_t_eichoic _a_cid _r_ibitol
Mini-Glossary
polyol
a compound having multiple hydroxyl functional groups
transesterification
the intra- or intermolecular nucleophilic attack of a TA d-alanyl ester by a free TA backbone hydroxyl
PBP
penicillin binding protein, which synthesizes peptidoglycan by elongating and crosslinking the glycan chains
synthetic lethality
synthetic lethality occurs when the simultaneous disruption of two nonessential genes or processes results in a loss of viability
autolysins
bacterial enzymes that hydrolyze peptidoglycan during cell growth and division; they enable incorporation of new material into the cell wall and promote cell separation (115)
virulence factor
a factor essential for bacterial colonization and pathogenesis, but non-essential for survival in vitro
biofilm
multilayered communities of bacterial cells embedded in a self-produced matrix (127)
traditional antibiotic target
a bacterial protein or pathway that is essential for survival so that small molecule inhibition is lethal
potentiator
a small molecule that increases the potency of an antibiotic when used in combination
Literature Cited
- 1.Allison SE, D'Elia MA, Arar S, Monteiro MA, Brown ED. Studies of the genetics, function, and kinetic mechanism of TagE, the wall teichoic acid glycosyltransferase in Bacillus subtilis 168. J Biol Chem. 2011;286:23708–23716. doi: 10.1074/jbc.M111.241265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aly R, Shinefield HR, Litz C, Maibach HI. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J Infect Dis. 1980;141:463–465. doi: 10.1093/infdis/141.4.463. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AJ, Green RS, Sturman AJ, Archibald AR. Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and concanavalin A, and its susceptibility to turnover. J Bacteriol. 1978;136:886–899. doi: 10.1128/jb.136.3.886-899.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre G, Deghorain M, Bron PA, van S, II, Kleerebezem M, et al. Fluorescence and atomic force microscopy imaging of wall teichoic acids in Lactobacillus plantarum. ACS Chem Biol. 2011;6:366–376. doi: 10.1021/cb1003509. [DOI] [PubMed] [Google Scholar]
- 5.Araki Y, Ito E. Linkage units in cell walls of gram-positive bacteria. Crit Rev Microbiol. 1989;17:121–135. doi: 10.3109/10408418909105745. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong JJ, Baddiley J, Buchanan JG, Carss B, Greenberg GR. Isolation and structure of ribitol phosphate derivatives (teichoic acids) from bacterial cell walls. Journal of the Chemical Society. 1958:4344–4354. [Google Scholar]
- 7.Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, et al. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proceedings of the National Academy of Sciences. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. Shows that S. aureus WTAs are required for proper localization of PBP4
- 8.Baddiley J. Structure, biosynthesis, and function of teichoic acids. Accounts of Chemical Research. 1970;3:98–105. [Google Scholar]
- 9.Badurina DS, Zolli-Juran M, Brown ED. CTP:glycerol 3-phosphate cytidylyltransferase (TarD) from Staphylococcus aureus catalyzes the cytidylyl transfer via an ordered Bi-Bi reaction mechanism with micromolar K(m) values. Biochim Biophys Acta. 2003;1646:196–206. doi: 10.1016/s1570-9639(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 10.Baptista C, Santos MA, São-José C. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J Bacteriol. 2008;190:4989–4996. doi: 10.1128/JB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baur S, Marles-Wright J, Buckenmaier S, Lewis RJ, Vollmer W. Synthesis of CDP-activated ribitol for teichoic acid precursors in Streptococcus pneumoniae. J Bacteriol. 2009;191:1200–1210. doi: 10.1128/JB.01120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol. 2007;189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal P, Zloh M, Taylor PW. Disruption of D-alanyl esterification of Staphylococcus aureus cell wall teichoic acid by the {beta}-lactam resistance modifier (−)-epicatechin gallate. J Antimicrob Chemother. 2009;63:1156–1162. doi: 10.1093/jac/dkp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhavsar AP, Beveridge TJ, Brown ED. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J Bacteriol. 2001;183:6688–6693. doi: 10.1128/JB.183.22.6688-6693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhavsar AP, Erdman LK, Schertzer JW, Brown ED. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J Bacteriol. 2004;186:7865–7873. doi: 10.1128/JB.186.23.7865-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhavsar AP, Truant R, Brown ED. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J Biol Chem. 2005;280:36691–36700. doi: 10.1074/jbc.M507154200. [DOI] [PubMed] [Google Scholar]
