The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier (original) (raw)
. Author manuscript; available in PMC: 2015 Mar 1.
Published in final edited form as: Biochim Biophys Acta. 2013 Sep 7;1841(3):401–408. doi: 10.1016/j.bbalip.2013.08.020
Summary
This review covers the background to discovery of the two key lipoxygenases (LOX) involved in epidermal barrier function, 12_R_-LOX and eLOX3, and our current views on their functioning. In the outer epidermis, their consecutive actions oxidize linoleic acid esterified in ω-hydroxy-ceramide to a hepoxilin-related derivative. The relevant background to hepoxilin and trioxilin biochemistry is briefly reviewed. We outline the evidence that linoleate in the ceramide is the natural substrate of the two LOX enzymes and our proposal for its importance in construction of the epidermal water barrier. Our hypothesis is that the oxidation promotes hydrolysis of the oxidized linoleate moiety from the ceramide. The resulting free ω-hydroxyl of the ω-hydroxyceramide is covalently bound to proteins on the surface of the corneocytes to form the corneocyte lipid envelope, a key barrier component. Understanding the role of the LOX enzymes and their hepoxilin products should provide rational approaches to ameliorative therapy for a number of the congenital ichthyoses involving compromised barrier function.
Keywords: Lipoxygenase, linoleic acid, hepoxilin, trioxilin, ceramide, ichthyosis, essential fatty acid
The mammalian epidermal water barrier: a short primer
One of the most important functions of the skin is the maintenance of a barrier to water vapor diffusion to the environment [1–3]. Substantial evidence demonstrates that the main determinant of the permeability properties of the skin is the lipid composition and organization in the outer layers of the epidermis, the stratum corneum [4, 5]. The mammalian stratum corneum (SC) consists of flat, dead cells, called corneocytes, surrounded by a protein layer, the cornified envelope (CE), in turn surrounded by a monolayer of lipids, the corneocyte lipid envelope (CLE), covalently attached to the CE. The spaces between corneocytes are filled with a matrix of intercellular lipids, mainly cholesterol, free fatty acids, and ceramides, which altogether organize in layers called lamellae in mammals. The backbones of the lamellae are the ceramides, molecules formed by a sphingoid base amide-linked to a fatty acid of varying chain length [6–12]. The intercellular lamellae are thought to impede excessive evaporation through the skin, as their disruption leads to increases in transepidermal water loss (TEWL) [13, 14]. Fundamental to the formation of a fully competent barrier is the need of linoleic acid, incorporated in a particular class of ceramides unique to the epidermis (EOS, esterified omega-hydroxyacyl-sphingosine) [5, 15, 16]. The activity of epidermal lipoxygenases (LOX) is crucial to understand the physiological process that links the need of essential fatty acids to ensure the normal formation and maintenance of the permeability barrier of the skin. Figure 1 illustrates the main structural components of the barrier relevant to the role of LOX enzymes.
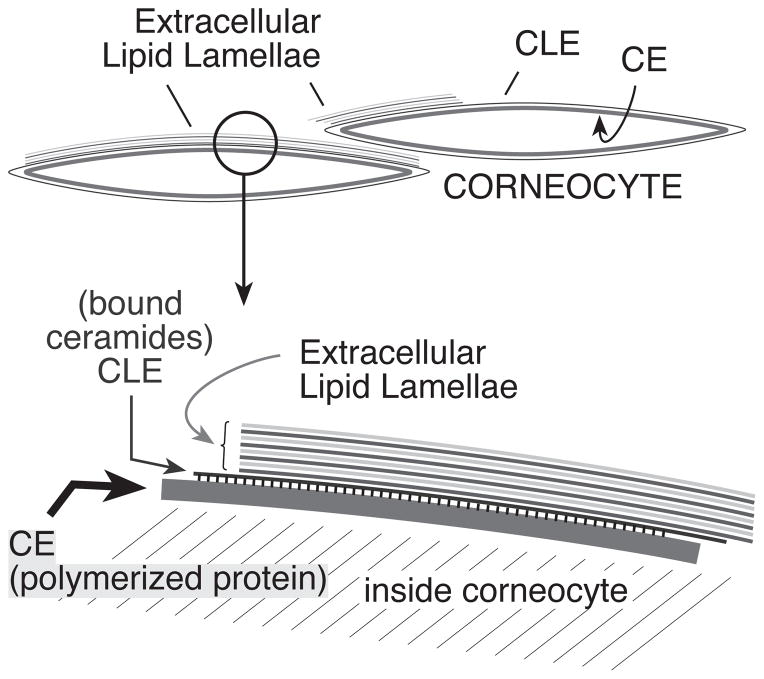
Figure 1. Structural components of the epidermal barrier.
This cartoon shows the arrangement in and around corneocytes in the barrier layer, with a closeup illustrated in the segment below. In the stratum corneum, a coat of polymerized protein, the corneocyte envelope (CE) lies just inside the periphery of the flattened corneocytes. Bonded to the CE is a monolayer of covalently-bound ceramides and fatty acids, the corneocyte lipid envelope (CLE). The CLE is considered to act as a scaffold for organization of the lamellae of extracellular lipids comprised of cholesterol, ceramides, and fatty acids. This figure was originally published in The Journal of Biological Chemistry [87] © the American Society for Biochemistry and Molecular Biology.
Barrier genes and ichthyosis
Genetic anomalies in the complex physiological process of epidermal barrier formation result in scaly skin diseases (ichthyoses) with medical complications like dehydration, infections, chronic blistering, and rapid-calorie loss [17–20]. Gene mutations implicated in the autosomal recessive congenital ichthyoses (ARCI) include ALOX12B, ALOX3, CYP4F22, ichthyin and TGM-1 [18, 21–24]. Cytochrome P450 CYP4F22 may be responsible for synthesis of the ω-hydroxyl of ω-hydroxy-acyl ceramides. ichthyin is a predicted transmembrane protein with undetermined function. TGM-1 cross-links the proteins forming the corneocyte envelope (CE), and is also implicated through in vitro studies and a knock-in mouse mutation in catalyzing the covalent bond between ω-hydroxy-acyl ceramides and the CE, forming the CLE [25, 26], (albeit with human studies showing that lamellar ichthyoses due to mutations in this isozyme have a normal CLE) [27]. The fact that inactivating mutations in all of these genes produce related skin phenotypes supports the idea that they are elements of the same physiological process.
The genetic evidence establishes that the integrity of the mammalian epidermal water barrier requires the normal functioning of 12_R_-lipoxygenase (12_R_-LOX, human gene ALOX12B) and epidermal lipoxygenase-3 (eLOX3, gene ALOXE3) [18, 21, 28–30]. LOX enzymes are dioxygenases that oxygenate lipids (polyunsaturated fatty acids and their esters), yet their specific role in skin barrier function is not well understood. In general terms, and taking parallels from better known LOX enzymes, their functions may be to produce oxidized lipids as signaling molecules, to induce structural changes through an enzyme-catalyzed lipid peroxidation, or rarely, to help initiate the mobilization of lipids (reviewed in ref. [31]). Our current model suggests a function mainly related to the second of these possibilities, the induction of structural changes.
The present paper stands as an update and major revision of an earlier review [32]. Before going into specifics on our current model of the workings of 12_R_-LOX and eLOX3 in epidermal barrier function, we present overviews of the background history of the two LOX enzymes and of their essential fatty acid substrate in the skin.
Historical perspective on 12_R_-Lipoxygenase
Mammalian 12_R_-LOX and eLOX3 remained undetected until the late 1990’s. The existence of LOX enzymes in the plant kingdom was recognized over 70 years ago [33], and the more modest levels of LOX enzyme activities in animal tissues were first identified in the mid-1970s. That work established the existence of a 12_S_-LOX in blood platelets [34, 35], a 5_S_-LOX in leukocytes (catalyzing the first steps in leukotriene biosynthesis) [36], and a 15_S_-LOX in reticulocytes [37]. Closer examination over the years revealed the expression of these enzymes in many other mammalian tissues [38].
