Hypertensive Retinopathy and Risk of Stroke (original) (raw)
. Author manuscript; available in PMC: 2014 Oct 1.
Abstract
Although assessment of hypertensive retinopathy signs has been recommended for determining end-organ damage and stratifying vascular risk in hypertensive persons, its value remains unclear. In this study, we examine whether hypertensive retinopathy predicts the long-term risk of stroke in hypertensives.
A total of 2907 hypertensive participants aged 50–73 at the 1993–1995 examination, who had gradable retinal photographs, no history of diabetes, stroke and coronary heart disease at baseline and data on incident stroke were included from the Atherosclerosis Risk in Communities (ARIC) Study. Retinal photographs were assessed for hypertensive retinopathy signs and classified as none, mild, and moderate/severe. Incident events of any stroke, cerebral infarction and hemorrhagic stroke were identified and validated.
After a mean follow-up period of 13.0 years, 165 persons developed incident stroke (146 cerebral infarctions and 15 hemorrhagic strokes). After adjusting for age, sex, blood pressure, and other risk factors, persons with moderate hypertensive retinopathy were more likely to have stroke (multivariable hazard ratios (HR), moderate versus no retinopathy: 2.37, 95%CI 1.39-4.02). In hypertensives on medication with good control of blood pressure, hypertensive retinopathy was related to an increased risk of cerebral infarction (HR, mild retinopathy: 1.96, 95%CI 1.09-3.55; moderate retinopathy: 2.98, 95%CI 1.01-8.83).
Hypertensive retinopathy predicts the long-term risk of stroke, independent of blood pressure, even in treated hypertensives with good hypertension control. Retinal photographic assessment of hypertensive retinopathy signs may be useful for assessment of stroke risk.
Keywords: Hypertension, Hypertensive retinopathy, Stroke, Cerebral infarction
INTRODUCTION
Despite the overwhelming evidence that hypertension represents the first risk factor for stroke and that prevention of stroke benefits the most from blood pressure lowering,1–3 it still remains difficult to predict among those with hypertension who will develop a stroke. Therefore, it is still pertinent to unravel other risk factors or signs that may provide additional information.
A fundus (retinal) examination to determine the presence and severity of retinopathy signs has been recommended as a means to determine the presence of end-organ damage in persons with hypertension and to stratify risk.4–6 However, the value of a retinal examination remains unclear as different classifications of hypertensive retinopathy (e.g., Keith Wagner Barker classification) are difficult to use in clinical practice,7 and a clinical ophthalmoscopic examination has low reliability and reproducibility.8 While a more simplified system of hypertensive retinopathy system (mild, moderate and severe) has been proposed,6 its use in predicting end-organ damage has not been validated.
Furthermore, it has been suggested that retinal photography, widely available in primary clinics, hospitals and even in the community (e.g. optical shops), may be a more precise means to document retinopathy signs.9 Recent studies have shown that retinopathy signs, are related to the risk of stroke,10–16 including MRI-defined cerebral infarcts, incident clinical stroke, ischemic strokes, and symptomatic and subclinical silent lacunar infarcts in healthy populations,12, 14–16 and Esubsequent vascular events in persons who have an acute stroke.17 However, a major gap in the literature is whether the simplified hypertensive retinopathy classification is predictive of stroke among subjects with hypertension.
In this paper, we examined in a cohort of persons with hypertension (without diabetes) the relationship between hypertensive retinopathy signs and long-term risk of stroke, its major subtype cerebral infarction, and whether this relationship is independent of hypertensive medication use and blood pressure control.
