Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis (original) (raw)
Graphical abstract
Keywords: Wolbachia, Filariasis, Pharmacopoeia, Drug discovery, Library screening, Anti-Wolbachia Consortium (A·WOL)
Highlights
- •
There is an urgent need to discover a macrofilaricide for filariasis. - •
The A·WOL approach is to target the Wolbachia bacteria of filarial nematodes. - •
The human pharmacopoeia was screened against Wolbachia for potential repurposing. - •
69 orally available hits from different drug categories were identified in vitro. - •
In vivo, the tetracyclines, fluoroquinolones and rifamycins were the most active.
Abstract
Lymphatic filariasis and onchocerciasis are debilitating diseases caused by parasitic filarial nematodes infecting around 150 million people throughout the tropics with more than 1.5 billion at risk. As with other neglected tropical diseases, classical drug-discovery and development is lacking and a 50 year programme of macrofilaricidal discovery failed to deliver a drug which can be used as a public health tool. Recently, antibiotic targeting of filarial Wolbachia, an essential bacterial symbiont, has provided a novel drug treatment for filariasis with macrofilaricidal activity, although the current gold-standard, doxycycline, is unsuitable for use in mass drug administration (MDA). The anti-Wolbachia (A·WOL) Consortium aims to identify novel anti-Wolbachia drugs, compounds or combinations that are suitable for use in MDA. Development of a Wolbachia cell-based assay has enabled the screening of the approved human drug-pharmacopoeia (∼2600 drugs) for a potential repurposing. This screening strategy has revealed that approved drugs from various classes show significant bacterial load reduction equal to or superior to the gold-standard doxycycline, with 69 orally available hits from different drug categories being identified. Based on our defined hit criteria, 15 compounds were then selectively screened in a Litomosoides sigmodontis mouse model, 4 of which were active. These came from the tetracycline, fluoroquinolone and rifamycin classes. This strategy of repurposing approved drugs is a promising development in the goal of finding a novel treatment against filariasis and could also be a strategy applicable for other neglected tropical diseases.
1. Introduction
Lymphatic filariasis (LF) and onchocerciasis (river blindness) are debilitating diseases caused by filarial nematodes, officially recognised as neglected tropical diseases (NTDs) (WHO, 2007). Although these nematode infections are currently being effectively managed using mass drug administration (MDA) of drugs donated by large pharmaceutical companies (Chu et al., 2010; Coffeng et al., 2013), elimination is hampered by several challenges including the incomplete efficacy of available drugs against the long-lived adult filarial worms (Liu and Weller, 1996; Richard-Lenoble et al., 2003; Bockarie and Deb, 2010; Mackenzie et al., 2012), problems associated with adverse events in areas of co-endemicity of Loa loa with either Wuchereria bancrofti or Onchocerca volvulus (Gardon et al., 1997; Bockarie and Deb, 2010; Taylor et al., 2010), and the risk that filarial worms will develop resistance to the drugs currently available for MDA (reviewed in Smits, 2009; Prichard et al., 2012).
Targeting the bacterial endosymbiont, Wolbachia, of these filarial nematodes offers solutions to these problems as the removal of Wolbachia, using tetracycline-based antibiotics, results in the slow death of the adult worm (reviewed in (Taylor et al., 2010) and, given that L. loa does not harbour these endosymbionts (McGarry et al., 2003), does not lead to adverse events following treatment (Wanji et al., 2009; Turner et al., 2010). The use of doxycycline in field trials has demonstrated that this antibiotic can be used successfully to permanently sterilise adult female worms and, if given for an appropriate length of time, lead to a macrofilaricidal effect (reviewed in Johnston and Taylor, 2007; Hoerauf, 2008; Taylor et al., 2010); an important improvement over current treatments. The current 4–6 weeks of daily treatment, is the main barrier to wide-spread scale-up of this treatment regimen into MDA programmes due to logistical constraints, although community-directed treatment with doxycycline for six weeks, achieving a therapeutic coverage of 73.8% and 98% compliance, is feasible and effective in restricted populations (Wanji et al., 2009; Tamarozzi et al., 2012). Doxycycline, however, also has limitations for mass use due to contraindications that make it unsuitable for treating children under eight and pregnant women (reviewed in Johnston and Taylor, 2007; Hoerauf, 2008).
The A·WOL Consortium was established to find a new anti-wolbachial drug or combination of drugs that is compatible with MDA with a secondary goal to optimise regimens using the currently known active antibiotics (doxycycline and rifampicin) (www.a-wol.com; Johnston et al., 2014; Taylor et al., 2014). Screening large chemical libraries to identify compounds with macrofilaricidal activity has been hindered in the past by the lack of efficient screening assays with available assays being labour intensive (Townson et al., 2000; Rao et al., 2002; Townson et al., 2006; Townson et al., 2007). To overcome this limitation the A·WOL Consortium developed a Wolbachia cell-based assay with a quantitative PCR (qPCR) readout which has been optimised as an in vitro drug screening tool. Here, we briefly describe the validation of this assay which utilises a _Wolbachia pipientis_-containing Aedes albopictus cell line (C6/36 (_w_AlbB)), in a 96-well format, and quantifies the 16S rRNA gene copy number of intracellular Wolbachia bacteria in the presence or absence of a drug, as well as an ATP–luminescence based cytotoxicity assay to examine off-target toxic effects of the drug on the mosquito host cells. The assay can be adapted to automated high throughput-screening and represents a rapid, sensitive and efficient assay for screening chemical libraries to identify anti-Wolbachia compounds. Hits from this primary in vitro cell-based screening assay are then selected for progression down the screening pipeline into both in vitro and in vivo nematode screening.
Repurposing or repositioning of drugs provides a less risky route to drug discovery given that candidates will already have well-known safety and pharmacokinetic profiles (Ashburn and Thor, 2004; Tobinick, 2009; Mucke, 2010; Grimberg and Mehlotra, 2011). Here, we describe screening efforts against Wolbachia using the A·WOL assay to screen a compound library of 2664 approved drugs, bioactive compounds and natural products (CRX; CombinatoRx Singapore). This strategy identified 121 hits that had anti-Wolbachia activity, of which 69 were orally available hits from different drug categories, and several drugs were progressed further down the screening pipeline into in vitro nematode screening assays and the primary in vivo screening model (Litomosoides sigmodontis mouse model). This approach has identified several classes of registered drugs with anti-Wolbachia activity, which has expanded the options for improving macrofilaricidal therapeutic regimes against onchcocerciasis and lymphatic filariasis.
