Probiotics in the Treatment and Prevention of Allergies in Children (original) (raw)
Abstract
Several studies on the pathogenesis of allergy both in man and experimental animals continue to show the importance of commensal bacteria in the gastrointestinal tract in stimulating and directing the immune system. The interest in modulating commensal bacteria flora with pre- and probiotics to prevent and treat food allergy has multiplied in recent years. We recently studied 230 infants with atopic dermatitis and suspected cow’s milk allergy. The infants were randomly allocated to groups which received_Lactobacillus_ GG (LGG), a mixture of four probiotic strains (MIX) or placebo for 4 weeks. We inferred that probiotics induce systemically detectable low-grade inflammation, which may explain the clinical effects and the secretion pattern of cytokines induced by PBMC. To study the ability of probiotics to prevent allergy in children, we recruited 1223 pregnant women carrying fetuses at increased risk of allergy for a double-blind placebo-controlled trial. Mothers used a mixture of four probiotic bacteria or a placebo from the 36th week of gestation. Their infants received the same probiotics plus prebiotic galacto-oligosaccharides for 6 months. At the 2-year follow-up, a total of 925 infants participated. The cumulative incidence of allergic disease did not differ significantly between the synbiotic and the placebo group. However, synbiotics significantly reduced eczema. The preventive effect of synbiotics was more pronounced against IgE-associated diseases. At the 5 year follow-up, 891(88%) of the 1018 intention-to-treat infants attended. In the probiotic and placebo groups, frequencies of allergic symptoms and IgE-associated allergic disease and sensitization were similar, and the frequencies of eczema did not differ between the groups. Atopic eczema, allergic rhinitis and asthma appeared equal frequency in the groups. However, less IgE-associated allergic disease occurred in the cesarean-delivered infants given probiotics. In cesarean-delivered childen, we noticed a delayed rise in bifidobacteria recovery in placebo-treated children which was corrected by pro- and prebiotic supplementation. Indications from studies of feces and blood at the age 6 months suggest that probiotics may enhance both inflammation and immune defence of the gut. The probiotic treatment further stimulated maturation of the immune system since the infants given probiotics showed increased resistance to respiratory infections and improved vaccine antibody responses.
Keywords: Probiotics, Prebiotics, Synbiotics, Lactobacillus rhamnosus strain GG, Allergic diseases, Cow’s milk allergy, Double-blind placebo-controlled trial, SCORAD index, Skin prick tests, IgE
The prevalence of allergic diseases in children has increased markedly in the past few decades. According to several published studies in Finland, the total prevalence of allergic symptoms during childhood has risen 8-fold from 1950 to 1995. In 1950, 5% of children had some type of allergic symptom while recent studies report a prevalence of 40% [1]. Similarly, an increase has taken place in all developed highly hygienic countries. In 1976 Canadian paediatrician Gerrard concluded that the increase in allergic diseases is the price to be paid for the relative freedom from diseases due to viruses, bacteria and helminths in infancy and early childhood [2]. The so-called hygiene hypothesis was formulated later by Strachan [3]. Based on the lower prevalence of allergy in families with large numbers of children than in small sibships, he proposed that high prevalence of infections in large families stimulates more Th1 cells, reciprocally inciting the Th2 population [4]. Epidemiologic studies show that a particularly high prevalence of infections such as hepatitis A in the gastrointestinal tract, is associated with lower prevalence of allergies [5]. Several environmental factors encountered in central European farms during infancy have resulted in the lower prevalence of allergic diseases and sensitization in children [6]. However, the defect in the stimulation of Th1 cells in the development of allergic diseases in the same environment and the prevalence of autoimmune diseases has increased in a similar manner [7]. Both arms of effector T cells have been shown to be regulated by T regulatory cells and these cell populations play an important role in the development of autoimmunity, self tolerance and allergic diseases [8].
Stimulation by the huge and active commensal bacterial flora in the intestine during early life is important in directing the development of regulatory T cells and tolerance. Their action is probably mediated by the innate immune system. The sterile gut of the newborn is gradually colonized by environmental bacteria. Vaginally born infants acquire the microbiota having the strongest association with mother’s colon [9]. Cesarean section delays colonization by Bifidobacteria,Lactobacilli, and Bacteroides [10, 11]. Later, the type of feeding influences the initial colonization [10]. Human milk oligosaccharides promote the growth and activity of Bifidobacteria and_Lactobacilli_ [12], which more abundantly colonize breast-fed than formula-fed infants [13]. In unhygienic environments, the commensal gut flora has a high diversity and a high turnover rate [14]. Such conditions, related to decreased risk of allergy, provide continuous exposure to an extensive array of bacteria in drinking water and in the soil and constantly stimulate the immune system [15].
