Pathophysiologic cascades in ischemic stroke (original) (raw)
. Author manuscript; available in PMC: 2014 Apr 14.
Abstract
Many advances have been achieved in terms of understanding the molecular and cellular mechanisms of ischemic stroke. But thus far, clinically effective neuroprotectants remain elusive. In this minireview, we summarize the basics of ischemic cascades after stroke, covering neuronal death mechanisms, white matter pathophysiology, and inflammation with an emphasis on microglia. Translating promising mechanistic knowledge into clinically meaningful stroke drugs is very challenging. An integrative approach that encompasses the multimodal and multicell signaling phenomenon of stroke will be required to move forward.
Keywords: microglia, neurovascular unit, penumbra, reperfusion, white matter
Introduction
Stroke and cerebrovascular disease is a major cause of mortality and disability worldwide (1,2). Ischemic stroke is caused by a reduction in blood flow to the brain. Hence, the decrease in cerebral blood flow (CBF) has received an effective answer: accelerated reperfusion via thrombolysis using recombinant tissue plasminogen activator (rt-PA) is associated with an improved clinical outcome. This achievement is now routinely transferred to practice. This ease of translation is due to the fact that the underlying conceptual model is simple: an arterial occlusion decreases CBF. So an effective treatment should increase CBF.
The best way to do this currently might be with rt-PA. However, due to the moderate recanalization rate, the limited time window, and the number of contraindications for thrombolysis, only minority of patients receive tPA so other treatments with potential additive effects are still urgently needed. Furthermore, even in patients who receive tPA, those with more severe initial strokes often do not significantly improve. In part, this might be related to some aspect of reperfusion injury. Overall, additive treatments that combine with rt-PA should be worth pursuing.
In terms of the blood flow distribution, cerebral ischemic strokes are often focal. In the central core regions of the insult, there is almost total CBF arrest. This area evolves rapidly toward death within minutes. Surrounding this core, CBF levels may fall below functional thresholds yet transiently lie above the threshold of cell death – this zone has been called the penumbra. The penumbra, a metastable zone, permits only cell survival for a certain period of time. Thus, this potentially salvageable tissue is the target for neuroprotective therapy. Over the past two decades, tremendous progress has been achieved in terms of understanding the intricate cellular and molecular mechanisms of stroke pathophysiology (3,4). However, to date, we still do not have a clinically effective neuroprotectant. In this minireview, we briefly survey the fundamentals of the ischemic cascade. Many excellent and more detailed reviews on this topic have been published (5–7). Our more limited scope here is focused on an attempt to discuss translational hurdles and identify promising targets.
Neuronal death mechanisms
The most upstream consequence of cerebral ischemia fundamentally is composed of an energetic problem. In the area of reduced blood supply, adenosine triphosphate (ATP) consumption continues despite insufficient synthesis, causing total ATP levels to drop and lactate acidosis to develop with concomitant loss of ionic homeostasis in neurons. Once this initial step has taken place, an ischemic cascade follows involving a multimodal and multicell series of downstream mechanisms. The reader is referred to several excellent reviews for an in-depth discussion of the molecular details (5,8). Here, we will summarize the key steps and focus on mechanisms of acute neuronal injury.
Severe cerebral ischemia leads to a loss of energy stores resulting in ionic imbalance and neurotransmitter release and inhibition of reuptake. It is especially the case for glutamate, the main excitotoxic neurotransmitter. Glutamate binds to ionotropic N-Methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (iGluRs), promoting a major influx of calcium. This calcium overload triggers phospholipases and proteases that degrade essential membranes and proteins (9). In addition, the glutamate receptors promote an excessive sodium and water influx with concomitant cell swelling, edema and shrinking of extracellular space (9). Massive calcium influx activates a catabolic process mediated by proteases, lipases, and nucleases. In experimental models, blockade of GluRs can reduce infarction volume via three ways: first, reducing calcium influx and its related proteases activations; second, attenuating cortical spreading depressions (CSDs); third, triggering in neurons the subfamily of metabotropic mGluRs toward prosurvival or prodeath signaling (10). This glutamate excitotoxicity concept raised major hope for stroke new therapies. Despite validation of the concept in many experimental models, all the trials targeting NMDA and AMPA receptors failed to improve outcome of the patients (11,12), likely for reasons beyond methodological shortcomings in study designs (13).