2. Materials and methods
2.1. In vitro Wolbachia cell-based screening assay
An A. albopictus cell line C6/36 (ATCC number CRL-1660) stably infected with _W. pipientis w_AlbB (C6/36 (_w_AlbB)) was routinely cultured in L15 Leibovitz medium containing 2 mM l-glutamine, 1% non-essential amino acids, 2% tryptose phosphate broth (Sigma-Aldrich, UK), and 5% heat-inactivated FCS (Cambrex Bio Science, Walkersville, MD) at 26 °C (Turner et al., 2006). A C6/36 (_w_AlbB) cell-based assay developed to screen drugs/compounds active against Wolbachia in vitro was used as previously described (Johnston et al., 2010). C6/36 (_w_AlbB) cells, sub-cultured 24 h previously, were seeded at 10,000 cells per well in 96-well flat bottom culture plates. Test compounds were dissolved in DMSO (Sigma) and diluted to appropriate concentration (μM) in culture medium, added to test wells and cells cultured in a total volume of 200 μl at 26 °C for 9 days. Medium alone and vehicle-treated (DMSO) medium were used as negative controls. Compounds and controls were added in triplicate and medium/drug was replaced on day 4. At the end of the screening assay, samples were collected by washing adherent cells once in sterile Dulbecco’s PBS (Sigma) and adding 150 μl Wizard® SV Lysis Buffer (Promega, UK) for genomic DNA (gDNA) extraction.
In total, 2664 compounds from the CRX library, plated onto 37 master plates (72 compounds per plate), were screened at 10 μM in comparison to the gold standard doxycycline (7 μM) (Sigma). Cytotoxicity was measured in parallel using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega), according to the manufacturer’s instructions. The level of cytotoxicity for each compound was determined by comparing the CellTiterGlo® luminescence readout against the vehicle-treated control wells, with compounds that reduced the % luminescence by 30% or greater being classed as cytotoxic. Screening was executed with a “front loading” of the library wherein approximately 200 anti-infectives were used in the first three master plates to maximise the number of hits in the first phase of screening. A pre-production run was done to evaluate assay performance and dynamic range with Z′ factor being used as a primary quality control tool for data generation/analysis. With acceptable Z′ factors achieved (greater than 80% of the plates showed Z′ factors of 0.4 and above), the assay was validated for use.
Hits were cherry-picked from master plates and examined in a titration series (6 doses from 10 to 0.3 μM) for dose response effects set up in quadruplicates (inter and intra plate duplicates) and were also tested in parallel for cytotoxicity. Hits were also validated using compounds sourced externally, where available. Prioritisation of hit compounds for further screening was based on the following criteria: (1) suitability/approval status, (2) potency in screening assay, (3) repeat validation (both library and sourced compounds), (4) paediatric use, and (5) pregnancy category (US–FDA categories, www.fda.gov).
2.2. In vitro Onchocerca gutturosa screening assay
Adult male O. gutturosa were dissected from the nuchal ligament connective tissues obtained from naturally infected cattle in The Gambia, as previously described (Townson et al., 2006). Worms were maintained individually in the wells of a 24-well plate in 2 ml of Minimum Essential Medium containing 10% heat-inactivated FCS, 200 U/ml penicillin, 200 μg/ml streptomycin and 50 μg/ml amphotericin B (Sigma), at 36.5 °C with 5% CO2 for 24 h until the addition of drugs. Compounds, dissolved in 99% DMSO, were prepared as previously described (Townson et al., 2006) in medium and each compound was tested against ten individual worms for 5 days. Daily microscopic observations were carried out to determine worm motility using a scale of 0 (immobile) to 10 (maximum motility) and the mean % motility reduction was derived by the comparison to untreated controls. At assay termination, each group of 10 worms (per compound) were transferred to RNAlater (Ambion, Applied Biosystems, UK) for 24 h at 4 °C and stored at −20 °C for gDNA extraction from individual worms at a later date.
2.3. In vivo L. sigmodontis screening assay
Treatment groups of BALB/c female mice (6–8 week old) received intraperitoneal (IP) injections with the test compounds, in comparison to doxycycline, at appropriate concentrations (MKD, mg/kg/day) for 14 days beginning the day after natural mite (Ornithonyssus bacoti) infection with L. sigmodontis. Compounds were formulated in appropriate delivery vehicles (eg. methacycline was formulated in 0.5% hydroxypropyl methylcellulose, HPMC) and doses calculated based on the recommended human dosage and in a volume of 10 ml/kg based on body weight. At 35 days post-infection, worms were recovered from the pleural cavity, counted, staged for development and measured for length (mm). Worms were frozen at −80 °C for gDNA extraction. All animal experiments were performed according to the European Union animal welfare guidelines. All protocols were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz, Cologne, Germany (AZ.: 8.87-50.10.35.08.024).
2.4. DNA isolation and quantitative real-time PCR (qPCR)
Genomic DNA was extracted from C6/36 (_w_AlbB) cell lysates using the Wizard® SV 96 Genomic DNA Purification System (Promega) according to the manufacturer’s instructions and eluted in 100 μl water. Quantification of the ribosomal genes; W. pipientis 16S and A. albopictus 18S, was performed as described previously (Makepeace et al., 2006) with modifications. Briefly, qPCR was carried out on a DNA Engine PTC-200 thermocycler (MJ Research, GRI, UK) with Chromo4 real-time PCR detection system (Bio-Rad Laboratories Ltd, UK) under the following conditions: 95 °C for 15 min, 40 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 15 s; and melting curve analysis between 50 and 95 °C to confirm the specificity of the amplification products. qPCR reactions were performed in 20 μl Quantitect SYBR Green (Qiagen, UK) reactions containing 1 μl gDNA for 18S or 2 μl gDNA for 16S, 0.2 μM of each primer (Supplementary Table S1) in 1× SYBR Green PCR reaction mix. Quantification was calculated by reference to a linear standard curve of log 10 diluted (5 × 106–5 × 100) full-length amplicons synthesised as single-stranded oligonucleotides (Sigma–Genosys, UK).
Following in vitro culture, gDNA was extracted from individual adult male O. gutturosa using the Wizard® SV 96 Genomic DNA Purification plate (Promega) and QiaAmp DNA mini-kit reagents (Qiagen) and eluted in 100 μl water. Quantification of the Wolbachia surface protein (wsp) and nematode glutathione _S_-transferase (Ov-gst) gene copy numbers was performed by qPCR carried out on a DNA Engine PTC-200 thermocycler (MJ Research) with Chromo4 real-time PCR detection system (Bio-Rad) under the following conditions: 95 °C for 15 min, 40 cycles of 95 °C for 15 s, 57 °C (gst) or 60 °C (wsp) for 30 s, and 72 °C for 15 s; and melting curve analysis between 60 and 97 °C. qPCR reactions were performed in 20 μl Quantitect SYBR Green (Qiagen) reactions containing 1 μl gDNA, 3 mM MgCl2 and 0.3 μM of each primer (Supplementary Table S1 for gst or 2 μl gDNA, 3.5 mM MgCl2 and 0.35 μM of each primer (Supplementary Table S1) for wsp, in 1× SYBR Green PCR reaction mix. The gene copy number was determined using a gene specific standard curve of plasmid DNA.