Several associations exist between commensal microbiota and the development of allergic diseases during childhood. In Estonia, at the time of this study, there was a low prevalence of allergy, and the microbiota of healthy infants was different from that of infants in Sweden, a country with a high prevalence of allergy [16]. In prospective studies, early fecal samples of infants who go on to develop allergies, compared to those who remain healthy, grew less Enterococci,Bifidobacteria, and Bacteroides, and more_Clostridia_ and Staphylococci [17]. In the feces of 5 year-old Estonian children, those with allergic diseases had fewer common Bifidobacteria than those without allergy, while_Clostridia_ was more common in allergic children [18]. Japanese infants developing early allergy have different_Bifidobacteria_ species compared to non-allergic infants; in particular, they have an adult type of Bifidobacterium (catenulatum) which has been described as appearing earlier than in other populations [19]. In an experimental animal model of food allergy, the gut microbiota and its stimulatory action on innate immune system by toll-like receptors (TLR), particularly TLR4, is of paramount importance. In germ-free mice food tolerance does not develop, but is inducible after colonisation of the intestine [20]. Mice susceptible to food allergies have a mutation in TLR4, blocking its signalling [21].
Altering the intestinal microbiota of an individual is a potential treatment for allergic symptoms as well as for the prevention of the development of allergies. Probiotic bacteria are “live micro-organisms, which administered in adequate amounts confer health benefits on the host” [22]. They are a heterogeneous group of bacteria with specific biological activities. Lactobacilli, Bifidobacteria, and Streptococci strains are most commonly selected from among human microbiota or dairy-product starters.Lactobacilli, Bifidobacteria, and_Propionibacteria_ belong to the lactic acid bacteria group.
Probiotics may directly affect the immune system of the host or change the microbiota of the host; and in that way may either prevent or ameliorate allergies. Prebiotics are indigestible substances that beneficially affect the host by selectively stimulating the growth and/ or the activity of a limited number of bacterial strains established in the gut; thereby having an impact on allergies. The term synbiotics may be used for a combination of these pre- and probiotics, and combination preparations have been used in the treatment and prevention of allergic diseases.
TREATMENT OF ALLERGIC DISEASES
Ten randomized clinical trials have compared the effect of probiotics with that of a placebo preparation in infants and children with eczema (Table 1). The first study by Majamaa and Isolauri [23] reported the effect of Lactobacillus rhamnosus strain GG (LGG) in the treatment of eczema in 42 infants referred to a hospital for suspected cow’s milk allergy (CMA). LGG was given open-label for one month to 11 breast-feeding mothers or directly to 15 infants receiving extensively hydrolyzed formula (EHF). In the control group delete, 16 infants received only EHF. In the final analysis, 37 of 42 infants undergoing a positive cow’s milk challenge after the intervention were included. Among these 37, the SCORAD index [24] improved significantly in the 13 formula-fed infants receiving LGG and in the 10 breast-fed infants whose mothers received LGG. In the 14 control infants, the index remained unchanged. However, at 2 months the eczema was healed in both study groups. Isolauri’s study included 27 infants suffering from eczema during exclusive breast-feeding. Nine were weaned onto EHF, 9 infants onto the same formula with added LGG, and 9 infants received the formula with added Bifidobacterium lactis Bb12. After 2 months, infants receiving the probiotic-containing formula showed less severe eczema, whereas in the placebo group no improvement was observed. Six months later, eczema had improved in all infants, with no difference in incidence between the study groups [25].
Table 1.
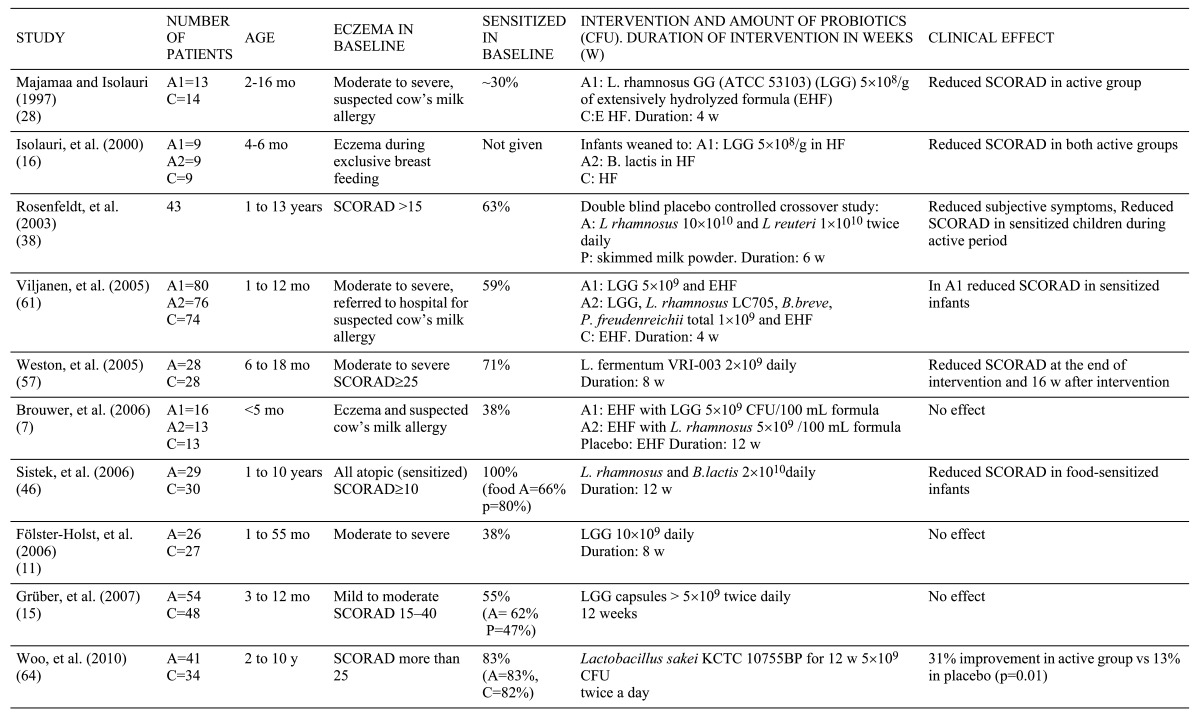
Rosenfedlt et al. [26] studied 43 children aged 1 to 13 with eczema, in a double-blind, placebo-controlled crossover setting with a combination of 2 strains of bacteria (Table 1). A significantly greater proportion (56%) of patients experienced improvement after active treatment than after placebo (15%). A greater decrease in SCORAD score was seen in patients with atopic constitution after probiotic treatment than after placebo.