- 17.Bierbaum G, Sahl HG. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 18.Biswas R, Martinez RE, Gohring N, Schlag M, Josten M, et al. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS One. 2012;7:e41415. doi: 10.1371/journal.pone.0041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boylan RJ, Mendelson NH, Brooks D, Young FE. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972;110:281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bracha R, Davidson R, Mirelman D. Defect in biosynthesis of the linkage unit between peptidoglycan and teichoic acid in a bacteriophage-resistant mutant of Staphylococcus aureus. J Bacteriol. 1978;134:412–417. doi: 10.1128/jb.134.2.412-417.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bron PA, Tomita S, van S, II, Remus DM, Meijerink M, et al. Lactobacillus plantarum possesses the capability for wall teichoic acid backbone alditol switching. Microb Cell Fact. 2012;11:123. doi: 10.1186/1475-2859-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown S, Meredith T, Swoboda J, Walker S. Staphylococcus aureus and Bacillus subtilis W23 make polyribitol wall teichoic acids using different enzymatic pathways. Chem Biol. 2010;17:1101–1110. doi: 10.1016/j.chembiol.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci U S A. 2012;109:18909–18914. doi: 10.1073/pnas.1209126109. Shows a tailoring modification of WTAs is required to maintain β-lactam resistance in MRSA
- 24.Brown S, Zhang Y-H, Walker S. A revised pathway proposed for Staphylococcus aureus wall teichoic acid biosynthesis based on in vitro reconstitution of the intracellular steps. Chemistry & Biology. 2008;15:12–21. doi: 10.1016/j.chembiol.2007.11.011. Biochemical reconstitution of S. aureus WTA biosynthesis leads to a revision of the pathway
- 25.Campbell J, Singh AK, Santa Maria JP, Kim Y, Brown S, et al. Synthetic Lethal Compound Combinations Reveal a Fundamental Connection between Wall Teichoic Acid and Peptidoglycan Biosyntheses in Staphylococcus aureus. ACS Chem. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell J, Singh AK, Swoboda JG, Gilmore MS, Wilkinson BJ, Walker S. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1810–1820. doi: 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee AN, Mirelman D, Singer HJ, Park JT. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969;100:846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childs WC, 3rd, Taron DJ, Neuhaus FC. Biosynthesis of D-alanyl-lipoteichoic acid by Lactobacillus casei: interchain transacylation of D-alanyl ester residues. J Bacteriol. 1985;162:1191–1195. doi: 10.1128/jb.162.3.1191-1195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin T, Burger MM, Glaser L. Synthesis of teichoic acids. VI. The formation of multiple wall polymers in Bacillus subtilis W-23. Arch Biochem Biophys. 1966;116:358–367. doi: 10.1016/0003-9861(66)90042-7. [DOI] [PubMed] [Google Scholar]
- 30.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 31.Cole RM, Popkin TJ, Boylan RJ, Mendelson NH. Ultrastructure of a temperature-sensitive rod-mutant of Bacillus subtilis. J Bacteriol. 1970;103:793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins LV, Kristian SA, Weidenmaier C, Faigle M, Van Kessel KPM, et al. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis. 2002;186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 33.D'elia MA, Henderson JA, Beveridge TJ, Heinrichs DE, Brown ED. The N-acetylmannosamine transferase catalyzes the first committed step of teichoic acid assembly in Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009;191:4030–4034. doi: 10.1128/JB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'elia MA, Millar KE, Beveridge TJ, Brown ED. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol. 2006;188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'elia MA, Millar KE, Bhavsar AP, Tomljenovic AM, Hutter B, et al. Probing teichoic acid genetics with bioactive molecules reveals new interactions among diverse processes in bacterial cell wall biogenesis. Chemistry & Biology. 2009;16:548–556. doi: 10.1016/j.chembiol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 36.D'elia MA, Pereira MP, Chung YS, Zhao W, Chau A, et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006;188:4183–4189. doi: 10.1128/JB.00197-06. Reports that late-stage WTA biosynthetic genes are conditionally essential