High calcium, sodium, and adenosine diphosphate (ADP) levels in ischemic cells stimulate excessive mitochondrial oxygen radical production within other sources of free radicals production such as prostaglandin synthesis and degradation of hypoxanthine. These reactive oxygen species (ROS) directly damage lipids, proteins, nucleic acid, and carbohydrates (3). They are especially toxic for the cells because baseline levels and any corresponding upregulation of antioxidant enzymes [superoxyde dismutase (SOD), catalase, glutathione] and scavenging mechanisms (α-tocopherol, vitamin C) are too slow to offset ROS production. Downstream of free radicals, other neuronal death mechanisms will also be induced involving, e.g. mitochondrial transition pore formation (14), the lipoxygenase cascade (15), the activation of poly ADP-ribose polymerase (PARP) (16) and amplified ionic imbalance via secondary recruitment of calcium-permeable transient receptor potential ion (TRPM) channels (17). Furthermore, ROS and reactive nitrogen species also has the potential of modifying endogenous functions of proteins, which may be neuroprotective (18). Ultimately, these multimodal cascades will result in a complex mix of neuronal death comprising, necrosis, apoptosis, and autophagy (19).
In parallel with the molecular events briefly outlined here, a physiologic process called cortical spreading depression (CSD) has also been recently identified as a candidate target for stroke. CSD is an intense depolarization of neuronal and glial membranes slowly propagating by way of gray matter contiguity at a speed of two- to six-millimeters per minute. It is characterized by near complete breakdown of ion gradients, near complete sustained depolarization, extreme shunt of neuronal membrane resistance, loss of electrical activity, and neuronal swelling and distortion of dendritic spines (20,21), associated with a shrinkage of extracellular volume. CSD occurs when extracellular K+ concentrations exceed a critical threshold. Glutamate, inhibitors of the sodium pump, hypoxia, hypoglycemia, ischemia, electrical stimulation, and status epilepticus are other well-described triggers. Gene mutations affecting either ions channels such as calcium channel (22) or the astrocytic sodium pump decrease the level for CSD (23). Interestingly, the Neurogenic locus notch homolog protein 3 (NOTCH3) gene mutation causing cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome (CADASIL) is also associated with susceptibility to CSD linking CSD with the neurovascular unit (24). Moreover, CSD is illustrating another aspect of the normal neurovascular coupling of regional cerebral blood flow (rCBF) and physiological neuronal activity. Under physiological conditions, CSD is associated with a major CBF rise in an attempt to match to the increase in metabolic demand. This sharp hyperemia is followed by a long lasting oligemia associated with abnormal vascular responses. Under pathological conditions such as ischemia, an inverse hemodynamic response characterized by a CBF reduction spreading ischemia can be observed (25). In ischemia, these waves of CSD originating in the peri infarct area can invade repeatedly peri-ischemic tissue, adding a major metabolic demand on penumbra, and at term, the spreading depolarization expands the volume of infarction (20,26,27). After decades of clinical doubt, CSD has now been demonstrated in patients suffering from brain trauma, sub-arachnoid hemorrhage, spontaneous intracerebral hematoma, and ischemic strokes (28–30).
It is tempting to target CSD as they increase the volume of infarction. NMDA antagonists can effectively suppress CSDs (31). But this class of drugs has failed before in many clinical stroke trials. Some differences between animal and human ischemic brains such as presence of gyri (32) or astrocytes–neurons ratio (33) may explain differences in occurrence and consequences of CSD. Nontraumatic methods to study CSD in humans and better methods to block CSD and improve stroke outcomes will have to be developed.
Ultimately, blocking integrated mechanisms of neuronal death in the ischemic cascade may be difficult. But this is not because these targets are invalid. Indeed, the fundamental neurobiology of these molecular mechanisms is sound. From a clinical perspective, blocking these early targets is difficult because the therapeutic time windows may be extremely narrow. Furthermore, the majority of these pathways do not necessarily occur during the period of arterial occlusion but when the tissue is reperfused or some significant blood flow still persists (34). Hence, the clinical rationale herein might not be driven by a stand-alone neuroprotective treatment but instead aimed at a combination therapy to be given along with thrombolytic or mechanical reperfusion. Indeed, a recent powerful study provides proof of concept. Tymianski and colleagues recently showed that a compound that blocks the post-synaptic density protein post-synaptic density protein-95 (PSD-95) was able to reduce infarction in a nonhuman primate model of transient focal cerebral ischemia (35).