The “_S_” in 12_S_-, 5_S_- and 15_S_-LOX enzymes refers to the mirror image form of the fatty acid hydroperoxide product. “_R_” chirality LOX enzymes were unknown. Then in the mid-1980s enzyme activities in invertebrate animals (corals, starfish, sea urchins) were characterized thatsynthesized “_R_” chirality fatty acid hydroperoxides [39]. In the mid-1990s, purification of one of these invertebrate _R_-LOX proteins and its molecular cloning revealed a close sequence homology to the plant and animal _S_-LOX enzymes [40]. Differences in substrate binding were proposed that could explain how structurally related enzymes can form products of one mirror image form or the other [40, 41].
The first indication of a role for oxidized polyunsaturated fatty acids in skin biology came in the mid 1970’s when an accumulation of 12-HETE (12-hydroxyeicosatetraenoic acid, i.e. 12-hydroxy-arachidonic acid) and free arachidonic acid was discovered in the involved epidermis of psoriasis [42]. At the time, it was presumed that the 12-HETE arose from the activity of 12_S_-LOX (subsequently identified in the germinal layer cells of the mammalian epidermis [43]). A decade later the psoriatic product was shown to be mainly 12_R_-HETE [44]. It took another decade of research before the “culprit” enzyme in this case was identified as the uncommon type of _R_-lipoxygenase, 12_R_-LOX, the only _R_-LOX in mammalian tissues [45, 46].
Historically the enzymatic oxidation of arachidonic acid in mammalian biology shows a strong association with the biosynthesis of pro-inflammatory lipid mediators [47], and this remains a focus of eicosanoids in skin inflammation and immunity [48]. Synthesis of the prostaglandin products of the cyclooxygenase pathway is inhibited by NSAIDs, and the leukotriene products of 5-LOX have their pro-inflammatory actions blocked by receptor antagonists such as Singulair® and Accolate® [47]. Initially therefore, it was natural to equate the synthesis of 12_R_-HETE in psoriasis with the inflammation in the epidermis. Yet 12_R_-HETE itself showed only modest pro-inflammatory activity [49]. From a current perspective, the abundance of 12_R_-HETE in psoriasis may reflect merely the co-occurrence of free arachidonic acid (not present normally, but a facet of the inflammation in psoriasis) with an over-abundance of the granular cells of the upper epidermis, the natural site of 12_R_-LOX expression in healthy skin. Having the arachidonic acid available as substrate together with high expression of 12_R_-LOX may account for the excess of 12_R_-HETE in psoriatic epidermis. In fact in normal epidermis, there is only one definitive account of the synthesis of 12_R_-HETE, barely detectable as a product of [14C]arachidonic acid metabolism in human hair roots [50].
In primary cultures of human keratinocytes there is no detectable 12_R_-LOX activity (they produce only traces of 15-HETE [51, 52]). The absence of 12_R_-LOX is likely attributable to its association with the late stages of keratinocyte differentiation, a condition that requires special long-term treatments to mimic in culture [53], and not studied so far with regard to LOX enzyme activities. (A prominent 12-LOX activity was detected in normal human epidermis, but the R/S chirality of the 12-HETE was not determined [54]). Despite the hard to detect activity, immunohistochemical evidence clearly defines the occurrence of 12_R_-LOX (and eLOX3) in the outermost cells of the stratum granulosum in normal mammalian epidermis [29, 30]. We also have evidence from in situ analysis in human skin that 12_R_-LOX mRNA expression occurs in the outermost epidermal cells that retain a nucleus (Figure 2). Although 12_R_-LOX expression is not entirely skin-specific, no symptomology or phenotype is reported in relation to the limited expression in other tissues. While mRNA transcripts for 12_R_-LOX are detectable by RT-PCR in several other tissues (and similarly for eLOX3) [55, 56], the only instance confirmed so far with catalytically active enzyme is a weak activity of 12_R_-LOX in human tonsils [57].
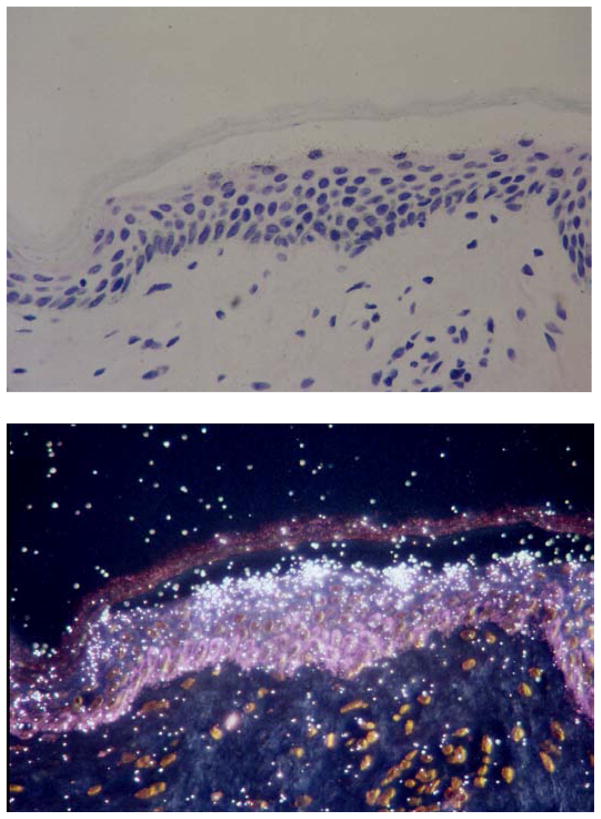
Figure 2. Expression of 12_R_-LOX mRNA in human epidermis.
Top: the bright-field view shows an H & E stained section of human abdominal skin. Below: the dark-field view shows hydridization of the antisense probe (radiolabeled cRNA specific for human 12_R_-LOX) over the outermost epidermal cells that retain a nucleus (upper stratum granulosum). Discarded human skin tissue was obtained according to protocols approved by Vanderbilt University Institutional Review Board. In situ was performed as described [98–100].
The role of 12_R_-LOX in healthy skin is quite different from the earlier implications of its appearance in psoriasis, and we now know that the natural substrate of 12_R_-LOX is a linoleate ester rather than arachidonic acid. As firmly established from analysis of the gene inactivation in humans and mice, the biological role of 12_R_-LOX is helping to seal the permeability barrier of the outer epidermis, an activity that entails intimate cooperation with eLOX3 in synthesis of linoleate-derived “hepoxilins”.
Hepoxilins
Fatty acid hydroperoxides are easily broken down through non-enzymatic transformations, including into fatty acids in which the original two oxygen atoms of the hydroperoxide moiety are rearranged in the molecule to form one epoxide and one hydroxyl group [58]. Non-enzymatically, these epoxy-hydroxy (also called epoxyalcohol) derivatives are produced as a mixture of isomers.
The name “hepoxilin” was coined in the mid-1980s to describe epoxyalcohol products of the 12_S_-LOX-derived arachidonic acid hydroperoxide, 12_S_-HPETE [59]. They were considered tobe derived enzymatically, although at the time there was scant evidence to support the contention. “Hepox” stood for hydroxy-epoxy and “ilin” came from an activity as an insulin secretagogue (albeit an activity not confirmed to be of physiological significance). All fatty acid hydroperoxides readily give rise to epoxyalcohol derivatives, although strictly only derivatives of the arachidonic acid hydroperoxide 12_S_-HPETE merit the connotation “hepoxilin”. Nonetheless, all the others can be considered as hepoxilin-related or hepoxilin-type fatty acid derivatives [32]. For example, the term is commonly applied to the epoxyalcohols formed by eLOX3 from linoleate hydroperoxides.