METHODS
Study Population
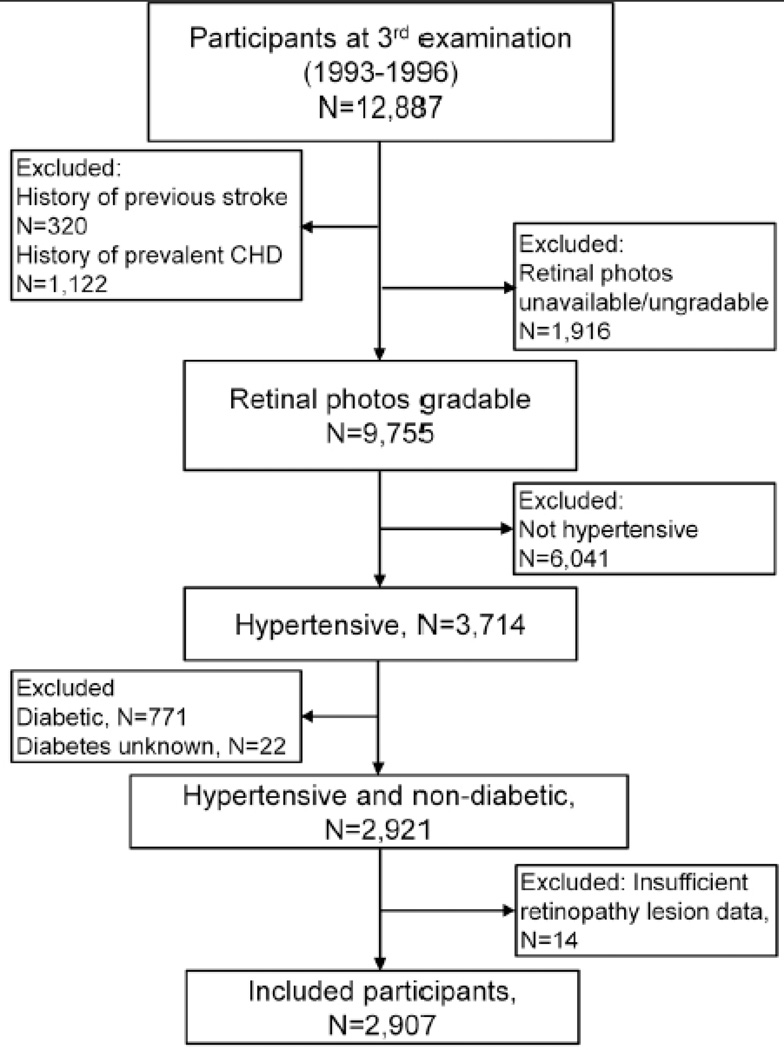
The Atherosclerosis Risk in Communities (ARIC) Study is a population-based study that included 15,792 participants aged 44–66 years from 1986 to 1990.18 Our study cohort consists of individuals who participated at the third examination (1993 to 1996), aged 49–73, when retinal photography was performed. Response rates of participants from the first to third examination have been previously reported.19 Of the 12,887 individuals who participated at the third examination, a total of 2907 participants with prevalent hypertension, with no prevalent stroke or coronary heart disease (CHD), without diabetes at the time of retinal photography, and had gradable retinal photographs were included into this study (Figure). Approval was given by institutional review boards at each study site, and informed consent obtained from all participants.
Figure 1.
Participant flow chart showing inclusion and exclusion criteria for this study.
Assessment of Hypertensive Retinopathy
Retinal photography and the grading procedure have been documented elsewhere.19 Briefly, a 45° non-mydriatic retinal photograph centered on the region of the optic disc and macula was taken from one randomly selected eye after five minutes of dark adaption. Trained graders masked to participant characteristics and clinical status evaluated photographs for the presence of retinopathy signs. Retinopathy signs were evaluated without assumption of cause, noting the following findings: retinal hemorrhages (blot and flame-shaped), microaneurysms, soft exudates, hard exudates, macular edema, intraretinal microvascular abnormalities, venous beading, new vessels at the disc or elsewhere, vitreous hemorrhage, disc swelling, and laser photocoagulation scars.14 Generalized arteriolar narrowing was determined as those with a central retinal arteriolar equivalent (CRAE) in the lowest quintile of the entire cohort (CRAE < 148.7micrometers). The reliability coefficient (for retinal arteriolar and venular caliber) and kappa statistics (for retinal lesions) within and between graders ranged between 0.61-1.00.19 Severity of hypertensive retinopathy was defined as none, mild, moderate and severe, as described previously (Table 1, Figure S1 for examples).6
Table 1.
