The APC tumor suppressor is required for epithelial cell polarization and three-dimensional morphogenesis (original) (raw)
. Author manuscript; available in PMC: 2016 Mar 1.
Published in final edited form as: Biochim Biophys Acta. 2015 Jan 8;1854(3):711–723. doi: 10.1016/j.bbamcr.2014.12.036
Abstract
The Adenomatous Polyposis Coli (APC) tumor suppressor has been previously implicated in the control of apical-basal polarity; yet, the consequence of APC loss-of-function in epithelial polarization and morphogenesis has not been characterized. To test the hypothesis that APC is required for the establishment of normal epithelial polarity and morphogenesis programs, we generated APC-knockdown epithelial cell lines. APC depletion resulted in loss of polarity and multi-layering on permeable supports, and enlarged, filled spheroids with disrupted polarity in 3D culture. Importantly, these effects of APC knockdown were independent of Wnt/β-catenin signaling, but were rescued with either full-length or a carboxy (c)-terminal segment of APC. Moreover, we identified a gene expression signature associated with APC knockdown that points to several candidates known to regulate cell-cell and cell-matrix communication. Analysis of epithelial tissues from mice and humans carrying heterozygous APC mutations further support the importance of APC as a regulator of epithelial behavior and tissue architecture. These data also suggest that the initiation of epithelial-derived tumors as a result of APC mutation or gene silencing may be driven by loss of polarity and dysmorphogenesis.
Keywords: Adenomatous Polyposis Coli, Epithelial Polarity, Madin Darby Canine Kidney
1. Introduction
Epithelial morphogenesis is a tightly coordinated process that requires extrinsic and intrinsic cues to couple cell-cell and cell-matrix interactions, polarity, proliferation, cell death, and differentiation. In contrast to traditional two-dimensional (2D) culture on glass or plastic, the organotypic 3D culture of epithelial cells in extracellular matrix (ECM), such as the reconstituted basement membrane Matrigel or collagen, is a powerful in vitro model that recapitulates many of the features of tissue polarity and architecture (reviewed in (Zegers et al., 2003a)). Common features of these organoid or spheroid models (conventionally referred to as “acini” for mammary cells and “cysts” for kidney cells) are that after a couple of cell divisions of plated single cells, they polarize to form a basal surface that contacts the ECM, a lateral surface between cells, and an apical surface which faces the lumen. Apoptosis will occur in those cells that do not contact the ECM, and cells that do not yet have an apical surface will generally form a lumen at the point of contact with other cells (reviewed in (Bryant and Mostov, 2008)). Recent insights into the molecular mechanisms that guide polarization and lumen formation, for example, have supported the importance of junction and polarity complexes, laminins, integrins, phosphoinositides and Rho GTPases family members in these processes (O'Brien et al., 2001; Yu et al., 2005; Yu et al., 2008; Zhan et al., 2008; Kim and Giardiello, 2011). Importantly, these polarity and morphogenesis programs are often disrupted or hijacked in pathological conditions such as chronic wounds, kidney fibrosis and cancer; therefore, a more complete understanding of the pathways and critical players involved has significant clinical relevance.
The Adenomatous Polyposis Coli (APC – by convention, the mouse gene is Apc, the human gene is APC, and the protein from either species is APC) tumor suppressor is a large, multifunctional protein that is frequently mutated or down-regulated in epithelial-derived cancers, including colorectal, breast and renal cancer, among many others (reviewed in (Prosperi, J.R. et al., 2011)). A current view of APC function is as a scaffold that facilitates the assembly or stability of multi-protein complexes, such as the β-catenin destruction complex. While this one well-characterized activity of APC controls β-catenin levels and signaling via the canonical Wnt signaling pathway, APC associates with many other protein partners. At its carboxy-terminus, which is invariably lost if APC is mutated, APC binds directly and indirectly to the microtubule (MT) and actin cytoskeleton (Munemitsu et al., 1994; Smith et al., 1994; Su et al., 1995; Morrison et al., 1998; Rosin-Arbesfeld et al., 2001; Moseley et al., 2007; Okada et al., 2010) as well as to the Dlg and Scrib basolateral domain identity proteins (Matsumine et al., 1996; Takizawa et al., 2006). APC also interacts with the Rac and Cdc42 guanine-exchange factors (GEFs) Asef- and Asef-2, the Rac and Cdc42 effector IQGAP1, the plus-end MT binding protein EB1, the kinesin family members KAP3, KIF3 and KIF17, and the formin mDia ((Prosperi and Goss, 2011) for review). It is likely that through these protein-protein interactions, and others, APC affects a broad set of activities in normal epithelial cells in addition to controlling the levels of β-catenin and Wnt signaling.
