Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial (original) (raw)
. Author manuscript; available in PMC: 2014 May 22.
Summary
Background
No available treatments slow or halt progression of multiple system atrophy, which is a rare, progressive, fatal neurological disorder. In a mouse model of multiple system atrophy, rifampicin inhibited formation of α-synuclein fibrils, the neuropathological hallmark of the disease. We aimed to assess the safety and efficacy of rifampicin in patients with multiple system atrophy.
Methods
In this randomised, double-blind, placebo-controlled trial we recruited participants aged 30–80 years with possible or probable multiple system atrophy from ten US medical centres. Eligible participants were randomly assigned (1:1) via computer-generated permuted block randomisation to rifampicin 300 mg twice daily or matching placebo (50 mg riboflavin capsules), stratified by subtype (parkinsonian vs cerebellar), with a block size of four. The primary outcome was rate of change (slope analysis) from baseline to 12 months in Unified Multiple System Atrophy Rating Scale (UMSARS) I score, analysed in all participants with at least one post-baseline measurement. This study is registered with ClinicalTrials.gov, number NCT01287221.
Findings
Between April 22, 2011, and April 19, 2012, we randomly assigned 100 participants (50 to rifampicin and 50 to placebo). Four participants in the rifampicin group and five in the placebo group withdrew from study prematurely. Results of the preplanned interim analysis (n=15 in each group) of the primary endpoint showed that futility criteria had been met, and the trial was stopped (the mean rate of change [slope analysis] of UMSARS I score was 0·62 points [SD 0·85] per month in the rifampicin group vs 0·47 points [0·48] per month in the placebo group; futility p=0·032; efficacy p=0·76). At the time of study termination, 49 participants in the rifampicin group and 50 in the placebo group had follow-up data and were included in the final analysis. The primary endpoint was 0·5 points (SD 0·7) per month for rifampicin and 0·5 points (0·5) per month for placebo (difference 0·0, 95% CI –0·24 to 0·24; p=0·82). Three (6%) of 50 participants in the rifampicin group and 12 (24%) of 50 in the placebo group had one or more serious adverse events; none was thought to be related to treatment.
Interpretation
Our results show that rifampicin does not slow or halt progression of multiple system atrophy. Despite the negative result, the trial does provide information that could be useful in the design of future studies assessing potential disease modifying therapies in patients with multiple system atrophy.
Funding
National Institutes of Health, Mayo Clinic Center for Translational Science Activities, and Mayo Funds.
Introduction
Multiple system atrophy is a sporadic multisystem progressive disorder characterised by autonomic failure with orthostatic hypotension, neurogenic bladder and erectile dysfunction, cerebellar ataxia, corticospinal dysfunction, and parkinsonism that might be poorly responsive to levodopa.1 Neuropathologically, multiple system atrophy is characterised by glial cytoplasmic inclusions of abnormally aggregated α-synuclein.2 Although glial cytoplasmic inclusions are the primary neuropathological hallmark of the disease, neuronal cytoplasmic and nuclear inclusions of α-synuclein have also been reported.2–5
Treatments for patients with multiple system atrophy are aimed at symptom control, since no treatments are available that halt or reverse disease progression. Many lines of evidence emphasise the pathological importance of α-synuclein aggregation.6,7 Disease progression might be causally linked to accumulation and aggregation of α-synuclein, hence much effort has gone into devising strategies to combat these processes.6 Much of the work has focused on the neuronal α-synuclein aggregation, characteristic of Parkinson’s disease, rather than on oligodendroglial aggregation seen in multiple system atrophy.7 Research has been advanced by the development of a transgenic mouse model expressing human α-synuclein under control of the myelin basic protein promoter; these transgenic mice develop oligodendroglial aggregates of α-synuclein and motor deficits characteristic of multiple system atrophy.8
Several agents have shown promise in combating α-synuclein aggregation in these transgenic mice.7 The antibiotic rifampicin inhibits the formation of α-synuclein fibrils and disaggregates fibrils already formed,9 which makes it of particular interest. These findings led to the hypothesis that this drug might delay progression or reverse neurological and autonomic dysfunction in multiple system atrophy.10 It has also been proposed as a potential disease-modifying agent for parkinsonism.9,11,12
As a result of these findings and anecdotal reports of potential efficacy in human beings,13 we aimed to compare the safety and efficacy of rifampicin with that of placebo in patients with multiple system atrophy.
Methods
Study design and participants
We undertook a randomised, double-blind, placebo-controlled, 12 month, safety and efficacy study of patients with multiple system atrophy of the parkinsonian or cerebellar type. Participants were recruited from ten US medical centres: Mayo Clinic (Rochester, MN, and Jacksonville, FL); University of Michigan (Ann Arbor, MI); Vanderbilt University (Nashville, TN); New York University Medical Center (New York, NY); University of California, Los Angeles (Los Angeles, CA); University of Texas Southwestern Medical Center (Dallas, TX); Beth Israel Deaconess Medical Center (Boston, MA); University of South Florida (Tampa, FL); and University of California, San Diego (La Jolla, CA).
