The Steroid Metabolome of Adrenarche (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 2.
Published in final edited form as: J Endocrinol. 2012 Jun 19;214(2):133–143. doi: 10.1530/JOE-12-0183
Abstract
Adrenarche is an endocrine developmental process whereby humans and select nonhuman primates increase adrenal output of a series of steroids, especially dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS). The timing of adrenarche varies between primates, but in humans, serum levels of DHEAS are seen to increase around 6 years of age. This phenomenon corresponds with the development and expansion of the zona reticularis (ZR) of the adrenal gland. The physiological phenomena that trigger the onset of adrenarche are still unknown; however the biochemical pathways leading to this event have been elucidated in detail. There are numerous reviews examining the process of adrenarche, most of which, have focused on the changes within the adrenal as well as the phenotypic results of adrenarche. This article reviews the recent and past studies that show the breadth of changes in the circulating steroid metabolome that occurs during the process of adrenarche.
1. Introduction
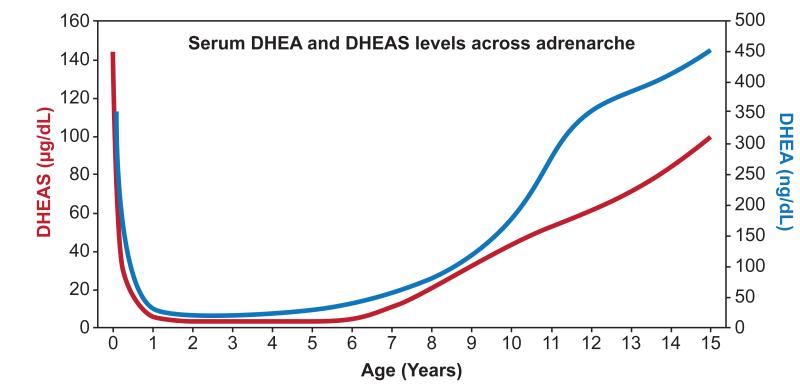
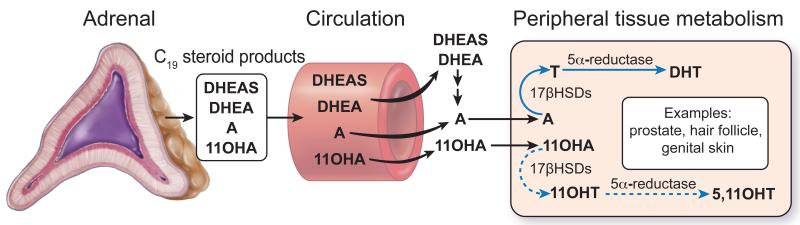
The human adrenal produces large quantities of the 19-carbon (C19) steroids DHEA and DHEAS during fetal development, but the production of these steroids falls rapidly after birth and remains low for the first few years of life. Adrenarche refers to the reemergence of adrenal C19 steroid production at around 6 years of age that results from the expansion and differentiation of the adrenal ZR (1-5) (Figure 1). Neither DHEA nor DHEAS are bioactive androgens, but they act as precursors for production of more potent androgens including testosterone in peripheral tissues that include hair follicles, genital skin and prostate (6-8) (Figure 2). The initiation of androgen-dependent growth of axillary and pubic hair (pubarche) is the phenotypic hallmark of adrenarche (4, 9, 10). The peripherally converted bioactive androgens also stimulate the development of apocrine glands in the skin to produce characteristic adult type body odor and can act on the sebaceous glands leading to initiate signs of acne (11, 12). There are numerous reviews examining the process of adrenarche, most of which, have focused on the adrenarche associated changes in adrenal steroidogenic enzymes and differentiation (1, 3-5, 9, 13). Herein, we have reviewed the recent and past studies that show the breadth of changes in the circulating steroid metabolome that occurs during the process of adrenarche.
Figure 1.
Serum DHEA and DHEAS levels before and after the onset of human adrenarche. Based on steroid levels from de Peretti and Forest 1976, 1978.
Figure 2.
Adrenal-derived C19 steroids act as precursors for the production of more potent androgens in peripheral tissues including hair follicles, genital skin and prostate. The classical pathway for bioactive androgen synthesis as well as a proposed alternative pathway using 11β-hydroxyandrostenedione (11OHA) is shown. A, androstenedione; T, testosterone; DHT, dihydrotestosterone; 11OHT, 11β-hydroxytestosterone; 5,11OHT, 5α,11β-hydroxytestosterone; 17βHSDs; 17β-hydroxysteroid dehydrogenases (type 5 or type 3)
2. C19 steroids and adrenal morphology
While most of our focus will be placed on the adrenarchal rise in adrenal production of C19 steroids, it should be noted that during development the fetal adrenal produces large amounts of DHEAS. During this period C19 steroids arise from a developmental specific fetal zone that comprises 80% of the fetal adrenal. Till birth, the fetal zone continues to secrete dramatic quantities of the C19 steroid DHEAS, which are utilized by the placenta for production of remarkably large amounts estrogens during pregnancy (14-16).
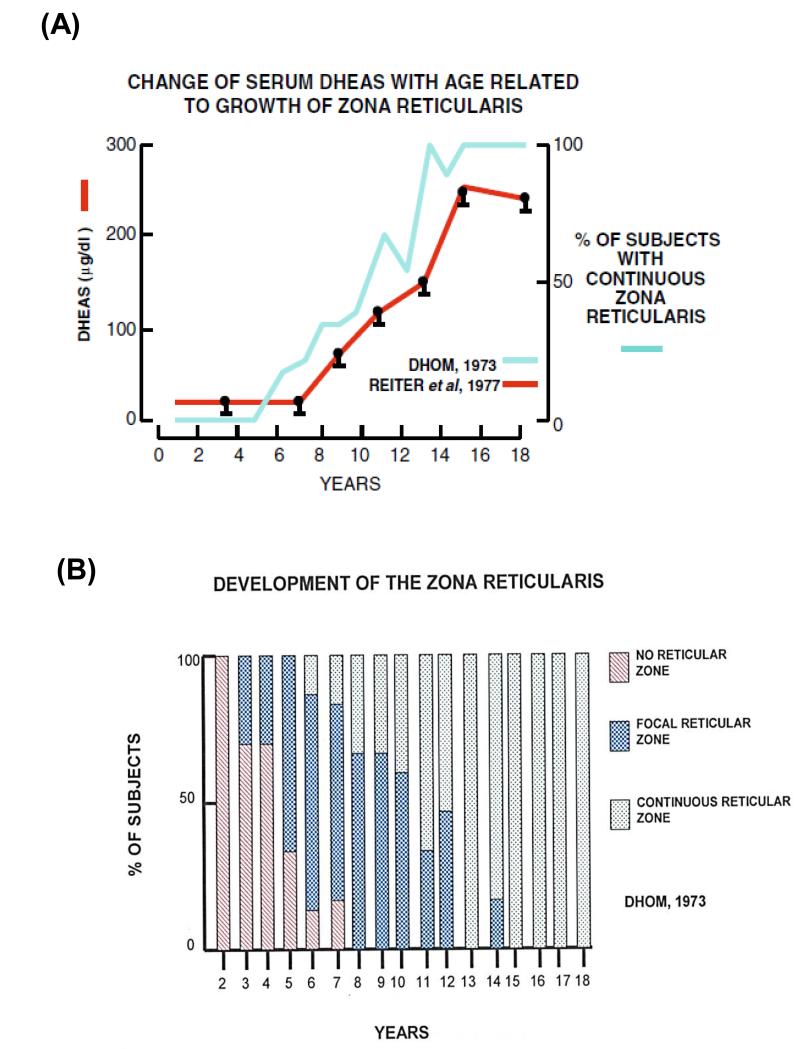
After birth there is a dramatic involution of the fetal zone, which accounts for the sharp decline in DHEAS synthesis in the first few months of life (Figure 1). The levels of DHEAS remain low until adrenarche commences as a result of the expansion of the ZR (17-19). This phenomenon was first described and given the name adrenarche by Albright and colleagues in1942 when they observed the growth of axillary and pubic hair in the absence of gonadal androgens due to congenital absence or malformation of ovaries (20). In 1973, Dhom characterized the morphological changes that occurred in the prepubertal and pubertal adrenal. According to this study, the adrenal cortex of the infant shows distinct ZG and ZF with little ZR (17). However, in the adrenals of children around age 3 years, focal islands of ZR appear, which expand at age 4-5 (17, 18). A continuous layer of ‘functional’ ZR is first seen at age 6, which continues to expand till age 12-13 (Dhom 1973; Hui, et al. 2009). The emergence of the developed ZR corresponds with the rise in circulating DHEAS (Dhom 1973). It has also been demonstrated that the thickness of the ZR is directly proportional to the production of DHEAS) (17, 21) (Figure 3).