Previous work from our laboratory and others has established a link of APC to polarity and tissue architecture. Endogenous and exogenous APC is concentrated in puncta at the ends of cell protrusions in motile cells, such as astrocytes, radial glia or subconfluent epithelial cells, where it associates with the microtubules and is required for front-rear polarity downstream of the Cdc42 RhoGTPase (Nathke et al., 1996; Barth et al., 2002; Etienne-Manneville and Hall, 2003; Etienne-Manneville et al., 2005; Reilein and Nelson, 2005; Kita et al., 2006; Yokota et al., 2009). In polarized epithelia in vitro and in vivo, APC localizes to the basal plasma membrane (Rosin-Arbesfeld et al., 2001; Mogensen et al., 2002; Prosperi et al., 2009) in an actin-dependent fashion (Rosin-Arbesfeld et al., 2001), where it controls the establishment of parallel arrays of microtubule bundles (Mogensen et al., 2002). However, there are also reports of APC localization to the apical membrane of polarized epithelial cell types, including in the differentiated mammary epithelium (Reinacher-Schick and Gumbiner, 2001; Prosperi et al., 2009). Our laboratory has demonstrated that heterozygous Apc mutation abrogates mammary lobuloalveologenesis by inhibiting proliferation during pregnancy, inducing apoptosis during lactation and severely altering epithelial integrity, including cell-cell interactions and polarity (Prosperi et al., 2009). Furthermore, knockdown of APC in Madin-Darby Canine Kidney (MDCK) cells perturbs mitotic spindle orientation (den Elzen et al., 2009) that can lead to monolayer disruption, and APC expression in EpH4 mammary epithelial cells was required for normal monolayer formation (Prosperi et al., 2009). APC also mediates directionality of cell extrusion from an epithelial monolayer through its control of microtubule dynamics (Marshall et al., 2011). However, key questions regarding the role of APC in epithelial morphogenesis and the mechanisms by which APC mediates these behaviors remain unanswered, and, importantly, it has not been established whether this is one of the essential ways in which APC acts as a tumor suppressor.
In the current study, we test the hypothesis that APC function is required for normal epithelial polarity and 3D morphogenesis. By establishing in vitro models of stable APC knockdown in multiple epithelial cell lines, we found that APC is required for monolayer formation in 2D and normal spheroid morphogenesis in 3D culture. The effects of APC depletion were rescued with overexpression of either full-length or a carboxy (c)-terminal fragment of APC, but not by a central region containing the β-catenin-binding domain. These data are consistent with the interactions between APC and cystoskeletal and/or polarity complex proteins being required for normal polarity and morphogenesis programs, but the phenotypes associated with APC knockdown do not involve activation of the Wnt signaling pathway. These data highlight the importance of APC as a regulator of epithelial behavior and tissue architecture, and suggest that tumor initiation as a result of APC mutation or inactivation may be driven by loss of proper apical-basal polarity and dysmorphogenesis.
2. Results
2.1 Polarity and morphogenesis are disrupted in mammary epithelial and colorectal cancer cells with APC knockdown
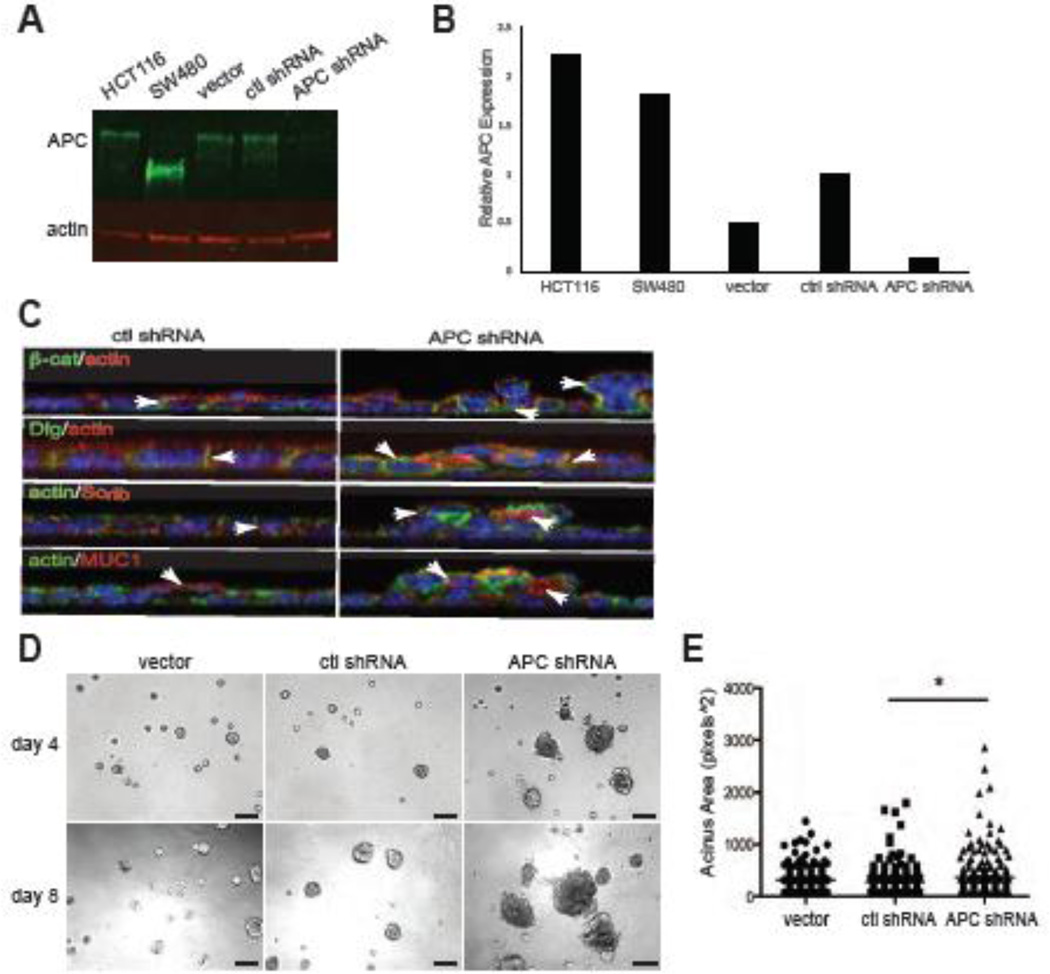