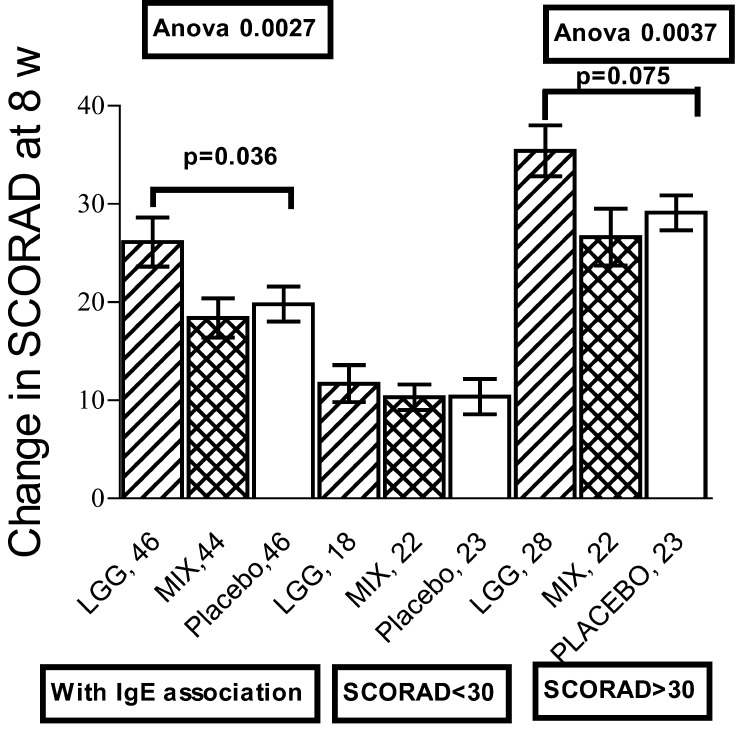
We invited infants below the age of 12 months with eczema and suspicion of CMA to participate in a double-blind placebo controlled study evaluating the effects of two probiotic treatments. Of the 230 participants, 80 received LGG, 76 a mixture of 4 bacteria strains and 74 placebo preparation for 4 weeks [27]. All were put on an EHF and effective topical care was advised at the beginning of the study. Patients’ SCORAD was evaluated before treatment, after 4 weeks of treatment and again after 4 weeks on EHF. At that time eczema was healed to the extent that a double-blind placebo controlled CM challenge (DBPC) test could be done; it was positive in 120. The reduction of SCORAD scores were comparable across the study groups: whether they had CMA or not, did not have an effect. IgE sensitization was studied by skin prick tests (SPT) and specific IgE concentrations (Pharmacia CAP system); 136 were sensitized. The treatments had significantly different effects if the infants had IgE sensitization; among them LGG gave superior results compared to placebo (p=0.036) (Fig. 1). The greatest effect of LGG was among sensitized patients with severe eczema (SCORAD>30). A large percentage of the patients had received antibiotics during treatment and their number differed in the treatment groups. When they were eliminated, the effect of LGG was more pronounced among patients with IgE-associated diseases and SCORAD >30 (p=0.008). Fecal samples proved that colonization with the supplemented probiotics was successful. Total counts of Lactobacilli increased in the probiotic groups, but decreased in the placebo group.
Fig. 1.
Effect of Lactobacillus rhamnosus strain GG (LGG), a mixture of 4 probiotic strains and placebo on the SCORAD score of infants with eczema and IgE sensitization. The columns show the mean reduction and +/- standard error of the mean in the all sensitized patients, those with SCORAD < 30 and those with SCORAD > 30.
Of the 10 published studies on the treatment of childhood eczema with probiotics, in 3 no effect was seen. These studies had the lowest proportion of patients with IgE sensitization (Table 1). In the remaining 7, the positive effect was associated with those sensitization, while the role of food allergy did not play any role in the success of the treatment. Probiotics have smaller effects on respiratory allergies in children. In a small preliminary study on the combined effect of probiotics and laser acupuncture a change in the peak flow variability was demonstrated in the treatment group, while the need of medication or quality of life were not changed [28]. A probiotic strain in fermented milk given for 12 months to 2-5 year-old children did not improve their asthma symptoms [29].
