Nest fidelity is driven by multi-scale information in a long-lived seabird (original) (raw)
Abstract
Although the reproductive success of most organisms depends on factors acting at several spatial scales, little is known about how organisms are able to synthesize multi-scale information to optimize reproduction. Using longitudinal data from a long-lived seabird, Monteiro's storm-petrel, we show that average breeding success is strongly related to oceanic conditions at the population level, and we postulate that (i) individuals use proximal information (their own reproduction outcome in year t) to assess the qualities of their mate and nest and to decide to retain them or not in year t + 1; (ii) the intensity of these responses depends on the quality of the oceanic environment in year t, which affects the predictability of reproduction outcome in year t + 1. Our results confirm that mate and nest fidelities are higher following successful reproduction and that the relationship between the success of a given pair and subsequent nest fidelity is stronger in years with unfavourable oceanic conditions, suggesting that individuals rely on distant information to modulate their use of proximal information and adjust their breeding strategy.
Keywords: breeding habitat selection, predictability, site fidelity, environmental stochasticity, Oceanodroma monteiroi
1. Introduction
Annual reproductive success is a major component of lifetime fitness in all living organisms. At the individual level, at a given point in time, it depends on the interaction between numerous genetic and ecological sources of variation and on the individual strategy adopted to respond to this variation [1]. Empirical work on a wide range of species has highlighted the complexity of the variation in reproductive success, which depends on factors acting at different scales (population, pair or individual; see [2]). A corollary is that the strategies selected to maximize reproductive success should reflect this complexity [3], which in turn necessitates the use of complex and multi-scale information by individuals.
In gonochoric animals, factors underlying variation in reproductive success between (e.g. in a given year) and within (e.g. between years) individuals can be categorized according to three levels of integration: (i) variation in environmental quality affecting the whole population (e.g. variation between patch quality at the interpopulation level or between years in a given patch, hereafter referred to as the distant extrinsic variation); (ii) variation in environmental quality affecting the individuals of a given population differently (e.g. heterogeneity of breeding site quality within the patch, hereafter, proximal extrinsic variation); (iii) variation in quality among reproducing pairs (hereafter referred to as the intrinsic variation), related either to the variance in the additive qualities of the pair members [4,5] or to their genetic compatibility [6].
These three levels of variation generate a high uncertainty in an individual's reproductive outcome, depending on whether the sources of variation are predictable or not. The strategies adopted by individuals to minimize this uncertainty rely on adaptive behaviour processes [7], in which animals use biological information (hereafter we will use the nomenclature and definitions proposed in [8]) to adjust their behaviour to the environmental and intrinsic factors related to reproductive success. Behavioural processes include dispersal versus philopatry (at either intra- or interpopulation level [9–11]), divorce versus mate retention [12,13], as well as habitat [14] and mate [15] selection.
One of the most commonly observed information-gathering process used by individuals to maximize their reproductive success is the ‘win–stay, lose–switch (WSLS)’ model, in which individuals tend to maintain the conditions encountered in a given year if they reproduced successfully in that year and change the conditions if they failed. This model can be applied to the proximal extrinsic and intrinsic scales described above, via nest change [11] or divorce [13] conditioned on individual reproductive failure. The reasoning underlying the WSLS model is that current success is a reliable predictor of future success [16]. At larger scales, similar logic underlies the use of the average reproductive success of conspecifics at the patch level in some bird species during the process of habitat selection [3,10,17]. However, such a process applies to spatial distant extrinsic variation (i.e. patch quality) in cases where variation is consistent in time (i.e. predictable [3,16]), but not to temporal variation (such as meteorological conditions), which is usually not or poorly predictable [18,19]. Nevertheless, temporal variation in environmental quality is another important, universal source of variation in annual reproductive success and empirical evidence suggests that some organisms are able to process predictive information about the quality of the breeding season and respond to this information. Such mechanisms have been described in relation to the survival cost of reproduction in long-lived animals, whereby individuals can delay first breeding or skip reproduction in bad years to maximize their lifetime reproductive success [20,21].
In spite of the widely recognized importance of these various scales at which the factors affecting reproductive success can act, very little is known about the way organisms synthesize the information coming from different scales. We postulate here that the use of proximal scale information should be modulated by larger scale information [22]. To test this general hypothesis, we investigated the relationship between distant extrinsic variation (year-to-year variation in climatic and oceanic conditions), reproductive success, and mate and individual site fidelity in an endangered, recently described [23], long-lived colonial seabird, Monteiro's storm-petrel Oceanodroma monteiroi. Reproductive success in petrels is simple to estimate and binary as breeding females lay a single egg per year. The environmental variables used are the North Atlantic Oscillation (NAO) index, the sea surface temperature (SST) and the chlorophyll-a (Chl-a) concentration, which have an influence on the yearly cycle and abundance of zooplankton [24] and subsequent seabird prey availability [25], and which have been found to be related to demographic processes in Monteiro's storm-petrel [26].