Participants were eligible if they had possible or probable multiple system atrophy,1 were aged 30–80 years, were diagnosed less than 4 years before baseline, had expected survival of at least 3 years, were able to give informed consent, and achieved a Mini-Mental State Examination score of 24 points or higher. Exclusion criteria were: modified Unified Multiple System Atrophy Rating Scale (UMSARS) I score14 of 17 or greater; use of investigational drugs within the 60 days before baseline; treatment with tetrabenazine, rasagiline, or selegiline within the 3 months before baseline; abnormal liver function tests or porphyrias; any medical condition that would interfere with study activities or outcome measures; treatment with neuroleptics within the 6 months before baseline; use of medications known to have significant interactions with rifampicin; and pregnancy, lactation, or not using an acceptable method of birth control (applied to women only).
Study activities were approved by institutional review boards at each participating site and written informed consent was obtained from all participants at enrolment. The study had an independent data and safety monitoring board appointed by the National Institute of Neurological Disorders and Stroke (NINDS) to review study progress and monitor data quality, participant safety, and trial integrity. Additionally, the study chair (WRG) appointed an NINDS-approved independent medical monitor, masked to treatment allocation to review serious adverse events in real time.
Randomisation and masking
Eligible participants were randomly assigned to either rifampicin or placebo in a 1:1 ratio according to a computer-generated permuted block randomisation scheme stratified by multiple system atrophy subtype (parkinsonian vs cerebellar), with a block size of four. At randomisation, participants were assigned a drug kit number that determined treatment, and were masked to treatment assignment. All clinical care providers who might have been involved in the decision to enrol a patient in the study or the assessment of the treatment outcome were also masked to the assigned treatment. Blinding was achieved by using riboflavin capsules as placebo, which produce a similar yellow-orange urine discoloration to that produced by rifampicin.15 Capsules of rifampicin and placebo were of an identical appearance and were tasteless.
Procedures
Participants assigned to rifampicin (Investigational New Drug application 107301) received 300 mg twice daily, and those assigned to placebo received 50 mg riboflavin capsules twice daily. Compliance was monitored by pill counting.
Participants were assessed according to UMSARS I, a functional score of symptoms and ability to undertake activities of daily living usually consisting of 12 questions (we omitted question 11 [sexual function] because the question was poorly designed for women).14 Each question was scored from 0 to 4, with a higher score indicating a lower functional status. We also assessed participants with UMSARS II (neurological examination consisting of 14 questions scored from 0 to 4),14 the Composite Autonomic Symptoms Scale (COMPASS)-select (a measure of autonomic symptoms and autonomic functional status consisting of 46 questions, leading to a total score between 0 and 125, with a higher score indicating greater impairment),16 and COMPASS-select-change (done at 6 and 12 months only), in which participants scored how much their autonomic symptoms had changed. Assessments, including safety tests, took place at baseline (at the study facility), 3 months (by telephone interview), 6 months (study facility), 9 months (telephone interview), and 12 months (study facility). Participants were also contacted by telephone 2 weeks after commencement of the drug. Home visits were permitted for post-baseline assessments at the discretion of the investigator if the participant was not able to come to the site (although no home visits actually took place). Safety assessments were done on four additional occasions, and included blood tests (local laboratory) and information obtained via telephone interviews; further tests were done if abnormalities were detected.
We placed great emphasis on reducing variability in outcome measurement with a training session, built upon previous experience.17 This training included instructions for investigators on how each question should be answered, with examples, and a decision about whether the investigator and participant should have access to scores from the previous assessment (they routinely did, so that the assessment was comparative). A particular emphasis was placed on avoiding over-reporting of minor changes. These instructions were reinforced with subsequent reminders and supervision by a management team.
Outcomes
The primary efficacy endpoint was the rate of change from baseline to 12 months (using slope analysis) in total UMSARS I score. Secondary endpoints were: change from baseline to 12 months in UMSARS I score, UMSARS II score, total UMSARS score (UMSARS I+UMSARS II), and COMPASS-select score; rate of change (slope analysis) from baseline to 12 months in total UMSARS score (UMSARS I+UMSARS II); and COMPASS-select-change score at 12 months. Other preplanned disability endpoints were whether or not a participant achieved a score of 3 points or more on question 1 (speech impairment), question 2 (swallowing impairment), and question 8 (falling) of UMSARS I; however, we did not analyse these disability endpoints following the results of the futility analysis.
Statistical analysis
Preliminary slope estimates of UMSARS I were available from a study by Lipp and colleagues16 for 38 participants with probable multiple system atrophy who completed at least one follow-up assessment. The mean rate of increase in UMSARS I score was 0·375 points per month. The SD of the slope estimates was 0·633. However, slope estimates tend to be much steeper for patients in the early stages of the disease (who typically have possible multiple system atrophy) than for those at a later stage.14,18 Geser and colleagues18 reported that the rate of change in UMSARS I score in untreated patients with possible multiple system atrophy is about +0·66 points per month. We selected patients with probable and possible multiple system atrophy who had an UMSARS I score of 17 or less, ensuring that they were at an early stage of disease and so would probably have a steeper slope. In our power calculations, we assumed that the within-group SD of the slope estimate would be 0·559 and that the average rate of increase in UMSARS I score in the placebo group would be 0·66 points per month. Under these assumptions, with 46 participants per group, we had 80% power to detect a reduction in rate of progression in the rifampicin group of 50% compared with that in the placebo group, with α=0·05. Since our primary outcome of interest was rate of change, only participants with at least one post-baseline measurement were assessable (ie, those with only baseline but no follow-up measurement were excluded from the analysis).