Classification of Hypertensive Retinopathy
| Grade | Retinal signs |
|---|---|
| None | No detectable signs |
| Mild | Presence of generalized arteriolar narrowing (first quintile of CRAE), focal arteriolar narrowing, arteriovenous nicking, or a combination |
| Moderate | Presence of blot, or flame-shaped hemorrhage, microaneurysm, soft exudates or a combination of these signs |
| Severe | Presence moderate hypertensive retinopathy signs; and optic disc swelling |
Assessment of Incident Stroke and Subtypes
Ascertainment and classification of stroke in ARIC has been previously described.20 Information about stroke events was obtained through annual follow-up telephone interviews, identifying hospitalizations and deaths in the previous year, and by reviewing local hospital discharge lists and death certification from state statistics offices.20 A hospitalization was considered eligible for possible validation as a stroke if it contained a discharge diagnosis code of cerebrovascular disease (International Classification of Diseases, 9th Revision, Clinical Modification codes 430 to 438). Out-of-hospital deaths coded as fatal strokes in the death certificate were also identified, but not validated and therefore excluded.
When a potential stroke was identified, a trained nurse was sent to abstract hospital records. Each eligible case was classified by a computer algorithm, and independently classified by an expert physician reviewer. Disagreements between the two were adjudicated by a second physician-reviewer. Details on quality assurance are presented elsewhere.20
For this analysis, incident stroke is defined to include only strokes that occurred between the time of retinal photography in 1993–1995 and December 31, 2008. These are categorized as cerebral infarctions (thrombotic or embolic brain infarction) or hemorrhagic strokes (subarachnoid or intracerebral hemorrhage).14
Definition of Hypertension and Blood Pressure
History of hypertension and use of anti-hypertensive medication were ascertained from examiner-ascertained questionnaires at the third examination (1993–1996). Blood pressures were taken with a random-zero sphygmomanometer and the mean of the last two of three measurements at each visit was used for analyses. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg at the third examination, or the use of anti-hypertensive medication during the previous 2 weeks from the third examination. Subjects on medication who had systolic and diastolic blood pressures below 140 mmHg and 90 mmHg respectively at the third examination were considered to have good control of their hypertension, while those with systolic or diastolic blood pressures above 140 mmHg and/or 90 mmHg respectively were considered to have poor control of hypertension. Mean arterial blood pressure was computed as 2/3 of the diastolic blood pressure plus 1/3 of the systolic blood pressure.
Definition of Cardiovascular Risk Factors
Participants underwent standardized evaluations of cardiovascular risk factors at each examination. The following variables included in this study were assessed during the third examination. History of diabetes, cigarette smoking, alcohol consumption, and use of anti-diabetic medication were ascertained from examiner-administered questionnaires. Diabetes was defined as fasting blood glucose ≥126 mg/dL or a self-reported history of treatment for diabetes, and diabetic participants were excluded from our analysis. Fasting blood samples were collected and processed for total cholesterol, HDL cholesterol, triglycerides, and glucose.21 Height and weight were measured for calculation of body mass index.
Statistical Analysis
Cox proportional hazard models were used to calculate hazard ratio (HR) and 95% confidence intervals (CI) for stroke by severity of hypertensive retinopathy. Participants were followed from the time of retinal photography to the stroke event, death, last contact or December 31, 2008, whichever came first. Kaplan-Meier failure curves were constructed for incident stroke by hypertensive retinopathy classification. We initially adjusted for age, sex, and race-center categories; and additionally for mean arterial blood pressure (mmHg) at third examination (forming the baseline for the present study), fasting glucose, total cholesterol and triglyceride levels (mg/dL), body mass index (kg/m2), cigarette smoking and alcohol consumption. Analyses were repeated, stratifying for use of hypertension-lowering medications. Proportional hazard assumptions were tested using Kaplan-Meier and predicted survival plots, and Schoenfeld residuals. All analyses were carried out using SPSS version 17.0 and STATA/SE 11.2.