Based on the WSLS model, and focusing our analysis on the pair (not individual) level, we predict that successful reproduction is a better indicator of pair or nest quality if it occurs in a year with bad distant environmental conditions and low reproductive success at the colony level, than if occurring in a good year with high average success. We make the following predictions at the pair level: (i) reproductive success is correlated with climatic and oceanic conditions; (ii) year-to-year site and mate fidelity are higher following successful breeding (SB) than following a failure; (iii) there is a negative interaction between the reproduction outcome at the pair level in a given year and the environmental quality in that year on the probability of retaining one's mate and nest the next year.
2. Material and methods
(a). Model species
Monteiro's storm-petrel is a small (45 g) procellariiform endemic to the Azores archipelago (subtropical northeastern Atlantic), where it is known to breed only on two mammal-free islets (Praia and Baixo) situated 5 km apart just off Graciosa Island. Total population size is estimated at 250–300 breeding pairs [23]. Like all procellariiform species, Monteiro's storm-petrel is socially monogamous, females lay a single egg per breeding attempt and both sexes participate in incubation and chick-rearing [27]. Laying occurs between late April and early July and chicks hatch between the second week of June and late July [23]. Individuals can start breeding when 2 years old [28]. Although Monteiro's storm-petrels leave the islets at the end of the breeding period, the species is considered to remain in the Azorean waters the whole year round [23].
(b). Data collection and monitoring protocol
(i). Demographic data
We used capture–mark–recapture data collected on Praia Islet (39.8030° N, 27.8570° W; 0.12 km2) between 1993 and 2012, based on banding of adults and chicks. However, reproduction and nest/mate fidelity at this locality were monitored only during the period 2000–2012. On Praia Islet, Monteiro's storm-petrels breed in some of the 150 artificial nests available and in natural nests [29,30]. All nest-boxes and all accessible natural nests (approx. 30 nests) were checked during incubation and before fledging in order to determine pair identity (based on the identification of the two pair mates) and breeding status (see below). Each nest (natural or artificial) had a unique identification code. Owing to laying asynchrony, three field sessions occurred each year, the first one during the first half of June (identifying the early breeders during incubation), the second one in late July–early August (identifying the late breeders during incubation and banding the early-hatched chicks) and the last one in early September (banding the late-hatched chicks). Based on these three sessions, the breeding status of each pair was eventually recorded as either non-breeder (NB, that is, no egg laid), unsuccessful breeder (UB, i.e. failure during incubation or chick-rearing) or successful breeder (SB, i.e. fledged chick).
(ii). Climatic and oceanic data
To characterize the general climatic and oceanographic conditions in the surroundings of the breeding colony (Praia Islet), we used quarterly composites of the NAO, the Chl-a concentration (in mg m−3) in the sea surface and the SST (in °C) for the period 2000–2012. NAO indices were downloaded from NOAA (http://www.cpc.ncep.noaa.gov). Sea-viewing Wide Field-of-view Sensor (SeaWiFS) products of Chl-a were downloaded from OceanColor (http://oceancolor.gsfc.nasa.gov). Advanced Very High-Resolution Radiometer Pathfinder V5 products of SST were downloaded from NOAA (http://www.nodc.noaa.gov). Both composite products were extracted as level-3 HDF files at a spatial resolution of 0.048 (approx. 4 km). Files were converted to raster images using the Marine Geospatial Ecology tools (GeoEco) for ArcGIS v. 9.2 (ESRI 2006). Mean monthly values were obtained for 500 km radius from Praia Islet. All GIS products were processed in the European Albers Equal Area Conic Projection.
(c). Statistical analyses
(i). Demographic indices
The breeding success (BS) was a binary variable computed for each pair in each year t. It was set to ‘1’ in case of SB and ‘0’ otherwise (NR and UB, n = 701). We also computed the average BS of the population in a given year (BSmean). Mate fidelity (FidMate) was a binary variable computed for each pair in each year t. It was set to ‘1’ when pairs reunited in year t + 1, and to ‘0’ when they divorced. Only cases where both individuals were alive and their status was known in year t + 1 were considered (n = 403). A pair was considered to have divorced when at least one of the former partners was found breeding with a new partner while its previous mate was still alive [31]. Nest fidelity (FidNest) was a binary variable computed for each pair in each year t. It was set to ‘1’ if at least one individual of the pair remained in the nest in year t + 1 and ‘0’ if none of the individuals remained in the nest in year t + 1, excluding cases where both individuals were dead or absent (that is, they had taken a ‘sabbatical’ year [32]), and cases where the status of the nest and/or pair members was uncertain in year t + 1 (n = 445). The detailed algorithm is presented in the electronic supplementary material, Appendix S1. This variable was constructed in order to maximize sample size while avoiding false interpretation (due to death or uncertain status).