We have previously shown that Apc mutation perturbed mammary epithelial polarity in vivo (Prosperi et al., 2009). Therefore, to identify the mechanisms involved, an in vitro model was generated in which APC was stably knocked down in the HC11 mouse mammary epithelial cell line using lentiviral infection of APC-specific shRNAs. Western blot analysis confirmed that APC expression was significantly reduced in APC shRNA cells compared to the vector and control scrambled shRNA cells (Figure 1A,B). In order to assess the impact of APC depletion on epithelial monolayer formation, control shRNA HC11 and APC shRNA HC11 cells were plated on Transwell filters and grown to confluence. The APC shRNA HC11 cells demonstrated a phenotype of multilayering with mislocalization of the polarity markers, Dlg, Scrib, MUC1, and β-catenin (Figure 1C, Supplemental Figure 1). Unlike control cells in which Dlg, Scrib and β-catenin are restricted to the basolateral surface and MUC1 is apically restricted, there was pronounced intracellular localization of these markers and staining all around the membrane in APC-knockdown cells. Because the phenotype is heterogenous, there are confounding issues in separation of the morphological defects from the mislocalization of apical and basal markers. However, it is of note that there is no evidence of β-catenin translocation to the nucleus observed in APC shRNA cells, which is consistent with our previous findings in the mammary glands of ApcMin/+ females (Prosperi et al., 2009). Similar to other breast epithelial cell lines, such as MCF-10A, HC11 cells will form acinar structures in 3D Matrigel culture conditions and can differentiate a normal versus invasive phenotype (Xian et al., 2005). To test the impact of APC loss on 3D morphogenesis, single cells were plated in Matrigel, and we found that the APC shRNA HC11 cells demonstrated a marked increased acinar size (Figure 1D,E). In addition, the APC shRNA HC11 acini develop more invasive structures with cell extensions protruding into the Matrigel, which is not observed in control acini (Figure 1D). No changes in cell proliferation or apoptosis were observed via phospho-Histone H3 or cleaved caspase 3 staining respectively (data not shown). These data suggest a role for APC in the polarization and morphogenesis of breast-derived epithelial cells.
Figure 1. APC knockdown disrupts polarization and morphogenesis in mammary epithelial cells.
A) APC protein expression in HC11 cells was analyzed by Western blot. B) Western Blot analysis of APC expression in HC11 cells normalized to actin expression. C) Control and APC-knockdown HC11 cells were plated on Transwell permeable supports for 5 d and analyzed for the localization of polarized protein markers, β-catenin (top row, green), Dlg (2nd row, green), Scribble (3rd row, red) and MUC1 (bottom row, red). All were co-stained with phalloidin and Hoechst (blue). Arrows show basolateral (β-catenin, Dlg and Scrib) and apical (MUC1) distribution of the markers in control cells and mislocalization and intracellular accumulation in the APC-knockdown cells. Multi-layering is apparent in the APC-knockdown cells. X-Z confocal planes are shown. Scale bars, 10 µm. D) Phase-contrast images of HC11 cells grown in 3D Matrigel cultures demonstrate larger, disorganized acini in cells with APC silencing compared to controls. Scale bars, 100 µm. E) Quantification of the acinus size indicated that APC-knockdown HC11 cells generated larger acini (*p < 0.05 via Fisher’s exact test). Representative images are shown of experiments, which were all performed three times.
2.2 APC knockdown disrupts cyst morphology and polarity in MDCK cells
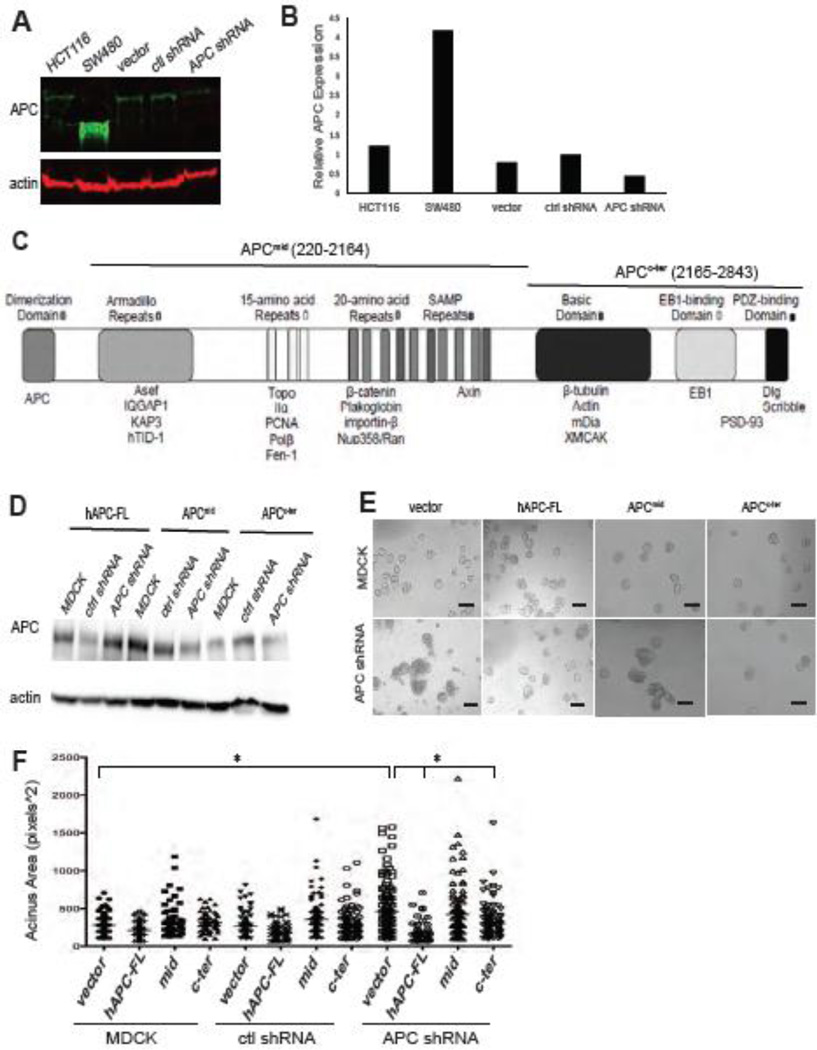
