The Acute Phase of Trypanosoma cruzi Infection Is Attenuated in 5-Lipoxygenase-Deficient Mice (original) (raw)
Abstract
In the present work we examine the contribution of 5-lipoxygenase- (5-LO-) derived lipid mediators to immune responses during the acute phase of Trypanosoma cruzi infection in 5-LO gene knockout (5-LO−/−) mice and wild-type (WT) mice. Compared with WT mice, the 5-LO−/− mice developed less parasitemia/tissue parasitism, less inflammatory cell infiltrates, and a lower mortality. This resistance of 5-LO−/− mice correlated with several differences in the immune response to infection, including reduced PGE2 synthesis; sustained capacity of splenocytes to produce high levels of interleukin (IL)-12 early in the infection; enhanced splenocyte production of IL-1_β_, IL-6, and IFN-γ; rapid T-cell polarization to secrete high quantities of IFN-γ and low quantities of IL-10; and greater numbers of CD8+CD44highCD62Llow memory effector T cells at the end of the acute phase of infection. The high mortality in WT mice was associated with increased production of LTB4/LTC4, T cell bias to produce IFN-γ, high levels of serum nitrite, and marked protein extravasation into the peritoneal cavity, although survival was improved by treatment with a cys-LT receptor 1 antagonist. These data also provide evidence that 5-LO-derived mediators negatively affect host survival during the acute phase of T. cruzi infection.
1. Introduction
Infection with Trypanosoma cruzi (T. cruzi), an obligate intracellular protozoan parasite, causes American trypanosomiasis or Chagas disease, a zoonosis endemic to Latin America. Approximately 60 million people live in areas with vector-borne transmission risk and the disease causes an estimated 14,000 deaths per year [1]. After entering the host, T. cruzi invades a variety of cell types, such as macrophages, heart muscle cells, skeletal muscle cells, and neurons, replicating within the cytoplasm [2]. The acute phase of the disease is characterized by a marked increase in parasite replication and migration to the blood, potentially leading to systemic infection. However, immunocompetent hosts are able to generate innate inflammatory and specific immune responses to acute secondary infection, thereby controlling the parasite burden [3]. These responses are primarily dependent on cytokine/chemokine mediated activation of infected phagocytes and/or tissue cells which leads to intracellular killing [4], although complete elimination of the parasite is rarely achieved. Parasite persistence in tissues is followed by an asymptomatic or indeterminate phase, and chronic chagasic immunopathology develops in approximately 25% of cases [5].
The factors governing immunological resistance to acute trypanosomiasis are not fully understood. Host genetic background and parasite strain differences might be relevant [6]. Early, partial control of parasites within infected tissue is achieved by local production of type 1 IFNs [7], IL-1_β_ [8], and _β_-chemokines [9]. Therefore, effective parasite control likely requires the participation of both innate and adaptive immune cells including macrophages, dendritic cells, and NK cells that secrete proinflammatory cytokines (e.g., IL-12 or IFN-γ) [10] and naive T cells for the generation of parasite-specific CD4+ and CD8+ effector T cells [11], which produce Th1 cytokines such as IFN-γ and, in lesser quantities, Th2 cytokines such as IL-4 and IL-10 [12, 13].
Although immune functions have been assigned to a number of polypeptide mediators (cytokines and chemokines) in host defense against T. cruzi, little attention has been paid to the role of lipid mediators. These lipid molecules are mainly eicosanoids that are generated through the effects of cyclooxygenases (COX) or 5-lipoxygenase (5-LO) and play a variety of roles in regulating host innate and adaptive immune responses [14]. The 5-LO pathway leads to the formation of two biologically relevant classes of leukotrienes (LTs): non-cysteinyl LTs such as LTB4; and cysteinyl-LTs (cys-LTs) such as LTC4, LTD4, and LTE4 [15]; and the activity of 5-LO seems to be a common step in LXA4 synthesis [16]. LTs have been established to play protective roles during infection with many microbial pathogens, including Salmonella typhimurium, Pseudomonas aeruginosa [17], Klebsiella pneumoniae [18], vesicular stomatitis virus encephalitis [19], and Histoplasma capsulatum [20]. However, in other settings 5-LO products have been shown to play contradictory roles, for example, in Mycobacterium tuberculosis infection models [21, 22]. In addition, in a cecal ligation and puncture model of peritonitis, LTs exhibited beneficial effects on local immunity but exhibited deleterious effects on hemodynamic responses [23]. Immunoregulatory lipids, such as the arachidonic acid-derived eicosanoids, are increasingly implicated in the pathogenesis of parasitic infections [24, 25]. The 5-LO pathway products have also been implicated in modulating the pathogenesis of several parasitic infections and the results have also been contradictory. In vitro, LTB4 and LTC4 potentiate macrophages to kill T. cruzi [26, 27] and Leishmania amazonensis [28]. However, these mediators have been implicated in conferring susceptibility to Schistosoma mansoni [29], Strongyloides venezuelensis [30], and cerebral malaria [31], thereby suggesting that LTs play conflicting roles during parasite infection.
The immunoregulatory effects of 5-LO pathway eicosanoids are complex and context dependent. While their net effects are beneficial to host defense against some microbial pathogens, this is not necessarily true for all infections. In light of the importance in regulating immune responses to parasitic infections, and the contrasting roles exhibited by LTs in several infection models, we asked whether the 5-LO pathway activity could modulate the T. cruzi infection. To address this issue, here we studied specifically the acute phase of T. cruzi infection in 5-LO−/− mice.
2. Materials and Methods
2.1. Animals
Male mice (18–20 g) were used; the 5-LO−/− (129-Alox5 tm1Fun /J) and strain-matched WT mice (129-SF2/J) were purchased from Jackson Laboratories (Bar Harbor, ME). The animal colony was bred and maintained under specific pathogen-free conditions at the Faculdade de Ciências Farmacêuticas de Ribeirão Preto (Universidade de São Paulo, Brazil). This study was approved and carried out in strict accordance with the guidelines of the Animal Care Committee of the Universidade de São Paulo and Biosafety Committees (Process nos. 05.1.592.53.2 and CQB-0019/97). All euthanasia was performed under CO2/O2 excess atmosphere, and all efforts were made to minimize suffering.
2.2. Parasite Infection and Pharmacological Treatment
Mice were infected intraperitoneally with 200 units of the blood form of T. cruzi (Colombian strain) in 0.2 mL of 0.15 M PBS. Control mice received the same volume of sterile PBS. Parasites were counted in 5 _μ_L of blood as previously described [32]. WT mice were previously subjected to T. cruzi infection [33]. In some experiments, the infected WT mice were treated with a cys-LT receptor 1 antagonist, montelukast (10 mg/kg, Singulair; Merck Sharp & Dohme, Campinas, Brazil) or its vehicle, carboxymethylcellulose (0.5% w/v), administered orally by gavage (300 _μ_L/animal) on postinoculation days 14–32, starting on day 14 and given every 2 days. T. cruzi soluble antigens were obtained from trypomastigote forms (Colombian strain) and used for in vitro experiments [32]. Briefly, trypomastigotes were washed twice in cold PBS, subjected to six freeze-thaw cycles, and centrifuged (9000 ×g, 10 min, 4°C). The supernatant was filtered through a 0.22 _μ_m pore size membrane filter, and the protein concentration was measured using a colorimetric assay (Pierce, Rockford, IL).
2.3. Histology and Quantitative Tissue Parasite Nest Determination
Histology and tissue parasite counts were performed as described elsewhere [11]. Briefly, tissue samples were fixed in 4% buffered formalin and processed for conventional paraffin embedding on day 16 after infection. Sections (8 _μ_m) were deparaffinized and stained with hematoxylin and eosin. Intact parasite nests were evaluated in blinded samples by counting the number of parasites nests in 100 microscopic fields/sample of nonconsecutive sections.
2.4. Eicosanoid Levels in Peritoneal Cell Supernatants
Peritoneal cells were collected by intraperitoneal injection of 4 mL of cold PBS from uninfected controls, infected WT, and 5-LO−/− mice at various time points of infection. Cell concentration was adjusted to 106 cells mL−1 in Hank's buffered salt solution (HBSS; Sigma, St. Louis, MO) with Ca+2 and Mg+2. Cells were stimulated with 0.5 _μ_M of the calcium ionophore A23187 (Sigma, Saint Louis, EUA) for 15 min at 37°C in a humidified atmosphere of 5% CO2. The supernatants were harvested and PGE2, LTB4, and LTC4 levels were determined by specific EIA kit, following the manufacturer's instructions (GE Healthcare, Little Chalfont, UK).
2.5. Spleen Cell Culture
Mice from experimental groups were euthanized on various days after inoculation. Single-cell suspensions were prepared by passing each spleen through a 70 _μ_m cell strainer (Falcon, Sollentuna, Sweden). The splenocytes were washed 3 times with HBSS, counted with a hemocytometer, assessed for viability, and suspended in RPMI 1640 medium supplemented with 10% FCS, penicillin (100 U/mL), streptomycin (100 _μ_g/mL), and gentamicin (50 _μ_g/mL) (Gibco-Invitrogen, Carlsbad, CA) or HBSS supplemented with 5% FCS. The cell concentration was adjusted to 107 cells/mL and cultured in 24-well plates (Nalge Nunc, Rochester, NY) in 1 mL of supplemented RPMI medium, with 5 μ_g of anti-CD3_ε or with 10–50 _μ_g of soluble T. cruzi antigens at 37°C in an atmosphere of 5% CO2 for 24–48 h. Supernatants were collected and stored at −70°C for further use.