PREVENTION OF ATOPIC DISEASE IN CHILDREN
The first study to examine the possibility of preventing allergy in high risk infants investigated 159 mothers with a high-risk child. The pregnant mothers were randomly allocated to two groups, one of which received LGG 4 weeks before delivery and after delivery during breastfeeding. LGG was given infants only when they started formula feeding. LGG was given up to the age of 6 months and 57% of the infants received LGG directly. At the age of 2, the cumulative incidence of eczema in the LGG group was 23%, and in the placebo group was 46%. The relative risk for developing eczema was significantly lower in the LGG group (Table 2) [30]. At the age of 4, 107 infants attended a follow-up examination. The cumulative incidence of eczema was 26% in the LGG group and 44% in the placebo group [31], and the relative risk for the development of eczema at age of 4 remained significantly reduced (Table 2). An equal number of children in each group had respiratory allergic symptoms, and prevalence of IgE sensitization was similar at both the ages of 2 and 4. Seventy-three percent of the children attended the 7-year follow-up. The cumulative incidence of eczema remained significantly lower in the LGG group, 43% vs. 66%. Positive SPT tests were detected in 32% of the children with no difference between the groups. The incidence of allergic airway diseases was low and similar in both the study groups [32]. Kopp et al. replicated the above study by giving LGG or placebo to 105 pregnant mothers of high-risk infants for 4 to 6 weeks before delivery and to their infants for 6 months. At the age of 2, they found similar incidences of eczema (28% vs. 27%) and of IgE sensitization, but LGG was associated with an increased rate of recurrent wheezing episodes (26% vs. 9%) [33].
Table 2.
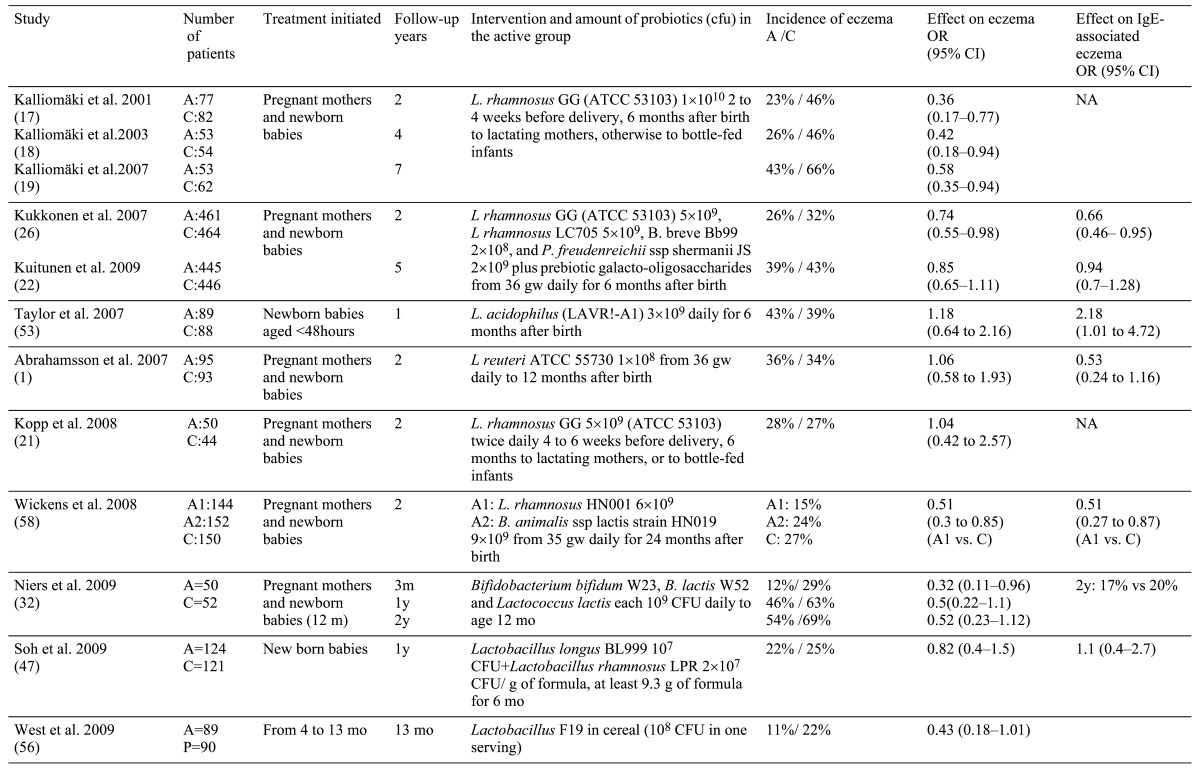
We conducted a double-blind, placebo-controlled trial involving 223 pregnant women carrying fetuses at increased risk of allergy [34]. The mothers recieved a mixture of 4 probiotic bacteria (Table 2) or placebo from their 36th week of gestation. Their infants were given the same probiotics. The infants also received prebiotic galacto-oligosaccharides, 0.8 g/day for 6 months. The strains given to the probiotic group were cultivated from feces. Significantly higher fecal counts for all Lactobacilli and_Bifidobacteria_ were found in the probiotic group than in the controls at the age of 6 months. The presence of probiotic strains in the feces was transient, and no differences in the colonization patterns were seen at 2 years.