- 37.Davison AL, Baddiley J. Glycerol Teichoic Acids in Walls of Staphylococcus Epidermidis. Nature. 1964;202:874. doi: 10.1038/202874a0. [DOI] [PubMed] [Google Scholar]
- 38.Debabov DV, Kiriukhin MY, Neuhaus FC. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in D-alanylation. J Bacteriol. 2000;182:2855–2864. doi: 10.1128/jb.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denapaite D, Bruckner R, Hakenbeck R, Vollmer W. Biosynthesis of teichoic acids in Streptococcus pneumoniae and closely related species: lessons from genomes. Microb Drug Resist. 2012;18:344–358. doi: 10.1089/mdr.2012.0026. [DOI] [PubMed] [Google Scholar]
- 40.Dengler V, Meier PS, Heusser R, Kupferschmied P, Fazekas J, et al. Deletion of hypothetical wall teichoic acid ligases in Staphylococcus aureus activates the cell wall stress response. FEMS Microbiol Lett. 2012;333:109–120. doi: 10.1111/j.1574-6968.2012.02603.x. [DOI] [PubMed] [Google Scholar]
- 41.Dreisbach A, van Dijl JM, Buist G. The cell surface proteome of Staphylococcus aureus. Proteomics. 2011;11:3154–3168. doi: 10.1002/pmic.201000823. [DOI] [PubMed] [Google Scholar]
- 42.Eberhardt A, Hoyland CN, Vollmer D, Bisle S, Cleverley RM, et al. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb Drug Resist. 2012;18:240–255. doi: 10.1089/mdr.2011.0232. [DOI] [PubMed] [Google Scholar]
- 43.Elbaz M, Ben-Yehuda S. The metabolic enzyme ManA reveals a link between cell wall integrity and chromosome morphology. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellwood DC. The wall content and composition of Bacillus substilis var. niger grown in a chemostat. Biochem J. 1970;118:367–373. doi: 10.1042/bj1180367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endl J, Seidl HP, Fiedler F, Schleifer KH. Chemical composition and structure of cell wall teichoic acids of Staphylococci. Arch Microbiol. 1983;135:215–223. doi: 10.1007/BF00414483. [DOI] [PubMed] [Google Scholar]
- 46.Endl J, Seidl PH, Fiedler F, Schleifer KH. Determination of cell wall teichoic acid structure of staphylococci by rapid chemical and serological screening methods. Arch Microbiol. 1984;137:272–280. doi: 10.1007/BF00414557. [DOI] [PubMed] [Google Scholar]
- 47.Eugster MR, Loessner MJ. Wall Teichoic Acids Restrict Access of Bacteriophage Endolysin Ply118, Ply511, and PlyP40 Cell Wall Binding Domains to the Listeria monocytogenes Peptidoglycan. J Bacteriol. 2012;194:6498–6506. doi: 10.1128/JB.00808-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farha MA, Leung A, Sewell EW, D'Elia MA, Allison SE, et al. Inhibition of WTA Synthesis Blocks the Cooperative Action of PBPs and Sensitizes MRSA to β-Lactams. ACS Chem Biol. 2012 doi: 10.1021/cb300413m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer W, Rosel P, Koch HU. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981;146:467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzgerald SN, Foster TJ. Molecular analysis of the tagF gene, encoding CDP-Glycerol:Poly(glycerophosphate) glycerophosphotransferase of Staphylococcus epidermidis ATCC 14990. J Bacteriol. 2000;182:1046–1052. doi: 10.1128/jb.182.4.1046-1052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Formstone A, Carballido-López R, Noirot P, Errington J, Scheffers D-J. Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis. J Bacteriol. 2008;190:1812–1821. doi: 10.1128/JB.01394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankel MB, Schneewind O. Determinants of murein hydrolase targeting to cross-wall of Staphylococcus aureus peptidoglycan. J Biol Chem. 2012;287:10460–10471. doi: 10.1074/jbc.M111.336404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geddes AM, Klugman KP, Rolinson GN. Introduction: historical perspective and development of amoxicillin/clavulanate. Int J Antimicrob Agents. 2007;30(Suppl 2):S109–S112. doi: 10.1016/j.ijantimicag.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Ginsberg C, Zhang Y-H, Yuan Y, Walker S. In vitro reconstitution of two essential steps in wall teichoic acid biosynthesis. ACS Chem. 2006;1:25–28. doi: 10.1021/cb0500041. [DOI] [PubMed] [Google Scholar]