2.6. Metabolic Assays
Splenocytes (4 × 105 cell/well) from different experimental groups were cultured in quintuplicate in flat 96-well microplates (Nalge Nunc, Rochester, NY) with supplemented RPMI medium. Cells were cultured alone or with anti-CD3_ε_ IgG (1 _μ_g/mL; BD Pharmingen, San Diego, CA) at 37°C in a humidified atmosphere of 5% CO2. After 60 h, 10 _μ_L (5 mg/mL) of MTT (Sigma, Saint Louis, EUA) was added to each well, and cells were incubated for an additional 4 h, followed by the addition of 50 _μ_L of 20% SDS in PBS and stored in the dark overnight. Absorbance was measured at 570 nm using an automated microplate reader (_μ_QUANT; BioTek Instruments, Winooski, VT).
2.7. Flow Cytometry
Spleen cells were isolated as described above and placed in ice-cold PBS supplemented with 5% FCS and 0.1% sodium azide. Staining was performed as previously described [11]. The following fluorochrome-conjugated monoclonal antibodies were used: anti-CD4 [H129.19]; anti-CD8 [53-6.7]; anti-CD19 [MB19-1]; anti-CD25 [7D4]; anti-CD44 [IM7]; anti-CD69 [H1.2F3]; anti-Gr-1/Ly6C/Ly6G [RB6-8C5]; anti-CD45RB [16A]; anti-CD62L [MEL-14]; anti-CD11b [M1/70] (BD Pharmingen, San Diego, CA); and anti-CD11c [HL3]—(Serotech, Raleigh, NC) anti-F4/80 [CI:A3-1] and anti-GITR [DTA-1] (eBioscience, San Diego, CA). After staining, the cells were fixed with 1% paraformaldehyde in PBS and analyzed using a FACSCanto (BD Biosciences, San Jose, CA), 50,000 events/sample recorded. Data were processed using FlowJo software (FlowJo LLC, Ashland, OR). Cell numbers were calculated using the percentage obtained by FACS analysis and the total numbers of leukocytes counted in a hemocytometer.
2.8. _T. cruzi_-Specific Antibodies
Specific IgG, IgG1, and IgG2a were determined in mouse sera by ELISA as previously described [32]. The individual titers were considered the highest serum dilutions that presented OD492 > 0.1.
2.9. Protein Extravasation
Protein extravasation was assessed as previously described [23]. Control mice or infected mice were i.v. injected with Evans blue dye (50 mg/kg in a volume of 0.1 mL; Sigma, Saint Louis, EUA). After 1 h, mice were euthanized by CO2 inhalation, and the peritoneal exudates were recovered by injecting 2 mL of PBS. The peritoneal exudates were centrifuged for 10 min at 200 ×g, and the supernatant was saved for colorimetric determinations. The OD was determined at 630 nm in the automated microplate reader.
2.10. In Vitro Macrophage Infection
Peritoneal cells from WT and 5-LO−/− mice were collected, washed twice, and counted and the cell concentration was adjusted to 106cells/mL in supplemented RPMI medium. Cells were attached on 13 mm-diameter glass coverslips placed to 24-well plates (Nalge Nunc, Rochester, NY), for 90 min at 37°C in an atmosphere of 5% CO2. The nonadherent cells were removed by washings in warm supplemented RPMI medium. Peritoneal macrophages (PM) isolated by this procedure were >90% pure as measured by staining for F4/80+ (data not shown). The PMs were stimulated for 6 h with 5 ng/mL of IFN-γ (BD Pharmingen, San Diego, CA) plus 0.1 _μ_g/mL of LPS from Escherichia coli (Sigma, Saint Louis, EUA) and infected at a parasite-to-macrophage ratio of 5 : 1/well. After 2 h, the glass coverslips were washed five times in PBS to remove free parasites, fixed in absolute methanol, stained with Panoptic stain (Laborclin, Pinhais, Brazil), dried, mounted on glass slides, and examined microscopically for association (parasite adhered to macrophages plus internalized parasites) as previously described [26]. For killing assay, noninternalized parasites of infected macrophages wells were removed 24 h later by three gentle washes with warm supplemented RPMI medium. Fresh supplemented RPMI medium was added to each well, and infected macrophages were cultured at 37°C, in an atmosphere of 5% CO2 for up to 10 days, 50% of the medium being removed and replaced with the same volume of supplemented RPMI medium every 48 h. After 7–11 days after infection, culture supernatants were collected daily to count the number of motile trypomastigotes/well.
2.11. Nitrite/Nitrate Concentration
Tail-vein blood samples were obtained at day 22 after inoculation. Nitrate in serum samples was converted to nitrite by nitrate reductase, and serum levels of nitrate/nitrite (Nitric oxide end-products or metabolites) were measured by absorbance using the Griess Reaction (Calbiochem, La Jolla, CA) [34]. The OD was determined at 540 nm in the _μ_QUANT automated microplate reader. The nitriteconcentration was determined by reference to a standard (1–100 _μ_M) sodium nitrite curve.
2.12. Cytokine ELISA
Levels of IL-1_β_, IL-2, IL-6, IL-10, IL-12, TNF-α, and IFN-γ were quantified by ELISA according to the manufacturer's instructions (BD Pharmingen, San Diego, CA) in the splenocytes culture supernatant. The lower limits of detection for those cytokines were 9.4 pg/mL.
2.13. Statistical Analysis
The results are presented as means ± SD, with the exception of those for parasitemia, shown as means ± SEM. The tests that were used to evaluate differences among groups are mentioned in the figure legends. Values of P < 0.05 were considered significant.
3. Results
3.1. Lipid Mediator Production by Infected Peritoneal Cells
Peritoneal cells from _T. cruzi_-infected mice (WT) released significantly more LTB4 and PGE2 upon calcium ionophore stimulation than did cells obtained from uninfected mice (Figures 1(a) and 1(b)). The potential of cells to produce LTB4 was elevated at an early time point after inoculation (day 5) and increased gradually throughout the infection, peaking on day 19. This potential then decreased drastically toward the late phase of acute infection (day 26; Figure 1(a)).
Figure 1.
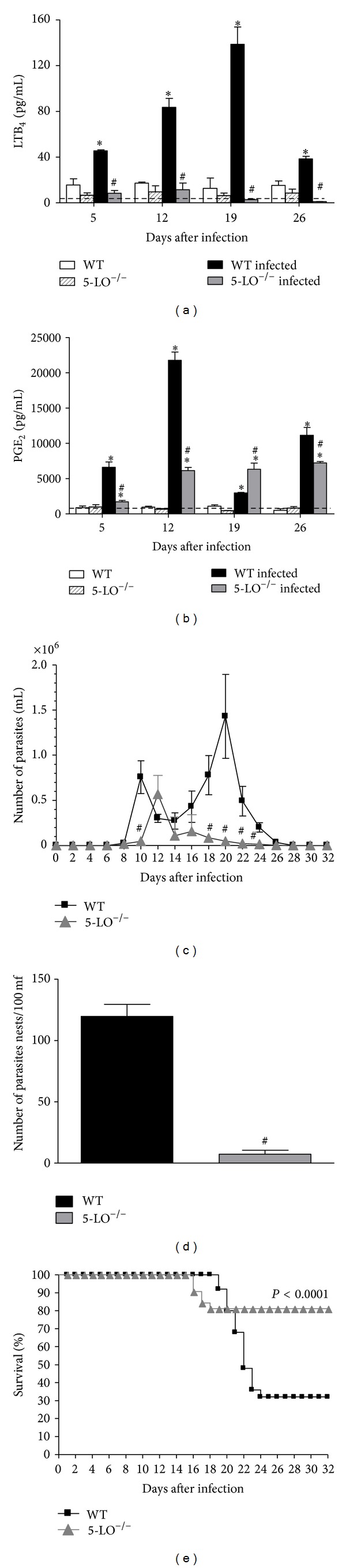
Lipid mediator production, parasitemia, tissue parasitism, and survival rate of WT and 5-LO−/− mice infected with T. cruzi: ((a) and (b)) LTB4 and PGE2: peritoneal cells were collected from control, infected WT, and infected 5-LO−/− mice (n = 10/group) and stimulated with calcium ionophore. *P < 0.01 versus uninfected group; # P < 0.01 versus WT infected mice. (c) Parasitemia (n = 10 mice/group). # P < 0.001 versus infected WT mice. (d) Parasite nests in heart tissue on postinoculation day 16. *P < 0.001 versus infected WT mice. (e) Survival: WT mice (squares) and 5-LO−/− mice (triangles), n = 10 animals/group. Wilcoxon signed-rank test (level of significance, P < 0.001). Data are representative of three independent experiments.
The potential of peritoneal cells from _T. cruzi_-infected mice to produce PGE2 was also increased (Figure 1(b)). Peritoneal cells from infected WT mice released PGE2 at early time points (day 5) and increased markedly on subsequent days, peaking on day 12. PGE2 levels decreased drastically by day 19 and increased again in the late phase of the acute infection by day 26. Peritoneal cells from infected 5-LO−/− mice also showed an enhanced production of PGE2 (Figure 1(b)) but the pattern was different than observed for WT cells and did not suggest shunting of arachidonic acid towards the cyclooxygenase pathway. PGE2 production by 5-LO−/− cells was not elevated on day 5 and increased on day 12 (albeit to levels below WT) to a level that was maintained throughout infection. Notably, the peritoneal cells from both WT and 5-LO−/− _T. cruzi_-infected mice appeared to produce more PGs than LTs, as evidenced by the fact that LTB4 levels (Figure 1(a)) were far lower than those of PGE2 (Figure 1(b)).