Hepoxilins are classified as A-type or B-type [60]. When a fatty acid hydroperoxide rearranges into epoxyalcohols, the new OH group can be separated from the new epoxy group by a double bond (hepoxilin A, HxA, chemically an “allylic” epoxide, readily hydrolyzed in mild acid e.g. pH 3), or the OH can be next to the epoxide (hepoxilin B, HxB, more stable to mild hydrolysis conditions) (Figure 3). Non-enzymatic synthesis produces a mixture of HxA- and HxB-type epoxyalcohols, and each of these is a mixture of _R_- and _S_-hydroxy isomers. So, non-enzymatically, four major hepoxilin products are formed from 12_S_-HPETE. Because of the differences in chemical stability, the HxB-type is the more commonly detected in tissue extracts, whereas the HxA-type may appear as the corresponding hydrolysis products, the trioxilins (Figure 3). The detection of hepoxilin and trioxilin derivatives in human epidermis occurred along these lines.
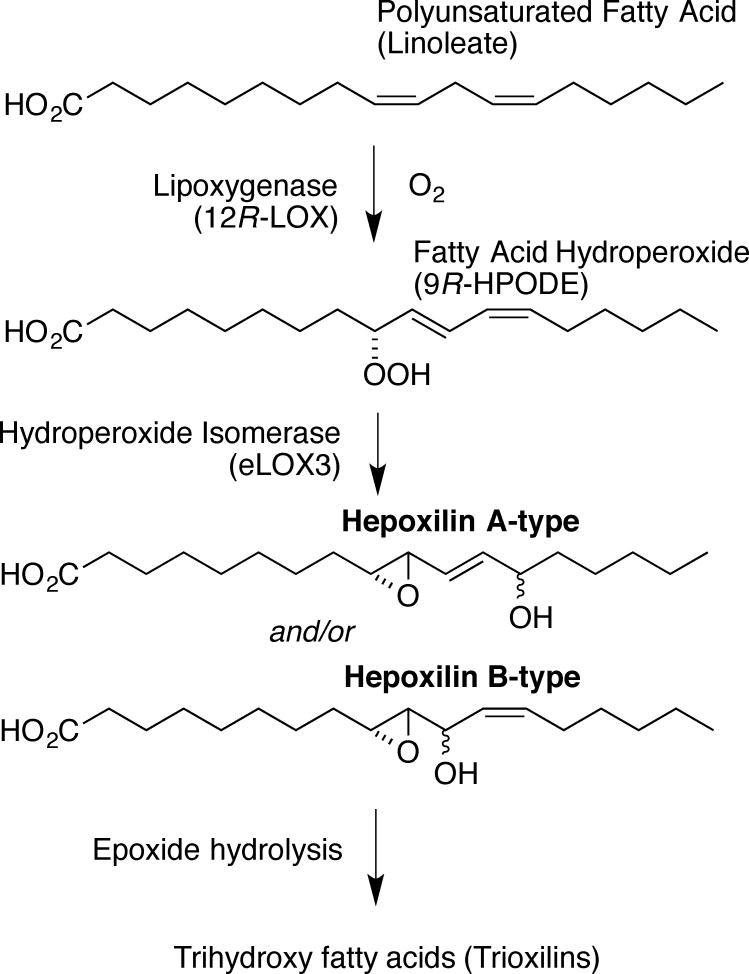
Figure 3. Lipoxygenase catalysis to hepoxilins and trioxilins.
In this example, linoleic acid (top) is metabolized by 12_R_-LOX to 9_R_-hydroperoxy-linoleic acid, a potential substrate for conversion to HxA-type of HxB-type hepoxilins by eLOX3. Hydrolysis of the epoxide gives trihydroxy fatty acids (Trioxilin-type triols).
Analysis of the hepoxilin/trioxilins in human and murine epidermis suggests that the majority of the free products are “true” hepoxilins originating from 12_S_-HPETE. Freshly isolated human epidermal cells catalyze the stereoselective biosynthesis of HxB3 from arachidonic acid and 12_S_-HPETE [54, 61], and the levels are greatly increased in the lesion epidermis in psoriasis, along with the corresponding trioxilins [62]. The recent studies in Aloxe3 knockout mice indicate that free hepoxilins are measureable in the normal murine epidermis (along with purely 12_S_-HETE) and that the synthesis is eLOX3-dependent [30].
Aside from hydrolysis promoted by the acidic conditions used for fatty acid extraction, formation of trioxilins in cells is likely to be catalyzed by epoxide hydrolases (EH). In 1989 a specifichepoxilin EH was isolated from rat liver with an observed preference for hepoxilin A3 over leukotriene A4 [63]. However, it was shown recently that this activity is indistinguishable from mammalian soluble epoxide hydrolase (sEH) [64]. Because moderate expression of sEH has been shown in human epidermis, the presence of trioxilins both from AA and LA is possible, warranting further investigation [65]. The substrate specificity with regard to possible EOS trioxilin moieties should also be examined. Novel candidate EH genes were described recently and the first of these enzymes to be characterized (termed EH3) is highly active in the hydrolysis of fatty acid epoxides [66]. “Skin” is among the tissues showing strong expression of EH3 [66], and clearly it is of interest to define the location of its expression more precisely.
A historical perspective on eLOX3
eLOX3 was cloned from mouse epidermis cDNA in the late 1990s by Fürstenberger, Krieg and colleagues as one of three newly discovered LOX genes [67, 68]. Two of the expressed proteins were characterized as the mouse 8_S_-LOX and 12_R_-LOX. No catalytic activity could be found with the third, so it could only be named as epidermal lipoxygenase-3 (eLOX3, human gene ALOXE3) [69].
The paradigm-shifting report that mutations in the human 12_R_-LOX or eLOX3 genes are associated with congenital ichthyosis included the insightful suggestion that because a mutation in either of the two LOX genes produces a similar phenotype, the two LOX enzymes may function in the same metabolic pathway [21]. This prompted further investigation of the biochemical activities of eLOX3, with the finding that the enzyme exhibits hydroperoxide isomerase activity, converting fatty acid hydroperoxides to specific hepoxilin-type derivatives (and also to fatty acid ketones) [70]. In contrast to the non-enzymatic hepoxilin synthesis discussed in the previous section, the hepoxilin products of eLOX3 are specific epoxyalcohol isomers, with just one chiral form of the new hydroxy group. For example, eLOX3 converts 12_R_-HPETE specifically to an 8_R_-hydroxy-11_R_,12_R_-epoxide, a single HxA-type epoxyalcohol [70]. Mechanistic studies on eLOX3 suggest that its unusual hydroperoxide isomerase activity is related to a lack of access of molecular oxygen within the active site, which allows redox cycling of the catalytic iron and efficient metabolism of fatty acid hydroperoxides [71, 72].
We hypothesize that it is linoleate esterified in epidermal-specific ceramides that are the natural substrates for 12_R_-LOX and eLOX3 and that these activities underlie the role of the LOX genes in skin barrier function. Actually, the linoleate hydroperoxides 9-HPODE and 13-HPODE are not outstandingly good substrates of eLOX3, at least not as the free fatty acids [73]. But this is not directly relevant according to our current hypothesis on the natural physiological substrates of 12_R_-LOX and eLOX3.
The crucial requirement for one essential fatty acid, linoleate
When the hydroperoxide isomerase activity of eLOX3 was first discovered it was shown that 12_R_-HPETE is a particularly good substrate [70]. If 12_R_-LOX and eLOX cooperate in their activities to produce a hepoxilin-type product, it appeared to make sense that the primary 12_R_-LOX product from arachidonic acid (12_R_-HPETE) should be the substrate for eLOX3. But in retrospect this was discounting a long line of evidence from studies of essential fatty acids and their role in the epidermis. The fact is that arachidonic acid is almost absent from the outermost cells of the mammalian epidermis. There is excellent biochemical evidence from fatty acid analyses and from feeding studies in animals that the physiologically relevant essential fatty acid in relation to mammalian epidermal barrier function is solely linoleic acid [74].