- 55.Gross M, Cramton SE, Götz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–3436. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haas R, Koch HU, Fischer W. Alanyl turnover from lipoteichoic acid to teichoic acid in Staphylococcus aureus. FEMS Microbiology Letters. 1984;21:27–31. [Google Scholar]
- 57.Hancock I, Wiseman G, Baddiley J. Biosynthesis of the unit that links teichoic acid to the bacterial wall: inhibition by tunicamycin. FEBS Lett. 1976;69:75–80. doi: 10.1016/0014-5793(76)80657-6. [DOI] [PubMed] [Google Scholar]
- 58.Heaton MP, Neuhaus FC. Biosynthesis of D-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the D-alanine-activating enzyme. J Bacteriol. 1992;174:4707–4717. doi: 10.1128/jb.174.14.4707-4717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heaton MP, Neuhaus FC. Role of the D-alanyl carrier protein in the biosynthesis of D-alanyl-lipoteichoic acid. J Bacteriol. 1994;176:681–690. doi: 10.1128/jb.176.3.681-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heptinstall S, Archibald AR, Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970;225:519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- 61.Holland L, Conlon B, O'Gara JP. Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development. Microbiology. 2010;157(Pt 2):408–418. doi: 10.1099/mic.0.042234-0. [DOI] [PubMed] [Google Scholar]
- 62.Hubscher J, McCallum N, Sifri CD, Majcherczyk PA, Entenza JM, et al. MsrR contributes to cell surface characteristics and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 2009;295:251–260. doi: 10.1111/j.1574-6968.2009.01603.x. [DOI] [PubMed] [Google Scholar]
- 63.James DB, Yother J. Genetic and Biochemical Characterizations of Enzymes Involved in Streptococcus pneumoniae Serotype 2 Capsule Synthesis Demonstrate that Cps2T (WchF) Catalyzes the Committed Step by Addition of β1–4 Rhamnose, the Second Sugar Residue in the Repeat Unit. J Bacteriol. 2012;194:6479–6489. doi: 10.1128/JB.01135-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenni R, Berger-Bächi B. Teichoic acid content in different lineages of Staphylococcus aureus NCTC8325. Arch Microbiol. 1998;170:171–178. doi: 10.1007/s002030050630. [DOI] [PubMed] [Google Scholar]
- 65.Karamata D, Pooley HM, Monod M. Expression of heterologous genes for wall teichoic acid in Bacillus subtilis 168. Mol Gen Genet. 1987;207:73–81. doi: 10.1007/BF00331493. [DOI] [PubMed] [Google Scholar]
- 66.Kawai Y, Marles-Wright J, Cleverley RM, Emmins R, Ishikawa S, et al. A widespread family of bacterial cell wall assembly proteins. EMBO J. 2011;30:4931–4941. doi: 10.1038/emboj.2011.358. identification and crystallization of the potential WTA ligases
- 67.Kern T, Giffard M, Hediger S, Amoroso A, Giustini C, et al. Dynamics characterization of fully hydrated bacterial cell walls by solid-state NMR: evidence for cooperative binding of metal ions. J Am Chem Soc. 2010;132:10911–10919. doi: 10.1021/ja104533w. [DOI] [PubMed] [Google Scholar]
- 68.Kiriukhin MY, Neuhaus FC. D-alanylation of lipoteichoic acid: role of the D-alanyl carrier protein in acylation. J Bacteriol. 2001;183:2051–2058. doi: 10.1128/JB.183.6.2051-2058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, et al. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohler T, Weidenmaier C, Peschel A. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J Bacteriol. 2009;191:4482–4484. doi: 10.1128/JB.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kojima N, Araki Y, Ito E. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J Bacteriol. 1985;161:299–306. doi: 10.1128/jb.161.1.299-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kovács M, Halfmann A, Fedtke I, Heintz M, Peschel A, et al. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J Bacteriol. 2006;188:5797–5805. doi: 10.1128/JB.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kristian S, Datta V, Weidenmaier C, Kansal R, Fedtke I, et al. D-alanylation of teichoic acids promotes group a Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kristian SA, Lauth X, Nizet V, Goetz F, Neumeister B, et al. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J Infect Dis. 2003;188:414–423. doi: 10.1086/376533. [DOI] [PubMed] [Google Scholar]