White matter mechanisms
As seen earlier, remarkable research efforts have been revealing basic mechanisms underlying neuronal death. Thus far, however, many drug candidates targeting the neuronal death pathways have all failed in clinical trials (36–38). Of course, there are many reasons for our translational difficulties (3,39,40). In this section, we address one of them, i.e. the question of white matter. The majority of our mechanisms and targets in experimental models remain mostly focused on neurons in gray matter. In human stroke, however, a significant portion of white matter injury is surely involved.
Nonneuronal cells and white matter are extremely vulnerable to ischemic stress (41–43). White matter is primarily composed of axonal bundles ensheathed with myelin. The cells forming these sheaths are the oligodendrocytes, which tend to be arranged in rows parallel to axonal tracts. Just before and after birth, oligodendrocyte precursor cells (OPCs) multiply rapidly, mature into OLGs, and develop processes for myelin formation. White matter blood flow is lower than in gray matter, and there is little collateral blood supply in the deep white matter (44). Hence, white matter ischemia will be typically severe with rapid cell swelling and tissue edema. White matter ischemia activates several kinds of proteases, which weaken the structural integrity of axons and myelin sheath (3). Demyelinated axons may be more susceptible to ischemic injury due to their heightened metabolic requirements caused by loss of energy-efficient salutatory conduction and leaky sodium channels (45). Moreover, mechanisms responsible for ischemic cell death may be different between gray matter and white matter. While an increase in intracellular Ca2+ is involved in both white and gray matter ischemia, the routes of Ca2+ entry might differ. In white matter, pathological Ca2+ entry occurs in part due to increased intracellular Na+ and membrane depolarization (46–48). In contrast, voltage-dependent Ca2+ channels and glutamate-activated ionic receptors in gray matter have been traditionally viewed as the primary routes of pathological Ca2+ entry (49,50). For a more detailed analysis of mechanistic and cell-based models of white matter injury, please refer to a previous review on this topic (3,51).
Although there are no validated drugs to prevent white matter injury in stroke, promising leads may perhaps be found in other fields. A noncompetitive NMDA receptor antagonist memantine is now licensed for moderate-to-severe Alzheimer’s disease in the United States and the European Union (52). At least two studies suggest that memantine may be protective to white matter (53,54). Furthermore, antioxidant drugs would be also potent therapeutic candidates for white matter stroke. Although the radical spin trap disufenton sodium (NXY)-059 failed in a final phase 3 clinical trial (55); it is now suspected that drug penetration through the blood–brain barrier might have been low for this specific compound. ROS are known to be deeply involved in white matter pathophysiology. Axons contain abundant mitochondria, which is an organelle for a source of ROS. Free radical scavengers significantly reduce white matter injury in rodent stroke models (56–59). Whether we can develop a radical scavenger that effectively penetrates white matter in large human brains remains to be seen. Another potential example may be found in the antiplatelet drug cilostazol, a type III phosphodiesterase (PDE3) inhibitor. Cilostazol is approved for the treatment of intermittent claudication and improves pain-free walking distance in patients with peripheral arterial disease (60). But recent report proposed that cilostazol may also be used for white matter protection (61). Future studies are warranted to dissect the mechanisms how PDE3 inhibition contributes to defend white matter from ischemic stress.
No matter how compelling our molecular targets might be, it may still be difficult to reach all stroke patients in time for acute therapy. Hence, we will also need to consider how to efficiently promote rehabilitation and repair after stroke. This may be especially relevant for white matter where ‘repairing or readapting the wiring’ in neuronal networks is essential for functional recovery. In the chronic phase after stroke, several endogenous responses should be induced for repairing white matter damage. Although the population of OPCs is relatively low in the adult white matter, OPCs may play substantial roles for remyelination after injury. During development, OPCs migrate from the ventricular zone to their final destination and then differentiate to from myelin sheaths (62,63). But, after brain injury, they are guided to the site for contributing to myelin repair (64). It remains to be fully elucidated how oligodendrogenesis occurs. But it is likely that multiple cell–cell trophic interactions may be involved. Recent findings suggest that there might exist an oligovascular niche in white matter wherein cell–cell trophic coupling between cerebral endothelium and oligodendrocyte helps sustain ongoing oligodendrogenesis and angiogenesis (65). In addition, astrocytes may secrete trophic factors to support OPC survival (66,67). Hence, cell–cell trophic coupling in white matter may provide an essential mechanism for repairing damaged white matter in the chronic stroke phase. In this regard, cell-based treatment would be promising as a restorative therapy in white matter stroke. Some experimental studies suggest that injected stem cells (and their derivatives) could survive, differentiate into not only neurons/astrocytes but also oligodendrocytes (68). Furthermore, cellular therapies may be additionally beneficial because these cells produce a rich broth of trophic factors that stimulate endogenous repair of host tissue.