(ii). Temporal autocorrelation
Temporal autocorrelation patterns of NAO, SST, Chl-a and BSmean were examined using the Box–Pierce test.
(iii). Modelling
We assessed the effects of environmental conditions on our three response variables BS, FidMate and FidNest using generalized mixed-effects models with a binomial error. The current year, pair identity and nest identity were included in all models as random effects variables. We first examined the relationships between BS and climatic and oceanic indices (fixed effects) by developing a candidate model set including all possible combinations of the independent variables. Our global model included quarterly composites of NAO, SST and Chl-a computed for the first three quarters of each year, as well as composites of the last quarter of the previous year for NAO only (10 fixed effects variables). We ranked models based on the Akaike information criterion corrected for small sample size (AICc), identified best models (i.e. ΔAICc from the best model of less than 2) and calculated associated Akaike weights (w) [33]. To assess environmental effects, we calculated model-averaged partial regression coefficients (β) for each covariate based on the set of best models. We determined the relative importance of each covariate based on the sum of w across the entire model set. We reported 95% confidence intervals (CIs) around β for each covariate and deemed an effect significant if unconditional CIs did not include zero. In parallel, in order to obtain the best single indices of environmental quality, we ran a series of univariate models.
In a second step, we examined whether nest and mate fidelities were associated with pair BS, using FidMate and FidNest (in year t + 1) as dependent variables and BS (in year t) as the independent variable.
Finally, to test our main predictions, we examined the interaction between ‘year quality’ (i.e. distant temporal variation) and pair BS on FidMate and FidNest. We used the same information-theoretic approach as for the BS analysis described above. For both FidMate and FidNest, our global model included (i) all climatic and oceanic covariates with significant effects on BS; (ii) BS; (iii) the average BS of the population, BSmean (computed for each pair, in each year, excluding the particular pair of interest to avoid dependency between BS and BSmean); (iv) the interaction between BS and each climatic/oceanic covariate; and (v) the BS × BSmean interaction.
The robustness of our results was assessed using sensitivity analyses where we (i) considered a more restricted definition of BS (excluding NBs); (ii) used an individual-based (instead of pair-based) approach, with separate analyses for males and females; (iii) used composite environmental variables; and (iv) checked that our results are related to within-pair variation and not variation between pairs (all additional results are presented in the electronic supplementary material). All statistical analyses were performed with R v. 3.0.2 [34], specifically with the lme4 [35] and MuMIn [36] packages.
3. Results
Although some of the climatic and oceanic parameters considered were correlated with each other, both within and between quarters (electronic supplementary material, Appendix S2), we considered all parameters in the results presented below, to discuss their relative influences on BS (an alternative analysis based on uncorrelated composite variables is presented in the electronic supplementary material, Appendix S3). No significant temporal autocorrelations were detected when considering average BS and environmental parameters (Box–Pierce test, p > 0.05 in all cases).
At the pair level, BS in a particular year was best predicted by the Chl-a concentration in the second and third quarters (Chl-a(q2) and Chl-a(q3)) and by the SST in the second quarter (SST(q2)), which had relatively high AICc weights and significantly positive model-averaged partial regression coefficients (table 1).
Table 1.
Model-averaged partial regression coefficients (β) and unconditional 95% CIs from generalized linear mixed-effects models of BS in the Monteiro's storm-petrel population monitored on Praia Islet (2000–2012, n = 701) in relation to climatic and oceanic conditions. NAO(qx), SST(qx) and Chl-a(qx), respectively, denote the NAO index, SST and Chl-a concentration computed for the _x_th quarter of the current year. NAO(_t_−1) denotes the NAO index computed for the fourth quarter of the previous year. Akaike weight (w) for a covariate indicates relative importance of the covariate based on summing weights across models where the covariate occurs. Random effects for pair identity and nest identity are fitted for all models. Variance components for the global model are 0.62 and 0.023 for pair identity and nest identity, respectively. Coefficients are in bold where CIs do not include zero. Only covariates occurring in the subset of best models (ΔAICc < 2) are presented.