The need for an essential fatty acid in barrier function was first recognized through the classic work of George and Mildred Burr, who discovered the dietary requirement for an “essential fatty acid” (EFA). They showed that one of the obvious symptoms of a dietary deficiency is increased TEWL and the development of a scaly skin phenotype [75, 76]. Topical application of EFA alleviates the skin symptoms [77]. Later it was shown that although feeding arachidonic acid cures the symptoms, the mechanism involves its retroconversion to linoleate [78]. The scaly skin also develops in EFA deficiency in humans and is reversible by adding linoleate back to the diet [79].
A key observation, highly relevant to the involvement of LOX enzymes, is that EFA deficiency is associated with a loss of linoleate (C18:2ω6) in the epidermis and its replacement with oleate (C18:1ω9). Oleate has only one double bond and is not a LOX substrate. Thus, eitherinactivating mutations in one of the key LOX enzymes or a deficiency in the epidermal LOX substrate (linoleate) results in loss of integrity of the epidermal water barrier.
EFA and ceramides, intimately linked in the epidermis
Linoleate is by far the most abundant EFA in the epidermis, wherein it is mainly esterified to the omega hydroxyl of the amide-linked very long-chain fatty acid (VLFA, C28–C36) in a class of ceramide specific to the epidermis, esterified omega-hydroxyacyl-sphingosine (EOS) [16]. EOS is an important structural ceramide in the intercellular lamellae. In addition, a portion is further converted to omega-hydroxyacyl-sphingosine (OS) and omega-hydroxy-VLFA that are covalently attached to the external face of the cornified cell envelope (CE) composed of cross-linked proteins (Figure 4) [25, 80]. These covalently bound lipids form the corneocyte lipid envelope (CLE) [81].
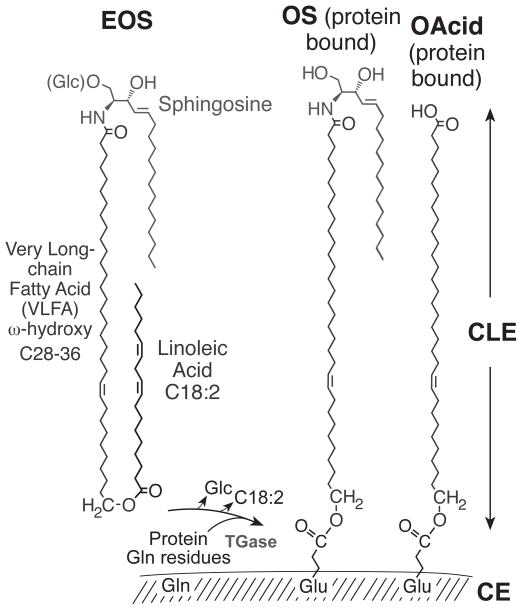
Figure 4. Epidermal specific ceramides.
EOS in the outer epidermis is esterified mainly with linoleic acid (C18:2) and is glucosylated at C-1 of the sphingosine (Glc-EOS) early in differentiation. After hydrolysis of the glycosidic and ester bonds, the resulting OS is esterified by transglutaminase (TGase) to glutamines in the cross-linked proteins of the CE [25]. The ester linkage in the resulting glutamates bonds the monomolecular lipid coating, the CLE, to the outer face of the CE. ω-Hydroxy-VLFA (OAcid, shown on the right side) are also ester-linked components of the CLE. This figure was originallypublished in The Journal of Biological Chemistry [87] © the American Society for Biochemistry and Molecular Biology.
Proposed role of linoleate and LOX enzymes in construction of the epidermal barrier
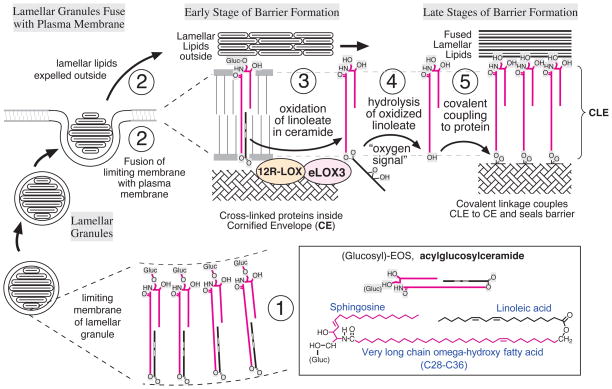
Figure 5 presents our proposal on the involvement of the two LOX enzymes in construction of the epidermal water barrier. The eponymous organelles of the stratum granulosum, the lamellar granules (or lamellar bodies), contain lipid precursors and a battery of hydrolytic enzymes [82, 83]. As illustrated at step 1 in Figure 5, glucosyl-EOS is a constituent of the limiting membrane of the lamellar bodies, with its glucose moiety on the inside [84, 85]. At stage 2, as the granules fuse with the plasma membrane, the molecules of glucosyl-EOS expose the glucose moieties on the outside, the very long chain fatty acid of EOS spans the bilayer, and in this rendering the molecule is folded in a U-shape with the linoleate chain in the membrane. As differentiation proceeds, the glucosyl-EOS is deglycosylated and the two LOX enzymes oxidize the linoleate tail (Figure 5, step 3), producing an “oxygen signal” as the more polar carbon chain no longer strongly favors the lipid environment. We hypothesize that the oxidized linoleate will serve as substrate for an esterase/hydrolase, which cleaves the oxidized linoleate to form OS with a free ω-hydroxyl (Figure 5, step 4). This lipase (not identified to date) could be released from the lamellar bodies and activated in the intercellular spaces of the SC (see ref [86]), or be intracellular, as currently assumed for the two LOX enzymes. Finally the ceramide OS will be covalently coupled to the polymerized protein of the CE via the action of transglutaminase, thusforming the corneocyte lipid envelope (CLE) of the completed epidermal barrier (Figure 5, step 5).
Figure 5. Ceramides, essential fatty acids and lipoxygenases and their proposed role on maturation of the epidermal water barrier.
(1) Lamellar granules contain lamellar lipid discs with acylglucosylceramides (Glc-EOS, with linoleate coupled to the omega-hydroxyl) in the limiting membrane. (2) Fusion combines the lamellar limiting membrane with the cell plasma membrane, an early stage in formation of the corneocyte lipid envelope (CLE, shown greatly expanded) and extrudes the lamellar contents extracellularly. (Glucosyl)-EOS has the very long chain fatty acid component (long red line) spanning the lipid bilayer, with linoleate (black line with two (white) double bonds) folded into the bilayer. (3) Progression towards the mature barrier entails LOX-catalyzed oxygenation of the linoleate in the ceramide by the consecutive actions of 12_R_-LOX and eLOX3. (4) The resultant “oxygen signal” permits esterase-catalyzed hydrolysis of the oxidized linoleate, freeing the ceramide omega-hydroxyl. (5) Transglutaminase-catalyzed covalent coupling of the CLE to the cross-linked proteins of the CE, thus helping to seal the barrier. A part of this figure was originally published in The Journal of Biological Chemistry [87] © the American Society for Biochemistry and Molecular Biology.
Evidence supporting the role of the LOX enzymes in barrier function
The skin phenotype in gene inactivation and knockout studies highlights the essential role of 12_R_-LOX and eLOX3 in barrier formation and prevention of transepidermal water loss, and the specific location of the LOX enzyme expression in the outer epidermis is consistent with this role. Furthermore, biochemical analyses show that linoleate (C18:2ω6, a LOX substrate) is the sole EFA required for skin barrier integrity and that its replacement by oleate (C18:1ω9, not a LOX substrate) in EFA deficiency results in barrier defects. Our working hypothesis of the involvement of the two LOX enzymes in the oxidation of linoleate-containing ceramides and formation of the CLE is supported by the following observations [87]:
- Normal pig and murine epidermis contain low levels of oxidized species of linoleate in the EOS ceramides.