At 35 days post-infection, L. sigmodontis worms were recovered from the pleural cavity and gDNA extracted using the QiaAmp DNA mini-kit (Qiagen) according to the manufacturer’s instructions and eluted in 50 μl water. Quantification of the Wolbachia ftsZ (wLs-ftsZ) and L. sigmodontis β-actin (Ls-act) gene copy numbers was performed by qPCR (Arumugam et al., 2008; Strübing et al., 2010) carried out on a RotorGene 3000 (Corbett Research, Sydney, Australia). The following cycling conditions were used: 95 °C for 15 min, 45 cycles of 95 °C for 15 s, 58 °C for 45 s, and 72 °C for 15 s; and melting curve analysis between 62 and 99 °C. qPCR reactions were performed in 10 μl reactions volumes using the following conditions: 1xPCR buffer (Qiagen), 0.2 mM dNTPs, 3 mM MgCl2, 0.1 μl SYBR Green (1:1000 dilution of stock in DMSO; Roche, Mannheim, Germany), 0.25 U HotStar Taq polymerase (Qiagen) and 2 μl DNA. The gene copy number (copy numbers/μl) was determined using a gene specific standard curve of plasmid DNA.
For all qPCR reactions results were expressed as Wolbachia gene:host gene ratios to normalise the data and obviate differences in the quality and quantity of DNA. The log drop in the ratio in comparison to the control gives a quantitative measure of the effect of the compound on Wolbachia.
2.5. Statistical analysis
Student _t_-test was performed for statistical analysis using Prism (GraphPad Software, LaJolla, CA).
3. Results
3.1. Development of a screening assay which identifies anti-Wolbachia activity in vitro
In this report, we describe the development, validation and use of an assay for the in vitro cell-based screening of anti-Wolbachia compounds. Using doxycycline as a gold-standard, critical features such as reproducibility, assay duration and dynamic range were evaluated. Initial experiments were conducted over 21 days in order to assess the dynamic range of the doxycycline response over time (Fig. 1). Assay quality and robustness were determined during the optimisation as well as during the screening process by calculation of the statistical parameters Z (and Z′) (Zhang et al., 1999), and having achieved acceptable Z′ factors the assay was validated for use (Supplementary Tables S2 and S3). Acceptable Z′ factors were achieved at day 9 but not at day 5 (Supplementary Table S2), demonstrating that the optimal duration of the assay was 9 days. A pre-production run of 8 replicate plates (master plate MS-250501) was used to calculate the intra-plate variability (Z′ factors) of the 16S qPCR assay read-out (Supplementary Table S3). Greater than 80% of the plates showed Z′ factors of 0.4 and above and signal window of 2 allowing us to be confident that the assay could be used for screening of the CombinatoRx (CRX) library.
Fig. 1.
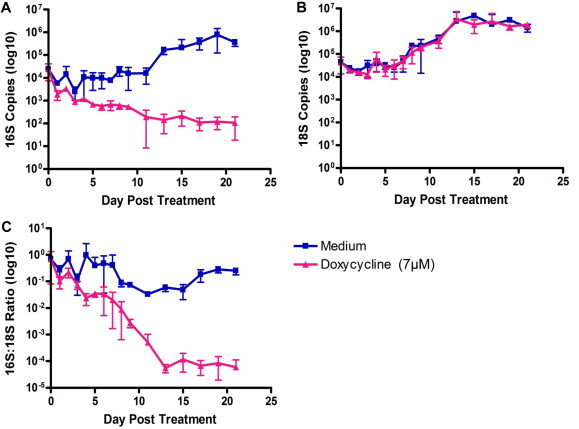
Dynamics of cell and Wolbachia response to doxycycline over 21 days. Wolbachia growth was assessed by qPCR targeting the 16S rRNA gene (A). C6/36 cell growth was assessed by qPCR targeting the 18S rRNA gene of Aedes albopictus (B). Data was normalised using the ratio of 16S copies to 18S copies (C).
3.2. Screening of the human pharmacopeia identified drugs with activity against Wolbachia
The CRX library of 2664 compounds, representing the approved human drug pharmacopoeia, was screened using the validated assay. Initial screening was executed with a “front loading” of the library where 200 anti-infectives were used in the first three master plates to maximise the number of hits in the first phase of screening and further validate the assay prior to screening the complete library. From the 2664 compounds tested, we identified 121 compounds that inhibited Wolbachia by 0.5 logs (70% inhibition at 10 μM); this represents a primary hit rate of 4.54%. We then further defined hits as those compounds that along with these in vitro hit criteria (⩾0.5 logs inhibition of Wolbachia 16S and ⩽30% cytotoxicity) are also available in an oral formulation in order to align our hit picking strategy to the Target Product Profile (TPP) criteria.
Of the 121 active compounds, 69 compounds (2.59% of the total screened), over several drug classes (Table 1), satisfied hit criteria (Table 2). Hits identified in the screening campaign were interesting and diverse (Table 1), and included anti-infective compounds (35%) such as antibiotics, anti-viral, anti-parasitic and anti-fungal compounds, as well as non anti-infective compounds (65%) constituting anti-psychotic compounds, natural products/nutraceuticals, receptor antagonists, anti-hypertensives, muscle relaxants, non-steroidal anti-inflammatory drugs and other drug classes, pointing towards potentially novel mechanisms of countering intra-cellular Wolbachia bacteria which could be exploited.
Table 1.
Distribution of drug classes across 69 hit anti-Wolbachia compounds. 121 compounds inhibited intracellular Wolbachia bacteria by 0.5 logs or more. Out of these, 69 belong to diverse classes of approved drugs and are available in an oral formulation and hence constitute hits for further analysis.
| Drug class | Number of hits | Percent of hits (%) |
|---|---|---|
| Anti-infectives | 24 | 35 |
| Anti-psychotics/anti-convulsants | 8 | 12 |
| Natural products/nutraceuticals | 7 | 10 |
| Receptor antagonists | 6 | 9 |
| Anti-hypertensives | 6 | 9 |
| Muscle relaxants | 5 | 7 |
| Others | 5 | 7 |
| Non-steroidal anti-inflammatories | 4 | 6 |
| Anti-neoplastic agents | 4 | 6 |
Table 2.
