A New Strategy for Public Health Surveillance at CDC: Improving National Surveillance Activities and Outcomes (original) (raw)
Public health surveillance is the cornerstone of public health practice and can be defined as the “… systematic, ongoing collection, management, analysis, and interpretation of data followed by the dissemination of these data to public health programs to stimulate public health action.”1 Stakeholders in the United States at all levels of government (i.e., federal and state, territorial, local, and tribal [STLT]), in academia and industry, and the general public rely on high-quality, timely surveillance data to detect and monitor diseases, injuries, and conditions; assess the impact of interventions; and assist in the management of large-scale disease incidents. Surveillance data are crucially important to inform policy changes, guide new program interventions, sharpen public communications, and help agencies assess research investments.
The public health surveillance enterprise in the U.S. is a long-term partnership that operates through thousands of agencies at the federal and STLT levels. The U.S. Centers for Disease Control and Prevention (CDC) generally does not collect public health surveillance information directly, but relies on state and local health departments and other systems to do so. CDC, however, plays an important collaborative role in aggregating, analyzing, and disseminating surveillance data; creating tools for surveillance; providing technical assistance to states and territories; researching surveillance policy; and funding surveillance activities. In the past few years, observers inside and outside CDC have identified some of the most important influences shaping surveillance in the 21st century (e.g., security concerns, technological advances, and health-care reform) and how these influences may affect the surveillance enterprise. Observers have touched on the need for ongoing evaluation of surveillance systems; standardization, with the goal of developing sustainable and integrated systems; and system and workforce adaptability to current demands. These observers have recognized many challenges that could impede progress, such as funding, workforce, information technology standards, patient confidentiality, and concerns about data access, quality, and sharing.1–3 For example, one fundamental challenge is the tension, both at the federal and STLT levels, between the needs of the whole surveillance enterprise and specific disease control programs, which require specialized surveillance data and are organized and funded along disease-specific lines.
CDC's overarching goal for federally supported surveillance activities is to get the right information into the right hands at the right time. In a fresh attempt to better achieve this goal, and in response to the observations and recommendations of experts,1–3 the agency launched the CDC Surveillance Strategy in February 2014.4 We describe the essential parts of the Strategy, which is not meant to be a national surveillance strategy; rather, it focuses on what CDC must do to impel progress and augment trust with the agency's surveillance partners in the field. It builds on previous work inside and outside CDC to arrive at a vision of public health surveillance for the 21st century.1–3 The Strategy outlines three goals and 10 specific aims to improve surveillance capabilities, outcomes, and public health. By embracing the current challenges as opportunities, fixing what needs to be fixed, and working closely with its STLT partners, CDC can help revitalize U.S. public health surveillance.
THE CDC SURVEILLANCE STRATEGY
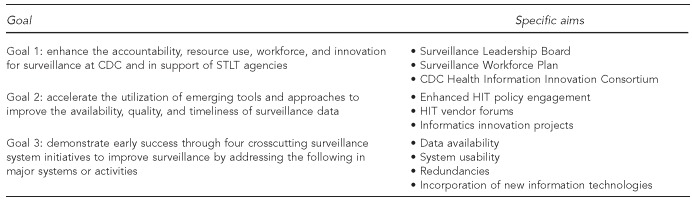
As shown in Figure 1, the Strategy addresses fundamental problems found through external review, stakeholder consultations, and internal assessments. Specific aims are contained within each of the three goals. Goal 1 focuses on establishing new structures, Goal 2 on processes, and Goal 3 on improvements in public health surveillance outcomes that can be accomplished relatively quickly. CDC aims to rapidly improve its activities in the short term, while laying the groundwork for ongoing evaluation and modification of surveillance systems in the long term. The Strategy will guide CDC efforts to make U.S. surveillance systems more adaptable to rapidly changing technology, more versatile in addressing evolving health threats, more adept at accessing and leveraging health-care data, and more capable of meeting demands for timely, population-specific, and geographically specific information. The Strategy also facilitates work to consolidate surveillance systems, eliminate unnecessary redundancies, reduce reporting burden, and improve data availability, quality, and timeliness for all stakeholders.4
Figure 1.
Goals and associated specific aims of the CDC Surveillance Strategy
CDC = Centers for Disease Control and Prevention
STLT = state, territorial, local, and tribal
HIT = health information technology
Specific aims of goal 1: establishing new structures
The Surveillance Leadership Board, comprising senior CDC leaders, was established in July 2014 to review, guide, and oversee the evolution of CDC surveillance systems in accordance with the overall Strategy and established recommendations.1–3 The Board, which is chaired by CDC's Deputy Director for Public Health Scientific Services, is expected to boost accountability to policy makers and public health partners. Rotating members are appointed from CDC operating divisions responsible for disease, injury, and condition surveillance. Initially, the Board is focusing on optimizing CDC investments in surveillance systems infrastructure by (1) assuring coordination among partners, (2) making transparent recommendations, (3) harmonizing CDC's efforts to work with health information technology (HIT) standards development organizations, (4) streamlining requests for public health reporting functionality in commercial electronic health record (EHR) systems, (5) monitoring new system implementation progress, (6) facilitating the use of best surveillance practices, and (7) evaluating overall Strategy implementation progress.