- These oxidized species of linoleate in the ceramides are chiral, with the hallmark of 12_R_-LOX and eLOX3 activity. The oxidized species are predominantly 9_R_-HODE, 9-keto-linoleate, and hepoxilin-related 9_R_,10_R_-epoxy-13_R_-hydroxy and/or 9_R_,10_R_-epoxy-13-oxo-linoleates.
- These oxidized ceramides are almost absent in the epidermis of 12_R_-LOX knockout mice.
- The epidermis of 12_R_-LOX knockout mice contains ~2-fold higher amounts of unoxidized EOS, indicating accumulation of the LOX substrate.
- Covalently bound ceramides in the epidermis of 12_R_-LOX knockout mice are severely reduced to only a few percent of the wild-type level, and in line with this, the CLE is largely absent in the epidermis of 12_R_-LOX knockout mice.
- eLOX3 knockout animals show a slightly less severe phenotype than the 12_R_-LOX knockouts (albeit neonatal lethal due to TEWL by ~10 hours), and this is associated with a reduction in covalently bound ceramides to about half of the normal levels [30].
Speculations on additional roles of oxidized linoleate
Structural versus signaling roles
Although ceramides EOS with oxygenated linoleate are present in the epidermis of pig and mouse [87], and probably in humans as well, it appears thatthe quantities present are insufficient to support a direct structural role. Indeed, if the hepoxilin (or trioxilin) tails serve as a signal for esterases and are hydrolyzed to form ceramides OS as we propose, then the majority of the 12_R_-LOX/eLOX3-derived hepoxilins should be present in the SC in their free form. The linoleate-related hepoxilins released in this manner might exert a signaling function in several processes related to components of the permeability barrier of the skin. This is also the case for the arachidonate-derived hepoxilins [30, 61, 62]. For example, it has been hypothesized that hepoxilins might be ligands for the plasma membrane receptor “ichthyin” [88]. Although the functions of ichthyin are not clear to date, ichthyin seems to interact with a fatty acid transporter of the endoplasmic reticulum, FATP4, which incorporates exogenous fatty acids into the cells [22, 89]. Patients with mutations in ALOX12B, the gene that codes for 12R-LOX, show a decrease in the expression of FATP4 but in an up-regulation of ichthyin [22]. Mutations in FATP4 itself are associated with ichthyosis prematurity syndrome [90, 91].
Potential interactions with epidermal PPARs
Hepoxilin-type products released from the ceramides have also been hypothesized to be activators of peroxisome proliferator activated receptors (PPARs). Although 12_R_-HPETE-derived (arachidonate) hepoxilins and trioxilins are activators of PPARα [92], this has not been tested using the linoleate-derived hepoxilins. PPARs in the skin are associated with various functions, including differentiation, proliferation, immune response, wound healing and lipid metabolism [93]. In relation to barrier function, Hanley and Feingold established an interesting role for PPARα as a transcriptional regulator of transglutaminase [94]. Quite possibly the LOX metabolism of linoleate may serve a dual purpose within skin: firstly, allowing cleavage of the linoleate moiety from the long chain ceramides, and secondly, by up-regulation of transglutaminase (responsible for ceramide coupling to corneocyte proteins), thus forming an efficient self-regulating system and reinforcing the crucial role of LOX within the skin via a novel-signaling pathway.
Barrier restoration
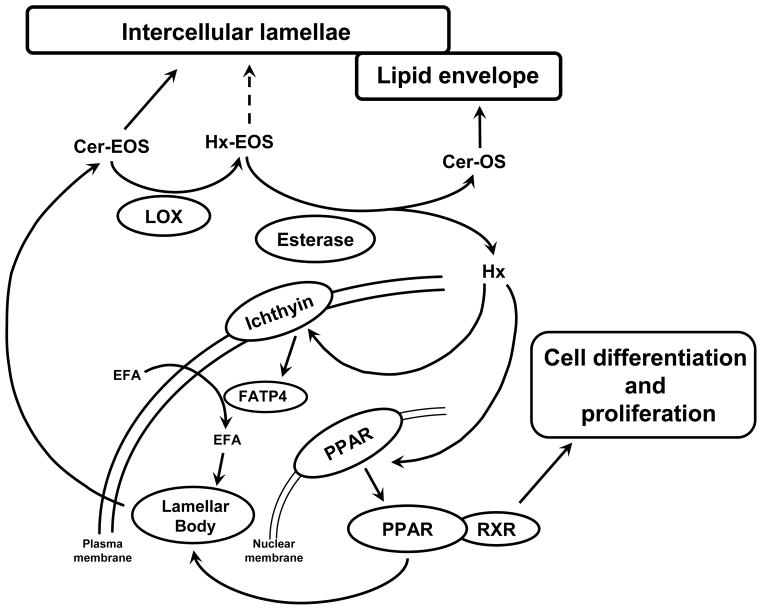
After disruption or following a change in environmental conditions that requires adjustment of TEWL, the permeability barrier needs to be built at a high rate. In this scenario, lamellar bodies will extrude their contents at a higher rate, and free hepoxilin concentration might be higher, which might promote the formation of lamellar bodies throughthe activation of fatty acid transporters, such as FATP4, via interaction with ichthyin. Activation of PPARs will also increase cell proliferation and differentiation (Figure 6). Altogether, these mechanisms will result in a prompt response of the permeability barrier of the skin to diverse environmental conditions.
Figure 6. Proposed pathway of the LOX products in the skin and its relationship with the formation of the permeability barrier.
LOX = Lipoxygenases; FATP4 = Fatty acid transport protein 4; PPAR = Peroxisome proliferator activated receptor; RXR = Retinoid X receptor, a molecule with which PPAR forms a heterodimer, which binds to the DNA of the target gene; EFA = Essential fatty acid; Cer-EOS = Ceramide EOS; Cer-OS = Ceramide OS; Hx = Hepoxilin; HX-EOS = Hepoxilin-EOS.
Future directions
Identification and characterization of the putative lipase
According to our working hypothesis on LOX enzymes and barrier function, 12_R_-LOX and eLOX3 oxidize the linoleate in EOS ceramide, allowing an esterase or hydrolase to recognize and cleave the oxidized linoleate ester, thus providing OS for synthesis of the CLE. The key lipase/esterase/ hydrolase enzyme that uses oxidized-EOS as substrate remains to be identified. Although it is well precedented that lipases will preferentially cleave a variety of oxidized lipids from cell membranes [95–97], the specificity implied in our working model is remarkable. The putative esterase/hydrolase has to recognize and cleave oxidized linoleate of a particular chirality and chemical structure, resulting from the coordinated action of both 12R-LOX and eLOX3 on ceramides EOS. There is no evidence so far for an esterase that will recognize and cleave a particular oxidized species, as opposed to merely peroxidized lipid, yet that is what might be inferred in this case.
Characterization of 12R-LOX/eLOX3 products in human epidermis
The enzymatic activity of 12R-LOX is relatively low in the conditions of the SC, and because the oxygenated hepoxilin-EOS ceramides are present in small concentrations in the epidermis, their detection and identification has posed a challenge. Epoxyalcohols, hydroxides and ketones of ceramides EOS have been detected in the epidermis of pig and mouse using HPLC coupled with UV and MS detection [87]. The presence of these molecules in human epidermis has not been firmly established yet. The qualitative and quantitative characterization of hepoxilin-EOS ceramides in normal and diseased human skin will have clinical implications in the search for a rational basis for treatment of several pathologies. If hepoxilin-EOS ceramides perform a structural function, they should be distributed throughout the entire depth of the SC. If they act exclusively as signals for esterase activity, they would be intermediates of a complex enzymatic chain reaction. In this case, only small amounts would remain in the lower layers of the SC, where 12R-LOX and eLOX3 are located.