Figure 3.
DHEAS and expansion of the adrenal reticularis. (A) The relationship between the development of the ZR and plasma DHEAS levels (Dhom 1973; Reiter et al. 1977). (B) The morphological development of the adrenal zona reticularis depicting the appearance of focal islands of reticular tissue and a continuous ‘functional’ ZR (17).
3. Steroidogenic enzymes and cofactors involved in C19 steroid production
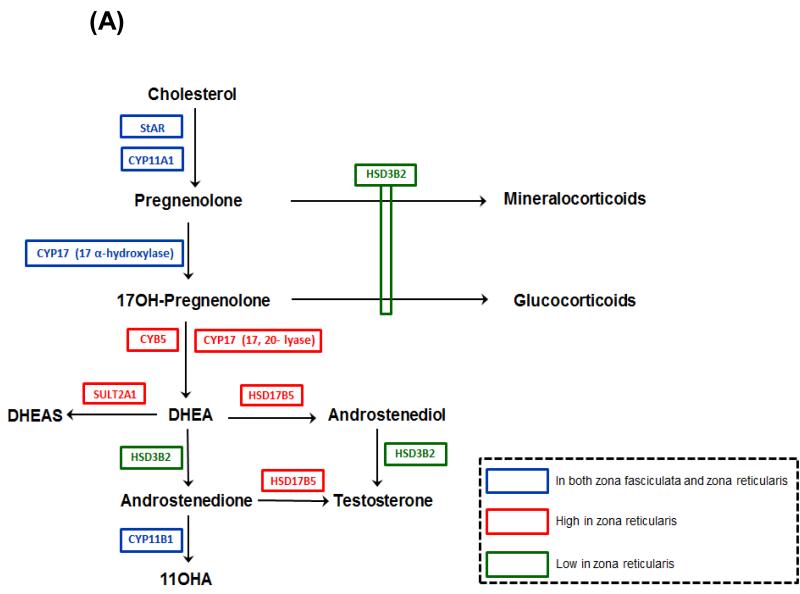
Although some steroidogenic enzymes and cofactor proteins are common to all zones of the cortex, the zone-specific production of steroids results in part due to differential expression of key steroidogenic enzymes. The pathway leading to the synthesis of DHEAS is quite simple and requires only three steroidogenic enzymes. However, across the period of adrenarche there are changes in the expression pattern of the steroidogenic enzymes and cofactors which facilitates C19 steroid production (Figure 4A). Immunohistochemistry studies have demonstrated the varied expression of key steroidogenic enzymes within the cortical zones (18, 19, 22, 23).
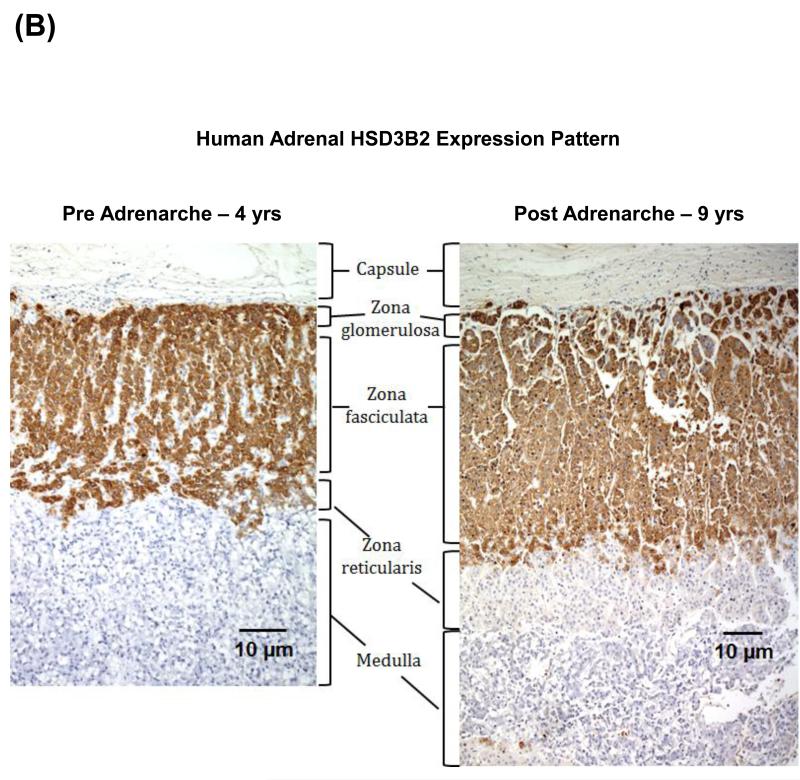
Figure 4.
(A) Steroid pathways for biosynthesis of adrenocortical steroids. StAR, steroidogenic acute regulatory protein; CYP11A1, cytochrome P450 cholesterol side-chain cleavage; CYP17,17α-hydroxylase/17,20-lyase; HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; CYB5, cytochrome b5; HSD17B5, 17β-hydroxysteroid dehydrogenase type 5; SULT2A1, steroid sulfotransferase type 2A1; CYP11B1, 11β-hydroxylase. (B) Immunohistochemical examination of HSD3B2 expression in the human adrenal cortex from a four year old child (pre adrenarche) and a nine year old child (post adrenarche). As observed in these micrographs the post adrenarche adrenal is characterized by the expansion of a HSD3B3 deficient ZR.
The initial conversion of cholesterol to pregnelonone is an essential step in all steroidogenic organs and is accomplished by the enzyme, cytochrome P450 cholesterol side-chain cleavage (CYP11A1). This enzyme is expressed in all zones of the adrenal and the expression pattern within the ZR does not change during adrenarche (13). CYP17, a single steroidogenic enzyme localized in the endoplasmic reticulum, is necessary for production of both cortisol and DHEA as it catalyzes two biosynthetic activities: 17α-hydroxylase and 17, 20-lyase (24-26). Thus, this enzyme is needed for both ZF and ZR function. However, Suzuki et al. reported changes in expression of CYP17 in the adrenal ZF and ZR of pre-adrenarche versus post-adrenarche children (19). Their semi-quantitative immunohisotchemical analysis showed that after age 5, the CYP17 protein appeared to increase in both zones and reached a plateau level after 13 years; suggesting that increased CYP17 could impact the capacity for DHEA synthesis.