RESULTS
Among the 2907 subjects, the most common sign of hypertensive retinopathy (excluding generalized arteriolar narrowing) was focal arteriolar narrowing (22.3%, 95%CI 20.8%-23.8%), arterio-venous nicking (17.5%, 95%CI 16.1%-18.9%) and other retinopathy signs (5.1%, 95%CI 4.3%-5.9%), which included microaneurysms, soft exudates, blot hemorrhages, and flamed-shaped hemorrhages. A total of 1406 (48.4%, 95%CI 46.5%-50.2%) had none, 1354 (46.6%, 95%CI 44.8%-48.4%) mild, 146 (5.0 %) moderate, and 1 severe hypertensive retinopathy. Since only 1 participant had severe hypertensive retinopathy, the participant was included into the moderate hypertensive retinopathy group (5.1%, 95%CI 4.3%-5.9%). Table 2 presents baseline characteristics of the subjects according to severity of hypertensive retinopathy.
Table 2.
Hypertensive participant characteristics according to retinopathy grade
| Retinopathy status | ||||
|---|---|---|---|---|
| Characteristics | None(n=1406, 48.4%) | Mild(n=1354, 46.6%) | Moderate/Severe(n=147, 5.1%) | P* |
| Age, years (sd) | 59.9 (5.6) | 61.0 (5.7) | 60.2 (6.1) | 0.559 |
| Men, n (%) | 525 (37.3) | 582 (43.0) | 67 (45.6) | 0.004 |
| African-Americans, n (%) | 475 (33.8) | 320 (23.6) | 76 (51.7) | <0.001 |
| Systolic blood pressure, mmHg (sd) | 133.6 (18.5) | 140.4 (18.4) | 142.5 (24.5) | <0.001 |
| Diastolic blood pressure, mmHg (sd) | 75.9 (10.5) | 78.9 (10.7) | 79.9 (13.1) | <0.001 |
| Blood glucose, mg/dL (sd) | 100.3 (10.4) | 100.2 (10.6) | 99.8 (9.7) | 0.564 |
| Body mass index, kg/m2 (sd) | 29.5 (5.6) | 29.6 (5.8) | 29.1 (5.9) | 0.435 |
| Total cholesterol, mg/dL(sd) | 209.8 (36.2) | 207.3 (37.1) | 208.0 (38.1) | 0.557 |
| HDL-cholesterol, mg/dL (sd) | 52.8 (17.7) | 52.7 (18.3) | 54.5 (19.0) | 0.596 |
| Total triglyceride, mg/dL (sd) | 145.0 (84.4) | 143.5 (83.1) | 133.5 (73.1) | 0.111 |
| Cigarette smoking, ever, n (%) | 774 (55.0) | 760 (56.1) | 77 (52.4) | 0.639 |
| Alcohol use, ever, n (%) | 1011 (71.9) | 1012 (74.7) | 102 (69.4) | 0.142 |
| Incident stroke, n (%) | 60 (4.3) | 86 (6.4) | 19 (12.9) | <0.001 |
| Incident cerebral infarction, n (%) | 51 (3.6) | 81 (6.0) | 14 (9.5) | <0.001 |
After a mean follow-up period of 13.0 years, there were 165 incident strokes, of which 146 were cerebral infarctions and 15 were hemorrhagic strokes. The incidence of stroke events for the whole population was 0.436 (95%CI 0.42-0.45) per 100 person-years, 0.322 (95%CI 0.305-0.339) per 100 person-years for the group with no retinopathy, and 0.493 (95%CI 0.466-0.519) per 100 person-years and 1.073 (95%CI 0.899 -1.246) per 100 person-years for the group with mild and moderate hypertensive retinopathy, respectively.
Kaplan-Meier failure curves constructed for incident stroke by hypertensive retinopathy classification (Figure S2) suggested that there was a significant difference in risk of stroke in the 3 groups, as pairwise Mantel-Cox Log Rank comparisons were all significant (p<0.05). Table 3 shows that in persons with hypertension, increasing severity of hypertensive retinopathy was associated with an increased risk of incident stroke, including cerebral infarction. Adjusted Cox regression models passed tests for proportional hazard assumptions. Furthermore, goodness-of–fit when evaluated using Cox-Snell residuals demonstrated reasonable fit with the data.