We report descriptive summaries as mean and SD for continuous variables, and frequencies and percentages for categorical variables. Each participant’s UMSARS I score was regressed against time (months) to estimate a participant-specific rate of increase in points per month. We used the resulting slope parameter estimate as the response feature19 for each participant to account for repeated measurements, and this slope parameter estimate was the primary endpoint. We did not do imputations for slope values for participants with no available post-baseline measurement. Since we did not identify any imbalances between the two groups at baseline, we used the Wilcoxon rank-sum test for the primary analysis of slope as an endpoint. We analysed secondary endpoints in a similar way, with either slope estimates or change from baseline to 12 months for outcomes of interest listed as outcome measures. All tests were two sided, and we regarded p values of less than 0·05 as statistically significant. We did all analyses using SAS software version 9.2.
An interim analysis was planned after the first 30 participants had completed the 12 month treatment period. The primary null hypothesis for the futility analysis was that rifampicin reduces the rate of progression by at least 50% compared with placebo. We tested this hypothesis against the futility alternative hypothesis that rifampicin reduces the rate of progression by less than 50% compared with placebo. A p value of less than 0·10 in the interim analysis was chosen a priori for rejection of this null hypothesis. With a p value of greater than 0·10, we would not reject the null hypothesis, and we would conclude that evidence of futility was insufficient, and proceed with the trial.20
This study is registered with ClinicalTrials.gov, number NCT01287221.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and the authors share the final responsibility for the decision to submit for publication.
Results
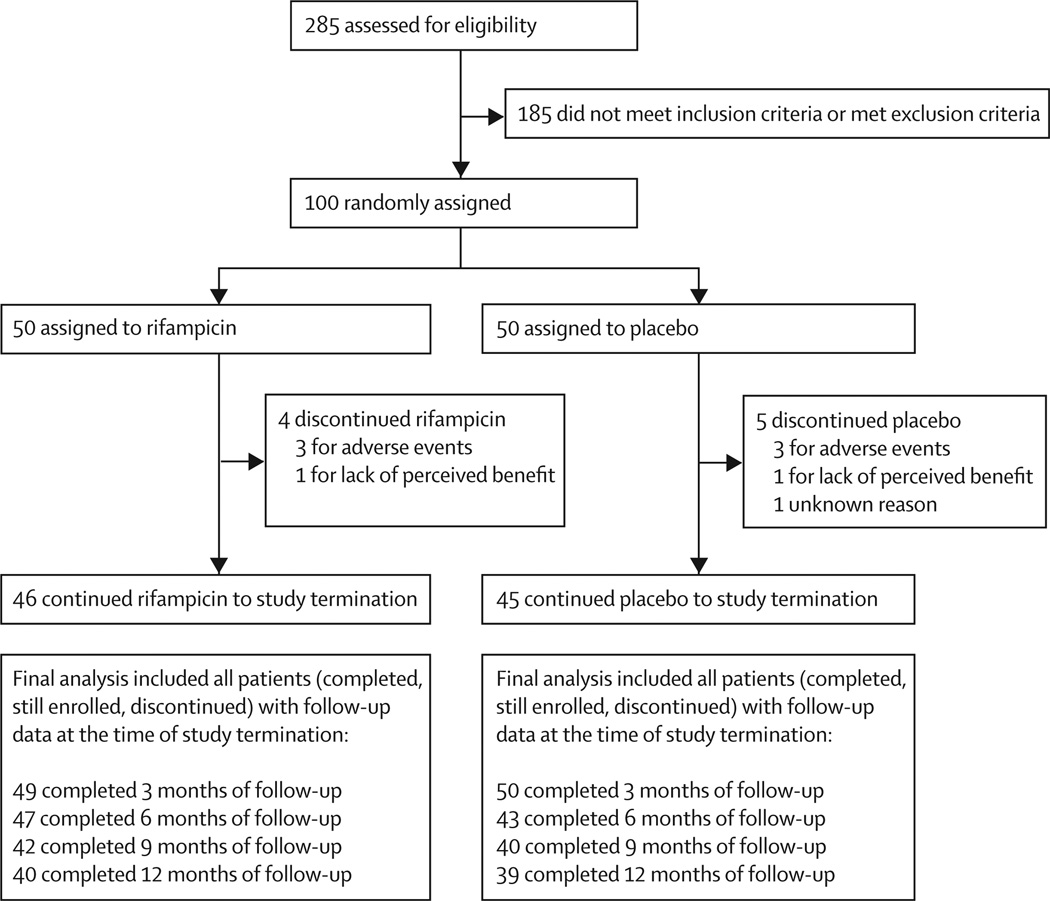
The first participant was screened and regarded as eligible on April 22, 2011, and was randomly assigned to a treatment group on April 25, 2011. Target enrolment was completed a year ahead of schedule, with the last participant starting treatment on April 19, 2012. The database was locked on March 19, 2013. Of 100 eligible and randomly assigned participants, 50 were assigned to placebo and 50 to rifampicin. By the final analysis, nine participants had left the study early (four in the rifampicin group and five in the placebo group; figure 1). The study population was balanced by age, race, phenotype (parkinsonian vs cerebellar), and in certitude of diagnosis (possible vs probable; table 1).
Figure 1. Trial profile.
Table 1.