The expression of the flavoprotein mediating the dual activity of CYP17, namely cytochrome P450 oxidoreductase (CPR) has also been studied across adrenarche and was found to increase in all the three adrenal zones, especially ZR (Suzuki, et al. 2000). The increased conversion of 17OHPreg to DHEA due to the elevated 17, 20-lyase activity of CYP17 (27) is central to understanding the mechanisms underlying adrenarche. The lyase activity of the enzyme is enhanced by the hemoprotein cytochrome b5 (CYB5) (25, 27-29). CYB5 expression was detected in the DHEAS-synthesizing fetal zone throughout gestation (29-31). Immunohistochemical studies indicated that CYB5 is weakly synthesized in all the adrenal zones of pre-adrenarchal children (Suzuki, et al. 2000). However, the CYB5 expression markedly rises in the ZR after age 5 years and plateaus at age 13 years, whereas the levels remain extremely low in the ZG and ZF at all ages (Suzuki, et al. 2000). A reduction in the proportion of the adrenal cortex that expresses CYB5 and DHEA levels was also detected with aging (32-34). These finding shed light on the significance of CYB5 in adrenarche.
The sulfoconjugation of steroids occurs extensively in the adrenal cortex and is primarily carried out by the enzyme SULT2A1, of the human hydroxysteroid sulfotransferase family (35-37). SULT2A1 has a broad substrate specificity, which includes DHEA, pregnenolone and 17OHPreg. Sulfonation of pregnenolone and 17OHPreg precludes metabolism by HSD3B2 and CYP17and thereby their utilization as precursors for the mineralocorticoid, glucocorticoid and androgen biosynthetic pathways. Also conversion of DHEA into its sulfate ester, i.e DHEAS prevents the transformation of the poor androgen DHEA into more potent androgens. Numerous studies have demonstrated that the transitional and fetal zones of the human fetal adrenal abundantly express SULT2A1 (38-40). Suzuki et al. carried out immunohistochemical studies in the postnatal period from infancy to old age, and established that SULT2A1 became highly discernible in the ZR at ages 5-13 years and reached a plateau thereafter; thus confirming it as marker for ZR development (19).
The sulfonation of steroids by SULT2A1 requires a sulfate donor, namely 3′-phosphoadenosine 5′-phosphosulfate (PAPS) (37, 41). In humans, PAPS synthesis requires two isoforms of the enzyme PAPS synthase, namely PAPSS1 and PAPSS2. Noordam et al. examined an 8-year-old girl with early pubic and axillary hair, and concluded that the cause of hyperandrogenism in this subject was a set of compound heterozygous mutations in PAPSS2 (42). Mutated PAPSS2 generated decreased amount of PAPS, which is a prerequisite for steroid sulfonation. This was consistent with impaired DHEA sulfonation; thereby making the unconjugated DHEA pool available for conversion into bioactive androgens like testosterone.
During adrenarche, as the ZR expands, this zone was found to have substantially lower levels of HSD3B2 compared to the adjacent ZF (Figure 4B). The relative lack of HSD3B2 expression/activity facilitates increased DHEAS synthesis because HSD3B2 competes with CYP17 for pregnenolone and 17OHPreg (31, 43). Thus HSD3B2 expression in the expanding ZR coincides with elevated adrenal DHEAS synthesis through the postnatal period to adult life is one of the driving forces of adrenarche (18, 19, 22, 23). The phenomenon of increased androgen production as a result of decreased HSD3B2 activity was also demonstrated by McCartin et al. when they reported the presence of premature adrenarche in an 11-year-old subject with a profound loss in HSD3B2 activity owing to a compound heterozygotic mutation in the HSD3B2 gene (44). However, it should be noted that there are cortical cells located at the border between ZF and ZR in normal adult adrenal glands that co-express HSD3B2 and CYB5, suggesting that these cells have the potential for direct production of androstenedione (45). Human ovarian theca cells also co-express HSD3B2, CYP17 and CYB5 and similarly have potential for androstenedione biosynthesis (29, 46). Nevertheless, these results await further investigations to clarify the regulatory mechanisms of co-expression of the two enzymes in this population of cortical cells.
4. Control of Adrenarche
The precise mechanisms that control adrenal androgen biosynthesis has not been clearly defined. Adrenocorticotropic hormone (ACTH) has been considered as accepted primary mediator of adrenarche for the past few decades (21, 47, 48). Clearly, dexamethasone suppression of adrenal androgens suggests a regulatory role for ACTH (49-51). Moreover children with an ACTH receptor defect fail to experience adrenarche (52) thereby supporting the postulate that ACTH plays an essential role in this phenomenon. However, several studies have demonstrated that ACTH and cortisol levels both remain constant even during adrenarche’s rise in adrenal androgens (3, 4, 53, 54). This is partly due to the tight regulatory feedback system between ACTH and cortisol, which keeps their levels within physiologic range throughout life (55, 56) as opposed to DHEA and other C19 steroids that have no clear feedback system. Thus, although ACTH affects both cortisol and adrenal androgen secretion there is a divergence in cortisol and androgen production at adrenarche. A related hypothesis was the proximal 18-amino acid hinge region (amino acids 79-96) of pro-opiomelanocortin (POMC) was a stimulator for adrenal androgen synthesis is not supported by in vitro studies (53, 54, 57). However, there have been numerous studies indicating that plasma levels of POMC-related peptides like β-lipotropin and β-endorphin correlated to the increasing levels of DHEAS during adrenarche (58-60). Several studies also indicated no positive correlation between other potential endocrine regulatory candidates including prolactin, insulin, insulin-like growth factor-I (IGF-I) with the DHEAS levels seen during adrenarche (61-64). Thus determination of the controlling factor of adrenarche still needs more investigation.
In 1980, Anderson postulated that adrenarche is triggered through an unknown mechanism involving the increased intra-adrenal levels of cortisol associated with the growth of the gland (65). Recent in vitro studies by Topor et al also established that cortisol inhibits HSD3B2 and stimulates the biosynthesis of DHEA at concentrations above 50 μM (66). Dickerman et al and Byrne et al confirmed that there were age-dependent increases in intra-adrenal concentrations of several C19 and C21 steroids and proposed that these concentrations were in the range of the Michaelis-Menten constant (Km) for HSD3B2 (67-69); might therefore inhibit the enzyme, and promote 17OHPreg metabolism to DHEA at adrenarche. However the same study demonstrated that 1μM cortisol did not inhibit HSD3B2 (68). Also, DHEAS levels appear to be high in adrenarchal children with untreated classic CAH (congenital adrenal hyperplasia) where intra-adrenal cortisol levels should be low (70). Thus the exact role for the modulation of HSD3B2 enzymatic activity by intra-adrenal steroid inhibitors remains unclear, while decreased expression of HSD3B2 in the post-adrenarche ZR appears clear.
Steroid metabolome in adrenarche
The difference in expression and activity of the various adrenal steroidogenic enzymes from infancy to adulthood suggest that there should also be age-related changes in serum concentrations of several Δ5- and Δ4-steroids that would underlie the physiologic processes of adrenarche.
5. Serum C21 steroids in adrenarche
As described earlier, it has been established that adrenarche is associated with the expansion of ZR having deficient expression of HSD3B2 along with increasing expression of CYP17. Also, 17OHProg and 17OHPreg are the key intermediates in cortisol and androgen metabolism, respectively, thereby making them the most analyzed C21 steroids in adrenarche. Abraham et al had demonstrated that the concentration of 17OHPreg was higher in neonates than preadolescents, adult males and nonpregnant women by radioimmunoassay (RIA) (71), presumably due to the low activity of HSD3B2 in neonatal adrenal cortex as compared to the adult cortex. Hughes et al had also observed age-related changes in serum concentrations of 17OHProg (72). However, a detailed age-dependent analysis of 17OHPreg and 17OHProg was described by Shimozawa et al in 1988 (73). They examined the levels of 17OHPreg and 17OHProg in 11 umbilical cord blood specimens and sera from 82 normal children of various ages and 20 normal adults by RIA (73). According to this study, the levels of these steroids are the highest in the cord blood owing to their metabolism by the feto-placental unit; and after birth they start declining to reach a nadir at 1-2 years age, followed by gradual increase from ages 3-6 years till adulthood. This is in agreement with the observation that CYP17 expression in the ZF and ZR increases during adrenarche (19).