We next sought to address the role for APC in establishing or maintaining polarity, and identify the molecular mechanisms responsible, in the MDCK cell line because it is a robust and very well studied model system for investigating epithelial polarization and morphogenesis. MDCK cells with stable expression of APC shRNA were generated using the same system as the HC11 cells. Western blot analysis confirmed that APC protein levels were markedly decreased in the APC shRNA cells compared to the controls (Figure 2A,B). In addition to APC knockdown in MDCK cells causing multi-layering on Transwells like HC11 cells (data not shown), these cells plated in 3D Matrigel cultures exhibited very large and highly disorganized cysts in the APC shRNA cells compared to the control cells by phase-contrast microscopy (Figure 2E). These qualitative differences were confirmed by morphometric analysis of the phase-contrast images and demonstrated that the cysts were larger in the APC shRNA cells compared to the control cells (Figure 2F). Not only were these phenotypes observed with multiple APC-specific shRNAs (data not shown), but also the stable introduction of full-length APC (hAPC-FL) was able to rescue this phenotype (Figure 2E,F) confirming that normal MDCK 3D morphogenesis is dependent on APC. For the rescue experiments, it is important to note that the construct contains the human APC cDNA and is not targeted by the APC shRNA.
Figure 2. MDCK 3D morphogenesis is perturbed by APC knockdown and restored by re-introduction of APC.
A) APC protein expression in MDCK cells was analyzed by Western blot. B) APC protein expression in MDCK cells was quantified and normalized to actin expression. C) Schematic of the full-length APC protein including functional domains (top) and binding partners (bottom) and APCmid and APCc-ter fragments. D) APC protein expression in MDCK cells with expression of human full-length APC (hAPC-FL), the central fragment of APC (APCmid), and the c-terminus of APC (APCc-ter) was analyzed by Western Blot and normalized to actin expression. E) Phase-contrast images of MDCK and APC-knockdown MDCK cysts show an increase in cyst size and altered morphology in APC-knockdown cell compared to controls (left panels). The 2nd-4th columns show the phenotype with ectopic expression of human full-length APC (hAPC-FL), APCmid, or APCc-ter. Scale bars, 100 µm. F) Cyst size was increased in APC-knockdown cells compared to controls, and this size difference was reversed with the introduction of hAPC-FL. The enhanced cyst size was significantly abrogated by introduction of the APCc-ter construct. Shown is a a bar graph with average cyst size. Representative images are shown of experiments, which were all performed three times.
To dissect the region of APC required for the altered cyst size, we generated cell lines stably expressing a large central segment of APC tagged with GFP (APCmid; residues 220–2164) and the c-terminus of APC tagged with GFP (APCc-ter; residues 2165–2843) in APC-knockdown and control cells (Figure 2C), and compared them to expression of the vector only and full-length APC (as a positive control for phenotype rescue as shown in Figure 2E,F). Expression of these fragments was confirmed through western blots of APC (Figure 2D), and immunofluorescence staining for GFP (Supplemental Figure 2). Notably, this central segment is necessary and sufficient for β-catenin binding and down-regulation (Rubinfeld et al., 1993; Munemitsu et al., 1995), and the c-terminal fragment associates with the MT and actin cytoskeleton, and Dlg and Scrib polarity proteins (Munemitsu et al., 1994; Matsumine et al., 1996; Askham et al., 2000; Takizawa et al., 2006; Moseley et al., 2007). While the APCmid fragment had minimal effect on cyst size, the APCc-ter significantly decreased the cyst size of the APC-knockdown cells compared to APC shRNA cells stably expressing the vector control (Figure 2E,F).