Prioritisation of hit compounds. The 69 hits obtained in single agent screening were first validated using either library or sourced compound, where available, and then prioritised for further screening based on (1) suitability/approval status, (2) potency in 16S assay, (3) repeat validation (both library and sourced compounds) (4) paediatric use and (5) pregnancy category (US-FDA pregnancy categories). Compounds were classed as top priority, second priority or deprioritised hits and are listed in the following table in rank order based on activity in the cell-based screen. nd = not determined, ? = evidence unclear.
| Compound | 16S log drop | Teratogenic/embryocidal | Pregnancy categorya | Use in pediatric indications | Validated-library compound | Validated-sourced compound | Characteristics | Comments |
|---|---|---|---|---|---|---|---|---|
| Top priority hits | ||||||||
| Methacycline hydrochloride | 1.8 | Yes | D | Not evaluated in children under 8 years | Yes | Yes | Anti-biotic | |
| Indomethacin | 1.7 | None observed | C | Not evaluated in children under 14 years | Yes | Yes | Non-steroidal anti-inflammatory | |
| Paromomycin sulfate | 1.7 | Yes | D | Approved | Yes | Yes | Anti-biotic | |
| Rifapentine | 1.7 | Yes | C | Not evaluated in children under 12 years | Yes | Yes | Anti-TB | |
| Minocycline | 1.6 | Yes | D | Not evaluated in children under 8 years | Yes | Yes | Anti-biotic | |
| Naftopidil | 1.2 | Not evaluated | ? | Not evaluated | Yes | Yes | Anti-hypertensive | Treatment of enlarged prostrate |
| Abacavir Sulfate | 1 | Yes? | C | Yes | Yes | Yes | Anti-viral | |
| Sparfloxacin | 1 | Yes | C | Not evaluated in children under 18 years | Yes | Yes | Anti-biotic | |
| Docusate Calcium | 0.8 | Yes | C | Not evaluated in children under 3 years | Yes | Yes | Laxative | |
| Loratadine | 0.8 | None observed | B | Not evaluated in children under 2 years | Yes | Yes | Allergy medication | |
| Ethoxzolamide | 0.7 | ? | ? | ? | Yes | Yes | Diuretic | |
| Bepridil | 0.6 | Yes | C | Not evaluated | Yes | Yes | Ca channel blocker | |
| Furazolidone | 0.6 | None observed | B | Contraindicated in infants < one month | Yes | Yes | Anti-protozoal | |
| Nefazodone hydrochloride | 0.6 | Yes | C | Not evaluated | Yes | yes | Anti-depressant | |
| Curcumin | 0.5 | None observed | ? | Yes? | Yes | Yes | Hepatoprotective agent | Experimental drug |
| Diacerein | 0.5 | Not evaluated | ? | Not evaluated | Yes | Yes | Osteoarthritis drug | |
| Isoniazid | 0.5 | Yes | C | Yes | Yes | Yes | Anti-TB | |
| 2nd Priority hits | ||||||||
| Ethosuximide | 2 | Yes | C | Not evaluated in children under 3 years | Yes | Anti-epileptic | ||
| Piracetam | 1.6 | Not evaluated | ? | Not evaluated | Yes | Nootropic | ||
| Sulfamethizole | 1.6 | Yes | Not safe | Not evaluated | Yes | Anti-biotic | ||
| Nevirapine | 1.5 | None observed | B | Approved | Yes | Anti-viral | ||
| Oxycodone hydrochloride | 1.4 | None observed | B | Not evaluated | Yes | Opioid agonist | ||
| Sulfaguanidine | 1.4 | Yes | ? | Not evaluated | Yes | Sulfa drug | ||
| Valacyclovir hydrochloride | 1.3 | None observed | B | Not evaluated in children under 2 years | Yes | Anti-viral | ||
| Ibuprofen | 1.2 | Yes? | C | Not evaluated | Yes | Non-steroidal anti-inflammatory | ||
| Phenytoin | 1 | Yes | D | Yes | Yes | Anti-eplieptic | ||
| Mefexamide hydrochloride | 0.9 | Not evaluated | ? | Not evaluated | Yes | Anti-depressant | ||
| Nitrazepam | 0.9 | Yes | D | Yes | Yes | Hypnotic | ||
| Benznidazole | 0.8 | Not evaluated | ? | Not evaluated | Yes | Anti-parasitic | ||
| Sorbic acid | 0.8 | None observed | B | ? | Yes | Anti-infective, food preservative | ||
| Acyclovir | 0.7 | None observed | B | Not evaluated in children under 2 years | Yes | Anti-viral | ||
| Tolterodine tartrate | 0.7 | Yes | C | not evaluated | Yes | Muscle relaxant | ||
| Trifluperidol | 0.7 | Not evaluated | ? | Not evaluated in children under 6 years | Yes | Anti-psychotic | ||
| Benzydamine hydrochloride | 0.6 | No contraindications | ? | ? | Yes | Non-steroidal anti-inflammatory | ||
| Bumetanide | 0.6 | Yes | C | Not evaluated in children under 18 years | Yes | Anti-hypertensive | ||
| Riboflavin | 0.6 | None observed | ? | ? | Yes | Micronutrient | Micronutrient | |
| Phytonadione | 0.5 | None observed | C | Not evaluated in pediatric populations | Yes | Micronutrient | ||
| Pyrimethamine | 0.5 | Yes | C | Yes | Yes | Anti-parasitic | ||
| Deprioritised | ||||||||
| Kitasamycin | 2.1 | ? | ? | ? | Yes | Anti-biotic, macrolide | Safe for lifestock | |
| Ciprofloxacin hydrochloride | 2 | None observed | C | Approved | Yes | Yes | Anti-biotic | Tested previously (Hoerauf et al., 2000) |
| Oxfendazole | 1.2 | Not evaluated | ? | Not evaluated | Yes | Anti-helmintic | Safe for lifestock | |
| Sodium Caseinate | 1.2 | None observed | A | Approved | Nd | Nutrient | ||
| Morantel Tartrate | 1.1 | Not evaluated | ? | Not evaluated | Yes | Anti-helminthic | Safe for lifestock | |
| Benactyzine (Hydrochloride) | 1 | Not evaluated | ? | Not evaluated | Yes | Yes | Anti-cholinergic | No longer widely used in medicine due to side effects |
| Neratinib | 1 | Not evaluated | ? | Not evaluated | Nd | Inhibitor of ErbB1 and ErbB2 | Phase I compound | |
| Eliprodil | 0.9 | Not evaluated | ? | Not evaluated | No | NMDA receptor antagonist | Other NMDA antagonists are category B | |
| Geftinib | 0.9 | Yes | D | Not evaluated | Yes | Yes | Anti-neoplastic | In clinical trials |
| Narasin | 0.9 | Not evaluated | ? | Not evaluated | Yes | Yes | Anti-biotic | Safe for lifestock |
| Dichlorophen | 0.7 | Not evaluated | ? | Not evaluated | Yes | Yes | Anti-parasitic | Safe for lifestock |
| L-Dopa | 0.8 | Yes | C | Not evaluated | No | Yes | Dopamine enhancer | |
| Selenium Powder | 0.8 | Safe | Safe | Not evaluated | No | Yes | Nutrient supplement | |
| Nitrofurantoin | 0.7 | None observed | B | Contraindicated in infants < one month | Nd | Anti-biotic | ||
| Quinidine | 0.7 | Yes | C | Yes | No | Na-antagonist | ||
| Ubenimex | 0.7 | Not evaluated | ? | Not evaluated | No | Aminopeptidase inhibitor | In clinical trials | |
| Baclofen | 0.6 | Yes | C | Not evaluated in children under 12 years | No | Yes | Muscle relaxant | |
| Chlorphenesin Carbamate | 0.6 | None observed | ? | Not evaluated | No | Muscle relaxant | ||
| Dasatinib | 0.6 | Yes | D | Not evaluated in children under 18 years | No | Anti-neoplastic | ||
| Nicarbazin | 0.6 | Not evaluated | ? | Not evaluated | Yes | Yes | Anti-biotic | Safe for lifestock |
| Sulfanitran | 0.6 | Not evaluated | ? | Not evaluated | Yes | Anti-protozoal | Safe for lifestock | |
| Trifluoperazine hydrochloride | 0.6 | Yes | C | Not evaluated in children under 6 years | No | Yes | Anti-psychotic | Long-term medication |