Inflammation and microglia
Inflammation is an important part of stroke pathophysiology, especially in the context of reperfusion. Restoring CBF is an obvious and primary goal. But as discussed earlier, ischemia–reperfusion itself can also set off numerous cascades of secondary injury. Reactive radicals will be generated, blood–brain barrier integrity may be compromised, and multimodal neuronal death processes composed of programmed necrosis, apoptosis, and autophagy may still continue unabated. Along with these central neuronal responses, an activation of peripheral immune responses is now known to occur as well. Over the course of days to weeks, a complex and orchestrated influx of inflammatory cells begins to take place. Proof of concept has been repeatedly obtained – blocking various steps in the inflammatory cascade prevents central accumulation of deleterious immune cells such as neutrophils and T cells, and improve outcomes, at least in experimental models, Many recent excellent reviews on this topic have been published and readers are referred to these for more in-depth discussions (69,70). In this section, we will focus on microglia, to ask whether these central responders in fact may comprise promising targets.
Microglia are resident immune cells of the central nervous system (CNS) and serve as sensors and effectors in the normal and pathologic brain (71,72). Microglia are involved in most CNS pathologies and constantly monitor the microenvironment and respond to any kind of pathologic change, thus exerting typical macrophagic functions, such as phagocytosis, secretion of proinflammatory cytokines, and antigen presentation. Microglia have important roles even in normal brain. In fact, it has been suggested that the term ‘resting microglia’ should be changed to ‘surveying microglia’ to describe how microglia continuously monitor the healthy CNS – these are not functionally silent cells (71,73). Microglial processes are highly dynamic in intact cortex (74,75). A recent imaging study demonstrated that the dynamic motility of ‘resting’ microglial processes in vivo may be because microglia constantly monitor and respond to the functional status of synapses (76). In the adult brain, microglia contribute to synapse remodeling and neurogenesis (77–80). Furthermore, microglia may also interact with axons (81), be involved in blood vessel formation (82), and act as phagocytes for the removal of dying cells during the process of programmed cell death (83).
After an ischemic lesion, resident microglia are activated within minutes of ischemia onset (84) and accumulate at the lesion site and in the penumbra. Postischemic microglial proliferation peaks at 48–72 h after focal cerebral ischemia and may last for several weeks after initial injury (85,86). Upon activation, microglia undergo proliferating, morphological change from a ramified to few and thicker processes or amoeboid appearance, producing cytokines, chemokines, and growth factors, generating reactive oxygen and nitrogen species, increasing expression of immunomodulatory surface markers, and having the ability of presenting antigen (87,88).
Traditionally, activated microglia would be viewed as deleterious actors in stroke. Overactivated microglia can be neurotoxic by releasing ROS via NADPH oxidase (89), proinflammatory cytokines [e.g. tumor necrosis factor (TNF)-α, interleukin (IL)-1β] (90–93), and neurovascular proteases such as matrix metalloproteinase (MMP-9) (94). But it is now increasingly recognized that microglial activation may not always be bad after stroke. Microglial activation is not a univalent state, and activated microglia can exhibit phenotypic and functional diversity depending on the nature, strength, and duration of the stimulus (87,95). At least two activated phenotypes, ‘classically activated’ (also called M1) or an ‘alternatively activated’ (also called M2), have been identified (96–99). Inflammatory microglia (M1) release a number of proinflammatory cytokines such as TNF-α and IL-1β, and ROS and nitrous oxide (NO). While anti-inflammatory microglia (M2) are considered less inflammatory than M1 cells, they are characterized by reduced NO production and increased production of anti-inflammatory cytokines and neurotrophic factors such as GDNF, BDNF, bFGF, insulin-like growth factor (IGF)-1, TGF-β, and VEGF (78,85,100–102).