3.2. Control of Parasite Dissemination and Host Survival after Infection
As shown in Figure 1(c), infected 5-LO−/− mice presented a delay in the appearance of blood-circulating parasites and very low parasite numbers at the second peak of parasitaemia compared to WT mice. In addition, the number of intact parasite nests in heart muscle tissue was considerably lower in 5-LO−/− mice (Figure 1(d)). Furthermore, about 17% of the 5-LO−/− mice died on postinoculation days 16–19, whereas WT mice did not begin to die until day 19 (Figure 1(e)). In the acute phase of infection, only 30% of WT mice were capable of surviving the infection. In contrast, 82.3% of 5-LO−/− mice controlled parasite efficiently and survived the acute phase of infection (Figure 1(e)).
3.3. Inflammatory Infiltrate and Tissue Parasitism during the Acute Phase of T. cruzi Infection
Analysis of the histological samples of heart muscle tissue collected after 16 days after inoculation revealed that WT mice presented more intact parasite nests and more amastigote forms within those nests (Figure 2(a)) than did 5-LO−/− mice (Figure 2(b)). In addition, there were greater inflammatory mononuclear cell infiltrates in WT mice (Figure 2(c)) than in 5-LO−/− mice (Figure 2(d)).
Figure 2.
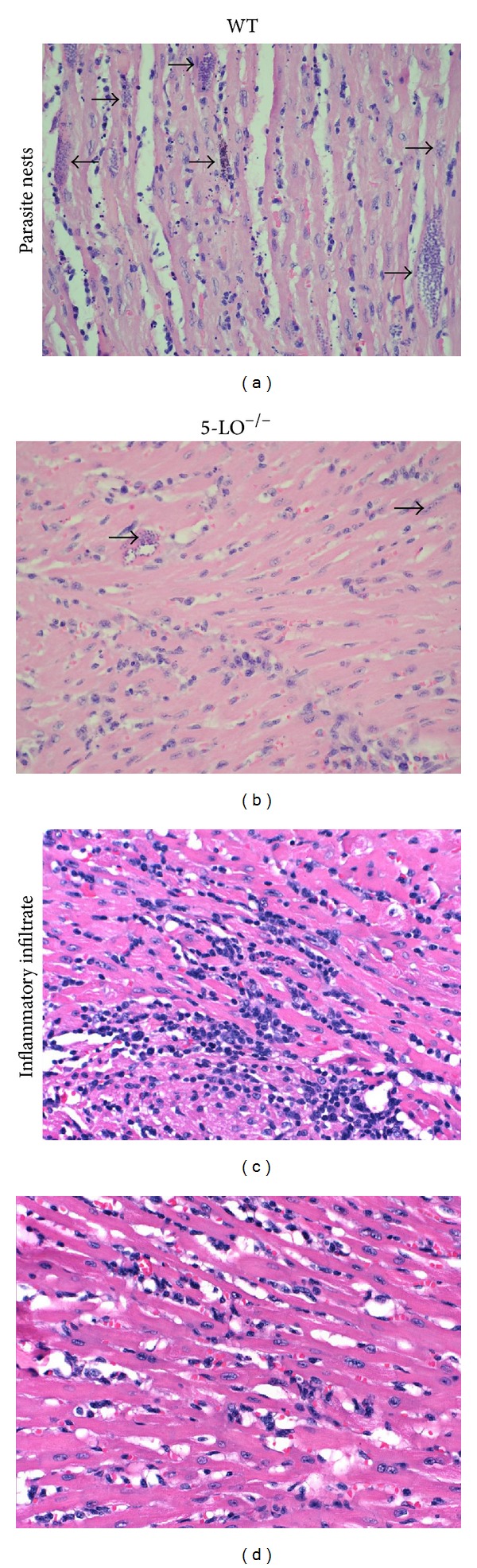
Histopathological analysis of heart tissue parasitism and inflammatory infiltrate in _T. cruzi_-infected WT and 5-LO−/− mice (n = 8/group) on postinoculation day 16 (staining with H&E; magnification, ×200): (a) section from a WT mouse, showing numerous large amastigote nests, (b) section from a 5-LO−/− mouse, showing fewer, smaller nests, (c) section from a WT mouse, showing intense mononuclear cell infiltration, (d) section from a 5-LO−/− mouse, showing less infiltration. Photomicrographs are from one experiment representative of three independent experiments.
3.4. Cytokine Production by Spleen Cells
In culture, the spleen cells of infected mice spontaneously produced IL-1_β_, IL-6, TNF-α, IL-10, IL-12, and IFN-γ (Figure 3). It is notable that the production of most of these cytokines was greater during the first two weeks of infection, peaking on day 12 after inoculation, correlating with parasitemia (Figure 1(c)). In general, the production of IL-6, IL-12, and IFN-γ was greater in spleen cells of infected 5-LO−/− than in WT cells. On day 5, the production of IL-1_β_, IL-6, IL-12, and IFN-γ was higher from 5-LO−/− cells than from WT cells but TNF-α production was lower. On day 12, IL-1_β_ levels were comparably high in cell cultures from both mouse strains, while spleen cells from infected 5-LO−/− showed significantly greater production of IL-6, IL-12, IFN-γ, and TNF-α compared to the infected WT cells. The production of IL-10 showed the opposite trend (Figure 3(d)). In the late phase of infection (on days 19 and 26), 5-LO−/− spleen cells, in contrast to what was observed for WT spleen cells, showed a sustained elevation in the production of IL-1_β_ (Figure 3(a)) and IL-10 (Figure 3(d)), higher levels of IL-12 (Figure 3(e)), and lower levels of IFN-γ (Figure 3(f)).
Figure 3.
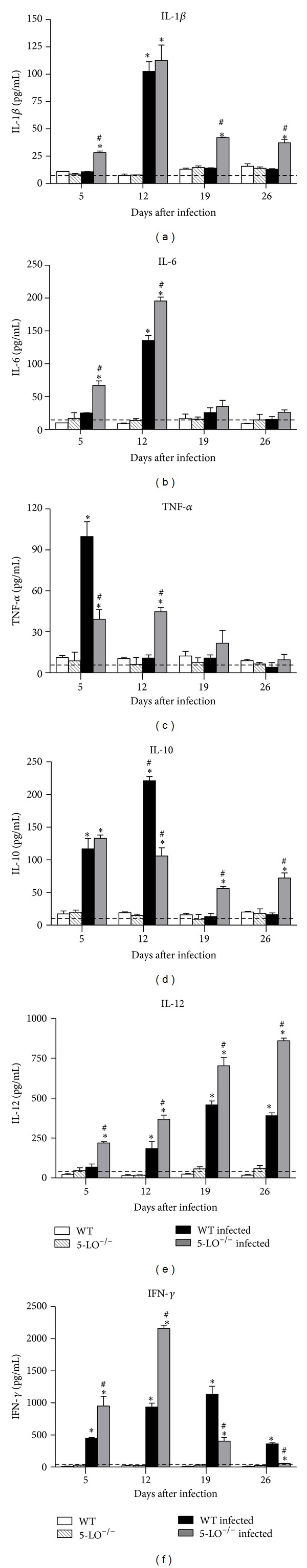
Cytokine production by spleen cells from _T. cruzi_-infected mice. Spleen cells from control, infected WT and infected 5-LO−/− mice (n = 10/group) cultured in medium alone. Data are from one of three independent experiments. Kruskal-Wallis test (*P < 0.01 versus uninfected group; # P < 0.05 versus infected WT mice).
3.5. Cell Populations in the Spleen of Infected Mice
Infection of mice with T. cruzi led to the accumulation of Gr1+ cells (i.e., neutrophils), Gr1+/CD11c+cells (i.e., plasmacytoid dendritic cells), CD11b+cells (i.e., myeloid lineage cells), and F4/80+cells (i.e., macrophages) in their spleens when compared with uninfected control mice (Figures 4(a)–4(d)). Greater numbers of Gr1+, Gr1+/CD11c+, CD11b+, and F4/80+ cells were present in 5-LO−/− than in WT spleens at day 12 after infection (Figures 4(a)–4(c)). Macrophage (F4/80+) numbers were higher in the spleens of 5-LO−/− mice on day 19 after infection compared with WT mice as well (Figure 4(d)). In addition, on day 5 after infection, plasmacytoid dendritic cell (Gr1+/CD11c+) counts were higher in infected 5-LO−/− mice, when compared with infected WT mice (Figure 4(b)). It is notable that myeloid lineage cells numbers were higher in the spleens of infected WT animals than the spleens of 5-LO−/− mice (Figure 4(c)).
Figure 4.
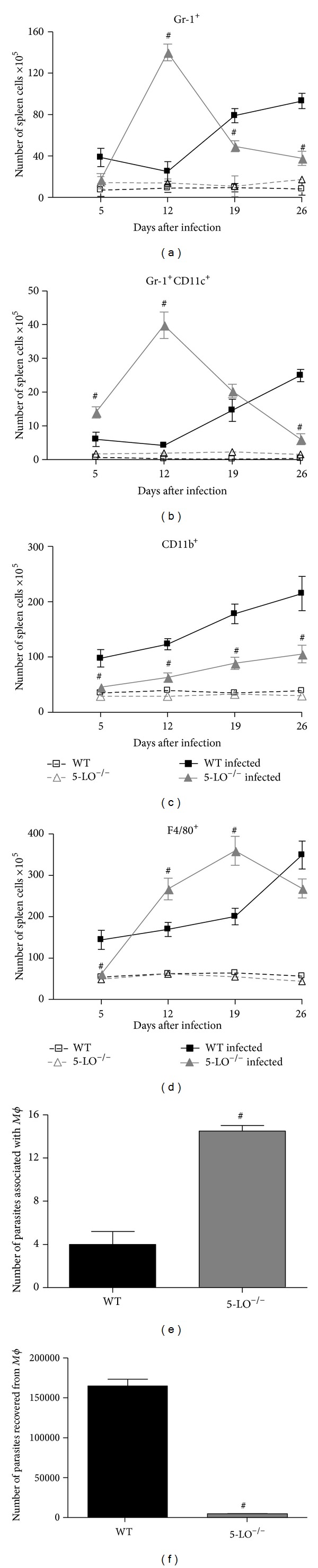
Quantitative and functional leukocyte responses to T. cruzi infection. (a) Gr1+ cell (neutrophils); (b) Gr1+CD11c+ cell (pDC cells); (c) CD11b+ cell (myeloid lineage cell marker, Mac-1); and (d) F4/80+ cell (macrophages) numbers in the spleen.