Implications for therapeutics
Based on our model of the actions of the LOX enzymes, rational approaches can be tested to correct of the skin symptomatology in ichthyosis patients with several different gene mutations. For patients with inactivating mutations in ALOX12B or ALOXE3, the prediction is that they fail to oxidize EOS, thus it is not cleaved, and consequently there is a lack of OS available for construction of the CLE. Thus, topical application of OS is predicted to be palliative. The same treatment could be effective for mutations in the cytochrome P450 gene CYP4F22. CYP4F enzymes are fatty acid omega-hydroxylases and the CYP4F22 mutations likely result in a deficit in omega-hydroxylated ceramides, although this remains to be shown. Again, topical application of OS (and for CYP4F22 mutations, also EOS) may by-pass the block in the pathway. In addition to the potential therapeutic benefits, the results should be informative on the roles of key players (ALOX12B, ALOXE3, CYP4F22, and ICHTHYIN) and the requirement for esterified or free hepoxilin-type products in barrier function.
Conclusions
We hypothesize that two epidermal lipoxygenases, 12_R_-LOX and eLOX3 act in tandem to oxidize the linoleic acid molecules of the ceramides EOS. Hepoxilins, as oxidation intermediates, would act as a signal which allows the recognition and hydrolysis of the linoleic acid and the conversion of ceramides EOS into ceramides OS. Thereafter, the ω-hydroxy fatty acid chain of the resulting ceramides OS covalently binds to proteins of the corneocytes to form the lipid envelope. This step is necessary to form a competent skin barrier. Understanding the role of hepoxilins in the epidermis can lead to a rational basis for therapy of diseases that disrupt the normal development of the barrier to water vapor diffusion of the skin, such as autosomal recessive congenital ichthyoses.
Highlights.
- The lipoxygenases 12_R_-LOX and eLOX3 act in tandem in epidermal barrier function
- They oxidize linoleic acid esterified in ω-hydroxyacyl ceramide to hepoxilin
- This acts as a signal for hydrolysis of the oxidized linoleate from the ceramide
- The free ceramide ω-hydroxyl is bonded to protein forming a key barrier component
- Understanding the role of hepoxilins can lead to therapy of skin diseases.
Acknowledgments
This work was supported by NIH grant AR-051968.
Abbreviations
ARCI
autosomal recessive congenital ichthyoses
CLE
corneocyte lipid envelope
EFA
essential fatty acids
LOX
lipoxygenase
eLOX3
epidermal lipoxygenase-3
EOS
esterified omega-hydroxyacyl-sphingosine
FATP4
fatty acid transport protein 4
H(P)ETE
hydro(pero)xyeicosatetraenoic acid
H(P)ODE
hydro(pero)xy-octadecadienoic acid
HxA
HxB, hepoxilin A or B
OS
omega-hydroxyacyl-sphingosine
VLFA
very long-chain fatty acid
References
- 1.Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 2.Lillywhite HB. Water relations of tetrapod integument. J Exp Biol. 2006;209:202–226. doi: 10.1242/jeb.02007. [DOI] [PubMed] [Google Scholar]
- 3.Menon GK, Kligman AM. Barrier functions of human skin: a holistic view. Skin Pharmacol Physiol. 2009;22:178–189. doi: 10.1159/000231523. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 5.Wertz PW. Lipids and barrier function of the skin. Acta Derm Venereol Suppl (Stockh) 2000;208:7–11. doi: 10.1080/000155500750042790. [DOI] [PubMed] [Google Scholar]
- 6.Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Iwai I, Han H, den Hollander L, Svensson S, Ofverstedt LG, Anwar J, Brewer J, Bloksgaard M, Laloeuf A, Nosek D, Masich S, Bagatolli LA, Skoglund U, Norlen L. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol. 2012;132:2215–2225. doi: 10.1038/jid.2012.43. [DOI] [PubMed] [Google Scholar]
- 8.Masukawa Y, Narita H, Sato H, Naoe A, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T. Comprehensive quantification of ceramide species in human stratum corneum. J Lipid Res. 2009;50:1708–1719. doi: 10.1194/jlr.D800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JR, Wertz PW. Molecular models of the intercellular lipid lamellae from epidermal stratum corneum. Biochim Biophys Acta. 2003;1616:121–126. doi: 10.1016/s0005-2736(03)00238-4. [DOI] [PubMed] [Google Scholar]
- 10.Swartzendruber DC, Wertz PW, Kitko DJ, Madison KC, Downing DT. Molecular models of the intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol. 1989;92:251–257. doi: 10.1111/1523-1747.ep12276794. [DOI] [PubMed] [Google Scholar]
- 11.van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52:1211–1221. doi: 10.1194/jlr.M014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wertz PW, van den Bergh B. The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem Phys Lipids. 1998;91:85–96. doi: 10.1016/s0009-3084(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 13.Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 2003;42:1–36. doi: 10.1016/s0163-7827(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 14.Meguro S, Arai Y, Masukawa Y, Uie K, Tokimitsu I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL) Arch Dermatol Res. 2000;292:463–468. doi: 10.1007/s004030000160. [DOI] [PubMed] [Google Scholar]
- 15.Bowser PA, Nugteren DH, White RJ, Houtsmuller UMT, Prottey C. Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim Biophys Acta. 1985;834:419–428. doi: 10.1016/0005-2760(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 16.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama M, Shimizu H. An update on molecular aspects of the non-syndromic ichthyoses. Exp Dermatol. 2008;17:373–382. doi: 10.1111/j.1600-0625.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 18.Eckl KM, de Juanes S, Kurtenbach J, Natebus M, Lugassy J, Oji V, Traupe H, Preil ML, Martinez F, Smolle J, Harel A, Krieg P, Sprecher E, Hennies HC. Molecular analysis of 250patients with autosomal recessive congenital ichthyosis: evidence for mutation hotspots in ALOXE3 and allelic heterogeneity in ALOX12B. J Invest Dermatol. 2009;129:1421–1428. doi: 10.1038/jid.2008.409. [DOI] [PubMed] [Google Scholar]
- 19.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobard F, Lefèvre C, Karaduman A, Blanchet-Bardon C, Emre S, Weissenbach J, Özgüc M, Lathrop M, Prud’homme JF, Fischer J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet. 2002;11:107–113. doi: 10.1093/hmg/11.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Vahlquist A, Torma H. Interactions between FATP4 and ichthyin in epidermal lipid processing may provide clues to the pathogenesis of autosomal recessive congenital ichthyosis. J Dermatol Sci. 2013;69:195–201. doi: 10.1016/j.jdermsci.2012.11.593. [DOI] [PubMed] [Google Scholar]
- 23.Lefèvre C, Bouadjar B, Ferrand V, Tadini G, Mégarbané A, Lathrop M, Prud’homme JF, Fischer J. Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet. 2006;15:767–776. doi: 10.1093/hmg/ddi491. [DOI] [PubMed] [Google Scholar]
- 24.Fischer J. Autosomal recessive congenital ichthyosis. J Invest Dermatol. 2009;129:1319–1321. doi: 10.1038/jid.2009.57. [DOI] [PubMed] [Google Scholar]