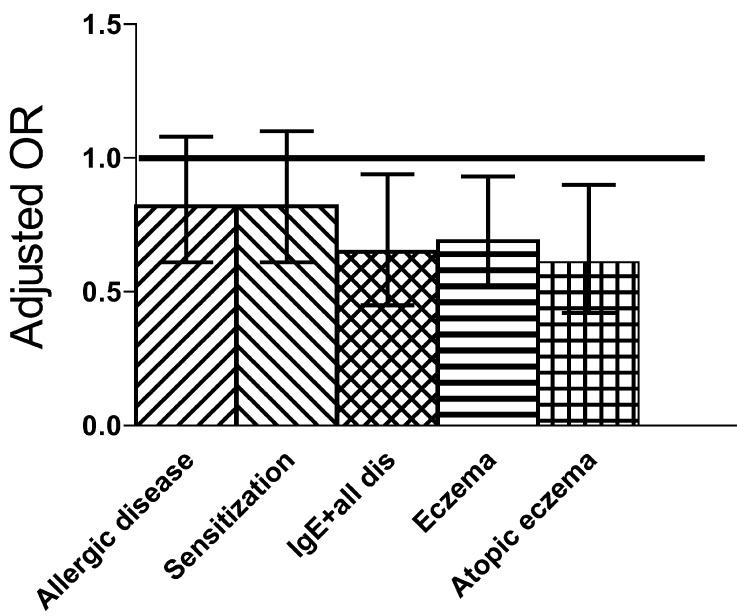
A total of 925 infants participated in the 2-year follow-up. The cumulative incidence of allergic diseases (food allergy, eczema, asthma, allergic rhinitis) did not differ significantly between the probiotic (32%) and the placebo (35%) groups, and IgE sensitization was not affected. However, compared to the placebo group, the probiotic group showed a reduction in all atopic (IgE-associated) diseases as assessed by SPT or specific IgE > 0.7 kU/l diseases (Fig. 2). Eczema constituted 88% of all allergic diseases by the age of 2 years, and occurred less frequently in the probiotic (26%) than in the placebo group (32%). The preventive effect of the probiotics was more pronounced against atopic (IgE-associated) eczema, the incidence of which in the probiotic group (12%) was significantly lower than in the placebo group (18%) (Fig. 2).
Fig. 2.
Odds ratios and their 95% confidence interval for the cumulative incidence of allergic symptoms, of IgE sensitization, of the combination of IgE sensitization and all allergic symptoms as well as that of eczema and eczema with IgE sensitization (atopic eczema) at the time when participants were 2 years old [26].
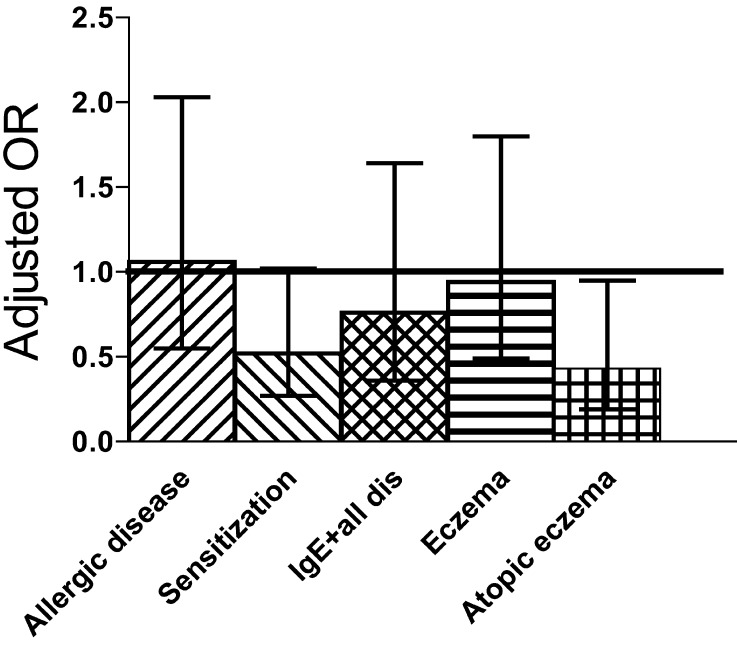
At the age of 5, 891 (88%) infants attended the follow-up examination [35]. The frequencies of allergic and IgE-associated allergic disease and sensitization in the probiotic and placebo groups were similar: 52.6 vs. 54.9%, 29.5 vs. 26.6% and 41.3% in both, respectively. Also, there were no differences in the cumulative incidences of eczema (39.3 vs. 43.3%), atopic eczema (24.0 vs. 25.1%), allergic rhinitis (20.7 vs. 19.1%) and asthma (13.0 vs. 14.1%) between the groups. Among the 148 children delivered by cesarean section, those who received probiotics had a lower frequency of IgE-associated allergic disease (Fig. 3), the cumulative prevalence for atopic eczema was significantly reduced (15.7 vs. 30.4%), and the prevalence of IgE antibodies to food allergens was significantly reduced (5% vs. 25%) [35]. We could also demonstrate that among the same infants the synbiotic preparation normalized the delayed development of_Bifidobacteria_ of the group. At the age of 6 months, the recovery of_Bifidobacteria_ from faeces of placebo group was 57%, while the probiotic group had the same 100% recovery as did both vaginally born groups.