3.6. Peritoneal Macrophage Infection
In the infected 5-LO−/− mice, PMs and IFN-γ both increased (Figures 4(d) and 3(f)). The in vitro infection of peritoneal LPS-plus IFN-_γ_-activated-macrophages from WT and 5-LO−/− mice showed differences in the association of macrophages with parasites (binding and internalization) and in their ability to kill intracellular parasites (Figures 4(e) and 4(f)). Compared with the activated PMs from WT mice, those from 5-LO−/− mice presented a greater capacity to associate with the blood form of the parasite, as evidenced by the higher numbers of bound and internalized parasites (Figure 4(e)). Activated PMs from 5-LO−/− mice were also more efficient at killing internalized parasites, as evidenced by the lower numbers of parasites recovered after in vitro infection as compared with WT PMs (Figure 4(f)).
3.7. Spleen B-Cell Counts and Serum Levels of Parasite-Specific Immunoglobulins
As shown in Figure 5(a), splenic CD19+ B-cell counts were greater in mice infected with T. cruzi than in control mice. On day 5 after inoculation, splenic CD19+ cell counts were elevated in infected WT mice and gradually returned to baseline values by the end of the acute phase of infection. In contrast, splenic CD19+ cell counts increased significantly less in infected 5-LO−/− mice during the first two weeks of infection, gradually becoming significantly more elevated than WT mice in the later phase of infection.
Figure 5.
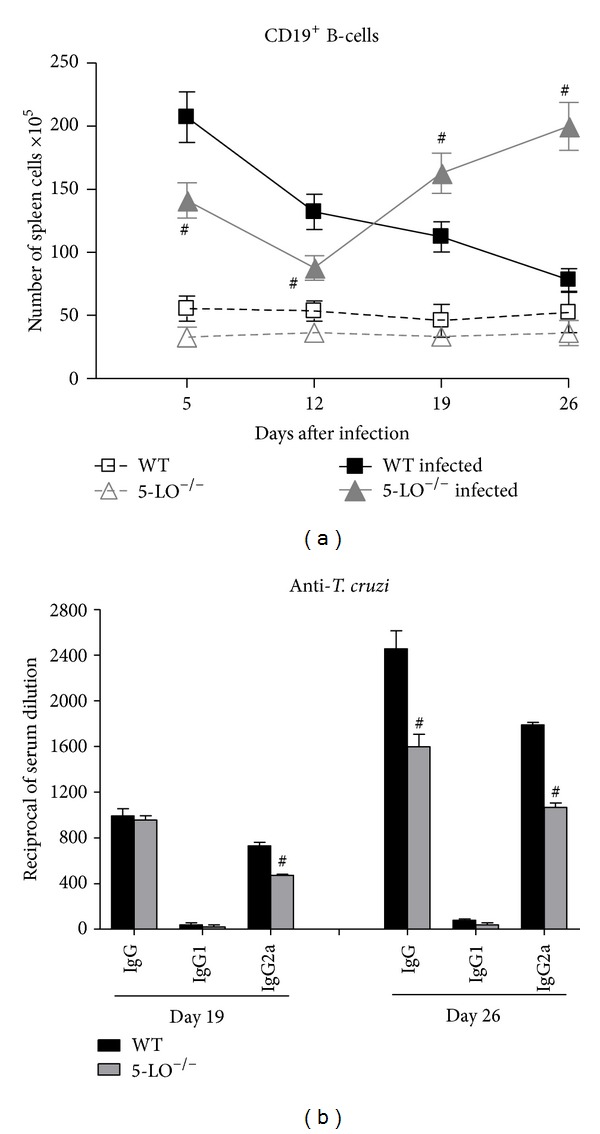
(a) Splenic CD19+ B-cell counts in control, infected WT and infected 5-LO−/− mice (n = 10/group). Data are from one of three independent experiments. Student's _t_-test (# P < 0.01 versus infected WT mice). (b) _T. cruzi_-specific serum antibody titers in infected WT and 5-LO−/− mice (n = 10/group). Data are from one of two independent experiments. # P < 0.001 versus infected WT mice.
Mice infected with T. cruzi produced detectable levels of parasite-specific IgG antibodies in a time-dependent manner during the later phase of infection (Figure 5(b)). The principal isotype produced during infection was IgG2a, and levels of IgG1 were low. Compared with WT mice, 5-LO−/− mice presented lower levels of parasite-specific IgG on day 26, as well as of parasite-specific IgG2a on days 19 and 26 of infection.
3.8. T-Cell Phenotypes in the Spleens of Infected Mice
As indicated in Figure 6, infected mice presented elevated CD4+ and CD8+ T-cell counts, the markers CD4+CD69+, CD8+CD69+, CD4+CD25+, CD4+CD44+, and CD8+CD44+, which indicate the presence of activated T-cells in the spleens of both WT and 5-LO−/− infected mice. In infected mice, the majority of the splenic T-cell populations presented the full/late T-cell activation markers CD4+CD44+ and CD8+CD44+ (Figures 6(c) and 6(d)). On day 5 after inoculation, numbers of all of these T-cell phenotypes were elevated in infected WT mice, gradually increasing over the course of infection and peaking on day 26, the study endpoint (Figure 6). In contrast, infected 5-LO−/− mice presented a delayed elevation in T-cell counts, and the increase of all of these activated T-cell phenotypes was less pronounced than observed in WT spleens. Infected 5-LO−/− mice presented a significant increase in CD4+CD44+ and CD8+CD44+ counts on day 12, a marked increase in the CD4+CD69+ count on day 19, and a slight but significant increase in CD8+CD69+ and CD4+CD25+ counts only on day 26.
Figure 6.
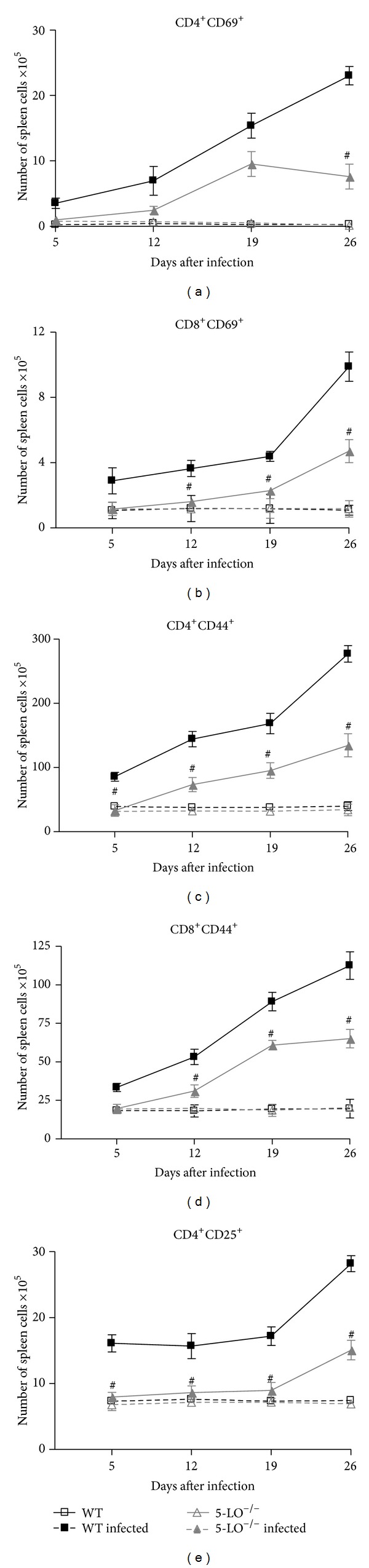
Splenic T-cell subpopulations in control, infected WT and infected 5-LO−/− mice (n = 8/group). Data are from one of three independent experiments. Student's _t_-test (# P < 0.01 versus infected WT mice).
3.9. T-Cells Properties, Cytokine Production, and CD4+ Memory T Cells Expressing CD45RBlow and CD44highCD62Llowin the Spleen
As shown in Figure 7(a), spleen cells from control mice proliferated after stimulation with anti-CD3_ε_, as expected, whereas spleen cells collected from infected WT mice on day 12 after inoculation and stimulated with anti-CD3_ε_ presented a dramatic reduction in proliferation. However, spleen cells from infected 5-LO−/− mice on day 12 and stimulated with anti-CD3_ε_ presented a partially restored proliferative capacity.
Figure 7.