CDC developed a federal and STLT workforce training and support plan in October 2014 that integrates Strategy goals with CDC workforce investments. This plan supports improved training of surveillance practitioners on new data sources, new technologies used by clinical health-care providers, and new commercial, governmental, and open-source surveillance system products. It addresses the short-term training needs of public health practitioners, but also looks forward to longer-term training and support of the surveillance workforce.
CDC created the CDC Health Information Innovation Consortium (CHIIC) in May 2014 to foster and promote creative solutions to surveillance challenges across CDC programs and in STLT agencies.5 This consortium aims to improve the availability, quality, and timeliness of surveillance data by accelerating the use of new tools and approaches. The CHIIC acts as a collaborative forum for sharing successes, learning from failures, and ensuring that informatics innovations are connected to current national HIT standards and policy framework.
Specific aims of goal 2: processes
The incorporation of new HIT tools and approaches by CDC and STLT partners can be improved through more effective policy and vendor engagement and through support of innovative projects. CDC has established two senior-level positions in the Office of Public Health Scientific Services to improve HIT policy engagement: the Chief Public Health Informatics Officer and the Senior Policy Advisor for Public Health Scientific Services. These leaders work with the Surveillance Leadership Board and CDC's various centers and offices, as well as other federal and STLT agencies, to contribute to surveillance-related HIT policy efforts. CDC is increasing its engagement with the Office of the National Coordinator for HIT (ONC) and other federal information technology regulators, specifically through increased interaction with the Department of Health and Human Services HIT Policy Committee and developers of the Federal Health Information Technology Strategic Plan, 2011–2015.6 CDC actively participated in crafting the most recent version of the Strategic Plan. CDC is also collaborating on projects with ONC to further STLT health agencies' capacity to implement HIT strategies.
CDC developed a forum in June 2014 to systematically engage HIT and EHR vendors regarding informatics technologies and tools that can advance surveillance systems and practice.7 Vendor engagement provides opportunities for CDC programs and STLT agencies to better communicate their needs to HIT and EHR vendors. In June 2014, CDC also began providing seed funding and technical support for small innovation project awards to advance innovations in data collection, transport, storage, analysis, visualization, and availability.
Specific aims of goal 3: improvements in public health surveillance outcomes
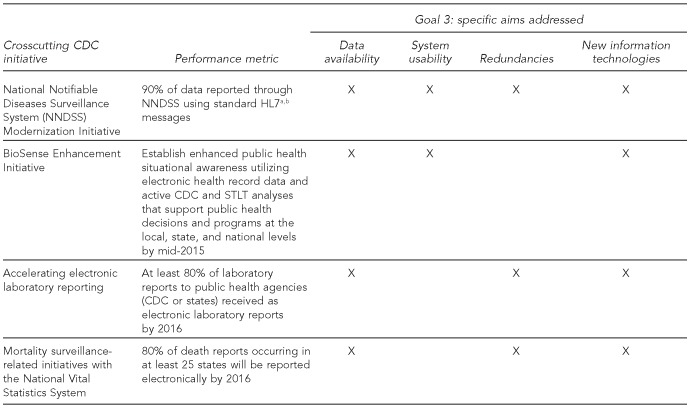
To demonstrate early success, CDC is pursuing four crosscutting initiatives intended to improve existing, widely applicable platforms or tools that can benefit STLT agencies and CDC programs. For example, CDC is creating robust, integrated platforms that can be adapted for new surveillance needs. Figure 2 summarizes these initiatives, their performance metrics, and how they address the specific aims of the Strategy.
Figure 2.
Initial crosscutting CDC initiatives, with established performance metrics and specific aims, under Goal 3 of the CDC Surveillance Strategy
aHealth Level Seven International. Introduction to HL7 standards [cited 2014 Jul 17]. Available from: URL: http://www.hl7.org/implement/standards/index.cfm?ref=nav
bPublic Health Information Network. Notifiable disease case notification guides [cited 2014 Jul 17]. Available from: URL: http://www.cdc.gov/phin/resources/PHINguides.html
CDC = Centers for Disease Control and Prevention
HL7 = Health Level Seven International Standards
STLT = state, territorial, local, and tribal
The National Notifiable Diseases Surveillance System (NNDSS) was established to monitor the occurrence and spread of diseases and conditions voluntarily reported by STLT agencies to CDC.8 NNDSS provides the infrastructure, standards, and incentives for rapid electronic reporting of surveillance data for notifiable conditions. NNDSS is used by numerous stakeholders to provide accurate and timely information for surveillance and response. An important component of NNDSS is the National Electronic Disease Surveillance System,9 which is used to facilitate data collection and reporting. In response to stakeholders' needs and evolving technologies, the NNDSS Modernization Initiative seeks to enhance surveillance capabilities by standardizing the exchange, processing, and provisioning of surveillance data.10
The BioSense program provides data for public health officials to monitor and respond to possible disease and hazardous conditions.11 It is an electronic surveillance system with standardized tools and procedures for rapidly collecting, sharing, and evaluating emergency department and other health-care-related data. A recent internal review highlighted the potential of BioSense, particularly regarding the increasing use of EHR, strengthening health-care partnerships, extending surveillance practices and methods, and reducing costs. The BioSense Enhancement Initiative builds on past successes, fixes problems, and improves the ability of public health agencies to analyze, compare, and act on program data.12
Electronic laboratory reporting (ELR) to public health agencies can improve surveillance for reportable diseases and conditions by improving report timeliness and completion. At the end of July 2013, approximately 62% of 20 million laboratory reports were received electronically, compared with 54% in 2012.13 This third initiative focuses on accelerating the adoption of ELR through collaboration among clinical laboratories, laboratory information management system vendors, and public health agencies.