| variable | w | β | lower CI | upper CI |
|---|---|---|---|---|
| Chl-a(q2) | 0.62 | 3.51 | 0.99 | 6.02 |
| SST(q2) | 0.59 | 0.71 | 0.04 | 1.39 |
| Chl-a(q1) | 0.57 | −4.19 | −10.8 | 2.48 |
| SST(q3) | 0.41 | −0.19 | −0.49 | 0.11 |
| Chl-a(q3) | 0.40 | 16.36 | 0.42 | 32.3 |
| NAO(_t_−1) | 0.38 | −0.038 | −0.57 | 0.49 |
| NAO(q2) | 0.36 | 0.23 | −0.12 | 0.58 |
| SST(q1) | 0.33 | 0.41 | −0.21 | 1.03 |
| NAO(q1) | 0.32 | −0.18 | −0.59 | 0.24 |
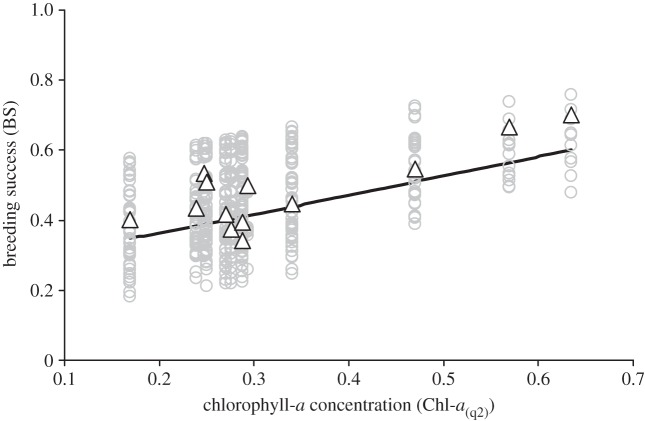
Three significant single environmental predictors of BS were obtained from univariate regressions: the NAO index in the second quarter and the Chl-a concentration in the second and third quarters (all were positively correlated with BS; see figure 1 for an illustration).
Figure 1.
BS in the Monteiro's storm-petrel population monitored on Praia Islet (2000–2012, n = 701) as a function of Chl-a concentration in the second quarter of each year (Chl-a(q2)). Grey circles are the predicted values obtained from univariate generalized linear mixed-effects model (see Material and methods) and open triangles are the observed values at the population scale (average annual BS). The black line is the regression line.
As expected, both mate fidelity (FidMate) and nest fidelity (FidNest) were significantly, positively correlated with BS (detailed results in the electronic supplementary material, Appendix S2).
In order to test our main prediction, we then examined the interaction between BS (i.e. reproduction outcome at the pair level) and the variables related to the reproduction outcome at the population level (i.e. the average breeding success (BSmean) and the three environmental indices related to success, Chl-a(q2), Chl-a(q3) and SST(q2)) on FidMate and FidNest. The analysis did not reveal any significant interaction between BS and predictors for FidMate. By contrast, for FidNest, the analysis revealed negative interactions between BS and Chl-a concentration variables, with a significant model-averaged partial regression coefficient for Chl-a(q2) (table 2). No BS × BSmean interaction was found. Further results obtained by running separate models of FidNest for successful pairs versus unsuccessful pairs indicated that: (i) in pairs that bred successfully in year t, nest fidelity between t and t + 1 increased with decreasing environmental quality in year t (i.e. lower Chl-a concentration) and (ii), by contrast, nest fidelity was unrelated to environmental quality in unsuccessful pairs (detailed results in the electronic supplementary material, Appendix S2). The interacting effects between indices of environmental quality and individual BS are illustrated in figure 2, for a single index of environmental quality (Chl-a(q2)) (in parallel univariate analyses, all three univariate indices of environmental quality Chl-a(q2), Chl-a(q3) and NAO(q2) exhibited significant negative interactions with BS on FidNest). No significant interaction was found when considering the predictors of environmental quality for year t + 1 (instead of t). All results were robust to our assumptions regarding the definitions of BS, nest fidelity, environmental predictors, scale of the analysis (i.e. individual versus pair) and the analysis of a subset of data indicated that our general result is related to within-pair variation and not variation between pairs (see all additional results in the electronic supplementary material, Appendix S3).
Table 2.
Model-averaged partial regression coefficients (β) and unconditional 95% CIs from generalized linear mixed-effects models of mate fidelity (FidMate, n = 403) and nest fidelity (FidNest, n = 445) in the Monteiro's storm-petrel population monitored on Praia Islet (2000–2012) in relation to (i) a reduced set of oceanic variables: SST computed for the second quarter of the year (SST(q2)) and Chl-a concentration computed for the second and third quarters (Chl-a(q2) and Chl-a(q3)); (ii) the BS at the pair level and (iii) the average breeding success at the colony level (BSmean); (iv) all first-order interactions between BS and other covariates. Akaike weight (w) for a covariate indicates relative importance of the covariate based on summing weights across models where the covariate occurs. Random effects for pair identity and nest identity are fitted for all models. For the FidMate global model, variance components are 3.1, 0.2 and 0.001 for pair identity, nest identity and year, respectively. For the FidNest global model, variance components are 0.0001, 0.23 and 0.02. Coefficients are in bold where CIs do not include zero. Only covariates occurring in the subset of best models (ΔAICc < 2) are presented.