Demographic and clinical characteristics at baselin
| Rifampicin(n=50) | Placebo(n=50) | |
|---|---|---|
| Age | 60·9 (7·8) | 61·1 (9·2) |
| Sex | ||
| Women | 23 (46%) | 16 (32%) |
| Men | 27 (44%) | 34 (68%) |
| Race | ||
| White | 46 (96%) | 44 (92%) |
| Non-white | 2 (4%) | 4 (8%) |
| Unknown or not reported | 2 (4%) | 2 (4%) |
| Multiple system atrophy type | ||
| Parkinsonian | 19 (38%) | 22 (44%) |
| Cerebellar | 31 (62%) | 28 (56%) |
| Certitude of diagnosis | ||
| Probable | 31 (62%) | 34 (68%) |
| Possible | 19 (38%) | 16 (32%) |
| UMSARS I score* | 13·1 (3·8) | 12·1 (3·4) |
| UMSARS II score | 16·6 (4·6) | 15·2 (4·8) |
| UMSARS IV score | 2·3 (0·8) | 2·0 (0·8) |
| COMPASS-select score | 32·9 (21·9) | 34·5 (18·5) |
At the interim analysis on Oct 18, 2012, with 15 participants per group, the prespecified futility criteria were met and the trial was stopped. The mean rate of change (slope) of UMSARS I was 0·62 points (SD 0·85) per month in the rifampicin group and 0·47 points (0·48) per month in the placebo group (futility p=0·032; efficacy p=0·76). The rapid rate of participant accrual resulted in complete recruitment by the time the decision to stop the study was made, and exploratory analyses of primary and secondary endpoints were feasible: 79 participants had completed 12 months, 82 participants had completed 9 months, 90 participants had completed 6 months, and 99 participants had completed 3 months (figure 1).
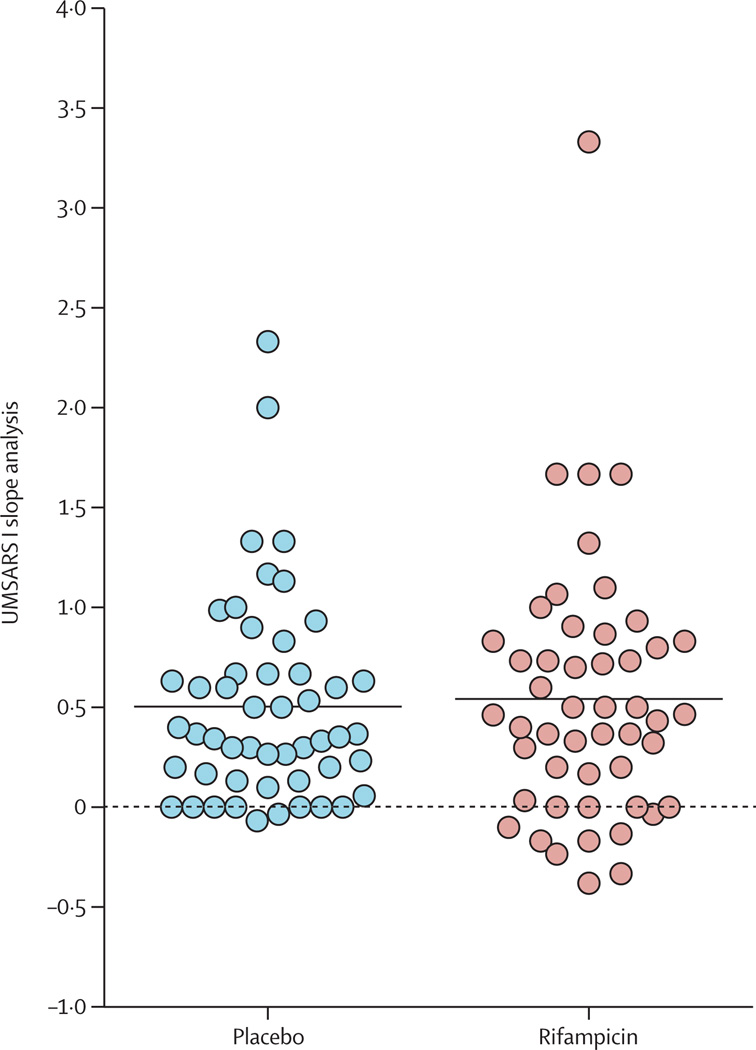
Analysis of the entire study cohort was undertaken and the results are summarised in table 2. The primary endpoint did not differ significantly between treatment groups (mean rate of change from baseline to study completion in UMSARS I score was 0·5 points [SD 0·7] per month in the rifampicin group and 0·5 points [0·5] per month in the placebo group; difference 0·0 [95% CI–0·24 to 0·24]; p=0·82; table 2). A scatterplot of the primary endpoint is shown in figure 2.
Table 2.