Table 3.
Hazard ratios (95% confidence intervals) for stroke and cerebral infarction by severity of hypertensive retinopathy grade
| HypertensiveRetinopathy Grade | Unadjusted | Model 1* | Model 2† | |||
|---|---|---|---|---|---|---|
| Stroke(n = 165) | Cerebralinfarction(n = 146) | Stroke(n = 165) | Cerebralinfarction(n = 146) | Stroke(n = 165) | Cerebralinfarction(n = 146) | |
| None (1406) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Mild (1354) | 1.53 (1.10–2.13) | 1.70 (1.20–2.41) | 1.50 (1.07–2.09) | 1.67 (1.17–2.38) | 1.35(0.96–1.89) | 1.52 (1.06–2.19) |
| Moderate/Severe (147) | 3.36 (2.00–5.63) | 2.86 (1.59–5.17) | 2.71 (1.61–4.56) | 2.29 (1.26–4.15) | 2.37 (1.39–4.02) | 2.01 (1.10–3.70) |
To assess whether hypertensive retinopathy was similarly associated with incident stroke over longer periods of time, we censored persons who had strokes within 5 years after retinal photography, and found that hazard ratios estimated from the multivariate model remained relatively unchanged for both incident stroke and cerebral infarction (mild hypertensive retinopathy: HR 1.39, 95%CI 0.91-2.11; for stroke and HR 1.68, 95%CI 1.06-2.64 for cerebral infarction; moderate hypertensive retinopathy: HR 2.20, 95%CI 1.11-4.37 for stroke and HR 2.46, 95%CI 1.18-5.10 for cerebral infarction).
Finally, we examined the association between hypertensive retinopathy and stroke in persons using anti-hypertensive medications (Table S1). We found that despite having good control of hypertension as defined by blood pressure levels at the time of the retinal examination, those with mild (HR 1.96, 95%CI 1.09-3.55), and moderate hypertensive retinopathy (HR 2.98, 95%CI 1.01-8.83) were at an increased risk of cerebral infarction. Additionally, interaction terms testing for interaction between hypertensive retinopathy grade and use of hypertensive medication, or good control of hypertension were not significant.
DISCUSSION
In this population-based study, we found that in persons with hypertension but without diabetes, hypertensive retinopathy was associated with stroke risk suggesting that the presence of these retinal microvascular changes is indicative of additional vascular risk beyond that conferred by traditional cardiovascular risk factors.
Histopathology of vascular lesions in retina and brain
Histopathological studies suggest that these hypertensive retinopathy lesions result from small vessel arteriolosclerosis,22 and continued elevated blood pressure results in retinal ischemia and breakdown of the blood-retina barrier.23 They parallel hypertensive microvascular changes described in the brain, such as concentric thickening of the arterial wall, intimal thickening, medial hyperplasia and increased vessel permeability due to blood-brain barrier breakdown,24–26 suggesting that retinal photography is a potential clinical tool to indirectly assess potential microvascular damage in the cerebral vasculature.
Hypertensive Retinopathy Classifications
Several attempts have been made to devise a classification system for retinopathy signs, and studies have related these signs to cardiovascular diseases and mortality. However, they are limited for several reasons. First, because they involved patients who had uncontrolled or untreated hypertension, generalization to contemporary populations of patients with lower blood-pressure levels may be problematic. Second, in studies conducted up to the 20th century, retinopathy was defined using only direct ophthalmoscopic examination.27–29 This technique is subject to high inter-observer variability.7 Third, although many earlier studies cite increased mortality among persons with hypertensive retinopathy, few have demonstrated associations between hypertensive retinopathy and specific cardiovascular outcomes such as incident stroke or have adequately controlled for relevant confounding factors. More recent population-based studies have adopted retinal photography and standardized protocols for the assessment of retinopathy signs. Using these procedures several studies have shown that retinal microvascular changes, including retinopathy signs, are related to subclinical and clinical cerebrovascular pathology .10–13, 15, 30, 31 However, these studies have mainly examined general elderly populations.30, 31
Possible clinical implications
In the present study, we focused on subjects with hypertension and found within this group that those with mild and moderate hypertensive retinopathy were at an additional increased risk of developing a stroke. Our current data suggest that within those who have hypertension, fundus examination may potentially provide additional information on long-term stroke risk stratification. The simplified 3-grade classification we used is easily implementable in both clinical and research settings with access to fundus exam procedures.