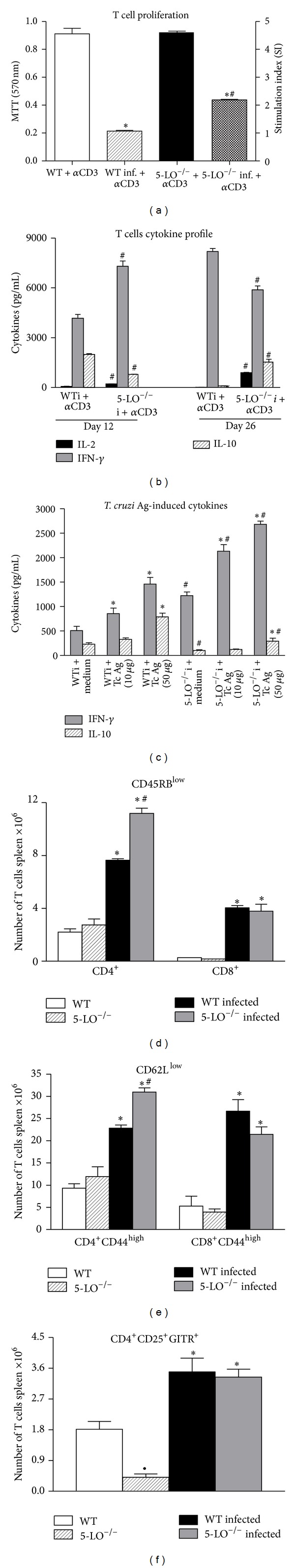
Splenic T-cell properties, effector/memory T cells, and regulatory T cells in control, infected WT and infected 5-LO−/− mice (n = 8/group): (a) proliferation; (b) cytokine production profile after anti-CD3 stimulation; and (c) Th1/Th2 cytokine recall response to soluble T. cruzi antigen. *P < 0.001 versus uninfected group; # P < 0.001 versus infected WT mice. (d) CD4+CD45RBlow and CD8+CD45RBlow; (e) CD4+CD44highCD62Llow and CD8+CD44highCD62Llow; and (f) CD4+CD25+GITR+. Stimulation index (SI) was generated by the ratio between the OD (570 nm) obtained in noninfected/infected cells. Data are from one of two independent experiments. Student's _t_-test (# P < 0.01 versus infected WT mice).
Spleen cells were collected from infected mice on days 12 and 26 after inoculation, after which they were stimulated with anti-CD3_ε_. The supernatants were tested for the presence of IFN-γ and IL-10, the most abundant of the cytokines secreted spontaneously by spleen cells from infected mice that we measured (Figure 3), as well as for IL-2. Spleen cells collected from infected WT mice on day 12 and stimulated with anti-CD3_ε_ produced significant amounts of the type 1 cytokine (Th1) IFN-γ, and the type 2 cytokine (Th2) IL-10, as well as very low levels of IL-2 (Figure 7(b)). In contrast, anti-CD3_ε_-stimulated spleen cells collected from infected 5-LO−/− mice on day 12 presented a bias to produce predominantly, and in greater quantities, Th1 cytokines, producing lower quantities of Th2 cytokines and greater quantities of IL-2.
On day 26 after inoculation, infected WT mouse spleen cells stimulated with anti-CD3_ε_ exhibited a bias to produce only the Th1 cytokine IFN-γ in quite high amounts (Figure 7(b)). However, infected 5-LO−/− mouse spleen cells receiving the same treatment presented no alterations in the cytokine production profile, a sustained capacity to produce detectable levels of IL-2, and high (and predominant) levels of Th1 cytokines, as well as low levels of Th2 cytokines, on day 12.
Since anti-CD3_ε_ promotes a polyclonal T-cell stimulation, we investigated whether IFN-γ and IL-10 were produced by primed _T. cruzi_-specific cells. We tested the recall responses of spleen cells collected on day 12 after inoculation and cultured with soluble T. cruzi antigens. _T. cruzi_-specific cells from infected WT mice cultured with nominal antigens produced IFN-γ and IL-10, whereas _T. cruzi_-specific cells from infected 5-LO−/− mice produced a recall response, characterized by high levels of IFN-γ and lower levels of IL-10, in the same culture.
These results regarding the recall response suggested the presence of functional parasite-specific effector/memory T cells. Therefore, we sought to study the populations of effector/memory T cells. As shown in Figure 7, infected mice exhibited increased numbers of splenic effector/memory T cells in CD4+ and CD8+ subsets, including CD4+CD45RBlow, CD4+CD44highCD62Llow, CD8+CD45RBlow, and CD8+CD44highCD62Llow, on day 26 after inoculation. Interestingly, the numbers of effector/memory T cells CD4+CD45RBlow and CD4+CD44highCD62Llow were higher in infected 5-LO−/− mice than in infected WT mice. However, we found no differences between infected 5-LO−/− mice and infected WT mice in terms of the numbers of CD8+ effector/memory T cells.
We questioned whether the kinetic differences between infected 5-LO−/− mice and infected WT mice in terms of the increase in CD4+CD25+ T cell numbers (Figure 6(e)) were related to the involvement of regulatory T cells in this model. Figure 7(f) shows that _T. cruzi_-infected mice exhibited increased numbers of CD4+CD25+GITR+regulatory T cells. However infected WT mice and 5-LO−/− mice exhibited similar numbers of splenic CD4+CD25+GITR+ regulatory T cells. Unexpectedly, the numbers of CD4+CD25+GITR+ in the spleen were lower in uninfected 5-LO−/− mice than in uninfected WT mice.
3.10. Levels of LTC4, Serum Nitrite Levels, and Protein Extravasation into the Peritoneal Cavity during the Acute Phase of T. cruzi Infection
The cys-LTs mediate detrimental vascular effects in systemic infections such as sepsis [23]. Vasoactive mediators are produced and may also be involved in the mortality of animals during the acute phase of T. cruzi infection [35]. Thus, we next examined the levels of some vasoactive mediators such as NO metabolites, LTC4, and measured the protein leak (as a marker of vascular permeability) at a time point when infected WT mice have a high mortality while infected 5-LO−/− mice do not. As illustrated in Figure 8, the capacity to produce LTC4 was markedly upregulated in the peritoneal cells of infected WT mice on day 22 after inoculation, although, as expected, those of infected 5-LO−/− mice produced no detectable levels of LTC4. At this time point, infected 5-LO−/− mice presented significantly lower serum nitrite levels than did infected WT mice. In addition, the peritoneal cavity protein extravasation assays (Figure 8(c)) indicated that on days 14 and 19, the degree of protein leakage was similar between the two groups of infected animals. Although the 5-LO−/− mice presented a tendency toward greater protein leakage than did the WT mice, the difference was not significant. However, on day 22, protein leakage in the peritoneal cavity was considerably greater in the infected WT mice than in the infected 5-LO−/− mice.
Figure 8.
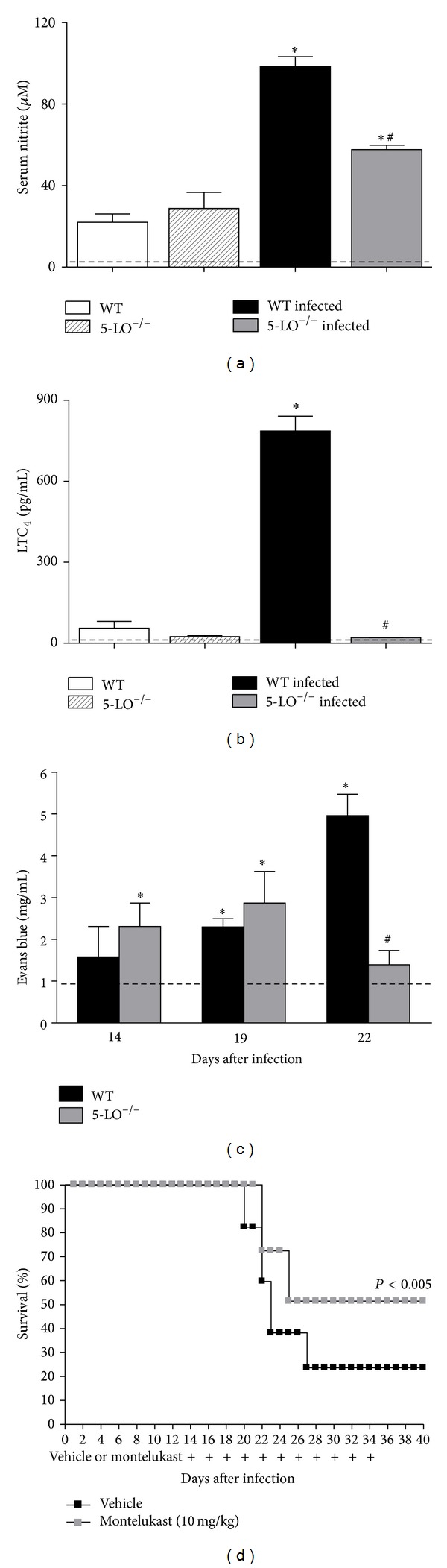
Levels of vasoactive/vasomotor mediators and relevance of cys-LTs in mice acutely infected with T. cruzi (n = 10/group): (a) total serum nitrite (nitrite/nitrate) levels and (b) LTC4 production by peritoneal cells upon stimulation with calcium ionophore. Data are from one of three independent experiments. # P < 0.001 versus infected WT mice. (c) Protein extravasation in the peritoneal cavity (colorimetric assay of extravascular dye leakage). Dotted line indicates the mean Evans blue D.O. found in control mice. Data are from one experiment representative of three separate experiments. # P < 0.05 versus infected WT mice. (d) Effect of cys-LT receptor antagonist on survival in _T. cruzi_-infected mice: (black squares) vehicle-treated infected WT mice and (gray squares) infected WT mice treated with montelukast (10 mg/kg) from postinoculation day 14 to postinoculation day 34 and monitored for 26 days. Data are from one experiment representative of two separate experiments. Wilcoxon signed-rank test # P < 0.005 versus vehicle-treated infected WT mice.