Summary of results for primary and secondary endpoint
| Rifampicin | Placebo | Difference (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| UMSARS I score, rate of change from baseline to termination (slope analysis)* | 49 | 0·5 (0·7) | 50 | 0·5 (0·5) | 0·0 (–0·24 to 0·24) | 0·82 |
| UMSARS I score, absolute change from baseline to completion | 39 | 6·2 (5·6) | 39 | 5·6 (5·0) | 0·6 (–1·76 to 2·96) | 0·62 |
| UMSARS II score, absolute change from baseline to completion | 36 | 7·0 (6·3) | 36 | 5·4 (6·6) | 1·6 (–1·38 to 4·58) | 0·23 |
| Total UMSARS score†, absolute change from baseline to completion | 36 | 12·9 (10·6) | 36 | 10·8 (10·7) | –2·2 (–7·2 to 2·9) | 0·31 |
| Total UMSARS score†, rate of change from baseline to termination (slope analysis) | 45 | 1·1 (1·0) | 43 | 1·2 (1·2) | –0·01 (–0·47 to 0·45) | 0·65 |
| COMPASS-select score‡, change from baseline to completion | 39 | 6·9 (16·5) | 38 | 4·6 (13·4) | 2·3 (–4·41 to 9·01) | 0·61 |
| COMPASS-select-change§, baseline to completion | 39 | 41·4 (33·8) | 38 | 32·1 (35·7) | 9·3 (–6·24 to 24·84) | 0·22 |
Figure 2. UMSARS I slope analysis, by treatment group.
Dots represent rate of change in UMSARS I score from baseline to termination in each individual patient. The horizontal black line through each cluster shows mean rate of change in each group. UMSARS=Unified Multiple System Atrophy Rating Scale.
Confining this analysis to the subgroup of participants who completed 12 months of follow-up (40 participants in the rifampicin group and 39 in the placebo group) resulted in similar findings (mean rate of change from baseline to study completion in UMSARS I score was 0·5 points [0·49] per month for rifampicin and 0·4 points [SD 0·42] per month for placebo; p=0·67). UMSARS II score (neurological deficits), total UMSARS score (combined symptoms and deficits), COMPASS-select score (autonomic symptoms and function), and COMPASS-select-change score were not improved with rifampicin (table 2).
223 adverse events were reported (table 3), with 18 regarded as serious (three serious adverse events occurred in three [6%] of 50 participants in the rifampicin group and 15 serious adverse events occurred in 12 [24%] of 50 participants in the placebo group). These 18 serious adverse events were ascertained by the site principal investigator and confirmed by the independent medical monitor as definitely or probably not related to the study drug.
Discussion
Our study, which is, to the best of our knowledge, the first double-blind, placebo-controlled treatment trial of rifampicin for multiple system atrophy, was stopped after a preplanned interim analysis of the primary endpoint revealed that futility criteria had been met; however, the successful recruitment of 100 participants within 12 months meant that exploratory analyses of primary and secondary endpoints were feasible. Our results clearly showed that rifampicin does not slow or halt progression of multiple system atrophy.
We chose a dose of rifampicin roughly equivalent to those used in animal studies (eg, 25 mg/kg administered intraperitoneally).10 Our selected dose (300 mg twice daily) was within the range that is used clinically to treat tuberculosis and leprosy,21 and is known to be well tolerated in human beings and to cross the blood–brain and blood–CSF barriers to exert its full effect.21–23 Blinding in a trial assessing rifampicin presents a challenge because the drug causes a yellow-orange discoloration of tears and urine.24 We therefore chose riboflavin as placebo because it causes a dark yellow discoloration of secretions. Toxicity is also a concern with rifampicin; however, no participants left the study early because of treatment-related adverse events or toxicity.
Although the finding of futility is disappointing, our study has provided new insights and filled some knowledge gaps. The results suggest a substantial gap between experimental multiple system atrophy studies and their clinical translation. Experimental studies have provided important insights into the sequence of changes from α-synuclein monomer to toxic oligomers and their aggregation, and beyond that, the roles of microglial activation and the inflammatory response.25–28 One distinct possibility for the absence of efficacy is that drugs such as rifampicin might be relatively ineffective in advanced disease. Strategies to overcome this possibility might include the identification and inclusion of patients at an even earlier stage of multiple system atrophy and the adoption of therapeutic approaches that block the pathogenetic pathway at several mechanistic sites.25,27,28
We note a major discrepancy between our clear negative results and the findings from an open-label study showing that rifampicin delays progression of multiple system atrophy.13 This discrepancy might be due to substantial limitations in the design of open-label studies, such as the absence of a control group and a lack of comprehensive assessments of neurological and autonomic symptoms and deficits. Most importantly, this discrepancy emphasises the need to control for observer bias and possible placebo response, which are limitations inherent in open-label studies.
Previous double-blind, placebo-controlled trials targeting progression of multiple system atrophy with lithium,29 rasagiline,30 mesenchymal stem cells,31 minocycline,32 riluzole,33 and growth hormone34 have been largely unsuccessful (panel). Mesenchymal stem cells were reported to delay progression of multiple system atrophy significantly in a single-centre study of 33 patients with cerebellar multiple system atrophy,31 but this study required intracarotid and intravertebral infusions, and cerebral ischaemia was a potential safety issue.40 Two studies were terminated before completion, one for toxicity (lithium)29 and the other for ineffectiveness (riluzole),33 and the others were inconclusive (growth hormone)34 or negative (minocycline, rasagiline).30,32 A randomised open-label study of oestrogen in combination with buspirone39 was also negative.