Indeed, beyond stroke per se, whether microglial activation is neurotoxic is a long-standing debate in neurobiology. Some degree of microglial activation reflects its important physiological function to protect neurons and the integrity of CNS, while overactivation or loss of microglial functions exacerbates a preexisting neuropathology or this function gets lost in neurodegenerative diseases (103). There is clear evidence shows that activated microglia can maintain and support neuronal survival (104,105) by release of neurotrophic and anti-inflammatory molecules, the clearance of toxic products or invading pathogens, as well as the guidance of stem cells to inflammatory lesion sites to promote neurogenesis (79,80,106). Hence, microglia can be potentially beneficial in some circumstances. Selective ablation of proliferating resident microglia results in a significant increase in the size of the infarction associated with an increase in apoptotic neurons and a decrease in the levels of IGF-1 in transient focal cerebral ischemia (85). Moreover, these results reveal a marked neuroprotective potential of proliferating microglia serving as an endogenous pool of neurotrophic molecules such as IGF-1. Exogenous microglia isolated from brain cultures injected into the subclavian artery of Mongolian gerbils results in increased numbers of surviving neurons in ischemic hippocampus (107). Administration of exogenous microglia increases the expression of BDNF and GDNF in the ischemic hippocampus, which may explain the positive effect of microglia on neuronal survival. Similarly, exogenous microglia microinjected into the ventricles of rats can migrate into brain parenchyma and significantly decrease neuronal loss induced by focal ischemia and reperfusion (108).
The most likely reason why the activation of microglia has contradictory influence on stroke outcome lies in the timing and the degree of the expression of the cytokines. TNF-α is a prime instance. Overexpression of TNF-α is detrimental to stroke outcome (109). However, some studies have shown that microglia-derived TNF-α can be neuroprotective in cerebral ischemia in mice (110) and that TNF-α-p55 receptor knockout mice have an increased infarction volume (110,111). It is important to understand the different states of microglia in diseases, and consequently, modulating the duration and the magnitude the expression of cytokines and growth factors will critically affect the results.
From a clinical perspective, targeting inflammation may not be easy. Previous clinical trials attempting to block intercellular adhesion molecule (ICAM)-1 on cerebral endothelium did not work and treated patients actually got worse (112,113). In part, this was because of complications that were not predicted in mouse models – the ‘humanized’ anti-ICAM antibodies ended up triggering deleterious complement activation that worsened injury after stroke (112). Differences in systemic blood and immune responses in animal models vs. human patients may make it difficult to safely and effectively broadly block inflammatory pathways. In this regard, searching for ‘druggable’ mechanisms in central compartment microglia may represent an alternative approach. However, careful attention will have to be paid to the notion that microglial responses after stroke might be biphasic as well, i.e. a mix of deleterious and beneficial effects that evolve over time.
Conclusions
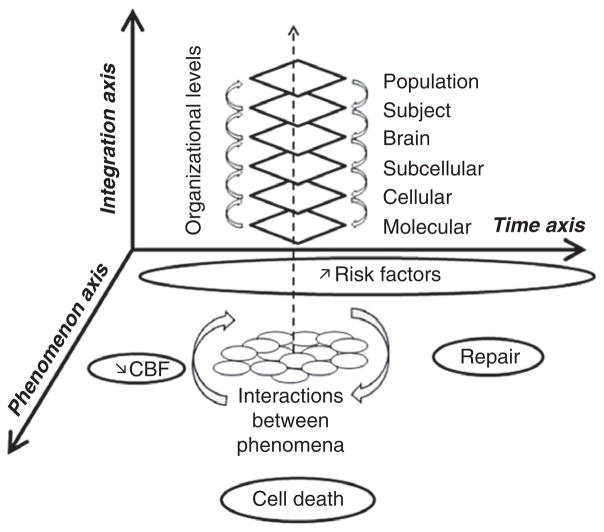
The search for effective acute stroke treatments has encountered many failures in clinical trials. In contrast, stroke prevention has gained major successes in recent years. For example, lowering blood pressure is associated with a significant reduction of stroke risk. Why? In part, this is because stroke prevention strategies address efficient targets that share an underlying linear and monotonic model. Taking hypertension as a paradigm, shear stress increase related to high blood pressure increases production of free radicals via NADPH activation and uncoupling of nitric oxide synthase, key steps in hypertension-related vascular oxidative stress (44). Once these proximal triggers are initiated, parallel downstream actions are induced. Loss of vasoregulatory nitric oxide leads to vasoconstriction, microvascular flow impairment, leukocytes adhesion, and smooth muscle cell proliferation. This integrated response then promotes cytokine and matrix metalloprotease-mediated inflammation in systemic and cerebral blood vessels (4). Thus, a ‘single’ vascular risk factor is able to trigger oxidative stress and inflammation to deleteriously affect the entire neurovascular unit (neuronal, glial, and vascular compartments) (3,4). From the molecular level to cellular injuries and vascular and brain consequences, each step of severity in risk factors amplifies the consequences, and can be integrated to the higher level (Fig. 1). These effects accumulate over time – the longer a subject is exposed to a risk factor, the more severe are the consequences. The effects of ‘risk factors’ are inherently complex. But these effects can be easily detected even in simplified animal models. For example, the free radical scavenger NXY-059 was much less effective in spontaneously hypertensive rats compared with normotensive rats (114). Hence, any translation of therapies from models to humans should require rigorous attention to how these targets are influenced by the entire range of risk factors inherent in stroke patients.