| Betazole hydrochloride | 0.5 | Not evaluated | ? | Not evaluated | No | Histamine analogue | Diagnostic agent | |
| Carbinoxamine maleate | 0.5 | Not evaluated | C | Yes | No | Anti-histamine | ||
| Diflunisal | 0.5 | Yes | C | Not evaluated in children under 12 years | No | Yes | Non-steroidal anti-inflammatory | |
| Fluoxetine hydrochloride | 0.5 | Yes | C | Not evaluated in children under 7 years | No | Yes | Anti-depressant | |
| Hydrochlorothiazide | 0.5 | None observed | C | Not evaluated in pediatric populations | No | Anti-hypertensive | ||
| Isoxsuprine hydrochloride | 0.5 | Not evaluated | ? | Not evaluated | Yes | Vasodilator | Safe for lifestock | |
| Nilutamide | 0.5 | Yes | C | Not evaluated | No | Yes | Androgen receptor blocker | |
| Scopolamine methylnitrate | 0.5 | Yes | C | Not evaluated in pediatric populations | No | Anti-cholinergic | ||
| Troleandomycin | 0.5 | Yes? | C | Not evaluated in pediatric populations | Nd | Anti-biotic |
Encouragingly, we identified hits among classes of antibiotics (the tetracyclines, rifamycins and fluoroquinolones) previously shown to reduce Wolbachia (Hoerauf et al., 2000; Townson et al., 2000; Hermans et al., 2001; Rao et al., 2002; Fenollar et al., 2003; Volkmann et al., 2003; Townson et al., 2006), thus giving us confidence in the screening outcome. Out of the 69 hits, 24 compounds inhibited Wolbachia by 1 log or more which corresponds to 90% inhibition. Moreover, 10 of these 24 compounds showed comparable or better activity than that of doxycycline (⩾1.6 logs or 95% inhibition). Compounds which were equivalent to or better than doxycycline in vitro were: ciprofloxacin hydrochloride, ethosuximide, indomethacin, kitasamycin, methacycline hydrochloride, minocycline, paromomycin sulfate, piracetam, rifapentine, and sulfamethizole (Table 2).
3.3. Validation and prioritisation of hits
To further characterise and validate the hit compounds, dose response assays were performed with 66 of the hit compounds to examine the dose-dependent effects. Of the re-tested compounds, 16 compounds failed to show any activity (>50% inhibition of Wolbachia) in the repeat assays and were termed as drop-outs (“validated – library compound” column, Table 2). The remaining compounds showed varying degrees of activity from 98% to 50% inhibition at 10 μM. In the dose response assays, 36 of the hits showed a dose response in the dose range tested (e.g. paromomycin sulfate and loratadine), while other hits did not show a dose response. Ten compounds were active at all tested concentrations to the same extent (e.g. methacycline hydrochloride and sulfaguanidine), while four compounds showed activity only at the highest concentration (10 μM) (e.g. ciprofloxacin hydrochloride and curcumin). In addition, hit compounds were further validated by re-screening using, where available, externally sourced compounds (30/69 compounds) (“validated–sourced compound” column, Table 2).
The hit compounds were then ranked based on the following criteria: (1) suitability/approval status, (2) potency in screening assay, (3) repeat validation (both library and sourced compounds), (4) paediatric use, and (5) pregnancy category (US–FDA categories, www.fda.gov), and then were prioritised for progression through the A·WOL screening pipeline into in vitro nematode screening assays and the primary in vivo screening model (L. sigmodontis mouse model) (Table 2). Seventeen compounds, that were validated using both library and sourced compounds, were classed as top priority hits. A further 21 compounds, that were validated using library compound only, were classed as second priority hits and ranked using the defined criteria. The remaining 31 hits were classed as de-prioritised hits for various reasons. For example, ciprofloxacin was de-prioritised as it had previously been tested in the in vivo mouse model (Hoerauf et al., 2000), as well as compounds that were subsequently found to be inactive, or variably active, in repeat screening. Furthermore, compounds that had not been validated using either library compound or sourced compound as well as hits that were anti-neoplastics, clinical trial compounds or compounds currently only used in livestock were also de-prioritised.
3.4. The effect of a selection of hits on the motility and Wolbachia load of filarial worms in vitro
Due to the promising results obtained in the in vitro cell-based assay screen some compounds were assessed more fully using in vitro nematode assays for proof-of-concept in our screening strategy. Further examination of active antibiotic classes that emerged as hits in the in vitro cell-based screening assay was conducted using an O. gutturosa adult nematode screen. A dose response assay using fourfold dilutions from 12.5 to 0.195 μM was used. All antibiotics tested showed only marginal or no effect on motility (data not shown) indicating that there was no direct toxicity against the nematode. The fluoroquinolones ciprofloxacin and moxifloxacin as well as the rifamycin rifapentine greatly reduced the Wolbachia load at all concentrations tested (Table 3). While doxycycline also reduced the Wolbachia load, the response across the different concentrations was more variable than the other compounds. Overall, no dose responses were observed for any of the compounds tested, suggesting a ceiling effect of the concentrations used. Taken together, these in vitro worm experiments demonstrate good translation of hits from the in vitro insect cell based assay to nematode Wolbachia within their natural hosts.
Table 3.
Comparison of different classes of antibiotic on O gutturosa Wolbachia loads.
| Compound | Class | Concentration (μM) | Reduction in Wolbachia (wsp:gst log drop from vehicle controls) |
|---|---|---|---|
| Doxycycline | Tetracycline | 12.5 | 0.80 |
| 3.125 | 0.21 | ||
| 0.781 | 0.14 | ||
| 0.195 | 0.77 | ||
| Ciprofloxacin | Fluoroquinolone | 12.5 | 0.67 |
| 3.125 | 0.79 | ||
| 0.781 | 0.80 | ||
| 0.195 | 0.39 | ||
| Moxifloxacin | Fluoroquinolone | 12.5 | 0.97 |
| 3.125 | 0.83 | ||
| 0.781 | 1.00 | ||
| 0.195 | 1.03 | ||
| Rifapentine | Rifamycin | 12.5 | 1.04 |
| 3.125 | 0.77 | ||
| 0.781 | 0.78 | ||
| 0.195 | 0.99 |
3.5. The effect of prioritised hits on the length and Wolbachia load of L. sigmodontis in vivo
Due to their in vitro activity the prioritised hits were tested in the _L. sigmodontis-_mouse model (Hoerauf et al., 1999 , 2000; Volkmann et al., 2003), using intra-peritoneal dosing. A total of 15 in vitro hit compounds were screened using doses calculated based on approved human doses and compared to doxycycline (Table 4). This included ciprofloxacin, which, although had been deprioritised due to previous work, was retested given its activity in the in vitro O. gutturosa screen. Dose responses were also performed on a selection of these compounds (Table 4).