The pattern of some of the unconjugated C21 steroids has been traced in humans from birth to adulthood. Toscano et al examined the levels of serum pregnenolone (50 ± 7 ng/dL), cortisol (13 ± 1.8 μg/dL) and 11-deoxycortisol (52 ± 3.6 ng/dL) in 20 children between ages 5.5 to 9 years (74). Several studies carried out in large cohorts of children between 2 to 12 years of age have established that serum cortisol levels do not increase in adrenarche (63, 75). Thus it is clear that the serum levels of most of the adrenal unconjugated C21 steroids are not grossly affected during the course of adrenarche.
The robust expression of the adrenal-related sulfotransferase enzyme, namely SULT2A1 (37), and its broad substrate specificity has also encouraged researchers to examine the sulfonated derivatives of C21 various steroids. De Peretti and Mappus traced the levels of pregnenolone sulfate (Preg-S) from birth to adulthood (76) and observed that Preg-S levels were high in cord plasma as a consequence of residual fetal zone activity. The levels started declining during the first year after birth and remained low thereafter. Also there was no detectable rise in Preg-S during the adrenarchal period as is seen for the classical markers of adrenarche, namely DHEA and DHEAS (76). Shimozawa et al demonstrated that 17α-hydroxypregnenolone sulfate (17OHPreg-S) exhibited an age-related change, wherein the 17OHPreg-S concentration was highest in cord blood and decreased to a minimum at 3-6 years, followed by a gradual increase from 7 year till adulthood as opposed to its non-sulfated form which showed a nadir at 1-2 years (73). These observations agree with what we now know regarding the adrenarchal expansion of the ZR which has high CYP17, SULT2A1 and low HSD3B2 expression. This would explain the ability of 17OHPreg-S to act as an additional steroid marker of adrenarche.
6. Serum C19 steroids in adrenarche
The weak C19 steroids DHEA and DHEAS are the characteristic markers of adrenarche (Figure 1). Extensive RIA studies tracing these steroids from birth to adolescence demonstrated that the plasma DHEA levels rapidly declined in the first two years of life, stayed low for the next few years and then dramatically increased after 6 years of age, corresponding to ‘adrenarche’ (10, 63, 76-79). DHEAS has been the most extensively studied steroid across adrenarche. A number of research groups traced the age-related changes in plasma DHEAS concentration and demonstrated that DHEAS decreased slowly during the first years of life, remained low till age 5 and then abruptly started to rise (51, 63, 78, 80, 81). Recent liquid chromatography-tandem mass spectrometry (LC-MS/MS) studies in a large cohort of children confirmed the same age-related pattern for serum DHEA levels (82). The patterns seen for both DHEA and DHEAS correlate well with intra-adrenal changes occurring during this period. Specifically, the high neonatal levels relate to the residual fetal zone that regresses over the first year leading to a nadir of DHEA/DHEAS and their rise is the circulation correlates well with the expansion of the reticularis.
The age-related patterns of secretion of several other C19 products have also been investigated but with varying results. While some immunoassay studies suggest no adrenarchal rise in androstenedione (63, 79), others show significant adrenarche-related increases in serum levels of androstenedione (Ducharme et al. 1976; Franckson et al. 1980; Likitmaskul et al. 1995; Tung et al. 2004). Recent LC-MS/MS analysis also showed a similar age-related rise in concentration of androstenedione (83). Parker et al did not find a relationship between serum 11β-hydroxyandrostenedione (11OHA) concentration and age (63); whereas Franckson et al found that serum 11OHA increased with bone age throughout prepubertal childhood (75). The major difference between the studies was data analysis. Because of the circadian association of 11OHA with cortisol, Franckson and colleagues utilized a 11OHA/ cortisol ratio to make steroid comparisons. Some immunoassay studies of testosterone in a large population of children established that this androgen did not rise demonstrably during the early stages of adrenarche (10, 79). Later studies using LC-MS/MS studies confirmed that the plasma levels of testosterone do not rise at adrenarche, but that its levels did rise as puberty approaches in boys (83). The exact role of direct adrenal secretion of testosterone remains unclear. Previous studies in adult women exhibiting signs of androgen excess demonstrated that the adrenal could secrete testosterone under pathologic conditions (84-86). In addition, the ZR expresses higher levels of the enzyme 17β-hydroxysteroid dehydrogenase type 5 (HSD17B5) than the adjacent ZF and its expression increases across adrenarche (18, 87). This enzyme has numerous activities, including the ability to convert androstenedione to testosterone (Figure 4A). Thus, while it is clear that DHEA/DHEAS represent the most consistent markers of adrenarche, the production of more potent androgens needs further study and the analysis of these androgens may require consideration of adrenal circadian secretion as well.
Studies carried out in the 1970’s and 80’s (88, 89) indicating that the conjugated androgen, androstane-3α,17β-diol glucuronide (Adiol-G) would be a good measure of testosterone transformation, led to further investigations that established the change in plasma steroid glucuronide levels at different ages (90, 91). Brochu et al determined the levels of androsterone glucuronide and Adiol-G across various age groups in males and concluded that, since these 5α-reduced conjugated steroids increase significantly before puberty, the adrenal C19 steroids like androstenedione and androstanedione must be getting converted into androsterone glucuronide, and potentially into testosterone which in turn is transformed into Adiol-G during prepubertal development (91). Their findings support the premise that androgenic manifestations like appearance of pubic and axillary hair are induced by the conversion of adrenal C19 steroids to active androgens that are further metabolized into their conjugated inactive products.
Urinary C19 steroids in adrenarche
Nathanson et al was one of the first groups of researchers to report the presence of androgen metabolites in the urine of a large cohort of children (92). The results of their study demonstrated that from ages 3-7 years, constant amounts of androgens are excreted; however at about 7 years, the rate of excretion of these steroids begins to progressively increase. Several studies were carried out to confirm the age-related changes in androgen excretion. It was also demonstrated that urinary total DHEA and DHEAS levels are elevated from ages 8 to 12 years (93-95). In 1991, the normal ranges for urinary steroid excretion were determined for the first time by gas chromatography and it was demonstrated that urinary steroid excretion rates in childhood positively correlate with growth and activity of the adrenal cortex (96). With the advent of better techniques and availability of commercial assays, Remer et al in 1994 demonstrated that urinary total 17-ketosteroid sulfate and DHEAS concentrations in 8 year old normal children is significantly higher as compared to preadrenarchal children (age 4 years) (97, 98). Extensive studies by gas chromatography-mass spectrometry (GC-MS) to determine the urinary markers of adrenarche were carried out in a large population of healthy children and adolescents between 3 to 18 years (99) which established that DHEA and its metabolites like 16α-hydroxy DHEA, 3β,16α,17α-androstenetriol and androstenediol show a continuous rise from ages 3-4 years to 17-18 years, thereby suggesting that adrenarche is a gradual process starting at an early age. The gradual nature of increasing steroids would appear to agree with Dhom’s histologic studies showing a gradual expansion of the adrenal ZR (Figure 3). Recent studies by Shi et al in 2009 also confirmed the use of the C19 steroids as urinary markers of adrenarche (36).
Future directions toward understanding the adrenarche steroid metabolome
In 2009, Hui et al, suggested that the process of adrenarche was associated with ZR increased expression of 17β-hydroxysteroid dehydrogenase type 5 (HSD17B5), an enzyme able to convert androstenedione to testosterone. They demonstrated the presence of HSD17B5 in the ZR of human adrenals around age 9, which tracks well with the onset of pubarche (18).