3.11. Treatment of Infected Mice with cys-LTs Receptor Antagonist and Mortality
To determine whether cysLTs are involved in the mortality of _T. cruzi_-infected mice, WT mice were subjected to infection with T. cruzi and treated with the cysLT receptor antagonist montelukast from day 14 to day 34 after inoculation. On day 40 after inoculation, when mortality among the vehicle-treated control WT mice was 82%, a moderately significant degree of protection (reduction to 48% mortality) was achieved after treatment with montelukast (Figure 8(d)).
4. Discussion
Leukotrienes, products of the 5-LO pathway of arachidonic acid metabolism, are potent immunomodulatory lipids that are increasingly recognized to regulate innate and adaptive immune responses to parasitic infections [36]. Despite the relevance of LTs in T. cruzi killing by macrophages in vitro as well as in controlling blood parasite numbers in vivo [26, 37, 38], the oxidative balance in 5-LO−/− T. cruzi infected mice has been related to be 5-LO-pathway independent [39]. Different results for NO and cytokines productions were observed during the acute phase of T. cruzi Y strain infection of 5-LO−/− mice and it has been related to resistance [40] or susceptibility [41]. The localized presence of TCD8+ or TCD4+ in the heart was demonstrated in T. cruzi 5-LO−/− infected mice [40], but other cell types and/or eicosanoid mediators were not investigated for a more complete immune response analysis. The novel finds in this study focus on the in vivo role of 5-LO metabolites in cells related to innate/adaptive immune responses, resistance, and mortality during the acute phase of T. cruzi murine infection. It is important to note that analysis performed after day sixteen after infection was done using infected mice that had survived up to that time point. Furthermore, we describe the results of 5-LO−/− mice infection, using T. cruzi Colombian strain, contributing to a better understanding of the immune response and pathology of the Chagas' disease.
Our data show that peritoneal cells from infected WT mice develop an enhanced capacity to produce LTB4, LTC4, and PGE2 compared with cells obtained from uninfected mice, implicating LTs and PGs in the host response to T. cruzi parasitic infection. Compared with WT, 5-LO−/− mice developed significantly reduced parasitemia, lower tissue parasitism, and less inflammatory cell infiltrates, as well as a significant improvement in survival. Our curve of parasitemia showed a different profile compared with two previous publications [40, 41]. However, these publications are also different between them, what could be due to different trypomastigotes that were used to infect the experimental groups of mice, since one work used cell culture-derived trypomastigotes [40] and other used mice-derived trypomastigotes [41]. Moreover, both works used the Y strain of T. cruzi, while we used the Colombian strain.
These scenarios suggest that LTs deficiency renders mice more resistant to T. cruzi infection and conversely that 5-LO products confer susceptibility to T. cruzi virulence. The production of proinflammatory cytokines IL-1_β_, IL-6, IL-12, TNF-α, and IFN-γ and the presence of parasite-specific T cells generating predominantly IFN-γ and low levels of IL-10 were associated with an increased efficiency of 5-LO−/− mice to control the infection within the blood and tissue compartments. In fact, in the later phases of infection, parasitemia and tissue parasitism were significantly reduced in 5-LO−/− mice and it is in accordance with previous studies showing that cytokines such as IL-1_β_, IL-6, IL-12, TNF-α, and IFN-γ play a relevant role in host killing mechanisms against T. cruzi [8].
Consistent with our data, there is evidence that LTs induce TNF-α [38] and PGE2 [42] release. In some models, it has been observed that drug-induced or genetic LTs deficiency increased PGE2 levels [43]. We observed that 5-LO−/− mice presented a sustained potential to produce PGE2 in the late phase of infection. In support of our findings that LTB4 induced IL-6, healthy patients subjected to inhalation of swine house dust and treated with 5-LO inhibitor showed elevated IL-6 serum levels [44]. In addition, LTB4 might induce IL-1_β_ production [45]; although it is not seen as a unique inducer, we can presume that, in 5-LO−/− mice, IL-1_β_ and IL-6 were induced by T. cruzi PAMPs (Pathogen Associated Molecular Patterns), as previously described [46].
In some murine models of fungal or bacterial infection, it was suggested that the pharmacological impairment of LT biosynthesis hindered the production of the Th1 cytokines IL-12 and IFN-γ [20, 21]. However, in our model, 5-LO−/− T. cruzi infected mice exhibited an increased capacity to produce IL-12 and IFN-γ. A similar result was obtained in other infection models using 5-LO−/− mice [22, 29, 30], and this capacity was found to be essential to achieving protective immunity against pathogens in these mice [22, 47]. Previous studies have demonstrated that the quality and quantity of inflammatory mediators such as IL-12, IFN-γ, and IL-10 released during the first two weeks of infection are critical to driving the generation of parasite-specific effector T cells [48] and we suggest that early IL-12 and IFN-γ production during infection was regulated by LTs. It is probable that splenic Gr-1+CD11c+ plasmacytoid dendritic cells, Gr-1+ neutrophils, and F4/80+ macrophages are sources of IL-12 and IFN-γ, since the numbers of these cells were found to be significantly higher in 5-LO−/− infected mice than in WT infected mice. These cell types have also been found to be increased by T. cruzi infection in other models [10, 49], and they are relevant source of IL-12 and IFN-γ in the setting of protozoal infection [50, 51]. Furthermore, IL-12-producing CD11c+ cells were found to be elevated in 5-LO−/− model of M. tuberculosis infection [22].
There is evidence that LTs contribute to the process of T-cell activation/migration in different models [52, 53]. Our results demonstrated the importance of 5-LO products in T-cell activation during T. cruzi infection. Although a reduction in the numbers of activated splenic T cells was achieved in the infected 5-LO−/− mice, the T cells from these animals, as opposed to those from WT mice, presented a partially recovered capacity to proliferate after anti-CD3_ε_ stimulation and also to produce IL-2 after anti-CD3_ε_ stimulation or in the presence of T. cruzi soluble antigens. We are in accordance with previous studies showing that LTs may also inhibit T cell proliferation and IL-2 production [54] and that susceptibility to T. cruzi infection is associated with elevated numbers of polyclonal activated T cells in the spleen [55] or with splenic T cell unresponsiveness to mitogens and inability to secrete IL-2 [56]. Furthermore, the resistance to T. cruzi infection of 5-LO−/− mice, in contrast to the susceptibility of WT mice, correlated with elevated numbers of splenic effector/memory T cells, including CD4+CD45RBlow and CD4+CD44high CD62Llow, at the end of the acute phase of infection. Elevated numbers of T cells expressing these phenotypes have been associated with IFN-γ production and resistance to T. cruzi infection [11].
The correlation between the resistance to infection and the Th1 bias of CD4+ T cells has been identified in 5-LO−/− mice infected with other pathogens, including M. tuberculosis [22] and T. gondii [57]. The bias towards IFN-γ production by T cells from 5-LO−/− mice was also observed following infections with typical Th2-inducing pathogens such as S. mansoni [29] and S. venezuelensis [30] leading these animals to become more resistant and susceptible to infection, respectively. The CD4+CD25+ T cells number abnormality found in T. cruzi 5-LO−/− infected mice led us to further investigate these CD4+CD25+ regulatory T cells. We found that WT and 5-LO−/− infected mice presented similar numbers of splenic CD4+CD25+GITR+ regulatory T cells. This does not completely rule out the involvement of CD4+CD25+ regulatory T cells in the present model. In fact, it was recently demonstrated that CD4+CD25+ regulatory T cells play a limited role during the acute and chronic phases of T. cruzi infection [58].
Phagocytes have long been known to play an important role in the T. cruzi killing process [59]. It is also known that IFN-γ is one of the major mediators conferring resistance to T. cruzi [60]. Macrophage (F4/80+) numbers and IFN-γ were found to be increased in T. cruzi 5-LO−/− infected mice. In vitro infection assays revealed that activated PMs from 5-LO−/− mice were strongly associated with more efficient parasite killing than WT macrophages. These results corroborate our in vivo findings that 5-LO−/− mice are more efficient at controlling parasitemia, but contrasts with previous in vitro findings showing LTs foster intracellular parasite killing [26, 38]. However, it is suggested in 5-LO−/− mice that an oxidative stress occurs by a leukotriene-independent pathway, since an increase in erythrocyte oxidative stress was observed in these animals [39]. Differences in experimental design between our study and previously published investigations warrant discussion. Some differences in results may be explained in part by the macrophage activation and/or responsiveness to LTs. We used classic activated macrophages (M1) in vitro [61], whereas previous studies showing enhanced pathogen-killing properties of LT-stimulated macrophages employed resident peritoneal macrophages [26, 37], thioglycollate-elicited macrophages [28, 38], or alveolar macrophages [18]. The functional differences among these different types of macrophages are remarkable and have been consistently described [62, 63]. The increased capacity of macrophages from 5-LO−/− mice to kill intracellular pathogens was previously described for M. tuberculosis [22]. These findings underscore the relevance of IFN-γ and the killing activity of macrophages in 5-LO−/− mice resistance to T. cruzi infection, although our data shed no light on whether LXA4 is involved in the parasite resistance of mice, as has been described for M. tuberculosis infection [22].