Furthermore, clinical guidelines strongly recommend that lowering blood pressure can lead to significant reduction of stroke risk,1, 2 however our findings suggest that despite having good control of blood pressure, patients with hypertensive retinopathy are at an increased risk of stroke. This suggests that closely monitoring blood pressures and medication compliance may not be sufficient for stroke prevention in hypertensives. Retinal assessment may be useful especially in those with good control of hypertension.
Methodological Considerations
Since hypertensive retinopathy is difficult to distinguish from diabetic retinopathy in patients with both comorbidities, with the actual cause of present retinopathy signs undeterminable, we excluded diabetic hypertensive participants in our analyses. As persons with a previous history of CHD may already be at an increased risk of stroke, including these subjects may confound the association between hypertensive retinopathy and incident stroke. Therefore, participants with CHD at baseline were excluded, which resulted in a smaller sample size and wider confidence intervals in the current study. As we did not have detailed information on other cardiovascular disease subtypes, we were unable to fully account for their confounding effect on the association between hypertensive retinopathy and the risk of stroke. We used a 45° non-stereoscopic fundus photograph taken through the non-dilated pupil of one eye, making retinopathy grading more variable. Unilateral retinopathy would be missed if the more involved eye was not examined. However, this misclassification of retinopathy is likely to be independent of a person developing a stroke and thus would result in bias towards the null, suggesting that the true association may be stronger. Due to the small number of hemorrhagic strokes we could not examine the association with this subtype. Furthermore, because blood pressure measurements were not obtained from participants after the examination visit in which retinal photography was performed, we could not adjust for blood pressure during the follow-up period. Several strengths of the current study include a large sample of subjects with hypertension, long follow-up for period of stroke and standardized procedures for the assessment of retinopathy.
PERSPECTIVES
Among persons with hypertension without diabetes, hypertensive retinopathy is associated to an increased long-term risk of stroke, independent of other vascular risk factors. Furthermore, among those who have seemingly good control of hypertension, persons with hypertensive retinopathy are nevertheless at an increased risk of developing cerebral infarction. These findings suggest that a retinal examination may be valuable for the assessment of stroke risk in patients with hypertension.
Supplementary Material
01
NOVELTY AND SIGNIFICANCE.
- What Is New
- Hypertensive retinopathy is associated with an increased long-term risk of stroke and cerebral infarction, independent of vascular risk factors in hypertensive persons.
- What Is Relevant?
- Hypertensive retinopathy in persons on medication with seemingly good control of blood pressure was nevertheless at an increased risk of developing cerebral infarction.
- Retinal fundus photography may be valuable tool for the assessment of long-term stroke risk in patients with hypertension.
- Summary
- Hypertensive retinopathy predicts long-term risk of stroke and cerebral infarction in hypertensives, independent of traditional risk factors.
ACKNOWLEDGEMENTS
YTO and MKI did the statistical analyses and wrote the manuscript. TYW formulated the main hypothesis and revised the manuscript. RK, BK, PM, and ARS helped review the report and provided additional intellectual content. TYW, ARS and DC provided statistical expertise and additional critical insight. All authors reviewed, edited, and approved the final version of the manuscript. YTO and MKI had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff and participants of the ARIC study for their important contributions.
SOURCES OF FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The funding sources had no role in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST / DISCLOSURES
None of the authors have a conflict of interest to disclose.