Fig. 1.
Schematic showing the nonlinear and highly integrative aspects of stroke pathophysiology. Mechanisms can be manifested at multiple levels of phenomenology, ranging from molecular and cellular biology to whole animal physiology and pharmacology to individual human subjects and population responses. The influence of genetics and risk factors adds to this complexity. And ultimately, these multifactorial processes connect into spatial and temporal gradients of tissue injury and repair. Altogether, this matrix represents a series of hurdles that must be considered before translation can take place. Finding clinically effective therapeutics for stroke prevention, treatment, and recovery is challenging. Multitarget integrative solutions may be required.
If such conceptual models work for stroke prevention, can they also work for our ongoing search for acute stroke treatments? The concept of the neurovascular unit has been useful in this regard. For perhaps far too long, we were focused only on the ‘neurobiology’ of stroke. But brain function (and dysfunction) is not only based on neurons but on cell–cell signaling between all cell types. Thus, stroke therapeutics should seek not only to prevent neuron death but to rescue functional crosstalk between all cells in the neurovascular unit (3,4). Embedded into this conceptual framework are the spatial and temporal aspects of stroke pathophysiology. Cell–cell interactions evolve depending on baseline risk factors and inflammation. And ultimately, the model is not monotonic – the same mediators can be deleterious or beneficial depending on the state of tissue injury and progression. Cell–cell interactions within the neurovascular unit underlie function, dysfunction, and remodeling (115). In this minireview, we have tried to briefly summarize the basics of stroke mechanisms, with a focus on neuronal death mechanisms, white matter pathophysiology, and inflammation. With a truly integrative approach that includes multimodel and multicell signaling that captures the gradients of both acute stroke injury and delayed stroke recovery, we may eventually make headway in this difficult disease.
Acknowledgments
The authors thank all their colleagues for many helpful discussions over the years and apologize to colleagues whose important work could not all be properly cited in this mini-review due to space limits. Note: ideas herein have been extensively drawn from our previous review papers – Lo et al., 2003 Nat Rev Neurosci; Lo, 2008 Nat Med, Arai et al., 2009 FEBS J; Arai and Lo, 2009 Biol Pharm Bull; Arai and Lo, 2009 Exp Transl Stroke Med, Arai et al., 2011 J Child Neurol; Hayakawa et al., in press Acta Neurochir Suppl. Supported in part by grants from the American Heart Association and the National Institutes of Health.