Analysis of B cells indicated that T. cruzi 5-LO−/− infected mice developed smaller increases in the numbers of splenic CD19+ B cells during the first two weeks than did WT mice, but this was followed by increased numbers of splenic CD19+ B cells in the following weeks. The elevated numbers of CD19+ B cells found in 5-LO−/− mice in the later phase of infection might indicate an accumulation of undifferentiated B cells in the splenic compartment. This hypothesis is supported by previous studies showing that antibody-secreting B cells lose their CD19 marker [64]. Previous in vitro findings showed that LTs are important in activating B cells in human and mouse models [65, 66] and suggest that this could be also relevant in vivo. The alteration in B cell activation observed in T. cruzi 5-LO−/− infected mice could explain the lower serum levels of parasite-specific IgG and IgG2a at the end of the acute phase of infection. It was previously described that LT deficiency altered specific-immunoglobulin class switching to specific pathogens in vivo [29, 30]. Indeed, LTs may affect parasite-specific antibody levels during infection and parasite-specific IgG and IgG2a might not be involved in 5-LO−/− mouse resistance to T. cruzi infection. This reinforces the previous observation that host resistance during the acute phase of T. cruzi infection can be achieved in the absence of B cells [67].
Animal mortality during the acute phase of T. cruzi infection has been associated with multiple factors, including parasite strain virulence [68], anemia [69], increased levels of TNF-α, and T-cell hyperactivity [70]. In fact, _T. cruzi_-infected mice have been shown to be extremely sensitive to sepsis-like inducers and to die with evidence of a shock syndrome [69]. We demonstrated that _T. cruzi_-infected WT mice presented, at the acute phase, an upregulated production of LTB4 and LTC4, as well as high serum nitrite levels. In addition, the analysis of protein extravasation in the peritoneal cavity revealed that infected WT mice exhibited stronger protein leakage as compared with 5-LO−/− mice. Previous studies showed that LTs induce NO production in macrophages and endothelial cells [38], and high levels of NO production have been associated with increased mortality in _T. cruzi_-infected mice [71]. LTs play a critical role in vascular events and mortality of mice subjected to the cecal ligation and puncture model of sepsis [23]. We observed that _T. cruzi-_infected WT mice treated with montelukast presented a significant reduction in mortality, providing evidence that cys-LTs are involved in vascular events associated with mortality of animals during the acute phase of T. cruzi infection. It is notable that montelukast treatment was less effective in increasing survival in contrast to what is observed in _T. cruzi-_infected 5-LO−/− mice. The deaths of some montelukast-treated animals might be attributable to the presence of other 5-LO products, such as LTB4, and its indirect effect of inducing vasoactive mediators such as NO [72] and thromboxane [73]. However, unlike WT, 5-LO−/− infected mice sustain the production of TNF-α and IL-6. This finding is not surprising, since these cytokines have been shown to have vasoactive properties [35].
One reasonable mechanism to explain WT mice mortality during the acute phase of T. cruzi infection involves their capacity to produce LTs, which lead to the extreme bias of spleen cells and T-cells that secrete high levels of Th1 cytokines, such as IFN-γ, and very low levels of IL-10. In addition, downregulated production of PGE2 might be relevant, since PGE2 has been shown to induce IL-10 production [74], inhibit the production of IFN-γ during T. cruzi infection, and promote the increased production of NO [75]. The advantage in survival of _T. cruzi_-5-LO−/− infected mice represents a complex contribution of various effects on host defense, including their capacity to efficiently control the parasites and produce detectable levels of IL-10, the increase in production of PGE2, and lesser amounts of NO, as well as their inability to produce cys-LTs.
5. Conclusions
Our findings demonstrated that 5-LO deficiency altered eicosanoids and cytokines production during T. cruzi infection and favored the generation/maintenance of protective immune responses. Also, they provided evidence that 5-LO-derived lipid mediators have a negative effect on host survival during the acute phase of infection.
Acknowledgments
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo). Adriana M. C. Canavaci is the recipient of a fellowship from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Adriana M. C. Canavaci and Carlos A. Sorgi contributed equally to this work.
References
- 1.Senior K. Chagas disease: moving towards global elimination. The Lancet infectious diseases. 2007;7(9):p. 572. doi: 10.1016/s1473-3099(07)70194-9. [DOI] [PubMed] [Google Scholar]
- 2.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: Location, invasion, retention. Nature Reviews Microbiology. 2005;3(10):819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 3.Brener Z, Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. International Archives of Allergy and Immunology. 1997;114(2):103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 4.Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β . European Journal of Immunology. 1992;22(10):2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 5.Santos-Buch CA. American trypanosomiasis: chagas' disease. International Review of Experimental Pathology. 1979;19:63–100. [PubMed] [Google Scholar]
- 6.Graefe SEB, Streichert T, Budde BS, et al. Genes from Chagas susceptibility loci that are differentially expressed in T. cruzi-resistant mice are candidates accounting for impaired immunity. PLoS ONE. 2006;1(1, article e57) doi: 10.1371/journal.pone.0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Avalos SV, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. The Journal of Biological Chemistry. 2002;277(1):639–644. doi: 10.1074/jbc.M109037200. [DOI] [PubMed] [Google Scholar]
- 8.Fichera LE, Albareda MC, Laucella SA, Postan M. Intracellular growth of trypanosoma cruzi in cardiac myocytes is inhibited by cytokine-induced nitric oxide release. Infection and Immunity. 2004;72(1):359–363. doi: 10.1128/IAI.72.1.359-363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado FS, Martins GA, Aliberti JCS, Mestriner FLAC, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102(24):3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro AC, Schmitz V, Svensjo E, et al. Cooperative activation of TLR2 and bradykinin B2 receptor is required for induction of type 1 immunity in a mouse model of subcutaneous infection by Trypanosoma cruzi . The Journal of Immunology. 2006;177(9):6325–6335. doi: 10.4049/jimmunol.177.9.6325. [DOI] [PubMed] [Google Scholar]
- 11.Nomizo A, Cardillo F, Postól E, de Carvalho LP, Mengel J. Vγ1 γδ T cells regulate type-1/type-2 immune responses and participate in the resistance to infection and development of heart inflammation in Trypanosoma cruzi-infected BALB/c mice. Microbes and Infection. 2006;8(3):880–888. doi: 10.1016/j.micinf.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356(6367):338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoft DF, Schnapp AR, Eickhoff CS, Roodman ST. Involvement of CD4+ Th1 cells in systemic immunity protective against primary and secondary challenges with Trypanosoma cruzi . Infection and Immunity. 2000;68(1):197–204. doi: 10.1128/iai.68.1.197-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. The Journal of Immunology. 2005;174(2):589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 15.Peters-Golden M, Brock TG. 5-Lipoxygenase and FLAP. Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69(2-3):99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demitsu T, Katayama H, Saito-Taki T, Yaoita H, Nakano M. Phagocytosis and bactericidal action of mouse peritoneal macrophages treated with leukotriene B4. International Journal of Immunopharmacology. 1989;11(7):801–808. doi: 10.1016/0192-0561(89)90134-3. [DOI] [PubMed] [Google Scholar]
- 18.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. Journal of Immunology. 1996;157(12):5221–5224. [PubMed] [Google Scholar]
- 19.Chen N, Restivo A, Reiss CS. Leukotrienes play protective roles early during experimental VSV encephalitis. Journal of Neuroimmunology. 2001;120(1-2):94–102. doi: 10.1016/s0165-5728(01)00415-5. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros AI, Sá-Nunes A, Soares EG, Peres CM, Silva CL, Faccioli LH. Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infection and Immunity. 2004;72(3):1637–1644. doi: 10.1128/IAI.72.3.1637-1644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peres CM, de Paula L, Medeiros AI, et al. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes and Infection. 2007;9(4):483–489. doi: 10.1016/j.micinf.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bafica A, Scanga CA, Serhan C, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. The Journal of Clinical Investigation. 2005;115(6):1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin CF, Canetti C, Cunha FQ, Kunkel SL, Peters-Golden M. Opposing and hierarchical roles of leukotrienes in local innate immune versus vascular responses in a model of sepsis. Journal of Immunology. 2005;174(3):1616–1620. doi: 10.4049/jimmunol.174.3.1616. [DOI] [PubMed] [Google Scholar]
- 24.D'Avila H, Freire-de-Lima CG, Roque NR, et al. Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E2 generation and increased parasite growth. Journal of Infectious Diseases. 2011;204(6):951–961. doi: 10.1093/infdis/jir432. [DOI] [PubMed] [Google Scholar]
- 25.Xiao L, Patterson PS, Yang C, Lal AA. Role of eicosanoids in the pathogenesis of murine cerebral malaria. American Journal of Tropical Medicine and Hygiene. 1999;60(4):668–673. doi: 10.4269/ajtmh.1999.60.668. [DOI] [PubMed] [Google Scholar]
- 26.Wirth JJ, Kierszenbaum F. Stimulatory effects of leukotriene B4 on macrophage association with and intracellular destruction of Trypanosoma cruzi. Journal of Immunology. 1985;134(3):1989–1993. [PubMed] [Google Scholar]