- 25.Nemes Z, Marekov LN, Fesus L, Steinert PM. A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation. Proc Natl Acad Sci USA. 1999;96:8402–8407. doi: 10.1073/pnas.96.15.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa N, Yamamoto M, Imai Y, Sakaguchi Y, Takizawa T, Ohta N, Yagi N, Hatta I, Hitomi K, Takizawa T, Takeda J, Tsuda T, Matsuki M, Yamanishi K. Knocking-in the R142C mutation in transglutaminase 1 disrupts the stratum corneum barrier and postnatal survival of mice. J Dermatol Sci. 2012;65:196–206. doi: 10.1016/j.jdermsci.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, Feingold KR, Holleran WM, Pharm D. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- 28.Moran JL, Qiu H, Turbe-Doan A, Yun Y, Boeglin WE, Brash AR, Beier DR. A mouse mutation in the 12(R)-lipoxygenase, Alox12b, disrupts formation of the epidermal permeability barrier. J Invest Dermatol. 2007;127:1893–1897. doi: 10.1038/sj.jid.5700825. [DOI] [PubMed] [Google Scholar]
- 29.Epp N, Fürstenberger G, Müller K, de Juanes S, Leitges M, Hausser I, Thieme F, Liebisch G, Schmitz G, Krieg P. 12R-Lipoxygenase deficiency disrupts epidermal barrier function. J Cell Biol. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieg P, Rosenberger S, de Juanes S, Latzko S, Hou J, Dick A, Kloz U, van der Hoeven F, Hausser I, Esposito I, Rauh M, Schneider H. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J Invest Dermatol. 2013;133:172–180. doi: 10.1038/jid.2012.250. [DOI] [PubMed] [Google Scholar]
- 31.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 32.Brash AR, Yu Z, Boeglin WE, Schneider C. The hepoxilin connection in the epidermis. The FEBS journal. 2007;274:3494–3502. doi: 10.1111/j.1742-4658.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- 33.Theorell H, Holman RT, Åkeson Å. Crystalline lipoxidase. Acta Chem Scand. 1947;1:571–576. doi: 10.3891/acta.chem.scand.01-0571. [DOI] [PubMed] [Google Scholar]
- 34.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugteren DH. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975;380:299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- 36.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 37.Schewe T, Rapoport SM, Kühn H. Enzymology and physiology of reticulocyte lipoxygenase: Comparison with other lipoxygenases. Adv Enzymol. 1986;58:191–271. doi: 10.1002/9780470123041.ch6. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 39.Schneider C, Brash AR. Lipoxygenase-catalyzed formation of R-configuration hydroperoxides. Prostaglandins Other Lipid Mediat. 2002;68–69:291–301. doi: 10.1016/s0090-6980(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 40.Brash AR, Boeglin WE, Chang MS, Shieh B-H. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J Biol Chem. 1996;271:20949–20957. doi: 10.1074/jbc.271.34.20949. [DOI] [PubMed] [Google Scholar]
- 41.Coffa G, Schneider C, Brash AR. A comprehensive model of positional and stereo control in lipoxygenases. Biochem Biophys Res Commun. 2005;338:87–92. doi: 10.1016/j.bbrc.2005.07.185. [DOI] [PubMed] [Google Scholar]
- 42.Hammarström S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2α in epidermis of psoriasis. Proc Natl Acad Sci USA. 1975;72:5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussain H, Shornick LP, Shannon VR, Wilson JD, Funk CD, Pentland AP, Holtzman MJ. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. Am J Physiol. 1994;266:C243–C253. doi: 10.1152/ajpcell.1994.266.1.C243. [DOI] [PubMed] [Google Scholar]
- 44.Woollard PM. Stereochemical difference between 12-hydroxyeicosatetraenoic acid in platelets and psoriatic lesions. Biochem Biophys Res Commun. 1986;136:169–176. doi: 10.1016/0006-291x(86)90891-0. [DOI] [PubMed] [Google Scholar]
- 45.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: Mechanistic evidence, molecular cloning and expression. Proc Natl Acad Sci USA. 1998;95:6744–6749. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun D, McDonnell M, Chen X-S, Lakkis MM, Li H, Isaacs SN, Elsea SH, Patel PI, Funk CD. Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. J Biol Chem. 1998;273:33540–33547. doi: 10.1074/jbc.273.50.33540. [DOI] [PubMed] [Google Scholar]
- 47.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 48.Kendall AC, Nicolaou A. Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res. 2013;52:141–164. doi: 10.1016/j.plipres.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Fretland DJ, Widomski DL, Zemaitis JM, Tsai BS, Djuric SW, Penning TD, Miyashiro JM, Bauer RF. 12(R)-hydroxyeicosatetraenoic acid is a neutrophil chemoattractant in the cavine, lapine, murine and canine dermis. Prostaglandins Leukot Essent Fatty Acids. 1989;37:79–81. doi: 10.1016/0952-3278(89)90102-6. [DOI] [PubMed] [Google Scholar]
- 50.Baer AN, Green FA. Fatty acid oxygenase activity of human hair roots. J Lipid Res. 1993;34:1505–1514. [PubMed] [Google Scholar]
- 51.Burrall BA, Wintroub BU, Goetzl EJ. Selective expression of 15-lipoxygenase activity by cultured human keratinocytes. Biochem Biophys Res Commun. 1985;133:208–213. doi: 10.1016/0006-291x(85)91862-5. [DOI] [PubMed] [Google Scholar]
- 52.Henneicke-von Zepelin HH, Schröder J-M, Smíd P, Reusch MK, Christophers E. Metabolism of arachidonic acid by human epidermal cells depends on the maturational stage. J Invest Dermatol. 1991;97:291–297. doi: 10.1111/1523-1747.ep12480558. [DOI] [PubMed] [Google Scholar]
- 53.Sando GN, Howard EJ, Madison KC. Induction of ceramide glucosyltransferase activity in cultured human keratinocytes. Correlation with culture differentiation. J Biol Chem. 1996;271:22044–22051. doi: 10.1074/jbc.271.36.22044. [DOI] [PubMed] [Google Scholar]
- 54.Antón R, Abian J, Vila L. Characterization of arachidonic acid metabolites through the 12-lipoxygenase pathway in human epidermis by high-performance liquid chromatography and gas chromatography/mass spectrometry. J Mass Spectrom Rapid Commun Mass Spectrom. 1995:S169–182. [PubMed] [Google Scholar]
- 55.Heidt M, Fürstenberger G, Vogel S, Marks F, Krieg P. Diversity of mouse lipoxygenases: Identification of a subfamily of epidermal isozymes exhibiting a differentiation-dependent mRNA expression pattern. Lipids. 2000;35:701–707. doi: 10.1007/s11745-000-0576-0. [DOI] [PubMed] [Google Scholar]
- 56.Krieg P, Marks F, Fürstenberger G. A gene cluster encoding human epidermis-type lipoxygenases at chromosome 17p13.1: cloning, physical mapping, and expression. Genomics. 2001;73:323–330. doi: 10.1006/geno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 57.Schneider C, Keeney DS, Boeglin WE, Brash AR. Detection and cellular localization of 12R-lipoxygenase in human tonsils. Arch Biochem Biophys. 2001;386:268–274. doi: 10.1006/abbi.2000.2217. [DOI] [PubMed] [Google Scholar]
- 58.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radical Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 59.Pace-Asciak CR, Martin JM. Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets. Prostaglandins Leukot Med. 1984;16:173–180. doi: 10.1016/0262-1746(84)90069-6. [DOI] [PubMed] [Google Scholar]
- 60.Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins. A review. Adv Exp Med Biol. 1999;447:123–132. [PubMed] [Google Scholar]
- 61.Antón R, Vila L. Stereoselective biosynthesis of hepoxilin B3 in human epidermis. J Invest Dermatol. 2000;114:554–559. doi: 10.1046/j.1523-1747.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 62.Antón R, Puig L, Esgleyes T, de Moragas JM, Vila L. Occurrence of hepoxilins and trioxilins in psoriatic lesions. J Invest Dermatol. 1998;110:303–310. doi: 10.1046/j.1523-1747.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 63.Pace-Asciak CR, Lee WS. Purification of hepoxilin epoxide hydrolase from rat liver. J Biol Chem. 1989;264:9310–9313. [PubMed] [Google Scholar]