Fig. 3.
Odds ratios and their 95% confidence interval for the cumulative incidence of allergic symptoms, of IgE sensitization, of the combination of IgE sensitization and all allergic symptoms as well as that of eczema and eczema with IgE sensitization (atopic eczema) in 149 children born by cesarean section when they were 5 years old [22].
Altogether, the synbiotic preparation to infants was safe and was not associated with any adverse symptoms such as abdominal pains, diarrhea or excessive crying. The groups had identical growth [36], in fact the administration of synbiotics conferred some advantages: fewer antibiotic treatments before the age of 6 months, respiratory infections between 6 to 24 months [36]. In addition, the proportion of infants given probiotics who showed t protective IgG titres to Haemophilus influenzae type b was higher at the age of 6 months [37].
Although 9 studies have been published on the potential of probiotics to prevent allergic diseases in childhood (Table 2), only one, Kalliomaki’s study, was fully successful, while four studies reported no significant effect. The four others reported partial success, either in a subgroup of patients, at some time point as in our studies, or only one bacterial strain being effective. One firm conclusion is that one should not make meta-analysis on the use of various types of probiotic bacteria in the prevention of allergy; only those using the exactly same strains and protocols may be compared. Both in the prevention as in treatment different strains of probiotic bacteria have quite different effects.
MODE OF ACTION OF PROBIOTICS
Several studies have described immunologic effects of probiotics on human cells or on experimental animals. However, the majority provide no information relevant to human_in vivo_ conditions. Effects of probiotic bacteria on isolated human cells do not reflect conditions in the intestine, where contact with bacteria takes place only with epithelial cells and with extensions of dendritic cells [38].
According to Majamaa and Isolauri, probiotics may reduce inflammation in the intestine [23]. The inflammatory cytokine, tumor-necrosis-factor-α (TNF- α), content was reduced in the fecal extracts from patients receiving LGG, while no change took place in the extracts from controls [23]. Probiotics have been suggested to act by reducing the permeability of the intestine [39]. In a double-blind, placebo-controlled cross-over study, probiotic treatment resulted in a lower ratio of lactulose/ mannitol in the urine [39]. We, however, did not find any change in intestinal permeability during the treatment of infants with eczema with either LGG or a combination of probiotic strains [40].
We found no difference in the TNF-α content in the feces of patients receiving either probiotics or placebo [41]. LGG treatment resulted in a greater increase in concentration of IgA after a positive CM challenge test of IgE-mediated CM allergic infants than that in controls [42]. In the prevention study, we discovered that high fecal IgA concentrations at the age of 6 months was associated with protection from atopic (IgE-associated) diseases by the age of 2 years. Probiotics associated with increased concentrations of inflammatory markers, fecal α1-antitrypsin and calprotectin, and tended to augment fecal IgA concentrations [43]. We therefore infer that in the intestine, probiotics may enhance both the inflammation and immune defence of the gut.
When we studied the secretion of various cytokines by peripheral blood mononuclear cells (PBMC) before and after treatment with probiotics and placebo, the secretion of interferon-γ (IFN-γ) was significantly lower in IgE-mediated CMA than in infants without CMA [44]. Treatment with LGG resulted in a significant increase in the ability of PBMC to secrete IFN- γ among patients with atopic eczema, the same group which benefited clinically from the treatment [44]. The same increase was observed for IFN- γ responses to mitogens and staphylococcal enterotoxin B(SEB) in infants with eczema given Lactobacillus fermentum VRI 003 [45].
Both in the treatment and prevention studies, we found evidence that probiotics give rise to low grade inflammation, which is probably associated with the healing/protective actions of the probiotics. During treatment of eczema with LGG, we found a significant increase in the blood concentration of C-reactive protein (CRP) in infants with IgE-associated eczema showing a favorable clinical effect. The LGG treatment affected the serum concentration of IL-6, which was significantly increased in the group showing significantly increased CRP. IL-6 may induce the secretion of CRP in the liver. In infants at high risk of allergy, the mixture of probiotics was associated with an increase in CRP at the age of 6 months; they also had higher IL-10 levels. Furthermore, they had higher levels of serum IgA and IgE levels than those given placebo [46]. We, therefore, infer that probiotics induce low-grade inflammation characterized by increases in CRP, total IgA, total IgE and IL-10 levels. These changes closely resemble those seen in helminth infections which are associated with induction of regulatory mechanisms and reduced incidence of allergy [47].
Commensal microbiota and their recognition by toll-like receptors (TLR) are important in host defence, in directing specific immune responses of the gut, and in the development of food allergies in experimental animals [48, 49]. Probiotic strains have the ability to adhere to gut epithelial cells, which may express TLRs [50], and stimulate these cells to produce cytokines. Extensions of dendritic cells in the intestinal lumen function in the development of immune responses in the gut [38]. These cells may be stimulated by probiotic bacteria. In vitro, isolated myeloid dendritic cells express TLR-2 and may be stimulated by LGG to express inflammatory cytokines [51]. We thus infer that stimulation of innate immunity may be the cause of the observed inflammatory signs and beneficial clinical effects.