Testosterone derived from the testes as well as the adrenals is converted to a more potent androgen, 5α-dihydrotetosterone (DHT) by the enzyme 5α-reductases type I and II (SRD5A) in androgen target tissues like genital skin and prostate (100, 101). Recent studies suggest that there are likely several pathways leading to DHT biosynthesis (102-106). The peculiarity about these pathways is the synthesis of 5α-androstane-3α,17β-diol (androstanediol) involving 5α -pregnane-3α,17α -diol-20-one as a key intermediate. Androstanediol, in turn, acts as precursor for DHT; thereby bypassing the need for testosterone, which is the ‘classical’ precursor for DHT production. Recently, Flück et al confirmed that both the classical and alternative pathways of testicular androgen production are involved in the formation of normal human male sexual differentiation (103), whereas another group suggested that synthesis of DHT in castration-resistant prostate cancer requires 5α-androstenedione and not testosterone as an obligate precursor (100).
Two derivatives of testosterone, 11β-hydroxytestosterone (11OHT) and 11-ketotestosterone (11KT), may represent unique adrenal-derived androgens. While testosterone and DHT are the primary human androgens, 11OHT and 11KT are the major androgens found in a variety of fish (107-109). These C19 steroids are also produced in small quantities by mouse gonads (109). The physiologic role of these steroids in humans has not been studied but circulating levels of these androgens have been examined in human blood (110, 111). 11OHT could be speculated to be an adrenal-derived androgen since it requires the activity of 11β-hydroxylation via the adrenal-specific enzyme CYP11B1 (110, 111). Because the adrenal produces large amounts of DHEA, DHEAS, androstenedione and 11OHA, it is likely that these androgen precursors will feed into both the classical and alternative peripheral metabolic pathways that can lead to active androgens.
Premature Adrenarche
Premature adrenarche (PA) can be defined as the early rise in adrenal androgen production that usually results in the appearance of pubic or axillary hair before age 8 years in girls and 9 years in boys, without the appearance of other secondary sex characteristics (112, 113). Girls with PA are susceptible to develop polycystic ovary syndrome (PCOS) with hirsutism, irregular menses and hyperandrogenism (114). PA is also an early onset predictor of hyperinsulinemia (115, 116). Children with PA have been shown to exhibit higher serum levels of DHEA, DHEAS, androstenedione and testosterone as well as their urinary metabolites (11, 79, 115, 117-121). Recently studied LC-MS/MS steroid profiles of infants with fine genital hair showed a mild elevation of DHEAS as compared to healthy pre-adrenarchal children, thus indicating that pubic hair in infancy may represent a mild and early onset variant of PA (122). Toscano et al observed increase in plasma levels of C21 steroids like pregnenolone and 17OHPreg along with the C19 steroids like DHEA, DHEAS and androstenedione but with no change in cortisol or 11-deoxycortisol in PA children as compared to controls. They interpreted these steroid changes to suggest that the PA adrenal had decreased HSD3B2 activity and increased CYP17 activity (74). They also indicated that PA might not be exclusively dependent on ACTH regulation (74). The levels of Adiol-G also increased in children with PA and showed a strong correlation with DHEA, DHEAS and androstenedione (115, 123-126). Thus it is clear that numerous adrenal-derived steroids can be used as markers in the diagnosis of PA.
Conclusions
Adrenarche is characterized by rising levels of C19 steroids like DHEA and DHEAS due to the expanding adrenal zona reticularis and in humans this occurs at ages 6-8 years. The phenotypic hallmark of adrenarche is the appearance of axillary and pubic hair. This phenomenon is driven by the concerted actions of the enzymes CYP17, SULT2A1 and the cofactor Cyb5, which are highly expressed in the expanding population of ZR cells seen during adrenarche. The low expression of HSD3B2 in the ZR also enables the increased production of androgens by the adrenal cortex. Herein, we have reviewed recent and past studies that discuss the changes in the circulating steroid metabolome that occurs during the process of adrenarche. It is clear that along with DHEA and DHEAS, serum levels of other C19 steroids like androstenedione, 11OHA and Adiol-G are elevated during adrenarche. The serum levels of most of the adrenal unconjugated C21 steroids also do not seem to be excessively affected during the course of adrenarche. It is still worth noting that although biochemical pathways leading to the formation of these steroids have been elucidated in detail, the primary signal(s) that drive adrenarche remains unknown. This makes the process of adrenarche one of the least understood of human endocrine developmental events.
Funding
This work was supported by the National Institutes of Health Grant DK-00058 to WER.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, Bioscientifica accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at http://joe.endocrinology-journals.org/content/214/2/133.long (2012)
References
- 1.Cutler GB., Jr. Loriaux DL 1980 Andrenarche and its relationship to the onset of puberty. Fed Proc. 39:2384–2390. [PubMed] [Google Scholar]
- 2.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 3.Parker LN. Adrenarche. Endocrinol Metab Clin North Am. 1991;20:71–83. [PubMed] [Google Scholar]
- 4.Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord. 2009;10:3–17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman FR, Stanczyk FZ, Matteri RK, Gentzschein E, Delgado C, Lobo RA. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertil Steril. 1990;54:251–254. [PubMed] [Google Scholar]
- 7.Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:223–228. doi: 10.1016/j.beem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc. 2005;10:205–208. doi: 10.1111/j.1087-0024.2005.10106.x. [DOI] [PubMed] [Google Scholar]
- 9.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme JR, Forest MG, De Peretti E, Sempe M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993;22:507–532. [PubMed] [Google Scholar]
- 12.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10:19–26. doi: 10.1007/s11154-008-9092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frandsen VA, Stakemann G. 1964 The Site of Production of Oestrogenic Hormones in Human Pregnancy. 3 Further Observations on the Hormone Excretion in Pregnancy with Anencephalic Foetus. Acta Endocrinol (Copenh) 47:265–276. [PubMed] [Google Scholar]
- 15.Bolte E, Wiqvist N, Diczfalusy E. Metabolism of dehydroepiandrosterone and dehydroepiandrosterone sulphate by the human foetus at midpregnancy. Acta Endocrinol (Copenh) 1966;52:583–597. doi: 10.1530/acta.0.0520583. [DOI] [PubMed] [Google Scholar]
- 16.Siiteri PK, MacDonald PC. The utilization of circulating dehydroepiandrosterone sulfate for estrogen synthesis during human pregnancy. Steroids. 1963;2:713–730. [Google Scholar]