Substantial uncertainty has surrounded the number of participants needed to power a treatment trial of multiple system atrophy. The best estimate for US studies was based on a prospective study of 67 patients with probable multiple system atrophy.41 UMSARS II as the primary endpoint required a sample of 400 patients to detect a 50% reduction in slope, and use of UMSARS I was estimated to require an even greater sample size.41 However, slope estimates in probable multiple system atrophy, which comprises late-stage disease, reach a plateau phase with a flat slope of 0·375 for UMSARS I.16 The slopes are much steeper in the milder cases of possible multiple system atrophy.18 As mentioned, Geser and colleagues18 reported that the increase in UMSARS I score in untreated patients with possible multiple system atrophy was about 0·66 points per month. In our study, mean rate of change in UMSARS I score in the placebo group was 0·5 points (SD 0·5) per month. Using these data and assuming an equal SD in the treatment group, we would have required 64 participants per group to detect a difference of 50% (ie, a slope of 0·5 points per month in the placebo group vs 0·25 points per month in the treatment group) with 80% power and an alpha level of 0·05 based on a two-sample t test. Required sample sizes for 40% and 30% reduction in slope would have been 100 participants per group and 176 participants per group, respectively.
These results support the assessment of interventions in patients with mild, early multiple system atrophy in future clinical trials, an approach that could be further strengthened by linking clinical phenotype with biomarkers. The results also provide an example of training investigators on how to score clinical scales. In a previous study, we showed that variability in scoring of neurological abnormalities in research studies is substantial42 and that this variability can be reduced by instructions and training on how scoring should be done.17
Another conclusion is the validation of the rare disease consortium infrastructure and multicentre design that we used. In multicentre studies, many centres, including international centres, recruit just one or two participants, which introduces excessive variability in acquired data. In our study, three sites together recruited 50% of the participants. We are optimally placed to undertake future studies in patients with multiple system atrophy, since we have identified key sites and have shown effective study implementation using our consortium infrastructure. Our study was also monitored inexpensively, using a combination of data audit, an error-trapping algorithm, and the use of a management team.
The main limitation of our study was the fact that it was terminated for futility before all participants had completed the planned 12 month follow-up, and therefore the preplanned efficacy endpoints could not be analysed for all participants. Owing to the rapid rate of recruitment, however, 90 of 100 participants had completed 6 months of follow-up when the study was terminated. Therefore, primary and secondary endpoints (defined as baseline to end of study) could be assessed and treatment effects could be analysed.
An additional limitation is that although MRI was routinely done, we had no prespecified imaging endpoint. Also, we included both patients with parkinsonian or cerebellar multiple system atrophy, which potentially reduced statistical power if the drug only worked for one subtype. Finally, the dose of rifampicin might have been too low; however, a higher dose would probably have been associated with higher toxicity.21 The lack of any suggestion of a beneficial effect on UMSARS score would argue against any efficacy of the drug.
In conclusion, our findings suggest that rifampicin does not provide any benefit to patients with multiple system atrophy in terms of halting or slowing disease progression. The trial does, however, provide important information regarding design and power estimations for future studies assessing potential disease-modifying therapies in patients with multiple system atrophy.
Table 3.
Adverse events by system, grade, and attributio
| Rifampicin (n=50) | Placebo (n=50) | p value* | |
|---|---|---|---|
| Adverse events by system | |||
| Gastrointestinal or hepatic | 23 (13 [26%]) | 18 (9 [18%]) | 0·33 |
| Genitourinary | 15 (12 [24%]) | 23 (16 [32%]) | 0·37 |
| Neurological | 12 (11 [22%]) | 14 (12 [24%]) | 0·81 |
| Cardiovascular | 9 (6 [12%]) | 9 (8 [16%]) | 0·56 |
| Respiratory | 7 (4 [8%]) | 7 (7 [14%]) | 0·34 |
| Musculoskeletal | 4 (3 [6%]) | 10 (7 [14%]) | 0·18 |
| Haematological | 6 (4 [8%]) | 6 (5 [10%]) | 1·00 |
| Dermatological | 2 (2 [4%]) | 6 (5 [10%]) | 0·44 |
| Endocrine | 2 (2 [4%]) | 3 (3 [6%]) | 1·00 |
| Constitutional or other | 22 (14 [28%]) | 25 (12 [24%]) | 0·65 |
| Adverse events by grade | |||
| 1 (mild) | 52 (22 [44%]) | 71 (21 [42%]) | 0·84 |
| 2 (moderate) | 38 (19 [38%]) | 32 (18 [36%]) | 0·84 |
| 3 (severe) | 10 (8 [16%]) | 10 (9 [18%]) | 0·79 |
| 4 (life-threatening or disabling) | 0 | 3 (3 [6%]) | 0·24 |
| 5 (fatal) | 2 (2 [4%]) | 5 (5 [10%]) | 0·44 |
| Adverse events by attribution to study treatment | |||
| Definitely not related | 23 (13 [26%]) | 39 (17 [34%]) | 0·38 |
| Probably not related | 28 (16 [32%]) | 48 (21 [42%]) | 0·30 |
| Possibly related | 20 (12 [24%]) | 21 (9 [18%]) | 0·46 |
| Probably related | 9 (6 [12%]) | 6 (4 [8%]) | 0·50 |
| Defi nitely related | 22 (11 [22%]) | 7 (6 [12%]) | 0·18 |
| Total | 102 (31 [62%]) | 121 (31 [62%]) | 1·00 |
Panel: Research in context.