- 75.Lazarevic V, Abellan F-X, Möller SB, Karamata D, Mauël C. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology (Reading, Engl) 2002;148:815–824. doi: 10.1099/00221287-148-3-815. [DOI] [PubMed] [Google Scholar]
- 76.Lazarevic V, Karamata D. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol Microbiol. 1995;16:345–355. doi: 10.1111/j.1365-2958.1995.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 77.Lee K, Campbell J, Swoboda JG, Cuny GD, Walker S. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorganic & Medicinal Chemistry Letters. 2010;20:1767–1770. doi: 10.1016/j.bmcl.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lovering AL, Lin LY-C, Sewell EW, Spreter T, Brown ED, Strynadka NCJ. Structure of the bacterial teichoic acid polymerase TagF provides insights into membrane association and catalysis. Nat Struct Mol Biol. 2010;17:582–589. doi: 10.1038/nsmb.1819. First crystal structure of a WTA polymerase
- 79.Maki H, Yamaguchi T, Murakami K. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J Bacteriol. 1994;176:4993–5000. doi: 10.1128/jb.176.16.4993-5000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matias VR, Beveridge TJ. Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol Microbiol. 2005;56:240–251. doi: 10.1111/j.1365-2958.2005.04535.x. [DOI] [PubMed] [Google Scholar]
- 81.Matias VR, Beveridge TJ. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J Bacteriol. 2006;188:1011–1021. doi: 10.1128/JB.188.3.1011-1021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matias VR, Beveridge TJ. Cryo-electron microscopy of cell division in Staphylococcus aureus reveals a mid-zone between nascent cross walls. Mol Microbiol. 2007;64:195–206. doi: 10.1111/j.1365-2958.2007.05634.x. [DOI] [PubMed] [Google Scholar]
- 83.Mauck J, Glaser L. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J Biol Chem. 1972;247:1180–1187. [PubMed] [Google Scholar]
- 84.May JJ, Finking R, Wiegeshoff F, Weber TT, Bandur N, et al. Inhibition of the D-alanine:D-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium's susceptibility to antibiotics that target the cell wall. FEBS J. 2005;272:2993–3003. doi: 10.1111/j.1742-4658.2005.04700.x. [DOI] [PubMed] [Google Scholar]
- 85.Meredith TC, Swoboda JG, Walker S. Late-stage polyribitol phosphate wall teichoic acid biosynthesis in Staphylococcus aureus. J Bacteriol. 2008;190:3046–3056. doi: 10.1128/JB.01880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mericl AN, Friesen JA. Comparative kinetic analysis of glycerol 3-phosphate cytidylyltransferase from Enterococcus faecalis and Listeria monocytogenes. Med Sci Monit. 2012;18:BR427–BR434. doi: 10.12659/MSM.883535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minnig K, Lazarevic V, Soldo B, Mauel C. Analysis of teichoic acid biosynthesis regulation reveals that the extracytoplasmic function sigma factor sigmaM is induced by phosphate depletion in Bacillus subtilis W23. Microbiology. 2005;151:3041–3049. doi: 10.1099/mic.0.28021-0. [DOI] [PubMed] [Google Scholar]
- 88.Nathenson SG, Ishimoto N, Anderson JS, Strominger JL. Enzymatic synthesis and immunochemistry of α- and β-N-acetylglucosaminylribitol linkages in teichoic acids from several strains of Staphylococcus aureus. J Biol Chem. 1966;241:651–658. [PubMed] [Google Scholar]
- 89.Naumova IB, Kuznetsov VD, Kudrina KS, Bezzubenkova AP. The occurrence of teichoic acids in Streptomycetes. Arch Microbiol. 1980;126:71–75. doi: 10.1007/BF00421893. [DOI] [PubMed] [Google Scholar]
- 90.Naumova IB, Shashkov AS, Tul'skaya EM, Streshinskaya GM, Kozlova YI, et al. Cell wall teichoic acids: structural diversity, species specificity in the genus Nocardiopsis, and chemotaxonomic perspective. FEMS Microbiol Rev. 2001;25:269–284. doi: 10.1111/j.1574-6976.2001.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 91.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Over B, Heusser R, McCallum N, Schulthess B, Kupferschmied P, et al. LytR-CpsA-Psr proteins in Staphylococcus aureus display partial functional redundancy and the deletion of all three severely impairs septum placement and cell separation. FEMS Microbiol Lett. 2011;320:142–151. doi: 10.1111/j.1574-6968.2011.02303.x. [DOI] [PubMed] [Google Scholar]