Table 4.
Testing of several prioritised hits in the L. sigmodontis in vivo model. The in vitro cell based Wolbachia reductions and cytotoxicity values are shown for comparison.
| Compound | Characteristics | 16S log drop in vitro | Cytotoxicity (%) | in vivo dose(s) (MKDa) | Ls length reduction in vivo (%) | wLs ftsz log drop in vivo |
|---|---|---|---|---|---|---|
| Doxycycline | Anti-biotic | 1.6 | 0 | 25,b 50 | 78.3, 79.4 | 2.2, 4.7 |
| Methacycline hydrochloride | Anti-biotic | 1.8 | 0 | 10, 50 | 73.4, 80.5 | 3.0, 5.7 |
| Minocycline | Anti-biotic | 1.6 | 0 | 25b | 81.7 | 3.78 |
| Paromomycin sulfate | Anti-biotic | 1.7 | 0 | 25 | 0 | 1.5 |
| Rifapentine | Anti-TB | 1.7 | 20 | 50 | 68.3 | 3.0 |
| Indomethacin | Non-steroidal anti-inflammatory | 1.7 | 0 | 15 | 0 | 1.0 |
| Abacavir sulfate | Anti-viral | 1 | 0 | 200 | 0 | N.D. |
| Sparfloxacin | Anti-biotic | 1 | 0 | 25,b 130 | 28.6, 77.4 | 0.6, 5.7 |
| Ciprofloxacin | Anti-biotic | 2 | 0 | 100 | 17.56 | 0.46 |
| Docusate calcium | Laxative | 0.8 | 0 | 200 | 20.0 | 1.0 |
| Loratadine | Allergy medication | 0.8 | 20.7 | 0.3, 1, 3 | 0, 0, 0 | 0.2, 0, 0.35 |
| Ethoxzolamide | Diuretic | 0.7 | 19 | 200 | 0 | 0 |
| Isoniazid | Anti-TB | 0.5 | 0.3 | 25 | 0 | 1.0 |
| Curcumin | Hepatoprotective agent | 0.5 | 0 | 100 | 0 | N.D. |
| Nilutamide | Androgen receptor blocker | 0.5 | 0.01 | 5 | 12.6 | 2.3 |
| Diacerein | Osteoarthritis drug | 0.5 | 0 | 100 | 0 | 1.3 |
Four compounds, methacycline, minocycline, rifapentine and sparfloxacin, significantly reduced the worm length and the Wolbachia load at the standard doses tested (P < 0.05) (Table 4). Methacycline treatment resulted in significant reductions in both worm length and Wolbachia load at both 50 and 10 MKD doses. Interestingly, when treated with methacycline at 50 MKD the reduction in Wolbachia load was significantly greater than from worms recovered from mice treated with the same dose of doxycycline (P < 0.05). In contrast, loratadine had no effect on either worm length or Wolbachia load as measured by Wolbachia ftsZ copies at any dose tested. Ciprofloxacin also did not reduce worm length or Wolbachia load (Table 4), confirming a previous report using this model (Hoerauf et al., 2000), while sparfloxacin did produce a significant reduction in both (P < 0.01). Further experiments using other members of this class have shown that levofloxacin is inactive, while moxifloxacin is active (data not shown), demonstrating diversity within this class. As part of the drive to reduce the duration of treatment for anti-Wolbachia therapy, a reduction in doses and treatment durations have also been investigated using this model. While the reduced regimen tested for sparfloxacin did not significantly affect worm length or Wolbachia numbers, a reduced minocycline dose and treatment duration (25 MKD for 10 days) significantly reduced the Wolbachia load (P < 0.0001) and this reduction was also significantly greater than Wolbachia reduction following the equivalent doxycycline treatment (P < 0.05) (Table 4).
4. Discussion
Here we describe the development of an in vitro Wolbachia screening assay and the subsequent use of this assay to screen the complete human pharmacopoeia, with a view to drug repurposing for filariasis. Repurposing or repositioning of drugs provides a less risky route to drug discovery given that candidates will already have well-known safety and pharmacokinetic profiles (Ashburn and Thor, 2004; Tobinick, 2009; Mucke, 2010; Grimberg and Mehlotra, 2011).
This study identified 121 compounds with in vitro activity against Wolbachia, 69 of which satisfied our hit criteria. These hits included, as expected, numerous anti-infective compounds (35%). These included drugs from classes known to show some efficacy against Wolbachia, namely the tetracyclines, rifamycins and fluoroquinolones (Hoerauf et al., 2000; Townson et al., 2000; Hermans et al., 2001; Rao et al., 2002; Fenollar et al., 2003; Volkmann et al., 2003; Townson et al., 2006). Interestingly, many were non anti-infective compounds (65%) encompassing several different drug classes, thereby pointing towards potentially novel mechanisms of action. Although mechanisms of action have not been investigated here, the number of non anti-infective compounds that demonstrated activity against Wolbachia in vitro offers several interesting avenues to pursue. As well as the possibility that these compounds are acting on the bacteria directly, a perturbation of the complex interplay between the Wolbachia and their host cells must also be considered. Indeed, interfering with the _Wolbachia_-host relationship through chemotherapy may be just as effective as targeting the bacteria themselves. The involvement of Wolbachia in the maintenance of host homeostasis has been referred to in previous studies, especially in relation to oxidative stress regulation (Brennan et al., 2008; Kremer et al., 2012). Antioxidants were among the compounds active against Wolbachia in this study and this class is currently being mined further to inform the potential repurposing and repositioning of these drugs. Autophagy, a conserved intracellular defence mechanism, has recently been demonstrated to be play a key role in controlling Wolbachia populations (Voronin et al., 2012) and therefore components of the pathways involved in this mechanism may be the targets of some of non anti-infective compounds that were hits. This aspect of the screening outcomes is also currently being investigated. Furthermore, the presence of these hits offers the potential for combining drugs, such as antibiotics and non-antibiotics for synergistic effect (Ejim et al., 2011).
As expected with any screening strategy, many of the compounds found to be hits in vitro failed at the in vivo model stage. Minocycline, methacycline, rifapentine and sparfloxacin demonstrated activity in the L. sigmodontis mouse screening assay. The drop-outs can be explained in a variety of ways. Firstly there may be differences in drug susceptibility between insect and nematode Wolbachia, but, more likely, they may also reflect issues of penetration across the nematode cuticle or bioavailability within the mouse model. As the compounds within the CRX library were registered drugs, the in vitro nematode screens described here were not a key decision-making checkpoint, as they were for the screening of other focused and diversity libraries containing novel chemical entities. The prioritised hits progressed directly into the primary in vivo screen, thus making it impossible to distinguish between issues of penetration or bioavailability. The lack of activity of loratadine on both L. sigmodontis length and Wolbachia load can potentially be explained by the dosage used. Generally, the recommended human dosages of anti-histamines are very low and, given that this dose of loratadine was used to calculate doses for this experiment, this could be a factor in its lack of activity. Alternatively, the relatively weaker activity of loratadine in the cell assay in comparison to the anti-bacterial hits may make it difficult to translate to the in vivo situation. Certainly, as the A·WOL screening process has been developed and improved, now utilising a high content imaging platform (Clare et al., in press), potency has become a more important driver of in vivo experiments.