Footnotes
Conflict of interest: None declared.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:2011–30. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Projections of mortality and burden of disease, 2002–2030. doi: 10.1371/journal.pmed.0030442. Available at http://www.who.int/healthinfo/global_burden_disease/projections2002/en/index.html. [DOI] [PMC free article] [PubMed]
- 3.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. 2009;10:644–54. [PubMed] [Google Scholar]
- 6.Gutierrez M, Merino JJ, de Lecinana MA, Diez-Tejedor E. Cerebral protection, brain repair, plasticity and cell therapy in ischemic stroke. Cerebrovasc Dis. 2009;27(Suppl 1):177–86. doi: 10.1159/000200457. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 8.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 9.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 10.Bruno V, Battaglia G, Copani A, et al. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–33. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–14. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 13.O’Collins VE, Macleod MR, Cox SF, et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab. 2011;31:962–75. doi: 10.1038/jcbfm.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–19. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 15.Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–9. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzhandaivel A, Nistri A, Mladinic M. Kainate-mediated excitotoxicity induces neuronal death in the rat spinal cord in vitro via a PARP-1 dependent cell death pathway (Parthanatos) Cell Mol Neurobiol. 2010;30:1001–12. doi: 10.1007/s10571-010-9531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aarts M, Iihara K, Wei WL, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 18.Sen N, Hara MR, Ahmad AS, et al. GOSPEL: a neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron. 2009;63:81–91. doi: 10.1016/j.neuron.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin AP, Zhang HL, Qin ZH. Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci Bull. 2008;24:117–23. doi: 10.1007/s12264-008-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–47. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 21.Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–62. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 22.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–10. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 23.De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–6. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 24.Eikermann-Haerter K, Yuzawa I, Dilekoz E, Joutel A, Moskowitz MA, Ayata C. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann Neurol. 2011;69:413–8. doi: 10.1002/ana.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–30. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Strong AJ, Dohmen C, et al. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain. 2010;133:1994–2006. doi: 10.1093/brain/awq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strong AJ, Anderson PJ, Watts HR, et al. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- 28.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–37. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 29.Strong AJ, Fabricius M, Boutelle MG, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–43. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- 30.Dohmen C, Sakowitz OW, Fabricius M, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–8. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 31.Sakowitz OW, Kiening KL, Krajewski KL, et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40:e519–22. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- 32.Bowyer SM, Tepley N, Papuashvili N, et al. Analysis of MEG signals of spreading cortical depression with propagation constrained to a rectangular cortical strip. II. Gyrencephalic swine model. Brain Res. 1999;843:79–86. doi: 10.1016/s0006-8993(99)01893-4. [DOI] [PubMed] [Google Scholar]
- 33.Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol. 1997;78:295–307. doi: 10.1152/jn.1997.78.1.295. [DOI] [PubMed] [Google Scholar]
- 34.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–17. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 36.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 37.Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs Aging. 2007;24:537–46. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy TP, Vinten-Johansen J. A review of the clinical use of anti-inflammatory therapies for reperfusion injury in myocardial infarction and stroke: where do we go from here? Curr Opin Investig Drugs. 2006;7:229–42. [PubMed] [Google Scholar]
- 39.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 40.Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies – the need for new approaches. Cerebrovasc Dis. 2004;17(Suppl 1):153–66. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 41.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–6. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 42.Volpe JJ. Cerebral white matter injury of the premature infant – more common than you think. Pediatrics. 2003;112:176–80. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 43.Alix JJ. Recent biochemical advances in white matter ischaemia. Eur Neurol. 2006;56:74–7. doi: 10.1159/000095543. [DOI] [PubMed] [Google Scholar]
- 44.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–4. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 46.Imaizumi T, Kocsis JD, Waxman SG. Anoxic injury in the rat spinal cord: pharmacological evidence for multiple steps in Ca(2+)-dependent injury of the dorsal columns. J Neurotrauma. 1997;14:299–311. doi: 10.1089/neu.1997.14.299. [DOI] [PubMed] [Google Scholar]
- 47.Stys PK, Ransom BR, Waxman SG. Tertiary and quaternary local anesthetics protect CNS white matter from anoxic injury at concentrations that do not block excitability. J Neurophysiol. 1992;67:236–40. doi: 10.1152/jn.1992.67.1.236. [DOI] [PubMed] [Google Scholar]
- 48.Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- 49.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–79. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi DW. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990;10:2493–501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009;1:6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipton SA. NMDA receptors, glial cells, and clinical medicine. Neuron. 2006;50:9–11. doi: 10.1016/j.neuron.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Bakiri Y, Hamilton NB, Karadottir R, Attwell D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56:233–40. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning SM, Talos DM, Zhou C, et al. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–8. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Proctor PH, Tamborello LP. SAINT-I worked, but the neuroprotectant is not NXY-059. Stroke. 2007;38:e109. doi: 10.1161/STROKEAHA.107.489161. author reply e110. [DOI] [PubMed] [Google Scholar]