- 93.Park JT, Shaw DR, Chatterjee AN, Mirelman D, Wu T. Mutants of Staphylococci with altered cell walls. Ann N Y Acad Sci. 1974;236:54–62. doi: 10.1111/j.1749-6632.1974.tb41481.x. [DOI] [PubMed] [Google Scholar]
- 94.Park YS, Sweitzer TD, Dixon JE, Kent C. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J Biol Chem. 1993;268:16648–16654. [PubMed] [Google Scholar]
- 95.Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. 1995;270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 96.Pereira MP, Brown ED. Bifunctional catalysis by CDP-ribitol synthase: convergent recruitment of reductase and cytidylyltransferase activities in Haemophilus influenzae and Staphylococcus aureus. Biochemistry. 2004;43:11802–11812. doi: 10.1021/bi048866v. [DOI] [PubMed] [Google Scholar]
- 97.Pereira MP, D'elia MA, Troczynska J, Brown ED. Duplication of teichoic acid biosynthetic genes in Staphylococcus aureus leads to functionally redundant poly(ribitol phosphate) polymerases. J Bacteriol. 2008;190:5642–5649. doi: 10.1128/JB.00526-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pereira MP, Schertzer JW, D'elia MA, Koteva KP, Hughes DW, et al. The wall teichoic acid polymerase TagF efficiently synthesizes poly(glycerol phosphate) on the TagB product lipid III. ChemBioChem. 2008;9:1385–1390. doi: 10.1002/cbic.200800026. [DOI] [PubMed] [Google Scholar]
- 99.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 100.Peschel A, Vuong C, Otto M, Götz F. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pooley HM, Karamata D. Incorporation of [2–3H]glycerol into cell surface components of Bacillus subtilis 168 and thermosensitive mutants affected in wall teichoic acid synthesis: effect of tunicamycin. Microbiology (Reading, Engl) 2000;146(Pt 4):797–805. doi: 10.1099/00221287-146-4-797. [DOI] [PubMed] [Google Scholar]
- 102.Potekhina N, Streshinskaya G, EM Ts, YI K, Senchenkova S, Shashkov A. Phosphate-containing cell wall polymers of Bacilli. Biochemistry (Mosc.) 2011;76:745–754. doi: 10.1134/S0006297911070042. [DOI] [PubMed] [Google Scholar]
- 103.Price N, Tsvetanova B. Biosynthesis of the Tunicamycins: A Review. J Antibiotics. 2007;60:485–491. doi: 10.1038/ja.2007.62. [DOI] [PubMed] [Google Scholar]
- 104.Qamar A, Golemi-Kotra D. Dual Roles of FmtA in Staphylococcus aureus Cell Wall Biosynthesis and Autolysis. Antimicrob Agents Chemother. 2012;56:3797–3805. doi: 10.1128/AAC.00187-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qian Z, Yin Y, Zhang Y, Lu L, Li Y, Jiang Y. Genomic characterization of ribitol teichoic acid synthesis in Staphylococcus aureus: genes, genomic organization and gene duplication. BMC Genomics. 2006;7:74. doi: 10.1186/1471-2164-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reichmann NT, Gründling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rubinchik E, Schneider T, Elliott M, Scott WR, Pan J, et al. Mechanism of action and limited cross-resistance of new lipopeptide MX-2401. Antimicrob Agents Chemother. 2011;55:2743–2754. doi: 10.1128/AAC.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, et al. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathog. 2012;8:e1002891. doi: 10.1371/journal.ppat.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schertzer JW, Brown ED. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J Biol Chem. 2003;278:18002–18007. doi: 10.1074/jbc.M300706200. [DOI] [PubMed] [Google Scholar]
- 110.Schertzer JW, Brown ED. Use of CDP-glycerol as an alternate acceptor for the teichoic acid polymerase reveals that membrane association regulates polymer length. J Bacteriol. 2008;190:6940–6947. doi: 10.1128/JB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schirner K, Stone LK, Walker S. ABC transporters required for export of wall teichoic acids do not discriminate between different main chain polymers. ACS Chem Biol. 2011;6:407–412. doi: 10.1021/cb100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, et al. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Molecular Microbiology. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. Argues WTAs are absent from S. aureus cross walls, enabling cross wall localization of autolysins