Pharmacological factors may also explain the more surprising differences observed between closely-related drugs within the same class. The fluoroquinolone antibiotics sparfloxacin and ciprofloxacin were demonstrated to be active in vitro. Ciprofloxacin has previously been shown to have either no activity (Hermans et al., 2001) or modest activity (Fenollar et al., 2003) in other cell-based Wolbachia assays, and the fact that this activity extended to the in vitro nematode screen in our screening strategy, suggests that the optimisation of our cell-based screening assay has increased the detection of active compounds. Despite this, of the two fluoroquinolone antibiotics tested, only sparfloxacin was active in vivo, thus demonstrating that pharmacological parameters must differ between members of the class in our screens. Further studies in the L. sigmodontis model conducted recently have extended this knowledge of diversity within the fluoroquinolone class by demonstrating that moxifloxacin is active, thereby confirming our result in the O. gutturosa in vitro model, yet levofloxacin is inactive (S. Specht, unpublished observations). The inconsistency in the activity of ciprofloxacin throughout previous work and the absence of studies using other fluoroquinolones has meant that this class has largely been overlooked as a potential source of novel anti-Wolbachia compounds. DNA gyrase can now be considered as a valid chemotherapeutic target of Wolbachia. The L. sigmodontis model demonstrated increased potency of minocycline over doxycycline in vivo, adding weight to a previous observation made using nematodes in vitro (Townson et al., 2006). Minocycline is considered to be more lipid-soluble than doxycycline (Barza et al., 1975) and this may therefore lead to higher concentrations of the drug reaching the appropriate tissues, such as the nematode hypodermal cords, in which the Wolbachia reside. Work is currently ongoing to determine whether any increased potency observed across the models with the fluoroquinolones and tetracyclines translates into reduced treatment duration: a potentially important improvement when considering implementation of an anti-Wolbachia treatment for mass drug administration programs. Further outcomes based on double and triple combinations are also progressing through the screening strategy.
The development of the 96-well in vitro cell-based assay described here has, in itself, been a major development in the study of the biology and chemotherapy of Wolbachia. Wolbachia are obligate intracellular bacteria and previous cell-based screening had used either flasks (Hermans et al., 2001) or 24-well plates (Fenollar et al., 2003) therefore deeming large-scale screening studies unachievable within the five-year project. The robustness of this assay has already allowed, prior to the publication of this report, other studies to provide further insights into Wolbachia biology (Johnston et al., 2010; Schiefer et al., 2012; Voronin et al., 2012). Furthermore, this screening assay has since been further optimised and up-scaled (Clare et al., in press) to the extent that the A·WOL consortium has now screened tens of thousands of compounds from both focused and diversity compound libraries for anti-Wolbachia activity, a selection of which are moving down the screening funnel. The funnel, too, has been further optimised to streamline the A·WOL screening strategy and thus maximise hit discovery (Johnston et al., 2014).
These experiments not only provided a proof of concept of our cell-based assay and screening platform but also identified potential lead candidates that are better than the gold standard doxycycline in reducing Wolbachia load in vivo. A·WOL is currently testing in clinical trials whether refined regimes of registered anti-Wolbachia drugs can translate into improved regimes for macrofilaricidal therapy of onchocerciasis and lymphatic filariasis.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
The A·WOL consortium is supported by a grant from the Bill and Melinda Gates Foundation awarded to the Liverpool School of Tropical Medicine.
Appendix A. Supplementary data
Supplementary data
Supplementary Tables S1–S3.
References
- Arumugam S., Pfarr K.M., Hoerauf A. Infection of the intermediate mite host with Wolbachia-depleted Litomosoides sigmodontis microfilariae: impaired L1 to L3 development and subsequent sex-ratio distortion in adult worms. Int. J. Parasitol. 2008;38:981–987. doi: 10.1016/j.ijpara.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Barza M., Brown R.B., Shanks C., Gamble C., Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob. Agents Chemother. 1975;8:713–720. doi: 10.1128/aac.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockarie M.J., Deb R.M. Elimination of lymphatic filariasis: do we have the drugs to complete the job? Curr. Opin. Infect. Dis. 2010;23:617–620. doi: 10.1097/QCO.0b013e32833fdee5. [DOI] [PubMed] [Google Scholar]
- Brennan L.J., Keddie B.A., Braig H.R., Harris H.L. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS One. 2008;3:e2083. doi: 10.1371/journal.pone.0002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B.K., Hooper P.J., Bradley M.H., McFarland D.A., Ottesen E.A. The economic benefits resulting from the first 8 years of the global programme to eliminate lymphatic filariasis (2000–2007) PLoS Negl. Trop. Dis. 2010;4:e708. doi: 10.1371/journal.pntd.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare, R., Cook, D.A., Johnston, K.L., Ford, L., Ward, S.A., Taylor, M.J., in press. Development and validation of a high throughput anti-Wolbachia whole cell screen: a route to macrofilaricidal drugs against onchocerciasis and lympatic filariasis. J. Biomol. Screen. [DOI] [PubMed]
- Coffeng L.E., Stolk W.A., Zoure H.G., Veerman J.L., Agblewonu K.B., Murdoch M.E., Noma M., Fobi G., Richardus J.H., Bundy D.A., Habbema D., de Vlas S.J., Amazigo U.V. African programme for onchocerciasis control 1995–2015: model-estimated health impact and cost. PLoS Negl. Trop. Dis. 2013;7:e2032. doi: 10.1371/journal.pntd.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejim L., Farha M.A., Falconer S.B., Wildenhain J., Coombes B.K., Tyers M., Brown E.D., Wright G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011;7:348–350. doi: 10.1038/nchembio.559. [DOI] [PubMed] [Google Scholar]
- Fenollar F., Maurin M., Raoult D. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 2003;47:1665–1671. doi: 10.1128/AAC.47.5.1665-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardon J., Gardon-Wendel N., Demanga N., Kamgno J., Chippaux J.P., Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- Grimberg B.T., Mehlotra R.K. Expanding the antimalarial drug arsenal-now, but how? Pharmaceuticals (Basel) 2011;4:681–712. doi: 10.3390/ph4050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P.G., Hart C.A., Trees A.J. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 2001;47:659–663. doi: 10.1093/jac/47.5.659. [DOI] [PubMed] [Google Scholar]