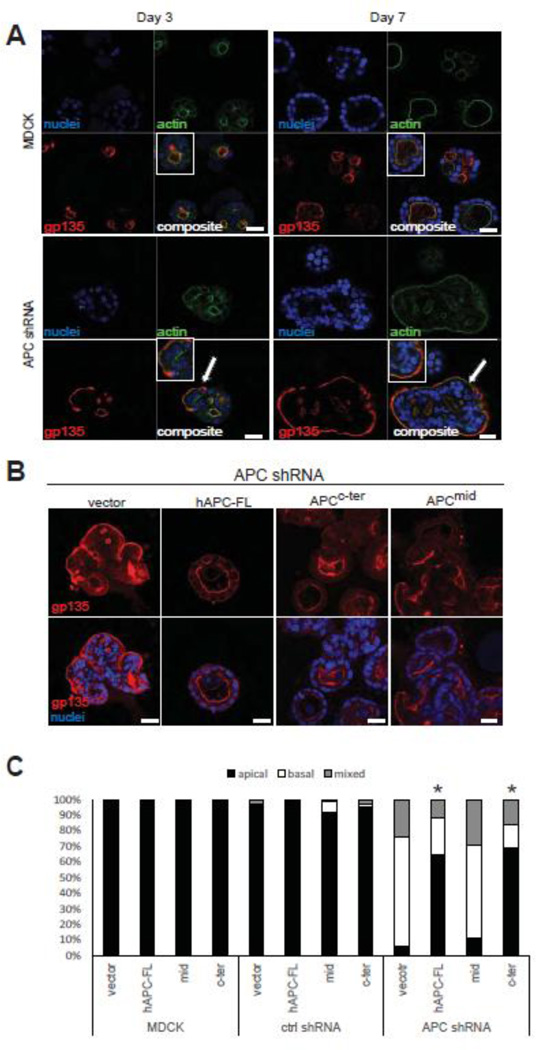
To further assess the impact of APC depletion on MDCK 3D morphogenesis and polarization, MDCK control and APC shRNA cells were plated in Matrigel over a time course of 7 days and analyzed by immunofluorescence and confocal microscopy for the localization of the apical marker gp135 (also referred to as podocalyxin) and phalloidin to stain F-actin. While the lumens observed at 3 days post-plating in the MDCK control cells were quite small, gp135 was localized to apical cell surface adjacent to these early lumens (Figure 3A), which is consistent with previous analysis of MDCK 3D morphogenesis in Matrigel (Engelberg et al., 2011; Kim and Giardiello, 2011). After 7 days in culture, the lumens of MDCK cell cysts were generally hollow, and most cysts exhibited only a single lumen. In striking contrast, the APC shRNA cells formed many cysts without lumens and gp135 was frequently localized to basal surface (Figure 3A), an effect that was observed as early as 3 days post-plating and very pronounced by day 7. Like the gross cyst morphological defects observed in APC-knockdown cells (i.e. increased size and non-spherical shape), the lack of discernible lumens and mis-localization of gp135 were abrogated by introduction of full-length APC (Figure 3B,C). Similar to the effect of the APC fragments on cyst size, the APCc-ter fragment, but not the APCmid fragment, partially rescued the polarized expression of membrane markers in the APC-knockdown cells (Figure 3B,C). These data demonstrate that the c-terminus of APC mediates epithelial polarization and morphogenesis, and suggest that one consequence of APC mutation and deletion of the c-terminus during tumorigenesis is loss of polarity and tissue architecture.
Figure 3. APC knockdown disrupts MDCK cell polarity in cysts grown 3D culture.
A) Control and APC-knockdown MDCK cells were grown in Matrigel for 3 or 7 d and stained for the apical marker gp135 (red) and phalloidin (green). Control MDCK cells show apical localization of gp135 over the time course, but basal localization (white arrows) of gp135 is observed as early as 3 d post-plating in the APC-knockdown MDCK cells and the cysts are generally larger and have a less spherical morphology. Insets are higher magnification images of interest. Scale bars, 20 µm. B) Cysts from APC rescue cells (using hAPC-FL, APCc-ter, and APCmid) were grown for 7 d and stained for the apical marker, gp135, which as shown in Figure 3A is inverted in the APC-knockdown cells. With re-introduction of full-length APC or the APCc-ter construct, gp135 localization is restored to the apical surface in knockdown cells. Scale bars, 20 µm. Shown is a bar graph (C) quantifying the polarity phenotypes as percent apical (normal), and basal or mixed (abnormal). 100 cysts were counted for each cell line with the various APC constructs re-introduced. Significance was determined using Fisher’s exact test (* p < .05 compared to APC shRNA vector cells). Representative images are shown of experiments, which were all performed three times.
2.3 The morphogenesis effects of APC knockdown are not associated with Wnt/β-catenin pathway activation
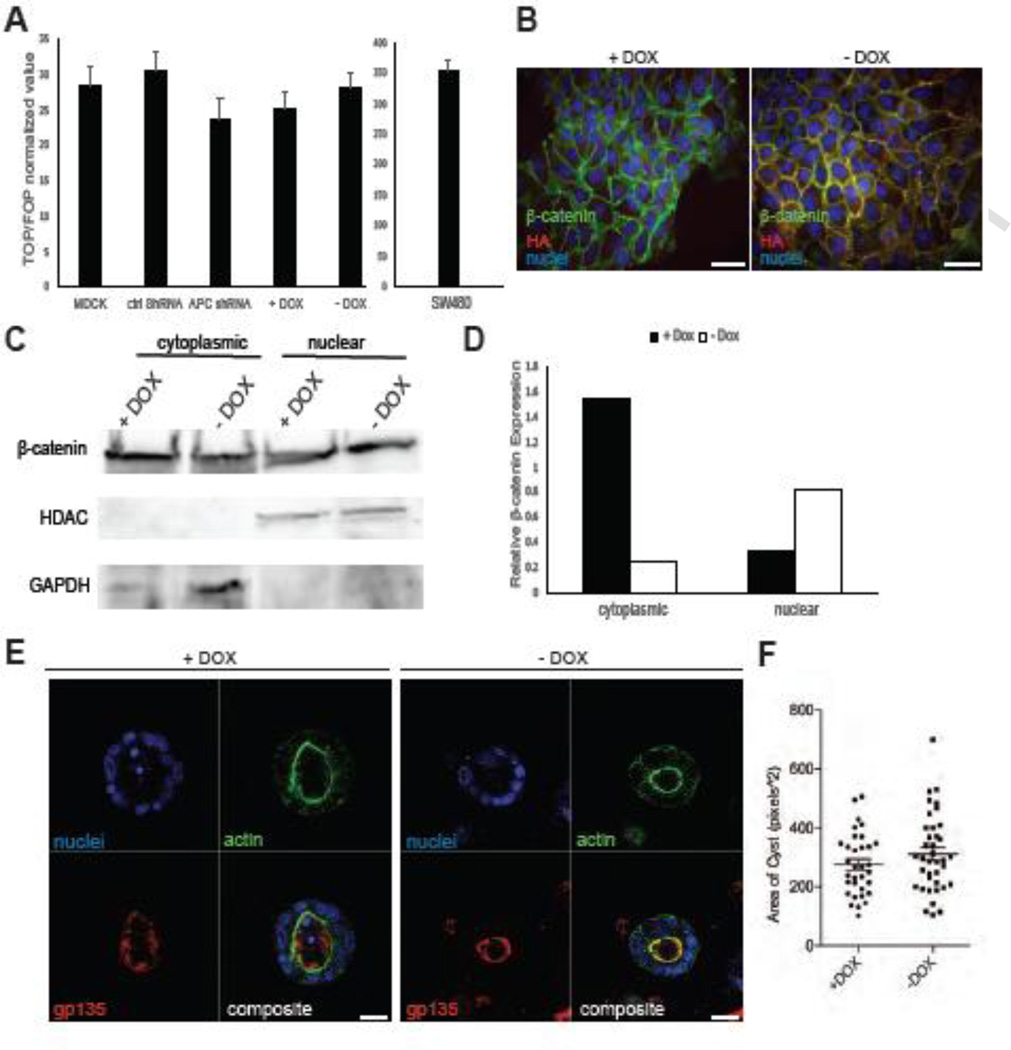
Because activation of the Wnt/β-catenin pathway is such a well-characterized consequence of APC mutation in tumor cells, we next addressed whether uncontrolled canonical Wnt signaling was involved in mediating the effect of APC knockdown on MDCK polarization and morphogenesis. A TOPflash reporter assay was performed in the cells (and _APC_-mutant SW480 colorectal cancer cells as a positive control) to assess the level of β-catenin/TCF-mediated transcriptional activity. In agreement with previous studies from our laboratory using mammary-derived epithelial and tumor cells (Prosperi et al., 