CONCLUSIONS
Studies using probiotics to treat and prevent allergy have shown promising, though highly variable results. Clearly the major variable between the studies has been the use of different bacterial strains; only results using the same strain and similar protocol are comparable.
We believe that the concept is of administering probiotics to treat and prevent allergy is valid:since the intestines of newborns and also older infants may be transiently colonized with bacteria given orally. These bacteria have an effect on the immune system of the recipient and also have clinical effects. Probiotics have been effective in the treatment of eczema in infants, though the results are modest. Regarding prevention, we saw the most long-lasting results in the subgroup of children born by cesarean section. In that event we can introduce probiotics to the intestine with low counts of bacteria to achieve higher counts of given strains. However, in all studies to date, the colonization achieved by given strains has been transient.
In attempts to prevent allergy in high-risk infants the results suggest that the intervention should start for mothers before delivery to make sure that the birth canal of the mother is colonized by probiotics. Whether both infants and mother should continue probiotics after birth is an open question, since giving probiotics directly to infants is proven to result in colonization.
Finding the most efficient strain of probiotics is a big challenge. In vitro studies may not simulate conditions in vivo, though some qualities of probiotic bacteria may be found in such studies. Experimental animals have a gut microbial flora, which e.g. in mice has less than 50% DNA identity with human microbiota. Therefore, much caution is needed in extending the interpretation of results from experimental animal studies to humans. Even in human experiments, we do not know what type of immune reaction will result from the ingestion of probiotics and need to prove their effect in allergy treatment and prevention. For more efficient and long-lasting effects we need more potent and long-lasting stimulation of the mucosal immune system. Maybe the intervention has to continue life-long, its type has to be changed or boosted at intervals. Challenges to find a safe and efficient intervention for the primary prevention of allergies are great.
REFERENCES
- 1.Siltanen M, Kajosaari M, Poussa T, Saarinen KM, Savilahti E. 2003. A dual long-term effect of breastfeeding on atopy in relation to heredity in children at 4 years of age. Allergy 58: 524–530 [DOI] [PubMed] [Google Scholar]
- 2.Gerrard JW, Geddes CA, Reggin PL, Gerrard CD, Horne S. 1976. Serum IgE levels in white and metis communities in Saskatchewan. Ann Allergy 37: 91–100 [PubMed] [Google Scholar]
- 3.Strachan DP. 1989. Hay fever, hygiene, and household size. BMJ 299: 1259–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strachan DP. 2000. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax 55:(Suppl 1): S2–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, Bonini S. 2000. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 320: 412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E. 2001. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358: 1129–1133 [DOI] [PubMed] [Google Scholar]
- 7.Bach JF. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347: 911–920 [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6: 345–352 [DOI] [PubMed] [Google Scholar]
- 9.Salminen SJ, Gueimonde M, Isolauri E. 2005. Probiotics that modify disease risk. J Nutr 135: 1294–1298 [DOI] [PubMed] [Google Scholar]
- 10.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegard IL, Wold AE. 2006. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res 59: 96–101 [DOI] [PubMed] [Google Scholar]
- 11.Grönlund MM, Lehtonen OP, Eerola E, Kero P. 1999. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 28: 19–25 [DOI] [PubMed] [Google Scholar]
- 12.Tuohy KM, Rouzaud GC, Bruck WM, Gibson GR. 2005. Modulation of the human gut microflora towards improved health using prebiotics–assessment of efficacy. Curr Pharm Des 11: 75–90 [DOI] [PubMed] [Google Scholar]
- 13.Brunser O, Figueroa G, Gotteland M, Haschke-Becher E, Magliola C, Rochat F, Cruchet S, Palframan R, Gibson G, Chauffard F, Haschke F. 2006. Effects of probiotic or prebiotic supplemented milk formulas on fecal microbiota composition of infants. Asia Pac J Clin Nutr 15: 368–376 [PubMed] [Google Scholar]
- 14.Adlerberth I, Jalil F, Carlsson B, Mellander L, Hanson LA, Larsson P, Khalil K, Wold AE. 1998. High turnover rate of Escherichia coli strains in the intestinal flora of infants in Pakistan. Epidemiol Infect 121: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Hertzen L, Laatikainen T, Pitkanen T, Vlasoff T, Makela MJ, Vartiainen E, Haahtela T. 2007. Microbial content of drinking water in Finnish and Russian Karelia–implications for atopy prevalence. Allergy 62: 288–292 [DOI] [PubMed] [Google Scholar]
- 16.Sepp E, Julge K, Vasar M, Naaber P, Bjorksten B, Mikelsaar M. 1997. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr 86: 956–961 [DOI] [PubMed] [Google Scholar]
- 17.Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. 2001. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 108: 516–520 [DOI] [PubMed] [Google Scholar]
- 18.Sepp E, Julge K, Mikelsaar M, Bjorksten B. 2005. Intestinal microbiota and immunoglobulin E responses in 5-year-old Estonian children. Clin Exp Allergy 35: 1141–1146 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Shimojo N, Tajiri Y, Kumemura M, Kohno Y. 2007. Differences in the composition of intestinal Bifidobacterium species and the development of allergic diseases in infants in rural Japan. Clin Exp Allergy 37: 506–511 [DOI] [PubMed] [Google Scholar]