- 56.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–54. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 57.Irving EA, Yatsushiro K, McCulloch J, Dewar D. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: involvement of free radicals. J Cereb Blood Flow Metab. 1997;17:612–22. doi: 10.1097/00004647-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Lin S, Rhodes PG, Lei M, Zhang F, Cai Z. alpha-Phenyl-n-tert-butyl-nitrone attenuates hypoxic–ischemic white matter injury in the neonatal rat brain. Brain Res. 2004;1007:132–41. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 59.Ueno Y, Zhang N, Miyamoto N. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–27. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 60.Barnett AH, Bradbury AW, Brittenden J, et al. The role of cilostazol in the treatment of intermittent claudication. Curr Med Res Opin. 2004;20:1661–70. doi: 10.1185/030079904X4464. [DOI] [PubMed] [Google Scholar]
- 61.Miyamoto N, Tanaka R, Shimura H, et al. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30:299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–21. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 63.Bhat MA. Molecular organization of axoglial junctions. Curr Opin Neurobiol. 2003;13:552–9. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 65.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–5. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corley SM, Ladiwala U, Besson A, Yong VW. Astrocytes attenuate oligodendrocyte death in vitro through an alpha(6) integrin-laminin-dependent mechanism. Glia. 2001;36:281–94. doi: 10.1002/glia.1116. [DOI] [PubMed] [Google Scholar]
- 67.Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J Neurosci Res. 2010;88:758–63. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders – time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–80. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 71.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 72.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–18. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 73.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–9. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 75.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–18. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 76.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 78.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 79.Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 80.Thored P, Heldmann U, Gomes-Leal W, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 81.Rochefort N, Quenech’du N, Watroba L, Mallat M, Giaume C, Milleret C. Microglia and astrocytes may participate in the shaping of visual callosal projections during postnatal development. J Physiol Paris. 2002;96:183–92. doi: 10.1016/s0928-4257(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 82.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 83.Caldero J, Brunet N, Ciutat D, Hereu M, Esquerda JE. Development of microglia in the chick embryo spinal cord: implications in the regulation of motoneuronal survival and death. J Neurosci Res. 2009;87:2447–66. doi: 10.1002/jnr.22084. [DOI] [PubMed] [Google Scholar]
- 84.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–8. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 85.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–53. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 87.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 88.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–62. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 89.Green SP, Cairns B, Rae J, et al. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–84. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Barone FC, Arvin B, White RF, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 91.Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119–35. doi: 10.1038/sj.jcbfm.9600014. [DOI] [PubMed] [Google Scholar]
- 92.Minami M, Kuraishi Y, Yabuuchi K, Yamazaki A, Satoh M. Induction of interleukin-1 beta mRNA in rat brain after transient forebrain ischemia. J Neurochem. 1992;58:390–2. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 93.Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. 1997;100:2648–52. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.del Zoppo GJ, Milner R, Mabuchi T, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–51. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 95.Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 97.Geissmann F, Auffray C, Palframan R, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 98.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 99.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 100.Narantuya D, Nagai A, Sheikh AM, et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merson TD, Binder MD, Kilpatrick TJ. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular Med. 2010;12:99–132. doi: 10.1007/s12017-010-8112-z. [DOI] [PubMed] [Google Scholar]
- 102.Kiefer R, Streit WJ, Toyka KV, Kreutzberg GW, Hartung HP. Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. Int J Dev Neurosci. 1995;13:331–9. doi: 10.1016/0736-5748(94)00074-d. [DOI] [PubMed] [Google Scholar]
- 103.Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–9. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 104.Harry GJ, McPherson CA, Wine RN, Atkinson K, Lefebvre d’Hellencourt C. Trimethyltin-induced neurogenesis in the murine hippocampus. Neurotox Res. 2004;5:623–7. doi: 10.1007/BF03033182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 106.Walton NM, Sutter BM, Laywell ED, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 107.Imai F, Suzuki H, Oda J, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab. 2007;27:488–500. doi: 10.1038/sj.jcbfm.9600362. [DOI] [PubMed] [Google Scholar]
- 108.Kitamura Y, Takata K, Inden M, et al. Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci. 2004;94:203–6. doi: 10.1254/jphs.94.203. [DOI] [PubMed] [Google Scholar]
- 109.Pettigrew LC, Kindy MS, Scheff S, et al. Focal cerebral ischemia in the TNFalpha-transgenic rat. J Neuroinflammation. 2008;5:47. doi: 10.1186/1742-2094-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–30. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–7. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Investigator EAST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–34. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 113.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6·5) in acute stroke. Curr Med Res Opin. 2002;18 (Suppl 2):s18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- 114.Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–9. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- 115.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16:1205–9. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]