References
- 1.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Thijs L, Staessen JA. Blood pressure lowering for primary and secondary prevention of stroke. Hypertension. 2006;48:187–195. doi: 10.1161/01.HYP.0000231939.40959.60. [DOI] [PubMed] [Google Scholar]
- 3.Ravenni R, Jabre JF, Casiglia E, Mazza A. Primary stroke prevention and hypertension treatment which is the first-line strategy. Neurol Int. 2011;3:e12. doi: 10.4081/ni.2011.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oprail S, Wright JT, Rocella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom SM BHS Guidelines Working Party. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): Summary. BMJ. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 7.van den Born, Hulsman CA, Hoekstra JB, Schlingemann RO, van Montfrans Value of routine funduscopy in patients with hypertension: Systematic review. BMJ. 2005;331:73. doi: 10.1136/bmj.331.7508.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimmitt SB, West JN, Eames SM, Gibson JM, Gosling P, Littler WA. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103–1106. doi: 10.1016/s0140-6736(89)92384-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet neurology. 2004;3:179–183. doi: 10.1016/s1474-4422(04)00682-9. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: A population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65:1005–1009. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 12.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37:82–86. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 13.Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, McNamara M, Thom SA, Klein R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981. doi: 10.1161/01.HYP.0000216717.72048.6c. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BEK, Liao D-P, Hubbard LD, Mosley TH ARIC Investigators. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 16.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BEK, Sharrett AR ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349–1355. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Silva D, Manzano JJ, Liu EY, Woon FP, Wong WX, Chang HM, Chen C, Lindley RI, Wang JJ, Mitchell P, Wong TY, Wong MC Multi-Centre Retinal Stroke Study Group. Retinal microvascular changes and subsequent vascular events after ischemic stroke. Neurology. 2011;77:896–903. doi: 10.1212/WNL.0b013e31822c623b. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 21.National Heart Lung Blood Institute. Atherosclerosis Risk in Communities Study Operations Manual No. 2, Cohort Component Procedures Version 20. Chapel Hill: ARIC Coordinating Center School of Public Health University of North Carolina; 1988. [Google Scholar]
- 22.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. Journal of Anatomy. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 24.Fredriksson K, Nordborg C, Kalimo H, Olsson Y, Johansson BB. Cerebral microangiopathy in stroke-prone spontaneously hypertensive rats. An immunohistochemical and ultrastructural study. Acta neuropathologica. 1988;75:241–252. doi: 10.1007/BF00690532. [DOI] [PubMed] [Google Scholar]
- 25.Fredriksson K, Kalimo H, Westergren I, Kåhrström J, Johansson BB. Blood-brain barrier leakage and brain edema in stroke-prone spontaneously hypertensive rats. Effect of chronic sympathectomy and low protein/high salt diet. Acta neuropathologica. 1987;74:259–268. doi: 10.1007/BF00688190. [DOI] [PubMed] [Google Scholar]
- 26.Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain pathology (Zurich, Switzerland) 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki N. Epidemiological evaluation of funduscopic findings in cerebrovascular diseases ii a multivariate analysis of funduscopic findings. JAPCIRCULATJ. 1975;39:271–282. doi: 10.1253/jcj.39.271. [DOI] [PubMed] [Google Scholar]
- 28.Okada H, Horibe H, Yoshiyuki O, Hayakawa N, Aoki N. A prospective study of cerebrovascular disease in japanese rural communities, akabane and asahi. Part 1: Evaluation of risk factors in the occurrence of cerebral hemorrhage and thrombosis. Stroke. 1976;7:599–607. doi: 10.1161/01.str.7.6.599. [DOI] [PubMed] [Google Scholar]
- 29.Svardsudd K, Wedel H, Aurell E, Tibblin G. Hypertensive eye ground changes prevalence relation to blood pressure and prognostic importance the study of men born in 1913. Acta Medica Scandinavica. 1978;204:159–167. [PubMed] [Google Scholar]
- 30.Longstreth W, Larsen EK, Klein R, Wong TY, Sharrett AR, Lefkowitz D, Manolio TA. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: The Cardiovascular Health Study. Am J Epidemiol. 2007;165:78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 31.Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, Kuller L, Burke G, Tracy RP, Polak JF, Gottdiener JS, Siscovick DS. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The Cardiovascular Health Study. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01