2009; Odenwald et al., 2013), APC knockdown was insufficient to activate reporter activity above baseline (normalized to the activity observed in cells transfected with a mutant FOPflash reporter) (Figure 4A). These findings are also consistent with a lack of cytosolic or nuclear β-catenin accumulation observed in APC-knockdown MDCK cells (data not shown) and failure of APC knockdown in HC11 cells (described earlier) to demonstrate TOPflash reporter activity (data not shown). Furthermore, treatment with LiCl (known to activate Wnt signaling) induced reporter activity in both MDCK control and APC knockdown cells (Supplemental Figure 3A). It is possible that the β-catenin/TCF transcriptional reporter assay and β-catenin immunofluorescence are not sensitive enough to detect subtle, but biologically relevant, levels of Wnt pathway activation subsequent to APC knockdown. Therefore, as a complementary approach, we generated an MDCK cell line with inducible Wnt/β-catenin pathway activity. The T23 “tetracycline-off” MDCK cells (Barth et al., 1997) were stably transfected with an expression vector containing a HA-tagged non-phosphorylatable, non-degradable, transcriptionally active S37Aβ-catenin mutant (Heinen et al., 2002) under the control of a tetracycline (or doxycycline, DOX)-responsive promoter. As expected, the removal of doxycycline from the culture media induced the expression of mutant β-catenin (Figure 4B, Supplemental Figure 3B), increased nuclear β-catenin expression (Figure 4C, D), and did not increase reporter activity compared to control cells (Figure 4A). To assess whether overexpression of stabilized β-catenin would disrupt MDCK morphogenesis and polarization similar to APC knockdown, the T23-S37A β-catenin-HA MDCK cells were plated in 3D culture using Matrigel in the presence or absence of doxycycline for 12 days and subjected to gp135 and phalloidin immunofluorescence and confocal microscopy. In both the presence and absence of doxycycline, we observed apical localization of gp135 with the stabilized β-catenin (Figure 4E) and no change in cyst size (Figure 4F). While 3D morphogenesis in the stabilized β-catenin cells was not completely normal (i.e. fewer cysts had hollow lumens), they did not phenocopy the APC-knockdown cells with respect to increased size, apical marker inversion and disrupted cyst morphology (Figures 2,3). Collectively, these data indicate that robust activation of β-catenin stabilization and β-catenin/TCF-mediated transcription are unlikely to account for cyst dysmorphogenesis and altered polarity observed in APC-depleted MDCK cells.
Figure 4. The phenotypes observed in APC-knockdown MDCK cells are not associated with Wnt/β-catenin pathway activation.
A) TOPflash reporter assays were performed and no change in TCF reporter activity in the APC-knockdown cells or T23-S37A with stabilized β-catenin was detected. Human colorectal cancer cells harboring an APC mutation, SW480, were used as a positive control. B) The T23-S37A β-catenin MDCK cells, expressing inducible stabilized β-catenin were stained with anti-β-catenin (green) and anti-HA (red) antibodies. The presence of doxycycline (DOX) suppresses stable β-catenin expression, and removal of DOX results in expression of HA-tagged mutant β-catenin. Scale bars, 20 µm. C) Nuclear expression of β-catenin was increased in T23-S37A in the absence of DOX. Expression was analyzed by Western blot and is shown in a bar graph (D). E) Cells grown in 3D Matrigel culture for 12 d were stained for the apical marker gp135 (red) or phalloidin (green) and show apical localization of gp135. Scale bars, 20 µm. F) Cyst size was unchanged in the T23-S37A β-catenin MDCK cells in the presence or absence of DOX. Representative images are shown of experiments, which were all performed three times.