| variable | Fidmate | FidNest | ||||||
|---|---|---|---|---|---|---|---|---|
| W | β | lower CI | upper CI | w | β | lower CI | upper CI | |
| BSmean | 0.75 | 13.16 | −4.71 | 31.04 | 0.39 | — | — | — |
| Chl-a(q2) | 0.69 | −0.09 | −23.59 | 23.41 | 0.65 | 6.31 | −0.92 | 13.55 |
| BS | 0.61 | −8.39 | −25.76 | 8.98 | 1.00 | 4.16 | 0.41 | 7.92 |
| BS × Chl-a(q2) | 0.61 | 35.65 | −28.99 | 100.30 | 0.56 | −7.60 | −14.67 | −0.54 |
| Chl-a(q3) | 0.55 | 123.45 | −113.71 | 360.61 | 0.98 | −36.82 | −82.28 | 8.65 |
| SST(q2) | 0.12 | — | — | — | 0.54 | −0.58 | −1.49 | 0.33 |
| BS × Chl-a(q3) | 0 | — | — | — | 0.54 | −35.41 | −83.89 | 13.07 |
Figure 2.
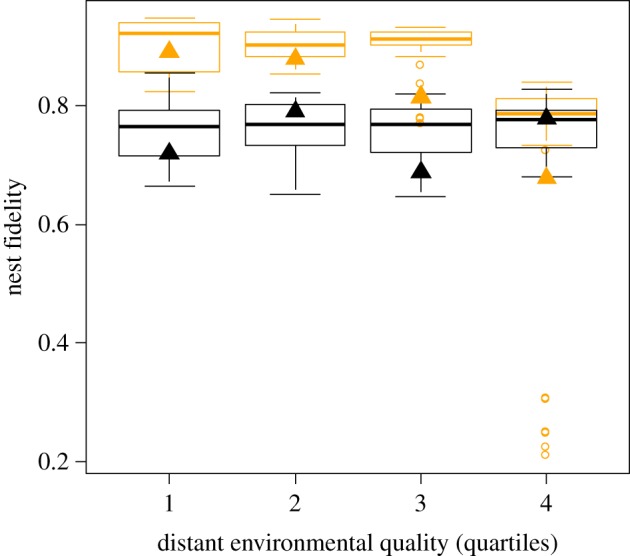
Boxplot representation of nest fidelity between years t and t + 1 (FidNest) in the Monteiro's storm-petrel population monitored on Praia Islet (2000–2012) for successful (orange boxes, n = 216) and unsuccessful (black boxes, n = 229) pairs in year t as a function of distant environmental quality (based on quartile cuts of Chl-a(q2), the Chl-a concentration in the second quarter of each year). Boxplots are based on the predicted values obtained from univariate generalized linear mixed-effects model (see Material and methods). Triangles are the observed values at the population level (weighted annual average proportions of nest fidelity for successful and unsuccessful breeding pairs in black and orange, respectively). (Online version in colour.)
4. Discussion
Our analysis of the reproduction in Monteiro's storm-petrel revealed that (i) reproductive success is strongly positively correlated with the oceanic Chl-a concentration; (ii) at the pair level, both mate and nest fidelity are higher following successful reproduction; (iii) the relationship between pair BS and subsequent nest fidelity is stronger in years with unfavourable distant extrinsic conditions (low Chl-a). No such interaction was found when considering mate fidelity. Although we found that BS significantly varied among nest sites (see the electronic supplementary material, Appendix S2), the physical or ecological characteristics of sites explaining this heterogeneity remain unknown. Previous work in our study population suggests that BS tends to be higher in the artificial nests installed to increase breeding numbers than in natural nests [30], mainly because artificial nests cannot be excavated by Cory's shearwaters, Calonectris borealis (another burrow-nesting petrel, which is much larger than Monteiro's storm-petrel) and cannot be flooded. However, chick productivity also shows great variations among natural nests and among artificial nests (J.B. 2012, unpublished data).
According to the habitat selection theory, individuals that settle and breed in high-quality sites achieve higher fitness than individuals settling in lower quality sites [1,37]. Thus, natural selection is expected to favour the development of complex methods for assessing site quality [3,10]. Because the factors acting on BS vary at different spatial and temporal scales corresponding to different levels of habitat selection [2], individuals should rely on these various scales to assess site quality. However, although recent works have revealed that combinations of information from several sources can be used by animals in other contexts [38], only a few studies have reported such processes in the context of breeding site selection.