- 113.Sewell EWC, Pereira MP, Brown ED. The wall teichoic acid polymerase TagF is non-processive in vitro and amenable to study using steady state kinetic analysis. J Biol Chem. 2009;284:21132–21138. doi: 10.1074/jbc.M109.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith T, Blackman S, Foster S. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 116.Soldo B, Lazarevic V, Karamata D. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology (Reading, Engl) 2002;148:2079–2087. doi: 10.1099/00221287-148-7-2079. [DOI] [PubMed] [Google Scholar]
- 117.Soldo B, Lazarevic V, Pooley HM, Karamata D. Characterization of a Bacillus subtilis thermosensitive teichoic acid-deficient mutant: gene mnaA (yvyH) encodes the UDP-N-acetylglucosamine 2-epimerase. J Bacteriol. 2002;184:4316–4320. doi: 10.1128/JB.184.15.4316-4320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki T, Campbell J, Swoboda JG, Walker S, Gilmore MS. Role of wall teichoic acids in Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2011;52:3187–3192. doi: 10.1167/iovs.10-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki T, Swoboda JG, Campbell J, Walker S, Gilmore MS. In Vitro Antimicrobial Activity of Wall Teichoic Acid Biosynthesis Inhibitors against Staphylococcus aureus Isolates. Antimicrobial Agents and Chemotherapy. 2011;55:767–774. doi: 10.1128/AAC.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swoboda JG, Meredith TC, Campbell J, Brown S, Suzuki T, et al. Discovery of a small molecule that blocks wall teichoic acid biosynthesis in Staphylococcus aureus. ACS Chem Biol. 2009;4:875–883. doi: 10.1021/cb900151k. A pathway-specific, cell-based screen exploiting conditional essentiality to identify an antibiotic inhibiting the WTA pathway
- 122.Theilacker C, Holst O, Lindner B, Huebner J, Kaczynski Z. The structure of the wall teichoic acid isolated from Enterococcus faecalis strain 12030. Carbohydr Res. 2012;354:106–109. doi: 10.1016/j.carres.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 123.Tomasz A, McDonnell M, Westphal M, Zanati E. Coordinated incorporation of nascent peptidoglycan and teichoic acid into Pneumococcal cell walls and conservation of peptidoglycan during growth. J Biol Chem. 1975;250:337–341. [PubMed] [Google Scholar]
- 124.Tomita S, Irisawa T, Tanaka N, Nukada T, Satoh E, et al. Comparison of components and synthesis genes of cell wall teichoic acid among Lactobacillus plantarum strains. Biosci Biotechnol Biochem. 2010;74:928–933. doi: 10.1271/bbb.90736. [DOI] [PubMed] [Google Scholar]
- 125.Torii M, Kabat EA, Bezer AE. Separation of teichoic acid of Staphylococcus aureus into two immunologically distinct specific polysaccharides with α- and β-N-acetylglucosaminyl linkages respectively. Antigenicity of teichoic acids in man. J Exp Med. 1964;120:13–29. doi: 10.1084/jem.120.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vadyvaloo V, Arous S, Gravesen A, Héchard Y, Chauhan-Haubrock R, et al. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology (Read, Engl) 2004;150:3025–3033. doi: 10.1099/mic.0.27059-0. [DOI] [PubMed] [Google Scholar]
- 127.Vergara-Irigaray M, Maira-Litrán T, Merino N, Pier GB, Penadés JR, Lasa I. Wall teichoic acids are dispensable for anchoring the PNAG exopolysaccharide to the Staphylococcus aureus cell surface. Microbiology (Reading, Engl) 2008;154:865–877. doi: 10.1099/mic.0.2007/013292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinogradov E, Sadovskaya I, Li J, Jabbouri S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr Res. 2006;341:738–743. doi: 10.1016/j.carres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 129.Volkman BF, Zhang Q, Debabov DV, Rivera E, Kresheck GC, Neuhaus FC. Biosynthesis of D-alanyl-lipoteichoic acid: the tertiary structure of apo-D-alanyl carrier protein. Biochemistry. 2001;40:7964–7972. [PubMed] [Google Scholar]