- 17.Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- 18.Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–252. doi: 10.1677/JOE-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 20.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am J Med Sci. 1942;204:625–648. [Google Scholar]
- 21.Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90:766–770. doi: 10.1016/s0022-3476(77)81244-4. [DOI] [PubMed] [Google Scholar]
- 22.Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR. Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocr Res. 1996;22:723–728. doi: 10.1080/07435809609043768. [DOI] [PubMed] [Google Scholar]
- 23.Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–3701. doi: 10.1210/jcem.83.10.5070. [DOI] [PubMed] [Google Scholar]
- 24.Yanase T, Simpson ER, Waterman MR. 17 alpha-hydroxylase/17,20-lyase deficiency: from clinical investigation to molecular definition. Endocr Rev. 1991;12:91–108. doi: 10.1210/edrv-12-1-91. [DOI] [PubMed] [Google Scholar]
- 25.Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- 26.Imai T, Globerman H, Gertner JM, Kagawa N, Waterman MR. Expression and purification of functional human 17 alpha-hydroxylase/17,20-lyase (P450c17) in Escherichia coli. Use of this system for study of a novel form of combined 17 alpha-hydroxylase/17,20-lyase deficiency. J Biol Chem. 1993;268:19681–19689. [PubMed] [Google Scholar]
- 27.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 28.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 29.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol. 2008;199:367–378. doi: 10.1677/JOE-08-0337. [DOI] [PubMed] [Google Scholar]
- 31.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 32.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Brissie RM, Parker CR., Jr. 2005 Effects of aging on cytochrome b5 expression in the human adrenal gland. J Clin Endocrinol Metab. 90:4357–4361. doi: 10.1210/jc.2005-0017. [DOI] [PubMed] [Google Scholar]
- 33.Parker CR., Jr. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids. 1999;64:640–647. doi: 10.1016/s0039-128x(99)00046-x. [DOI] [PubMed] [Google Scholar]
- 34.Parker CR, Jr., Slayden SM, Azziz R, Crabbe SL, Hines GA, Boots LR, Bae S. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:48–54. doi: 10.1210/jcem.85.1.6265. [DOI] [PubMed] [Google Scholar]
- 35.Luu-The V, Bernier F, Dufort I. Steroid sulfotransferases. J Endocrinol. 1996;150(Suppl):S87–97. [PubMed] [Google Scholar]
- 36.Shi L, Wudy SA, Buyken AE, Hartmann MF, Remer T. Body fat and animal protein intakes are associated with adrenal androgen secretion in children. Am J Clin Nutr. 2009;90:1321–1328. doi: 10.3945/ajcn.2009.27964. [DOI] [PubMed] [Google Scholar]
- 37.Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 38.Barker EV, Hume R, Hallas A, Coughtrie WH. Dehydroepiandrosterone sulfotransferase in the developing human fetus: quantitative biochemical and immunological characterization of the hepatic, renal, and adrenal enzymes. Endocrinology. 1994;134:982–989. doi: 10.1210/endo.134.2.8299591. [DOI] [PubMed] [Google Scholar]
- 39.Korte K, Hemsell PG, Mason JI. Sterol sulfate metabolism in the adrenals of the human fetus, anencephalic newborn, and adult. J Clin Endocrinol Metab. 1982;55:671–675. doi: 10.1210/jcem-55-4-671. [DOI] [PubMed] [Google Scholar]
- 40.Parker CR, Jr., Falany CN, Stockard CR, Stankovic AK, Grizzle WE. Immunohistochemical localization of dehydroepiandrosterone sulfotransferase in human fetal tissues. J Clin Endocrinol Metab. 1994;78:234–236. doi: 10.1210/jcem.78.1.8288708. [DOI] [PubMed] [Google Scholar]
- 41.Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB. Sulfation and sulfotransferases 1: Sulfotransferase molecular biology: cDNAs and genes. FASEB J. 1997;11:3–14. [PubMed] [Google Scholar]
- 42.Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009;360:2310–2318. doi: 10.1056/NEJMoa0810489. [DOI] [PubMed] [Google Scholar]
- 43.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 44.McCartin S, Russell AJ, Fisher RA, Wallace AM, Arnhold IJ, Mason JI, Varley J, Mendonca BB, Sutcliffe RG. Phenotypic variability and origins of mutations in the gene encoding 3beta-hydroxysteroid dehydrogenase type II. J Mol Endocrinol. 2000;24:75–82. doi: 10.1677/jme.0.0240075. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura Y, Xing Y, Hui XG, Kurotaki Y, Ono K, Cohen T, Sasano H, Rainey WE. Human adrenal cells that express both 3beta-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production. J Steroid Biochem Mol Biol. 2011;123:122–126. doi: 10.1016/j.jsbmb.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 47.Rosenfeld RS, Hellman L, Roffwarg H, Weitzman ED, Fukushima DK, Gallagher TF. Dehydroisoandrosterone is secreted episodically and synchronously with cortisol by normal man. J Clin Endocrinol Metab. 1971;33:87–92. doi: 10.1210/jcem-33-1-87. [DOI] [PubMed] [Google Scholar]
- 48.Rosenfield RL, Grossman BJ, Ozoa N. Plasma 17-ketosteroids and testosterone in prepubertal children before and after ACTH administration. J Clin Endocrinol Metab. 1971;33:249–253. doi: 10.1210/jcem-33-2-249. [DOI] [PubMed] [Google Scholar]
- 49.Kim MH, Hosseinian AH, Dupon C. Plasma levels of estrogens, androgens and progesterone during normal and dexamethasone-treated cycles. J Clin Endocrinol Metab. 1974;39:706–712. doi: 10.1210/jcem-39-4-706. [DOI] [PubMed] [Google Scholar]
- 50.Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- 51.Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P. Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab. 1981;52:1129–1136. doi: 10.1210/jcem-52-6-1129. [DOI] [PubMed] [Google Scholar]
- 52.Weber A, Clark AJ, Perry LA, Honour JW, Savage MO. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clin Endocrinol (Oxf) 1997;46:431–437. doi: 10.1046/j.1365-2265.1997.1580969.x. [DOI] [PubMed] [Google Scholar]
- 53.Mellon SH, Shively JE, Miller WL. Human proopiomelanocortin-(79-96), a proposed androgen stimulatory hormone, does not affect steroidogenesis in cultured human fetal adrenal cells. J Clin Endocrinol Metab. 1991;72:19–22. doi: 10.1210/jcem-72-1-19. [DOI] [PubMed] [Google Scholar]
- 54.Penhoat A, Sanchez P, Jaillard C, Langlois D, Begeot M, Saez JM. Human proopiomelanocortin-(79-96), a proposed cortical androgen-stimulating hormone, does not affect steroidogenesis in cultured human adult adrenal cells. J Clin Endocrinol Metab. 1991;72:23–26. doi: 10.1210/jcem-72-1-23. [DOI] [PubMed] [Google Scholar]
- 55.Reader SC, Daly JR, Alaghband-Zadeh J, Robertson WR. Negative feedback effects on ACTH secretion by cortisol in Cushing’s disease. Clin Endocrinol (Oxf) 1983;18:43–49. doi: 10.1111/j.1365-2265.1983.tb03185.x. [DOI] [PubMed] [Google Scholar]
- 56.Reader SC, Alaghband-Zadeh J, Daly JR, Robertson WR. Negative rate-sensitive feedback effects on adrenocorticotrophin secretion by cortisol in normal subjects. J Endocrinol. 1982;92:443–448. doi: 10.1677/joe.0.0920443. [DOI] [PubMed] [Google Scholar]
- 57.Parker L, Lifrak E, Shivley J. Human adrenal gland cortical androgen-stimulating hormone (CASH) is identical with a portion of the joining peptide of pituitary pro-opiomelanocortin (POMC); 71 st Annual Meet of The Endocrine Society; 1989; 1989. al. e. [Google Scholar]