In the colonial black-legged kittiwake, Rissa tridactyla, Danchin et al. [17] found that breeders tend to recruit to the previous year's most productive cliffs and to emigrate from the least productive ones. Individual success does not influence the probability of dispersing for birds breeding on cliffs with high local reproductive success, whereas individual success is highly, negatively related to dispersal for birds breeding on cliffs with lower local reproductive success, suggesting that the information provided by the average success of conspecifics is sufficient to override individual or pair experience. Schjørring et al. [39] found similar results in the great cormorant, Phalacrocorax carbo, in which the fidelity of females to their nesting site increases with both their own success and the increasing success of neighbouring pairs (BS was found to be spatially autocorrelated within the colony). Such double, hierarchical influence of social public and personal information on nesting site selection has been experimentally confirmed in a non-colonial short-lived passerine bird, the collared flycatcher, Ficedula albicollis [10].
Here, we hypothesized a completely different process in which broad scale information (i.e. oceanic conditions in a given year) does not allow assessing patch or individual site quality, but rather allows assessing lower scale information quality (i.e. predictability of future reproduction outcome given information on current individual success). Theoretical work has shown that the relationship between individual reproductive success and site fidelity should be stronger in more predictable habitats, or, more generally, when the predictability of the reproductive outcome increases [16]. In our context, individual success occurring in a year with bad conditions can be considered as a reliable indicator of nest quality, thus improving the predictability of the reproductive outcome at the pair level (complementary results indicate that the predictability of the reproductive outcome is higher following years with bad conditions than following years with good conditions; see the electronic supplementary material, Appendix S3, for details). These results further imply that a particular ‘potential information’ (e.g. individual success in a given year) can be ‘realized’ differently according to another information coming from a different scale (e.g. oceanic conditions in this year) to modulate a behavioural response (see [8,40] for definition and discussion on the potential versus realized information). Our analysis uncovered much stronger interactions between individual (pair) success and environmental factors than between individual success and average colony success to explain site fidelity, suggesting that the information used by breeders for assessing wide scale environmental quality does not rely on social public information. Supporting this, Monteiro's storm-petrel is a burrow- or cavity-nesting bird, so that information on pair BS is unlikely to be public. Under these conditions, distant environmental quality might be better reflected by personal information [7], such as personal foraging effort [41], than by social public information—unless such pelagic birds also have access to public information on collective foraging effort at sea. However, whether these storm-petrels forage in a restricted common marine area is still unknown.
Although we found a strong relationship between mate fidelity and individual reproductive success, as predicted by the WSLS model [13], contrary to site fidelity, we did not find any interaction of pair reproductive performance and environmental conditions on mate fidelity. Although mate choice follows apparently the same kind of information-gathering process as site (or patch) selection [42], it is considered by some authors to be a by-product of site fidelity ([11,43], but see [44]), or driven by completely different processes, such as genetic incompatibility [45] or the search for a partner with good parental abilities, especially in monogamous birds with obligate biparental care [46]. Our results are consistent with the view that mate retention is an active process relying on information that is at least partly distinct from the information used for nest fidelity in Monteiro's storm-petrel. Further research is needed to disentangle individual (or pair) quality from nest quality when examining the relationships between distant environmental conditions, reproductive performance and breeding dispersal. Nevertheless, this study highlights the part played by factors operating far from the breeding locality on nest fidelity, confirming the complexity of the processes underlying breeding dispersal in birds
Supplementary Material
Appendix S1
Supplementary Material
Appendix S2
Supplementary Material
Appendix S3
Acknowledgements
V. C. Neves, R. Medeiros, A. Campos, B. Hothersall, L. Aguiar, M. Laranjo, M. C. Magalhães, M. Villafane, P.-A. Crochet, M.-P. Dubois, M. Antunes, R. Fontaine, P. Visicchio and M. Andris provided field assistance. We thank the Regional Environment Directorate from the Azores (DRA) for allowing us to conduct fieldwork, and the DRA, M. Melo and R. Oliveira for transportation to the islet. We are grateful to Dr I. Lovette and two anonymous referees for their thoughtful and detailed reviews of this paper.
Data accessibility
Demographic and environmental data are available at Dryad (doi:10.5061/dryad.kp497).
Funding statement
This study was funded by grants from the Fundação para a Ciência e a Tecnologia (PRAXIS/C/BIA/13194/98, POCTI-BIA-13194/98 and PTDC/BIABDE/67286/2006) and FEDER (FCOMP-01-0124-FEDER-007061), and also received support from the programmes ‘MARE’ (Life contract B4-3200/98-509), ‘OGAMP’ (Interreg IIIB-MAC/4.2/A2), ‘MARMAC’ (Interreg IIIB/FEDER/MARMAC/003-4/2005-6 and Interreg IIIB-05/MAC/4.2/A4) and MoniAves (programme launched by the Regional Environment Directorate of the Azores) coordinated by R. S. Santos. IMAR-DOP/UAç is funded by FCT and DRCT-Azores (Research Unit no. 531 and Associate Laboratory no. 9-ISR-Lisbon).