- 27.Wirth JJ, Kierszenbaum F. Effects of leukotriene C4 on macrophage association with and intracellular fate of Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1985;15(1):1–10. doi: 10.1016/0166-6851(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 28.Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. Journal of Immunology. 2006;177(5):3201–3208. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 29.Secor WE, Powell MR, Morgan J, Wynn TA, Funk CD. Mice deficient for 5-lipoxygenase, but not leukocyte-type 12- lipoxygenase, display altered immune responses during infection with Schistosoma mansoni. Prostaglandins and Other Lipid Mediators. 1998;56(5-6):291–304. doi: 10.1016/s0090-6980(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 30.Machado ER, Ueta MT, Lourenço EV, et al. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. The Journal of Immunology. 2005;175(6):3892–3899. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Patterson PS, Yang C, Lal AA. Role of eicosanoids in the pathogenesis of murine cerebral malaria. The American Journal of Tropical Medicine and Hygiene. 1999;60(4):668–673. doi: 10.4269/ajtmh.1999.60.668. [DOI] [PubMed] [Google Scholar]
- 32.Cardillo F, Cunha FQ, Tamashiro WMSC, Russo M, Garcia SB, Mengel J. NK 1.1+ Cells and T-cell activation in euthymic and thymectomized C57B1/6 mice during acute Trypanosoma cruzi infection. Scandinavian Journal of Immunology. 2002;55(1):96–104. doi: 10.1046/j.1365-3083.2002.01034.x. [DOI] [PubMed] [Google Scholar]
- 33.Abrahamsohn IA, da Silva APG, Coffman RL. Effects of interleukin-4 deprivation and treatment on resistance to Trypanosoma cruzi. Infection and Immunity. 2000;68(4):1975–1979. doi: 10.1128/iai.68.4.1975-1979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infection and Immunity. 1994;62(11):5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hölscher C, Mohrs M, Dai WJ, et al. Tumor necrosis factor α-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infection and Immunity. 2000;68(7):4075–4083. doi: 10.1128/iai.68.7.4075-4083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogerio AP, Anibal FF. Role of leukotrienes on protozoan and helminth infections. Mediators of Inflammation. 2012;2012:13 pages. doi: 10.1155/2012/595694.595694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth JJ, Kierszenbaum F. Effects of leukotriene C4 on macrophage association with and intracellular fate of Trypanosoma cruzi . Molecular and Biochemical Parasitology. 1985;15(1):1–10. doi: 10.1016/0166-6851(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 38.Talvani A, Machado FS, Santana GC, et al. Leukotriene B4 induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infection and Immunity. 2002;70(8):4247–4253. doi: 10.1128/IAI.70.8.4247-4253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borges CL, Cecchini R, Tatakihara VLH, et al. 5-Lipoxygenase plays a role in the control of parasite burden and contributes to oxidative damage of erythrocytes in murine Chagas' disease. Immunology Letters. 2009;123(1):38–45. doi: 10.1016/j.imlet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Pavanelli WR, Gutierrez FRS, Mariano FS, et al. 5-Lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes and Infection. 2010;12(8-9):587–597. doi: 10.1016/j.micinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Panis C, Mazzuco TL, Costa CZF, et al. Trypanosoma cruzi: effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Experimental Parasitology. 2011;127(1):58–65. doi: 10.1016/j.exppara.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Rossi A, Acquaviva AM, Iuliano F, di Paola R, Cuzzocrea S, Sautebin L. Up-regulation of prostaglandin biosynthesis by leukotriene C4 in elicited mice peritoneal macrophages activated with lipopolysaccharide/ interferon-γ . Journal of Leukocyte Biology. 2005;78(4):985–991. doi: 10.1189/jlb.1004619. [DOI] [PubMed] [Google Scholar]
- 43.Byrum RS, Goulet JL, Griffiths RJ, Koller BH. Role of the 5-lipoxygenase-activating protein (FLAP) in murine acute inflammatory responses. Journal of Experimental Medicine. 1997;185(6):1065–1075. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsson BM, Kumlin M, Sundblad BM, Larsson K, Dahlén SE, Palmberg L. Effects of 5-lipoxygenase inhibitor zileuton on airway responses to inhaled swine house dust in healthy subjects. Respiratory Medicine. 2006;100(2):226–237. doi: 10.1016/j.rmed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Kageyama Y, Koide Y, Miyamoto S, Yoshida TO, Inoue T. Leukotrien B4-induced interleukin-1β in synovial cells from patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology. 1994;23(3):148–150. doi: 10.3109/03009749409103049. [DOI] [PubMed] [Google Scholar]
- 46.Talvani A, Ribeiro CS, Aliberti JCS, et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-γ as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes and Infection. 2000;2(8):851–866. doi: 10.1016/s1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 47.Aliberti J, Hieny S, Reis Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature Immunology. 2002;3(1):76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 48.da Silva APG, de Almeida Abrahamsohn I. Interleukin-12 stimulation of lymphoproliferative responses in Trypanosoma cruzi infection. Immunology. 2001;104(3):349–354. doi: 10.1046/j.1365-2567.2001.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goño O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1+)CD11b+ immature myeloid suppressor cells. International Immunology. 2002;14(10):1125–1134. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- 50.Liu CH, Fan YT, Dias A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. Journal of Immunology. 2006;177(1):31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. Journal of Leukocyte Biology. 2003;74(6):1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 52.Prinz I, Gregoire C, Mollenkopf H, et al. The type 1 cysteinyl leukotriene receptor triggers calcium influx and chemotaxis in mouse αβ- and γδ effector T cells. The Journal of Immunology. 2005;175(2):713–719. doi: 10.4049/jimmunol.175.2.713. [DOI] [PubMed] [Google Scholar]
- 53.Tager AM, Bromley SK, Medoff BD, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nature Immunology. 2003;4(10):982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 54.Goodwin JS. Regulation of T cell activation by leukotriene B4. Immunologic Research. 1986;5(3):233–248. doi: 10.1007/BF02919204. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira N, Ellis J, Chaplan S, Cohn Z. Trypanosoma cruzi: In vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Experimental Parasitology. 1981;51(3):325–334. doi: 10.1016/0014-4894(81)90120-x. [DOI] [PubMed] [Google Scholar]
- 56.Harel-Bellan A, Joskowicz M, Fradelizi D, Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. Journal of Experimental Medicine. 2002;196(9):1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotner J, Tarleton R. Endogenous CD4+ CD25+ regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infection and Immunity. 2007;75(2):861–869. doi: 10.1128/IAI.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams DM, Sawyer S, Remington JS. Role of activated macrophages in resistance of mice to infection with Trypanosoma cruzi . Journal of Infectious Diseases. 1976;134(6):610–614. doi: 10.1093/infdis/134.6.610. [DOI] [PubMed] [Google Scholar]
- 60.Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-γ is required for resistance to acute Trypanosoma cruzi infection in mice. Journal of Immunology. 1991;146(10):3626–3632. [PubMed] [Google Scholar]
- 61.Adams DO, Hamilton TA. Molecular transductional mechanisms by which IFNγ and other signals regulate macrophage development. Immunological Reviews. 1987;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 62.Kita Y, Takahashi T, Uozumi N, Nallan L, Gelb MH, Shimizu T. Pathway-oriented profiling of lipid mediators in macrophages. Biochemical and Biophysical Research Communications. 2005;330(3):898–906. doi: 10.1016/j.bbrc.2005.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Q, Feng Y, Yang Y, et al. Kinetics of the phenotype and function of murine peritoneal macrophages following acute inflammation. Cellular & Molecular Immunology. 2004;1(1):57–62. [PubMed] [Google Scholar]
- 64.Caligaris-Cappio F, Bergui L, Tesio L, et al. Identification of malignant plasma cell precursors in the bone marrow of multiple myeloma. The Journal of Clinical Investigation. 1985;76(3):1243–1251. doi: 10.1172/JCI112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaoka KA, Claesson HE, Rosen A. Leukotriene B4 enhances activation, proliferation, and differentiation of human B lymphocytes. Journal of Immunology. 1989;143(6):1996–2000. [PubMed] [Google Scholar]
- 66.Phillips C. Induction of leukotriene production before antigen challenge enhances antibody affinity in genetically selected mice. Cellular Immunology. 1991;136(1):173–184. doi: 10.1016/0008-8749(91)90392-o. [DOI] [PubMed] [Google Scholar]
- 67.Burgess DE, Hanson WL. Trypanosoma cruzi: the T-cell dependence of the primary immune response and the effects of depletion of T cells and Ig-bearing cells on immunological memory. Cellular Immunology. 1980;52(1):176–186. doi: 10.1016/0008-8749(80)90410-4. [DOI] [PubMed] [Google Scholar]
- 68.Andrade V, Barral-Netto M, Andrade SG. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Brazilian Journal of Medical and Biological Research. 1985;18(4):499–506. [PubMed] [Google Scholar]
- 69.Marcondes MCG, Borelli P, Yoshida N, Russo M. Acute Trypanosoma cruzi infection is associated with anemia, thrombocytopenia, leukopenia, and bone marrow hypoplasia: reversal by nifurtimox treatment. Microbes and Infection. 2000;2(4):347–352. doi: 10.1016/s1286-4579(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 70.Hamano S, Himeno K, Miyazaki Y, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 71.Cardillo F, Nomizo A, Postól E, Mengel J. NK1.1 cells are required to control T cell hyperactivity during Trypanosoma cruzi infection. Medical Science Monitor. 2004;10(8):BR259–BR267. [PubMed] [Google Scholar]
- 72.Arndt H, Russell JB, Kurose I, Kubes P, Granger DN. Mediators of leukocyte adhesion in rat mesenteric venules elicited by inhibition of nitric oxide synthesis. Gastroenterology. 1993;105(3):675–680. doi: 10.1016/0016-5085(93)90882-d. [DOI] [PubMed] [Google Scholar]
- 73.Sakata K, Dahlén S-E, Bäck M. The contractile action of leukotriene B4 in the guinea-pig lung involves a vascular component. British Journal of Pharmacology. 2004;141(3):449–456. doi: 10.1038/sj.bjp.0705641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. Journal of Experimental Medicine. 1994;180(6):2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michelin MA, Silva JS, Cunha FQC. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Experimental Parasitology. 2005;111(2):71–79. doi: 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]