2.4 Gene profiling experiments identify candidates for mediating APC-regulated epithelial morphogenesis
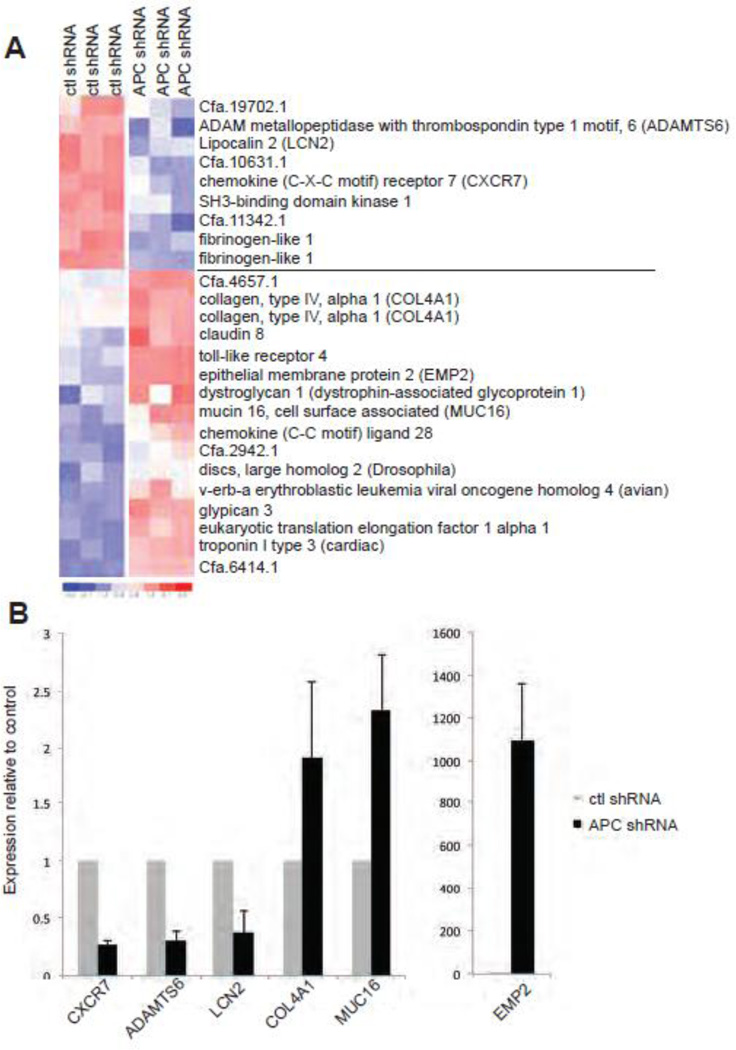
Given that the effects of APC-knockdown in MDCK cells could not be fully explained by Wnt/β-catenin activation, we addressed other potential molecular mechanisms responsible by gene expression profiling to identify candidate pathways or molecules dysregulated upon APC knockdown. In addition, we sought to address whether the APC-knockdown cells could be used as a tool to identify a gene signature associated with APC deficiency and epithelial polarity and morphogenesis. Gene expression microarray analysis was performed on RNA from control shRNA and APC shRNA MDCK cells grown in 3D Matrigel culture for 5 days, a time point when the polarity inversion and dysmorphogenesis phenotypes are pronounced in APC-knockdown cells (n=3 per cell line). Surprisingly, comparison of APC shRNA to control shRNA MDCK cells at this time point demonstrated that only 125 genes were significantly differentially expressed (i.e. >1.2-fold change; 75 up-regulated and 50 down-regulated). A representative heat-map of the signature is shown (Figure 5A), and the differential expression of multiple genes was validated by real-time RT-PCR ( Figure 5B). Consistent with the reporter assays described earlier, no characterized β-catenin/TCF target genes were contained in this APC-knockdown signature. Some of the genes in the signature had been previously linked to APC loss-of-function, and are generally implicated in mediating epithelial cell-cell or cell-matrix interactions. For example, epithelial membrane protein 2 (EMP2), which has been shown to regulate the activity and phosphorylation of focal adhesion kinase (FAK) through interaction with β1 integrin (Morales et al., 2009a; Morales et al., 2009b; Fu et al., 2011) was significantly up-regulated in the APC shRNA MDCK cells. This alteration in gene expression is of particular interest given our previous observations that mutation or loss of APC controls FAK activity in breast tumor cells (Prosperi, J. R. et al., 2011), and that inhibition of β1 integrin signaling, like APC depletion, confers an inverted polarity phenotype in MDCK cells (Ojakian and Schwimmer, 1994; Yu et al., 2005). The transmembrane glycoprotein MUC16 was also up-regulated in the APC shRNA MDCK cells. MUC16 has been implicated in early tumor development in both pancreatic and ovarian models (Bast et al., 1983; Chauhan et al., 2006; Haridas et al., 2011), and we have previously shown that APC is required for the localization of a related membrane-associated mucin, MUC1 (Prosperi et al., 2009). Interestingly, down-regulation of Lipocalin2 (LCN2), a marker for kidney injury (Viau et al., 2010), implicated in kidney epithelial cell morphogenesis (Gwira et al., 2005) and previously shown to be dysregulated in ApcMin/+ intestinal adenomas (Reichling et al., 2005), was observed in the APC shRNA MDCK cells. Other dysregulated genes including the alpha 1 subunit of type IV collagen (COL4A1, a basement membrane component), C-X-C chemokine receptor type 7 (CXCR7), and ADAM metallopeptidase with thrombospondin type 1, motif 6 (ADAMTS6), are implicated in controlling cell-cell or cell-matrix interactions (Kuhn, 1995; Bevitt et al., 2005; Hou et al., 2010; Aikio et al., 2012). These data collectively support a model in which APC loss-of-function in epithelial cells (through mutation and deletion of its c-terminus or gene silencing) leads to loss of polarity and tissue architecture, and subsequent tumor initiation, via altered communication between neighboring cells and the substratum.