References
- 1.Fretwell SD, Lucas HL., Jr 1970. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 14, 16–36. [Google Scholar]
- 2.Morris DW. 1987. Spatial scale and the cost of density-dependent habitat selection. Evol. Ecol. 1, 379–388. ( 10.1007/BF02071560) [DOI] [Google Scholar]
- 3.Boulinier T, Danchin E. 1997. The use of conspecific reproductive success for breeding patch selection in territorial migratory species. Evol. Ecol. 11, 505–517. ( 10.1007/s10682-997-1507-0) [DOI] [Google Scholar]
- 4.Curio E. 1983. Why do young birds reproduce less well? Ibis 125, 400–404. ( 10.1111/j.1474-919X.1983.tb03130.x) [DOI] [Google Scholar]
- 5.Wilson AJ, Nussey DH. 2010. What is individual quality? An evolutionary perspective. Trends Ecol. Evol. 25, 207–214. ( 10.1016/j.tree.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 6.Tregenza T, Wedell N. 2000. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 9, 1013–1027. ( 10.1046/j.1365-294x.2000.00964.x) [DOI] [PubMed] [Google Scholar]
- 7.Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. ( 10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 8.Wagner RH, Danchin E. 2010. A taxonomy of biological information. Oikos 119, 203–209. ( 10.1111/j.1600-0706.2009.17315.x) [DOI] [Google Scholar]
- 9.Haas CA. 1998. Effects of prior nesting success on site fidelity and breeding dispersal: an experimental approach. Auk 115, 929–936. ( 10.2307/4089511) [DOI] [Google Scholar]
- 10.Doligez B, Danchin E, Clobert J. 2002. Public information and breeding habitat selection in a wild bird population. Science 297, 1168–1170. ( 10.1126/science.1072838) [DOI] [PubMed] [Google Scholar]
- 11.Bai M, Severinghaus LM. 2012. Disentangling site and mate fidelity in a monogamous population under strong nest site competition. Anim. Behav. 84, 251–259. ( 10.1016/j.anbehav.2012.05.004) [DOI] [Google Scholar]
- 12.Bried J, Jouventin P. 2002. Site and mate choice in seabirds: an evolutionary approach. In Biology of marine birds (eds Schreiber EA, Burger J.), pp. 263–305. Boca Raton, FL: CRC Press. [Google Scholar]
- 13.Dubois F, Cézilly F. 2002. Breeding success and mate retention in birds: a meta-analysis. Behav. Ecol. Sociobiol. 52, 357–364. ( 10.1007/s00265-002-0521-z) [DOI] [Google Scholar]
- 14.Wiens JA. 1976. Population responses to patchy environments. Annu. Rev. Ecol. Syst. 7, 81–120. ( 10.1146/annurev.es.07.110176.000501) [DOI] [Google Scholar]
- 15.Bateson PPG. 1983. Mate choice. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Switzer PV. 1993. Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 7, 533–555. ( 10.1007/BF01237820) [DOI] [Google Scholar]
- 17.Danchin E, Boulinier T, Massot M. 1998. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology 79, 2415–2428. ( 10.1890/0012-9658(1998)079[2415:CRSABH]2.0.CO;2) [DOI] [Google Scholar]
- 18.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 19.Stireman JO, III, et al. 2005. Climatic unpredictability and parasitism of caterpillars: implications of global warming. Proc. Natl Acad. Sci. USA 102, 17 384–17 387. ( 10.1073/pnas.0508839102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erikstad KE, Fauchald P, Tveraa T, Steen H. 1998. On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79, 1781–1788. ( 10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2) [DOI] [Google Scholar]
- 21.Cubaynes S, Doherty PF, Jr, Schreiber EA, Gimenez O. 2011. To breed or not to breed: a seabird's response to extreme climatic events. Biol. Lett. 7, 303–306. ( 10.1098/rsbl.2010.0778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira MAR, Lee HKH. 2007. Multiscale modeling: a Bayesian perspective. New York, NY: Springer. [Google Scholar]
- 23.Bolton M, Smith AL, Gómez-Díaz E, Friesen VL, Medeiros R, Bried J, Roscales JL, Furness RW. 2008. Monteiro's Storm-petrel Oceanodroma monteiroi: a new species from the Azores. Ibis 150, 717–727. ( 10.1111/j.1474-919X.2008.00854.x) [DOI] [Google Scholar]
- 24.Bertram DF, Mackas D, McKinnell SM. 2001. The season cycle revisited: interannual variation and ecosystem consequences. Progr. Oceanogr. 49, 283–307. ( 10.1016/S0079-6611(01)00027-1) [DOI] [Google Scholar]
- 25.Schreiber EA. 2002. Climate and weather effects on seabirds. In Biology of marine birds (eds Schreiber EA, Burger J.), pp. 179–215. Boca Raton, FL: CRC Press. [Google Scholar]