- Hoerauf A. Filariasis: new drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr. Opin. Infect. Dis. 2008;21:673–681. doi: 10.1097/QCO.0b013e328315cde7. [DOI] [PubMed] [Google Scholar]
- Hoerauf A., Nissen-Pahle K., Schmetz C., Henkle-Duhrsen K., Blaxter M.L., Buttner D.W., Gallin M.Y., Al-Qaoud K.M., Lucius R., Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A., Volkmann L., Nissen-Paehle K., Schmetz C., Autenrieth I., Buttner D.W., Fleischer B. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health. 2000;5:275–279. [PubMed] [Google Scholar]
- Johnston K.L., Taylor M.J. Wolbachia in filarial parasites: targets for filarial infection and disease control. Curr. Infect. Dis. Rep. 2007;9:55–59. doi: 10.1007/s11908-007-0023-2. [DOI] [PubMed] [Google Scholar]
- Johnston K.L., Wu B., Guimaraes A., Ford L., Slatko B.E., Taylor M.J. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasit. Vectors. 2010;3:99. doi: 10.1186/1756-3305-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K.L., Ford L., Taylor M.J. Overcoming the challenges of drug discovery for neglected tropical diseases: the A-WOL experience. J. Biomol. Screen. 2014;19:335–343. doi: 10.1177/1087057113511270. [DOI] [PubMed] [Google Scholar]
- Kremer N., Charif D., Henri H., Gavory F., Wincker P., Mavingui P., Vavre F. Influence of Wolbachia on host gene expression in an obligatory symbiosis. BMC Microbiol. 2012;12(Suppl 1):S7. doi: 10.1186/1471-2180-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.X., Weller P.F. Antiparasitic drugs. N. Engl. J. Med. 1996;334:1178–1184. doi: 10.1056/NEJM199605023341808. [DOI] [PubMed] [Google Scholar]
- Mackenzie C.D., Homeida M.M., Hopkins A.D., Lawrence J.C. Elimination of onchocerciasis from Africa: possible? Trends Parasitol. 2012;28:16–22. doi: 10.1016/j.pt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Makepeace B.L., Rodgers L., Trees A.J. Rate of elimination of Wolbachia pipientis by doxycycline in vitro increases following drug withdrawal. Antimicrob. Agents Chemother. 2006;50:922–927. doi: 10.1128/AAC.50.3.922-927.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry H.F., Pfarr K., Egerton G., Hoerauf A., Akue J.P., Enyong P., Wanji S., Klager S.L., Bianco A.E., Beeching N.J., Taylor M.J. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. 2003;2:9. doi: 10.1186/1475-2883-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke, H.A.M., 2010. Drug repositioning: extracting value from prior R&D investments. Insight Pharma Rep. <www.insightpharmareports.com>.
- Prichard R.K., Basanez M.G., Boatin B.A., McCarthy J.S., Garcia H.H., Yang G.J., Sripa B., Lustigman S. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl. Trop. Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.U., Moussa H., Weil G.J. Brugia malayi: effects of antibacterial agents on larval viability and development in vitro. Exp. Parasitol. 2002;101:77–81. doi: 10.1016/s0014-4894(02)00019-x. [DOI] [PubMed] [Google Scholar]
- Richard-Lenoble D., Chandenier J., Gaxotte P. Ivermectin and filariasis. Fundam. Clin. Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Schiefer A., Schmitz A., Schaberle T.F., Specht S., Lammer C., Johnston K.L., Vassylyev D.G., Konig G.M., Hoerauf A., Pfarr K. Corallopyronin a specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 2012;206:249–257. doi: 10.1093/infdis/jis341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H.L. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev. Anti Infect. Ther. 2009;7:37–56. doi: 10.1586/14787210.7.1.37. [DOI] [PubMed] [Google Scholar]
- Strübing U., Lucius R., Hoerauf A., Pfarr K.M. Mitochondrial genes for heme-dependent respiratory chain complexes are up-regulated after depletion of Wolbachia from filarial nematodes. Int. J. Parasitol. 2010;40:1193–1202. doi: 10.1016/j.ijpara.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Tamarozzi F., Tendongfor N., Enyong P.A., Esum M., Faragher B., Wanji S., Taylor M.J. Long term impact of large scale community-directed delivery of doxycycline for the treatment of onchocerciasis. Parasit. Vectors. 2012;5:53. doi: 10.1186/1756-3305-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., Hoerauf A., Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Hoerauf A., Townson S., Slatko B.E., Ward S.A. Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis. Parasitol. 2014;141:119–127. doi: 10.1017/S0031182013001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinick E.L. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 2009;22:119–125. doi: 10.1358/dnp.2009.22.2.1303818. [DOI] [PubMed] [Google Scholar]
- Townson S., Hutton D., Siemienska J., Hollick L., Scanlon T., Tagboto S.K., Taylor M.J. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann. Trop. Med. Parasitol. 2000;94:801–816. doi: 10.1080/00034980020027988. [DOI] [PubMed] [Google Scholar]
- Townson S., Tagboto S., McGarry H.F., Egerton G.L., Taylor M.J. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006;5:4. doi: 10.1186/1475-2883-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson S., Ramirez B., Fakorede F., Mouries M.A., Nwaka S. Challenges in drug discovery for novel antifilarials. Expert Opin. Drug Discov. 2007;2:S63–73. doi: 10.1517/17460441.2.S1.S63. [DOI] [PubMed] [Google Scholar]
- Turner J.D., Langley R.S., Johnston K.L., Egerton G., Wanji S., Taylor M.J. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 2006;177:1240–1249. doi: 10.4049/jimmunol.177.2.1240. [DOI] [PubMed] [Google Scholar]
- Turner J.D., Tendongfor N., Esum M., Johnston K.L., Langley R.S., Ford L., Faragher B., Specht S., Mand S., Hoerauf A., Enyong P., Wanji S., Taylor M.J. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial. PLoS Negl. Trop. Dis. 2010;4:e660. doi: 10.1371/journal.pntd.0000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann L., Fischer K., Taylor M., Hoerauf A. Antibiotic therapy in murine filariasis (Litomosoides sigmodontis): comparative effects of doxycycline and rifampicin on Wolbachia and filarial viability. Trop. Med. Int. Health. 2003;8:392–401. doi: 10.1046/j.1365-3156.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- Voronin D., Cook D.A., Steven A., Taylor M.J. Autophagy regulates Wolbachia populations across diverse symbiotic associations. Proc. Natl. Acad. Sci. USA. 2012;109:E1638–1646. doi: 10.1073/pnas.1203519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanji S., Tendongfor N., Nji T., Esum M., Che J.N., Nkwescheu A., Alassa F., Kamnang G., Enyong P.A., Taylor M.J., Hoerauf A., Taylor D.W. Community-directed delivery of doxycycline for the treatment of onchocerciasis in areas of co-endemicity with loiasis in Cameroon. Parasit. Vectors. 2009;2:39. doi: 10.1186/1756-3305-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2007. Global plan to combat neglected tropical diseases 2008–2015. http://whqlibdoc.who.int/hq/2007/WHO\_CDS\_NTD\_2007.2003\_eng.pdf.
- Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary Tables S1–S3.