Systematic review
We searched Medline for articles published in any language with the search terms “multiple system atrophy”, “olivopontocerebellar atrophies”, “Shy-Drager syndrome”, or “striatonigral degeneration” and “randomized” as a keyword, to identify publications on randomised trials of interventions in multiple system atrophy. Our last search was done on Jan 9, 2014. We found no placebo-controlled trials assessing rifampicin in patients with multiple system atrophy, but we did find five double-blind, placebo-controlled clinical trials assessing long-term effects of other interventions29,31–34 and one recent report (a double-blind, placebo-controlled study) of rasagiline in multiple system atrophy30 (abstract only). All were either inconclusive (growth hormone,34 primary endpoint of Unified Parkinson’s Disease Rating Scale, n=43), negative (minocycline,32 primary endpoint of Unified Multiple System Atrophy Rating Scale [UMSARS] II, n=60; rasagiline,30 UMSARS I+II, n=174), or terminated prematurely (riluzole,33 primary endpoint of survival, n=760; lithium,29 primary endpoint of adverse events, n=9), except for the study on mesenchymal stem cells31 (primary endpoint of UMSARS I and II, n=33). We did not include four small studies that aimed to assess short-term symptomatic effects (paroxetine,35 riluzole,36 amantadine,37 and occupational therapy38) and a randomised but open-label study (oestrogen in combination with buspirone39) in our review.
Interpretation
With the exception of mesenchymal stem cells, all randomised blinded treatment trials assessing long-term therapeutic benefit in multiple system atrophy have failed to show efficacy. Despite promising preclinical data, our trial of rifampicin has to be added to this list of negative studies. Mesenchymal stem cell treatment holds promise for the future, although passage across the blood–brain barrier and safety concerns with intra-arterial injections (reported cerebral ischaemia)31,40 are obstacles that need to be addressed in future studies.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH; NS 44233 Pathogenesis and Diagnosis of Multiple System Atrophy, U54 NS065736 Autonomic Rare Disease Clinical Consortium, K23NS075141), Mayo CTSA (UL1 TR000135), and Mayo Funds. The Autonomic Diseases Consortium is a part of the NIH Rare Diseases Clinical Research Network. Funding and programmatic support for this project has been provided by U54 NS065736 from the National Institute of Neurological Diseases and Stroke and the NIH Office of Rare Diseases Research. We also acknowledge the participants for their time, effort, and encouragement.
Footnotes
Contributors
PAL, DR, SG, HK, WS, IB, RF, JM, WDD, and WRG were responsible for the study concept and design, and PAL, DR, SG, HK, WS, IB, and RF obtained the funding. PAL, DR, SG, HK, WS, IB, RF, SP, RAH, WC, SL, and SV were responsible for the acquisition of data, and PAL, DR, SG, HK, WS, IB, RF, SP, RAH, WC, SL, SV, and WRG for the analysis and interpretation of data. JM and WDD were responsible for the statistical analysis. PAL, DR, SG, HK, WS, IB, RF, JM, and WDD drafted the manuscript and all authors contributed to the critical revision. PAL, DR, SG, HK, WS, IB, RF, TC, and WRG provided administrative, technical or material support, and PAL, DR, SG, HK, WS, IB, RF, TC, and WRG supervised the study.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Phillip A Low, Mayo Clinic, Rochester, MN, USA.
David Robertson, Vanderbilt University, Nashville, TN, USA.
Sid Gilman, University of Michigan, Ann Arbor, MI, USA.
Horacio Kaufmann, University Medical Center, New York, NY, USA.
Wolfgang Singer, Mayo Clinic, Rochester, MN, USA.
Italo Biaggioni, Vanderbilt University, Nashville, TN, USA.
Roy Freeman, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Susan Perlman, University of California, Los Angeles Medical Center, Los Angeles, CA, USA.
Robert A Hauser, University of South Florida, Tampa, FL, USA.
William Cheshire, Mayo Clinic, Jacksonville, FL, USA.
Stephanie Lessig, University of California, San Diego, La Jolla, CA, USA.
Steven Vernino, Texas Southwestern Medical Center, Dallas, TX, USA.
Jay Mandrekar, Mayo Clinic, Rochester, MN, USA.
William D Dupont, Vanderbilt University, Nashville, TN, USA.
Thomas Chelimsky, Medical College of Wisconsin, Milwaukee, WI, USA.