- 26.Robert A, Paiva VH, Bolton M, Jiguet F, Bried J. 2012. The interaction between reproductive cost and individual quality is mediated by oceanic conditions in a long-lived bird. Ecology 93, 1944–1952. ( 10.1890/11-1840.1) [DOI] [PubMed] [Google Scholar]
- 27.Warham J. 1990. The petrels. Their ecology and breeding systems. London, UK: Academic Press. [Google Scholar]
- 28.Bried J, Bolton M. 2005. An initial estimate of age at first return and breeding in Madeiran storm petrels Oceanodroma castro. Atlantic Seabirds 7, 71–74. [Google Scholar]
- 29.Bolton M, Medeiros R, Hothersall B, Campos A. 2004. The use of artificial breeding chambers as a conservation measure for cavity-nesting procellariiform seabirds: a case study of the Madeiran storm petrel (Oceanodroma castro). Biol. Conserv. 116, 73–80. ( 10.1016/S0006-3207(03)00178-2) [DOI] [Google Scholar]
- 30.Bried J, Magalhães MC, Bolton M, Neves VC, Bell E, Pereira JC, Aguiar L, Monteiro LR, Santos RS. 2009. Seabird habitat restoration on Praia Islet, Azores archipelago. Ecol. Restor. 27, 27–36. ( 10.3368/er.27.1.27) [DOI] [Google Scholar]
- 31.Black JM. 1996. Introduction: pair bonds and partnerships. In Partnerships in birds: the study of monogamy (ed. Black JM.), pp. 3–20. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Mougin JL, Jouanin C, Roux F. 1997. Intermittent breeding in Cory's Shearwater Calonectris diomedea of Selvagem Grande, North Atlantic. Ibis 139, 40–44. ( 10.1111/j.1474-919X.1997.tb04502.x) [DOI] [Google Scholar]
- 33.Burnham K, Anderson D. 2003. Model selection and multimodel inference, 2nd edn New York, NY: Springer. [Google Scholar]
- 34.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ (accessed 15 May 2013). [Google Scholar]
- 35.Bates DM, Sarkar D. 2007. lme4: linear mixed-effects models using S4 classes. R package v. 99875–99876. http://cran.r-project.org/web/packages/lme4/(accessed 1 March 2014).
- 36.Barton K.2011. MuMIn: multi-model inference. R package v. 1.6.5. http://CRAN.R-project.org/package=MuMIn. (accessed 8 August 2014).
- 37.Bernstein CM, Krebs JR, Kacelnik A. 1991. Distribution of birds amongst habitats: theory and relevance to conservation. In Bird population studies: relevance to conservation and management (eds Perrins CM, Lebreton JD, Hirons GJM.), pp. 317–345. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Peake TM, Terry AMR, McGregor PK, Dabelsteen T. 2002. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proc. R. Soc. Lond. B 269, 1925–1929. ( 10.1098/rspb.2002.2112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schjørring S, Bregnballe T, Gregersen J. 2000. Sex difference in criteria determining fidelity towards breeding sites in the great cormorant. J. Anim. Ecol. 69, 214–223. ( 10.1046/j.1365-2656.2000.00385.x) [DOI] [Google Scholar]
- 40.Blanchet S, Clobert J, Danchin E. 2010. The role of public information in ecology and conservation: an emphasis on inadvertent social information. Ann. NY Acad. Sci. 1195, 149–168. ( 10.1111/j.1749-6632.2010.05477.x) [DOI] [PubMed] [Google Scholar]
- 41.Giraldeau LA. 1997. The ecology of information use. In Behavioural ecology (eds Krebs JR, Davies NB.), pp. 42–68. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 42.Sullivan MS. 1994. Mate choice as an information gathering process under time constraint: implications for behaviour and signal design. Anim. Behav. 47, 141–151. ( 10.1006/anbe.1994.1016) [DOI] [Google Scholar]
- 43.Naves LC, Cam E, Monnat JY. 2007. Pair duration, breeding success and divorce in a long-lived seabird: benefits or mate familiarity? Anim. Behav. 73, 433–444. ( 10.1016/j.anbehav.2006.10.004) [DOI] [Google Scholar]
- 44.Bried J, Pontier D, Jouventin P. 2003. Mate fidelity in monogamous birds: a re-examination of the Procellariiformes. Anim. Behav. 65, 235–246. ( 10.1006/anbe.2002.2045) [DOI] [Google Scholar]
- 45.Robert A, Couvet D, Sarrazin F. 2005. Inbreeding effects on pair fecundity and population persistence. Biol. J. Linn. Soc. 86, 467–476. ( 10.1111/j.1095-8312.2005.00545.x) [DOI] [Google Scholar]
- 46.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Data Availability Statement
Demographic and environmental data are available at Dryad (doi:10.5061/dryad.kp497).