- 20.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 159: 1739–1745 [PubMed] [Google Scholar]
- 21.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088> [DOI] [PubMed] [Google Scholar]
- 22.FAO/WHO 2002. Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Guidelines for the evaluation of probiotics in food: report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London Ontario, Canada. http://www.who.int// foodsafety/publications/fs_management/probiotics2/ en/index.html
- 23.Majamaa H, Isolauri E. 1997. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 99: 179–185 [DOI] [PubMed] [Google Scholar]
- 24.SCORAD1993. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 186: 23–31 [DOI] [PubMed] [Google Scholar]
- 25.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. 2000. Probiotics in the management of atopic eczema. Clin Exp Allergy 30: 1604–1610 [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, Paerregaard A. 2003. Effect of probiotic_Lactobacillus_ strains in children with atopic dermatitis. J Allergy Clin Immunol 111: 389–395 [DOI] [PubMed] [Google Scholar]
- 27.Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. 2005. Probiotics in the treatment of atopic eczema/ dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy 60: 494–500 [DOI] [PubMed] [Google Scholar]
- 28.Stockert K, Schneider B, Porenta G, Rath R, Nissel H, Eichler I. 2007. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of Traditional Chinese Medicine. Pediatr Allergy Immunol 18: 160–166 [DOI] [PubMed] [Google Scholar]
- 29.Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, Radaelli G. 2007. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res 62: 215–220 [DOI] [PubMed] [Google Scholar]
- 30.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357: 1076–1079 [DOI] [PubMed] [Google Scholar]
- 31.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361: 1869–1871 [DOI] [PubMed] [Google Scholar]
- 32.Kalliomäki M, Salminen S, Poussa T, Isolauri E. 2007. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1019–1021 [DOI] [PubMed] [Google Scholar]
- 33.Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. 2008. Lactobacillus GG has in vitro effects on enhanced interleukin-10 and interferon-gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clin Exp Allergy 38: 602–610 [DOI] [PubMed] [Google Scholar]
- 34.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. 2007. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 119: 192–198 [DOI] [PubMed] [Google Scholar]
- 35.Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Haahtela T, Savilahti E. 2009. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 123: 335–341 [DOI] [PubMed] [Google Scholar]
- 36.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. 2008. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics 122: 8–12 [DOI] [PubMed] [Google Scholar]
- 37.Kukkonen K, Nieminen T, Poussa T, Savilahti E, Kuitunen M. 2006. Effect of probiotics on vaccine antibody responses in infancy–a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol 17: 416–421 [DOI] [PubMed] [Google Scholar]
- 38.Mowat AM. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3: 331–341 [DOI] [PubMed] [Google Scholar]
- 39.Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. 2004. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr 145: 612–616 [DOI] [PubMed] [Google Scholar]
- 40.Kuitunen M, Viljanen M, Savilahti E. 2007. Probiotics and intestinal permeability in infants with cow’s milk allergyand eczema. Int J Probiotics Prebiotics 2: 239–244 [Google Scholar]
- 41.Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, Vaarala O, Savilahti E. 2005. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J Allergy Clin Immunol 115: 1254–1259 [DOI] [PubMed] [Google Scholar]
- 42.Viljanen M, Kuitunen M, Haahtela T, Juntunen-Backman K, Korpela R, Savilahti E. 2005. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol 16: 65–71 [DOI] [PubMed] [Google Scholar]
- 43.Kukkonen K, Kuitunen M, Haahtela T, Korpela R, Poussa T, Savilahti E. 2010. High intestinal IgA indicates reduced risk for IgE-associated allergic disease. Pediatr Allergy Immunol. 21: 67–73 [DOI] [PubMed] [Google Scholar]
- 44.Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, Savilahti E. 2004. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J Allergy Clin Immunol 114: 131–136 [DOI] [PubMed] [Google Scholar]
- 45.Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, Richmond P. 2005. Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin Exp Allergy 35: 1557–1564 [DOI] [PubMed] [Google Scholar]
- 46.Marschan E, Kuitunen M, Kukkonen K, Poussa T, Sarnesto A, Haahtela T, Korpela R, Savilahti E, Vaarala O. 2008. Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin Exp Allergy 38: 611–618 [DOI] [PubMed] [Google Scholar]
- 47.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 202: 1199–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241 [DOI] [PubMed] [Google Scholar]
- 49.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. 2004. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 172: 6978–6987 [DOI] [PubMed] [Google Scholar]
- 50.Cario E. 2005. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 54: 1182–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veckman V, Miettinen M, Matikainen S, Lande R, Giacomini E, Coccia EM, Julkunen I. 2003. Lactobacilli and streptococci induce inflammatory chemokine production in human macrophages that stimulates Th1 cell chemotaxis. J Leukoc Biol 74: 395–402 [DOI] [PubMed] [Google Scholar]