- 64.Cronin A, Decker M, Arand M. Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res. 2011;52:712–719. doi: 10.1194/jlr.M009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52:447–454. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- 66.Decker M, Adamska M, Cronin A, Di Giallonardo F, Burgener J, Marowsky A, Falck JR, Morisseau C, Hammock BD, Gruzdev A, Zeldin DC, Arand M. EH3 (ABHD9): the first member of a new epoxide hydrolase family with high activity for fatty acid epoxides. J Lipid Res. 2012;53:2038–2045. doi: 10.1194/jlr.M024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krieg P, Kinzig A, Heidt M, Marks F, Fürstenberger G. cDNA cloning of a 8-lipoxygenase and a novel epidermis-type lipoxygenase from phorbol ester-treated mouse skin. Biochim Biophys Acta. 1998;1391:7–12. doi: 10.1016/s0005-2760(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 68.Krieg P, Siebert M, Kinzig A, Bettenhausen R, Marks F, Fürstenberger G. Murine 12(R)-lipoxygenase: functional expression, genomic structure and chromosomal localization. FEBS Lett. 1999;446:142–148. doi: 10.1016/s0014-5793(99)00196-9. [DOI] [PubMed] [Google Scholar]
- 69.Kinzig A, Heidt M, Fürstenberger G, Marks F, Krieg P. cDNA cloning, genomic structure, and chromosomal localization of a novel murine epidermis-type lipoxygenase. Genomics. 1999;58:158–164. doi: 10.1006/geno.1999.5816. [DOI] [PubMed] [Google Scholar]
- 70.Yu Z, Schneider C, Boeglin WE, Marnett LJ, Brash AR. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc Natl Acad Sci USA. 2003;100:9162–9167. doi: 10.1073/pnas.1633612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Brash AR. Dioxygenase activity of epidermal lipoxygenase-3 unveiled: typical and atypical features of its catalytic activity with natural and synthetic polyunsaturated fatty acids. J Biol Chem. 2010;285:39866–39875. doi: 10.1074/jbc.M110.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y, Brash AR. On the role of molecular oxygen in lipoxygenase activation: comparison and contrast of epidermal lipoxygenase-3 with soybean lipoxygenase-1. J Biol Chem. 2010;285:39876–39887. doi: 10.1074/jbc.M110.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Z, Schneider C, Boeglin WE, Brash AR. Human and mouse eLOX3 have distinct substrate specificities: implications for their linkage with lipoxygenases in skin. Arch Biochem Biophys. 2006;455:188–196. doi: 10.1016/j.abb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hansen HS. The essential nature of linoleic acid in mammals. Trends Biochem Sci. 1986;11:263–265. [Google Scholar]
- 75.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. [Google Scholar]
- 76.Burr GO, Burr MM. On the nature and rôle of the fatty acids essential in nutrition. J Biol Chem. 1930;86:587–621. [Google Scholar]
- 77.Houtsmuller UMT, van der Beek A. Effects of topical application of fatty acids. Prog Lipid Res. 1981;20:219–224. doi: 10.1016/0163-7827(81)90041-2. [DOI] [PubMed] [Google Scholar]
- 78.Hansen HS, Jensen B, von Wettstein-Knowles P. Apparent in vivo retroconversion of dietary arachidonic to linoleic acid in essential fatty acid-deficient rats. Biochim Biophys Acta. 1986;878:284–287. doi: 10.1016/0005-2760(86)90158-x. [DOI] [PubMed] [Google Scholar]
- 79.Hansen AE, Haggard ME, Boelsche AN, Adam DJ, Wiese HF. Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency. J Nutr. 1958;66:565–576. doi: 10.1093/jn/66.4.565. [DOI] [PubMed] [Google Scholar]
- 80.Wertz PW, Downing DT. Covalently bound omega-hydroxyacylsphingosine in the stratum corneum. Biochim Biophys Acta. 1987;917:108–111. doi: 10.1016/0005-2760(87)90290-6. [DOI] [PubMed] [Google Scholar]
- 81.Swartzendruber DC, Wertz PW, Madison KC, Downing DT. Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol. 1987;88:709–713. doi: 10.1111/1523-1747.ep12470383. [DOI] [PubMed] [Google Scholar]
- 82.Grayson S, Johnson-Winegar AG, Wintroub BU, Isseroff RR, Epstein EH, Jr, Elias PM. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol. 1985;85:289–294. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz G, Müller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 84.Elias PM, Fartasch M, Crumrine D, Behne M, Uchida Y, Holleran WM. Origin of the corneocyte lipid envelope (CLE): observations in harlequin ichthyosis and cultured human keratinocytes. J Invest Dermatol. 2000;115:765–769. doi: 10.1046/j.1523-1747.2000.00124-5.x. [DOI] [PubMed] [Google Scholar]
- 85.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 86.Menon GK, Grayson S, Elias PM. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J Invest Dermatol. 1986;86:591–597. doi: 10.1111/1523-1747.ep12355263. [DOI] [PubMed] [Google Scholar]
- 87.Zheng Y, Yin H, Boeglin WE, Elias PM, Crumrine D, Beier DR, Brash AR. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: A proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J Biol Chem. 2011;286:24046–24056. doi: 10.1074/jbc.M111.251496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lefèvre C, Bouadjar B, Karaduman A, Jobard F, Saker S, Ozguc M, Lathrop M, Prud’homme JF, Fischer J. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet. 2004;13:2473–2482. doi: 10.1093/hmg/ddh263. [DOI] [PubMed] [Google Scholar]
- 89.Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem. 2005;280:11948–11954. doi: 10.1074/jbc.M412629200. [DOI] [PubMed] [Google Scholar]
- 90.Klar J, Schweiger M, Zimmerman R, Zechner R, Li H, Torma H, Vahlquist A, Bouadjar B, Dahl N, Fischer J. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet. 2009;85:248–253. doi: 10.1016/j.ajhg.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: Implications for health and disease. Dermatoendocrinol. 2011;3:53–61. doi: 10.4161/derm.3.2.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 93.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 94.Hanley K, Jiang Y, He SS, Friedman M, Elias PM, Bikle DD, Williams ML, Feingold KR. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARα. J Invest Dermatol. 1998;110:368–375. doi: 10.1046/j.1523-1747.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 95.Sevanian A, Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med. 1985;1:263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- 96.Feussner I, Kühn H. The lipid body lipoxygenase from cucumber seedlings exhibits unusual reaction specificity. FEBS Lett. 1995;367:12–14. doi: 10.1016/0014-5793(95)00531-d. [DOI] [PubMed] [Google Scholar]
- 97.Belkner J, Stender H, Holzhutter HG, Holm C, Kuhn H. Macrophage cholesteryl ester hydrolases and hormone-sensitive lipase prefer specifically oxidized cholesteryl esters as substrates over their non-oxidized counterparts. Biochem J. 2000;352(Pt 1):125–133. [PMC free article] [PubMed] [Google Scholar]
- 98.Keeney DS. Radiolabeled cRNA and in situ hybridization. In: Sundberg JP, Bogess D, editors. Systematic Approach to Evaluation of Mouse Mutations. CRC Press; Boca Raton: 1999. pp. 145–167. [Google Scholar]
- 99.Keeney DS, Waterman MR. Two novel sites of expression of NADPH cytochrome P450 reductase during murine embryogenesis: limb mesenchyme and developing olfactory neuroepithelia. Dev Dyn. 1999;216:511–517. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<511::AID-DVDY19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 100.Shappell SB, Keeney DS, Zhang J, Page R, Olson SJ, Brash AR. 15-Lipoxygenase-2 (15-LOX-2) expression in benign and neoplastic sebaceous glands and other cutaneous adnexa. J Invest Dermatol. 2001;117:36–43. doi: 10.1046/j.1523-1747.2001.01378.x. [DOI] [PubMed] [Google Scholar]