- 58.Genazzani AR, Facchinetti F, Petraglia F, Pintor C, Bagnoli F, Puggioni R, Corda R. Correlations between plasma levels of opioid peptides and adrenal androgens in prepuberty and puberty. J Steroid Biochem. 1983;19:891–895. doi: 10.1016/0022-4731(83)90030-4. [DOI] [PubMed] [Google Scholar]
- 59.O’Connell Y, McKenna TJ, Cunningham SK. beta-Lipotropin-stimulated adrenal steroid production. Steroids. 1996;61:332–336. doi: 10.1016/0039-128x(96)00002-5. [DOI] [PubMed] [Google Scholar]
- 60.Genazzani AR, Facchinetti F, Pintor C, Puggioni R, Parrini D, Petraglia F, Bagnoli F, Corda R. Proopiocortin-related peptide plasma levels throughout prepuberty and puberty. J Clin Endocrinol Metab. 1983;57:56–61. doi: 10.1210/jcem-57-1-56. [DOI] [PubMed] [Google Scholar]
- 61.Aubert ML, Grumbach MM, Kaplan SL. Heterologous radioimmunoassay for plasma human prolactin (hPRL); values in normal subjects, puberty, pregnancy and in pituitary disorders. Acta Endocrinol (Copenh) 1974;77:460–476. doi: 10.1530/acta.0.0770460. [DOI] [PubMed] [Google Scholar]
- 62.Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the GH/IGF-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal boys. J Clin Endocrinol Metab. 2002;87:1162–1169. doi: 10.1210/jcem.87.3.8330. [DOI] [PubMed] [Google Scholar]
- 63.Parker LN, Sack J, Fisher DA, Odell WD. The adrenarche: prolactin, gonadotropins, adrenal androgens, and cortisol. J Clin Endocrinol Metab. 1978;46:396–401. doi: 10.1210/jcem-46-3-396. [DOI] [PubMed] [Google Scholar]
- 64.Smith CP, Dunger DB, Williams AJ, Taylor AM, Perry LA, Gale EA, Preece MA, Savage MO. Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. J Clin Endocrinol Metab. 1989;68:932–937. doi: 10.1210/jcem-68-5-932. [DOI] [PubMed] [Google Scholar]
- 65.Anderson DC. The adrenal androgen-stimulating hormone does not exist. Lancet. 1980;2:454–456. doi: 10.1016/s0140-6736(80)91889-9. [DOI] [PubMed] [Google Scholar]
- 66.Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96:E31–39. doi: 10.1210/jc.2010-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byrne GC, Perry YS, Winter JS. Kinetic analysis of adrenal 3 beta-hydroxysteroid dehydrogenase activity during human development. J Clin Endocrinol Metab. 1985;60:934–939. doi: 10.1210/jcem-60-5-934. [DOI] [PubMed] [Google Scholar]
- 68.Byrne GC, Perry YS, Winter JS. Steroid inhibitory effects upon human adrenal 3 beta-hydroxysteroid dehydrogenase activity. J Clin Endocrinol Metab. 1986;62:413–418. doi: 10.1210/jcem-62-2-413. [DOI] [PubMed] [Google Scholar]
- 69.Dickerman Z, Grant DR, Faiman C, Winter JS. Intraadrenal steroid concentrations in man: zonal differences and developmental changes. J Clin Endocrinol Metab. 1984;59:1031–1036. doi: 10.1210/jcem-59-6-1031. [DOI] [PubMed] [Google Scholar]
- 70.Brunelli VL, Chiumello G, David M, Forest MG. Adrenarche does not occur in treated patients with congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 1995;42:461–466. doi: 10.1111/j.1365-2265.1995.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 71.Abraham GE, Buster JE, Kyle FW, Corrales PC, Teller RC. Radioimmunoassay of plasma pregnenolone, 17-hydroxypregnenolone and dehydroepiandrosterone under various physiological conditions. J Clin Endocrinol Metab. 1973;37:140–144. doi: 10.1210/jcem-37-1-140. [DOI] [PubMed] [Google Scholar]
- 72.Hughes IA, Winter JS. The application of a serum 17OH-progesterone radioimmunoassay to the diagnosis and management of congenital adrenal hyperplasia. J Pediatr. 1976;88:766–773. doi: 10.1016/s0022-3476(76)81112-2. [DOI] [PubMed] [Google Scholar]
- 73.Shimozawa K, Saisho S, Yata J, Kambegawa A. Age-related changes in serum 17-hydroxypregnenolone and 17-hydroxypregnenolone sulfate concentrations in human infancy and childhood. Endocrinol Jpn. 1988;35:189–195. doi: 10.1507/endocrj1954.35.189. [DOI] [PubMed] [Google Scholar]
- 74.Toscano V, Balducci R, Adamo MV, Mangiantini A, Cives C, Boscherini B. Changes in steroid pattern following acute and chronic adrenocorticotropin administration in premature adrenarche. J Steroid Biochem. 1989;32:321–326. doi: 10.1016/0022-4731(89)90271-9. [DOI] [PubMed] [Google Scholar]
- 75.Franckson JRM, Lejeune-Lenain C, Wolter R. Adrenal androgens. Raven Press; 1980. Basal level and responsiveness of 11β-hydroxyandrostenedione secretion in normal prepubertal children. [Google Scholar]
- 76.de Peretti E, Mappus E. Pattern of plasma pregnenolone sulfate levels in humans from birth to adulthood. J Clin Endocrinol Metab. 1983;57:550–556. doi: 10.1210/jcem-57-3-550. [DOI] [PubMed] [Google Scholar]
- 77.de Peretti E, Forest MG. Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in human: the use of a sensitive radioimmunoassay. J Clin Endocrinol Metab. 1976;43:982–991. doi: 10.1210/jcem-43-5-982. [DOI] [PubMed] [Google Scholar]
- 78.de Peretti E, Forest MG. Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab. 1978;47:572–577. doi: 10.1210/jcem-47-3-572. [DOI] [PubMed] [Google Scholar]
- 79.Korth-Schutz S, Levine LS, New MI. Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J Clin Endocrinol Metab. 1976;42:117–124. doi: 10.1210/jcem-42-1-117. [DOI] [PubMed] [Google Scholar]
- 80.Korth-Schutz S, Levine LS, New MI. Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion. J Clin Endocrinol Metab. 1976;42:1005–1013. doi: 10.1210/jcem-42-6-1005. [DOI] [PubMed] [Google Scholar]
- 81.Tung YC, Lee JS, Tsai WY, Hsiao PH. Physiological changes of adrenal androgens in childhood. J Formos Med Assoc. 2004;103:921–924. [PubMed] [Google Scholar]
- 82.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 83.Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95:2399–2409. doi: 10.1210/jc.2009-1670. [DOI] [PubMed] [Google Scholar]
- 84.Stahl NL, Teeslink CR, Beauchamps G, Greenblatt RB. Serum testosterone levels in hirsute women: a comparison of adrenal, ovarian and peripheral vein values. Obstet Gynecol. 1973;41:650–654. [PubMed] [Google Scholar]
- 85.Greenblatt RB, Colle ML, Mahesh VB. Ovarian and adrenal steroid production in the postmenopausal woman. Obstet Gynecol. 1976;47:383–387. [PubMed] [Google Scholar]
- 86.Stahl NL, Teeslink CR, Greenblatt RB. Ovarian, adrenal, and peripheral testosterone levels in the polycystic ovary syndrome. Am J Obstet Gynecol. 1973;117:194–200. doi: 10.1016/0002-9378(73)90632-7. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94:2192–2198. doi: 10.1210/jc.2008-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mauvais-Jarvis P, Charransol G, Bobas-Masson F. Simultaneous determination of urinary androstanediol and testosterone as an evaluation of human androgenicity. J Clin Endocrinol Metab. 1973;36:452–459. doi: 10.1210/jcem-36-3-452. [DOI] [PubMed] [Google Scholar]
- 89.Moghissi E, Ablan F, Horton R. Origin of plasma androstanediol glucuronide in men. J Clin Endocrinol Metab. 1984;59:417–421. doi: 10.1210/jcem-59-3-417. [DOI] [PubMed] [Google Scholar]
- 90.Belanger A, Brochu M, Cliche J. Plasma levels of steroid glucuronides in prepubertal, adult and elderly men. J Steroid Biochem. 1986;24:1069–1072. doi: 10.1016/0022-4731(86)90361-4. [DOI] [PubMed] [Google Scholar]
- 91.Brochu M, Belanger A. Increase in plasma steroid glucuronide levels in men from infancy to adulthood. J Clin Endocrinol Metab. 1987;64:1283–1287. doi: 10.1210/jcem-64-6-1283. [DOI] [PubMed] [Google Scholar]