Finally, modernizing and transforming the National Vital Statistics System (NVSS) into a system capable of supporting near-real-time public health surveillance has been a long-standing need. This fourth initiative focuses on the substantial mortality surveillance-related efforts needed to fully realize NVSS's potential as a surveillance tool.14
PUBLIC HEALTH SURVEILLANCE IMPLICATIONS
During the next 3–5 years, the CDC Surveillance Strategy will increasingly guide program and agency-wide surveillance activities. Through it, the agency aims to use its resources more efficiently and valuably for all stakeholders. By reducing the number of standalone systems and increasing the use of robust, integrated platforms, CDC seeks to increase functionality and decrease unnecessary redundancies and reporting burdens on STLT agencies. By leveraging emerging technologies in a more coordinated fashion with public health partners, CDC strives to realize the vision of a digital infrastructure that provides data and information to those who need it, when they need it, and in a form that enables them to act upon it.
Footnotes
The authors thank Nedra Garrett and Brian Lee at the U.S. Centers for Disease Control and Prevention (CDC) Center for Surveillance, Epidemiology, and Laboratory Services for assisting with the preparation of this article. The views contained in this article are those of the authors and do not necessarily represent the views of CDC.
REFERENCES
- 1.CDC's vision for public health surveillance in the 21st century. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):1–40. [Google Scholar]
- 2.Mirza N, Reynolds T, Coletta M, Suda K, Soyiri I, Markle A, et al. Steps to a sustainable public health surveillance enterprise: a commentary from the International Society for Disease Surveillance. Online J Public Health Inform. 2013;5:210. doi: 10.5210/ojphi.v5i2.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PF, Hadler JL, Stanbury M, Rolfs RT, Hopkins RS CSTE Surveillance Strategy Group. “Blueprint version 2.0:” updating public health surveillance for the 21st century. J Public Health Manag Pract. 2013;19:231–9. doi: 10.1097/PHH.0b013e318262906e. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (US) Surveillance strategy [cited 2014 Jul 18] Available from: URL: http://www.cdc.gov/ophss/docs/CDC-Surveillance-Strategy-Final.pdf.
- 5.phConnect. Health Information Innovation Consortium (CHIIC) [cited 2014 Jul 9] Available from: URL: http://www.phconnect.org/group/chiic.
- 6.Department of Health and Human Services (US), Office of the National Coordinator for Health Information Technology. Federal health information technology strategic plan 2011–2015. 2011. [cited 2014 Jul 9]. Available from: URL: http://www.healthit.gov/sites/default/files/utility/final-federal-health-it-strategic-plan-0911.pdf.
- 7.phConnect. Public health—EHR vendors collaboration initiative [cited 2014 Jul 9] Available from: URL: http://www.phconnect.org/group/public-health-ehr-vendors-collaboration-initiative.
- 8.Centers for Disease Control and Prevention (US) National Notifiable Diseases Surveillance System [cited 2014 Jul 17] Available from: URL: http://wwwn.cdc.gov/nndss.
- 9.Centers for Disease Control and Prevention (US) NEDSS/NBS [cited 2014 Jul 17] Available from: URL: http://wwwn.cdc.gov/nndss/script/nedss.aspx.
- 10.Centers for Disease Control and Prevention (US) NNDSS Modernization Initiative [cited 2014 Jul 17] Available from: URL: http://wwwn.cdc.gov/nndss/script/NNDSS_Modernization_Initiative.aspx.
- 11.Centers for Disease Control and Prevention (US) BioSense program [cited 2014 Jul 17] Available from: URL: http://www.cdc.gov/biosense.
- 12.Department of Health and Human Services (US) Memorandum: CDC BioSense data use agreement between CDC and the jurisdictions [cited 2014 Jul 17] Available from: URL: http://api.ning.com/files/WA*Dv5OWc2iiB-xsUvqzNF4F3m1dRET-WdUBjc4gHAmZhFraionWybQLV3qwO7-H4CRpfs3GcCgz6bAXb6GfzAO06UjKQRIl/DUA.CDC_Memo.pdf.
- 13.Progress in increasing electronic reporting of laboratory results to public health agencies—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(38):797–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Bancroft EA, Lee S. Use of electronic death certificates for influenza death surveillance. Emerg Infect Dis. 2014;20:78–82. doi: 10.3201/eid2001.130471. [DOI] [PMC free article] [PubMed] [Google Scholar]