Wendy R Galpern, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Gilman S, Wenning GK, Low PA, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 3.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain. 2006;129:2688–2696. doi: 10.1093/brain/awl109. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 6.Windisch M, Wolf HJ, Hutter-Paier B, Wronski R. Is alpha-synuclein pathology a target for treatment of neurodegenerative disorders? Curr Alzheimer Res. 2007;4:556–561. doi: 10.2174/156720507783018343. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto M, Rubenstein E, Masliah E. Transgenic models of a-synuclein pathology: past, present and future. Ann N Y Acad Sci. 2003;991:171–188. [PubMed] [Google Scholar]
- 8.Shults CW, Rockenstein E, Crews L, et al. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Ubhi K, Rockenstein E, Mante M, et al. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. Neuroreport. 2008;19:1271–1276. doi: 10.1097/WNR.0b013e32830b3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradbury J. New hope for mechanism-based treatment of Parkinson’s disease. Drug Discovery Today. 2005;10:80–81. doi: 10.1016/S1359-6446(04)03340-9. [DOI] [PubMed] [Google Scholar]
- 12.Kapurniotu A. Targeting alpha-synuclein in Parkinson’s disease. Chem Biol. 2004;11:1476–1478. doi: 10.1016/j.chembiol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Ohyagi M, Ishikawa K, Ota K, Sato N, Ishibashi S, Mizusawa H. Efficacy of oral rifampicin in multiple system atrophy. Neurology. 2013;80(suppl 1) P04.159 (abstr). [Google Scholar]
- 14.Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 15.Unna K, Greslin JG. Studies on the toxicity and pharmacology of riboflavin. J Pharmacol Exp Ther. 1942;76:75–80. [Google Scholar]
- 16.Lipp A, Sandroni P, Ahlskog JE, et al. Prospective differentiation of multiple system atrophy from Parkinson’s disease, with and without autonomic failure. Arch Neurol. 2009;66:742–750. doi: 10.1001/archneurol.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck PJ, Overland CJ, Low PA, et al. “Unequivocally abnormal” vs “usual” signs and symptoms for proficient diagnosis of diabetic polyneuropathy: Cl vs N Phys trial. Arch Neurol. 2012;69:1609–1614. doi: 10.1001/archneurol.2012.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geser F, Wenning GK, Seppi K, et al. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG) Mov Disord. 2006;21:179–186. doi: 10.1002/mds.20678. [DOI] [PubMed] [Google Scholar]
- 19.Dupont WD. Statistical modeling for biomedical researchers. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 20.Levy G, Kaufmann P, Buchsbaum R, et al. A two-stage design for a phase II clinical trial of coenzyme Q10 in ALS. Neurology. 2006;66:660–663. doi: 10.1212/01.wnl.0000201182.60750.66. [DOI] [PubMed] [Google Scholar]
- 21.Mindermann T, Zimmerli W, Gratzl O. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob Agents Chemother. 1998;42:2626–2629. doi: 10.1128/aac.42.10.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mindermann T, Landolt H, Zimmerli W, Rajacic Z, Gratzl O. Penetration of rifampicin into the brain tissue and cerebral extracellular space of rats. J Antimicrob Chemother. 1993;31:731–737. doi: 10.1093/jac/31.5.731. [DOI] [PubMed] [Google Scholar]
- 23.Yulug B, Kilic U, Kilic E, Bahr M. Rifampicin attenuates brain damage in focal ischemia. Brain Res. 2004;996:76–80. doi: 10.1016/j.brainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Snider DE, Jr, Farer LS. Rifampin and red urine. JAMA. 1977;238:1628. [PubMed] [Google Scholar]
- 25.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ubhi K, Low P, Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34:581–590. doi: 10.1016/j.tins.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. α-synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanova N, Bücke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 29.Sacca F, Marsili A, Quarantelli M, et al. A randomized clinical trial of lithium in multiple system atrophy. J Neurol. 2013;260:458–461. doi: 10.1007/s00415-012-6655-7. [DOI] [PubMed] [Google Scholar]
- 30.Poewe W, Barone P, Gliadi N, et al. A randomized, placebo-controlled clinical trial to assess the effects of rasagiline in patients with multiple system atrophy of the parkinsonian subtype. Mov Disord. 2012;27:1182. [Google Scholar]
- 31.Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 32.Dodel R, Spottke A, Gerhard A, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial) Mov Disord. 2010;25:97–107. doi: 10.1002/mds.22732. [DOI] [PubMed] [Google Scholar]
- 33.Bensimon G, Ludolph A, Agid Y, et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132:156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmberg B, Johansson JO, Poewe W, et al. Safety and tolerability of growth hormone therapy in multiple system atrophy: a double-blind, placebo-controlled study. Mov Disord. 2007;22:1138–1144. doi: 10.1002/mds.21501. [DOI] [PubMed] [Google Scholar]
- 35.Friess E, Kuempfel T, Modell S, et al. Paroxetine treatment improves motor symptoms in patients with multiple system atrophy. Parkinsonism Relat Disord. 2006;12:432–437. doi: 10.1016/j.parkreldis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Seppi K, Peralta C, Diem-Zangerl A, et al. Placebo-controlled trial of riluzole in multiple system atrophy. Eur J Neurol. 2006;13:1146–1148. doi: 10.1111/j.1468-1331.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 37.Wenning GK for the Working Group on Atypical Parkinsonism of the Austrian Parkinson’s Society. Placebo-controlled trial of amantadine in multiple-system atrophy. Clin Neuropharmacol. 2005;28:225–227. doi: 10.1097/01.wnf.0000183240.47960.f0. [DOI] [PubMed] [Google Scholar]
- 38.Jain S, Dawson J, Quinn NP, Playford ED. Occupational therapy in multiple system atrophy: a pilot randomized controlled trial. Mov Disord. 2004;19:1360–1364. doi: 10.1002/mds.20211. [DOI] [PubMed] [Google Scholar]
- 39.Heo JH, Lee ST, Chu K, Kim M. The efficacy of combined estrogen and buspirone treatment in olivopontocerebellar atrophy. J Neurol Sci. 2008;271:87–90. doi: 10.1016/j.jns.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Low PA, Gilman S. Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, are they effective? Ann Neurol. 2012;72:4–5. doi: 10.1002/ana.23655. [DOI] [PubMed] [Google Scholar]
- 41.May S, Gilman S, Sowell BB, et al. Potential outcome measures and trial design issues for multiple system atrophy. Mov Disord. 2007;22:2371–2377. doi: 10.1002/mds.21734. [DOI] [PubMed] [Google Scholar]
- 42.Dyck PJ, Overland CJ, Low PA, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs NPhys trial. Muscle Nerve. 2010;42:157–164. doi: 10.1002/mus.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]