- 92.Nathanson IT, Towne LE, Aub JC. Normal Excretion of Sex Hormones in Childhood. Endocrinology. 1941;28:851–865. [Google Scholar]
- 93.Kelnar CJ, Brook CG. A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clin Endocrinol (Oxf) 1983;19:117–129. doi: 10.1111/j.1365-2265.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 94.Tanner JM, Gupta D. A longitudinal study of the urinary excretion of individual steroids in children from 8 to 12 years old. J Endocrinol. 1968;41:139–156. doi: 10.1677/joe.0.0410139. [DOI] [PubMed] [Google Scholar]
- 95.Gupta D. A longitudinal study of steroid excretion patterns in children during adolescent growth. Steroidologia. 1970;1:267–294. [PubMed] [Google Scholar]
- 96.Honour JW, Kelnar CJ, Brook CG. Urine steroid excretion rates in childhood reflect growth and activity of the adrenal cortex. Acta Endocrinol (Copenh) 1991;124:219–224. doi: 10.1530/acta.0.1240219. [DOI] [PubMed] [Google Scholar]
- 97.Remer T, Hintelmann A, Manz F. Measurement of urinary androgen sulfates without previous hydrolysis: a tool to investigate adrenarche. Determination of total 17-ketosteroid sulfates. Steroids. 1994;59:16–21. doi: 10.1016/0039-128x(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 98.Remer T, Pietrzik K, Manz F. Measurement of urinary androgen sulfates without previous hydrolysis: a tool to investigate adrenarche. Validation of a commercial radioimmunoassay for dehydroepiandrosterone sulfate. Steroids. 1994;59:10–15. doi: 10.1016/0039-128x(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 99.Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3-18 years. J Clin Endocrinol Metab. 2005;90:2015–2021. doi: 10.1210/jc.2004-1571. [DOI] [PubMed] [Google Scholar]
- 100.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108:13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr Rev. 1993;14:577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 102.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Fluck CE, Meyer-Boni M, Pandey AV, Kempna P, Miller WL, Schoenle EJ, Biason-Lauber A. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89:201–218. doi: 10.1016/j.ajhg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 105.Wilson JD. The role of androgens in male gender role behavior. Endocr Rev. 1999;20:726–737. doi: 10.1210/edrv.20.5.0377. [DOI] [PubMed] [Google Scholar]
- 106.Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003;144:575–580. doi: 10.1210/en.2002-220721. [DOI] [PubMed] [Google Scholar]
- 107.Rosenblum PM, Yamada L, Callard IP, Callard GV. Validation of radioimmunoassay systems for the measurement of 11-keto- and 11 beta-hydroxytestosterone in teleost blood. Comp Biochem Physiol B. 1985;82:659–665. doi: 10.1016/0305-0491(85)90504-8. [DOI] [PubMed] [Google Scholar]
- 108.Brantley RK, Wingfield JC, Bass AH. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm Behav. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- 109.Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008;149:1786–1792. doi: 10.1210/en.2007-1015. [DOI] [PubMed] [Google Scholar]
- 110.Kley HK, Schlaghecke R, Kruskemper HL. The measurement of androst-4-en-17 beta-ol-3,11-dione (11-oxotestosterone) by radioimmunoassay in human plasma. J Clin Chem Clin Biochem. 1984;22:461–466. doi: 10.1515/cclm.1984.22.7.461. [DOI] [PubMed] [Google Scholar]
- 111.Schlaghecke R, Kley HK, Kruskemper HL. The measurement of 11 beta, 17 beta-dihydroxy-4-androsten-3-one (11 beta-hydroxytestosterone) by radioimmunoassay in human plasma. J Clin Chem Clin Biochem. 1986;24:577–581. doi: 10.1515/cclm.1986.24.8.577. [DOI] [PubMed] [Google Scholar]
- 112.Talbot NB, Butler AM, Berman RA, Rodriguez PM, Maclachlan EA. Excretion of 17-keto steroids by normal and abnormal children. Am J Dis Child. 1943;65:364–375. [Google Scholar]
- 113.Silverman SH, Migeon C, Rosemberg E, Wilkins L. Precocious growth of sexual hair without other secondary sexual development; premature pubarche, a constitutional variation of adolescence. Pediatrics. 1952;10:426–432. [PubMed] [Google Scholar]
- 114.Ibanez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinye M, Carrascosa A, Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1599–1603. doi: 10.1210/jcem.76.6.8501168. [DOI] [PubMed] [Google Scholar]
- 115.Ibanez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche--normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 116.Oppenheimer E, Linder B, DiMartino-Nardi J. Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab. 1995;80:614–618. doi: 10.1210/jcem.80.2.7852529. [DOI] [PubMed] [Google Scholar]
- 117.Doberne Y, Levine LS, New MI. Elevated urinary testosterone and androstanediol in precocious adrenarche. Pediatr Res. 1975;9:794–797. doi: 10.1203/00006450-197510000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Korth-Schutz S, Levine LS, New MI. Evidence for the adrenal source of androgens in precocious adrenarche. Acta Endocrinol (Copenh) 1976;82:342–352. doi: 10.1530/acta.0.0820342. [DOI] [PubMed] [Google Scholar]
- 119.Rosenfield RL, Rich BH, Lucky AW. Adrenarche as a cause of benign pseudopuberty in boys. J Pediatr. 1982;101:1005–1009. doi: 10.1016/s0022-3476(82)80033-4. [DOI] [PubMed] [Google Scholar]
- 120.Voutilainen R, Perheentupa J, Apter D. Benign premature adrenarche: clinical features and serum steroid levels. Acta Paediatr Scand. 1983;72:707–711. doi: 10.1111/j.1651-2227.1983.tb09798.x. [DOI] [PubMed] [Google Scholar]
- 121.Likitmaskul S, Cowell CT, Donaghue K, Kreutzmann DJ, Howard NJ, Blades B, Silink M. ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin Endocrinol (Oxf) 1995;42:265–272. doi: 10.1111/j.1365-2265.1995.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 122.Kaplowitz P, Soldin SJ. Steroid profiles in serum by liquid chromatography-tandem mass spectrometry in infants with genital hair. J Pediatr Endocrinol Metab. 2007;20:597–605. doi: 10.1515/jpem.2007.20.5.597. [DOI] [PubMed] [Google Scholar]
- 123.Balducci R, Finocchi G, Mangiantini A, Bianchi P, Guglielmi R, Toscano V. Plasma 3 alpha-androstanediol glucuronide in precocious adrenarche. J Endocrinol Invest. 1993;16:117–121. doi: 10.1007/BF03347661. [DOI] [PubMed] [Google Scholar]
- 124.Balducci R, Finocchi G, Mangiantini A, Maggi C, Bianchi P, Guglielmi R, Toscano V. Lack of correlation between sex hormone binding globulin, adrenal and peripheral androgens in precocious adrenarche. J Endocrinol Invest. 1992;15:501–505. doi: 10.1007/BF03348790. [DOI] [PubMed] [Google Scholar]
- 125.Montalto J, Funder JW, Yong AB, Whorwood CB, Connelly JF. Serum levels of 5-androstene-3 beta,17 beta-diol sulphate, 5 alpha-androstane-3 alpha, 17beta-diol sulphate and glucuronide, in late onset 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 1990;37:593–598. doi: 10.1016/0960-0760(90)90406-b. [DOI] [PubMed] [Google Scholar]
- 126.Riddick LM, Garibaldi LR, Wang ME, Senne AR, Klimah PE, Clark AT, Levine LS, Oberfield SE, Pang SY. 3 alpha-Androstanediol glucuronide in premature and normal pubarche. J Clin Endocrinol Metab. 1991;72:46–50. doi: 10.1210/jcem-72-1-46. [DOI] [PubMed] [Google Scholar]