- 130.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 131.Walter J, Loach DM, Alqumber M, Rockel C, Hermann C, et al. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract. Environmental Microbiology. 2007;9:1750–1760. doi: 10.1111/j.1462-2920.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Gill C, Lee S, Mann P, Zuck P, et al. Discovery of novel wall teichoic acid inhibitors as effective anti-MRSA β-lactam combination agents. Chem & Biol. 2013 doi: 10.1016/j.chembiol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ward JB. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiological Reviews. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wecke J, Madela K, Fischer W. The absence of D-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology. 1997;143:2953. doi: 10.1099/00221287-143-9-2953. [DOI] [PubMed] [Google Scholar]
- 135.Weidenmaier C, Kokai-Kun J, Kristian S, Chanturiya T, Kalbacher H, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. Demonstration that WTAs play a critical role in S. aureus pathogenesis
- 136.Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, et al. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol. 2008;298:505–513. doi: 10.1016/j.ijmm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 137.Weidenmaier C, Kristian SA, Peschel A. Bacterial resistance to antimicrobial host defenses--an emerging target for novel antiinfective strategies? Curr Drug Targets. 2003;4:643–649. doi: 10.2174/1389450033490731. [DOI] [PubMed] [Google Scholar]
- 138.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 139.Weidenmaier C, Peschel A, Xiong Y-Q, Kristian SA, Dietz K, et al. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis. 2005;191:1771–1777. doi: 10.1086/429692. [DOI] [PubMed] [Google Scholar]
- 140.Wickham JR, Halye JL, Kashtanov S, Khandogin J, Rice CV. Revisiting magnesium chelation by teichoic acid with phosphorus solid-state NMR and theoretical calculations. J Phys Chem B. 2009;113:2177–2183. doi: 10.1021/jp809313j. [DOI] [PubMed] [Google Scholar]
- 141.Wyke AW, Ward JB. Biosynthesis of wall polymers in Bacillus subtilis. J Bacteriol. 1977;130:1055–1063. doi: 10.1128/jb.130.3.1055-1063.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xayarath B, Yother J. Mutations blocking side chain assembly, polymerization, or transport of a Wzy-dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. J Bacteriol. 2007;189:3369–3381. doi: 10.1128/JB.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia G, Corrigan RM, Winstel V, Goerke C, Gründling A, Peschel A. Wall teichoic A=acid-dependent adsorption of Staphylococcal siphovirus and myovirus. J Bacteriol. 2011;193:4006–4009. doi: 10.1128/JB.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010;300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 145.Xia G, Maier L, Sanchez-Carballo P, Li M, Otto M, et al. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. Journal of Biological Chemistry. 2010;285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamamoto H, Miyake Y, Hisaoka M, Kurosawa S-I, Sekiguchi J. The major and minor wall teichoic acids prevent the sidewall localization of vegetative DL-endopeptidase LytF in Bacillus subtilis. Molecular Microbiology. 2008;70:297–310. doi: 10.1111/j.1365-2958.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 147.Yokoyama K, Miyashita T, Araki Y, Ito E. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur J Biochem. 1986;161:479–489. doi: 10.1111/j.1432-1033.1986.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 148.Young M, Mauël C, Margot P, Karamata D. Pseudo-allelic relationship between non-homologous genes concerned with biosynthesis of polyglycerol phosphate and polyribitol phosphate teichoic acids in Bacillus subtilis strains 168 and W23. Mol Microbiol. 1989;3:1805–1812. doi: 10.1111/j.1365-2958.1989.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 149.Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol Rev. 2008;32:361–385. doi: 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y, Ginsberg C, Yuan Y, S W. Acceptor substrate selectivity and kinetic mechanism of Bacillus subtilis TagA. Biochemistry. 2006;45:10895–10904. doi: 10.1021/bi060872z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zolli M, Kobric D, Brown E. Reduction precedes cytidylyl transfer without substrate channeling in distinct active sites of the bifunctional CDP-ribitol synthase from Haemophilus influenzae. Biochemistry. 2001;40:5041–5048. doi: 10.1021/bi002745n. [DOI] [PubMed] [Google Scholar]