Figure 5. An APC-knockdown gene signature is associated with altered cell-cell and cell-matrix communication.
A) Microarray analysis was performed on RNA from cells grown in 3D Matrigel culture for 5 d. Four wells were pooled to generate a single RNA sample, and 3 independent RNA samples per cell line were utilized in these studies. Global gene expression changes are shown in the heat map where overexpression is shown in red and underexpression is blue. B) Real-time PCR was used to validate some key gene expression differences, including CXCR7, ADAMTS6, LCN2, COL4A1, MUC16, and EMP2. Normalized expression relative to 18S rRNA is shown for real-time data.
2.5 APC mutation in vivo perturbs kidney and colonic epithelial architecture
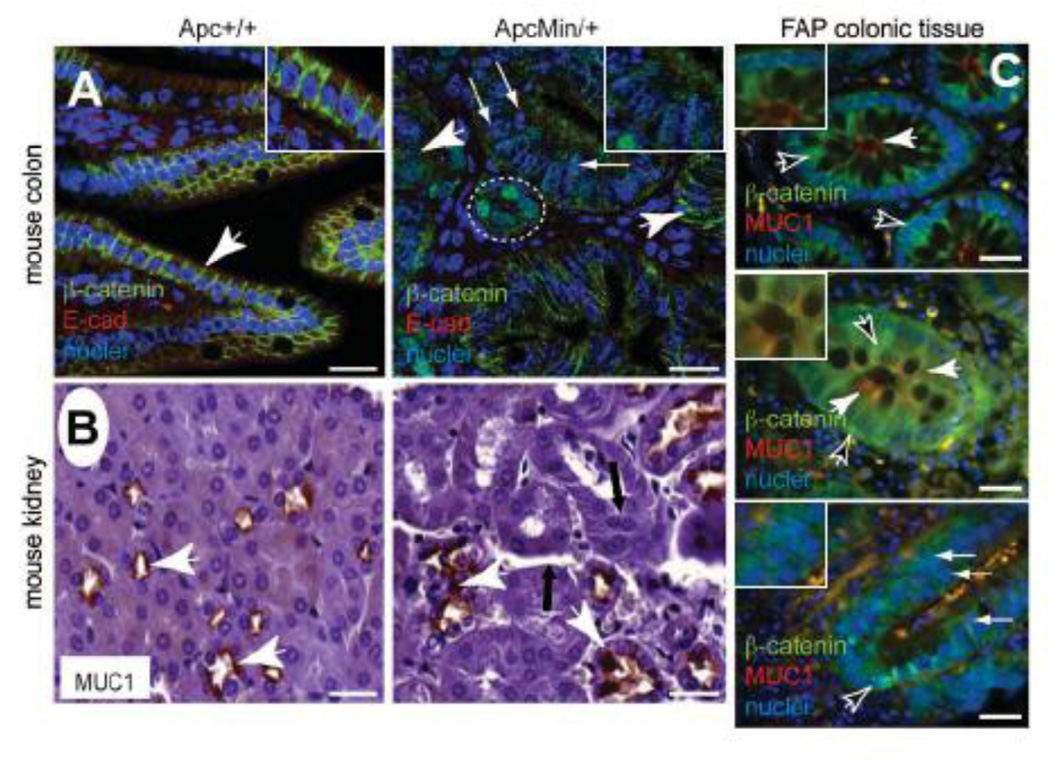
These data demonstrating that APC is required for epithelial cell line monolayer and 3D spheroid formation provide an in vitro correlate to our previous observations that epithelial integrity was compromised in germline heterozygous _Apc_-mutant mammary tissues (Prosperi et al., 2009). To address how generalizable this role for APC is in epithelia and whether our in vitro models are physiologically relevant, we turned back to the ApcMin/+ mouse model and used immunohistochemistry and immunofluorescence to analyze the localization of polarized membrane markers in intestinal and kidney tissues. In Apc+/+ colon tissues, the localization of the adherens junction components β-catenin and E-cadherin was restricted to the basolateral domain between adjacent differentiated epithelial cells as expected (Figure 6A, Supplemental Figure 4A). However, in non-adenomatous ApcMin/+ colon tissues, the localization of these markers was heterogeneous: it was restricted to the basolateral domain in some cells, was more diffuse at the membrane in others and cytosolic in many (Figure 6A, Supplemental Figure 4A). Moreover, multi-layering of the epithelium was observed, and only very focal nuclear β-catenin accumulation, as a surrogate for Wnt signaling, was detectable in microadenomas (Figure 6A, Supplemental Figure 4A). In the kidney tissues from wild-type mice, apical localization of MUC1 is pronounced in the distal convoluted tubules (Figure 6B) as has been described previously (Xing et al., 1998). In contrast, the localization of MUC1 is more diffuse and intracellular in ApcMin/+ kidney tissues as described in lactating mammary tissues from these mice (Prosperi et al., 2009). Moreover, defects in the kidney tissue architecture are characterized by gaps between tubules, some epithelial multi-layering and disorganization of the tubule structure (Figure 6B). Finally, we examined tissue architecture in non-adenomatous regions of colon tissue isolated from Familial Adenomatous Polyposis (FAP) patients by analyzing β-catenin and MUC1 localization by immunofluorescence (Figure 6C, Supplemental Figure 4B). While some areas retained normal histology, β-catenin was diffusely cytosolic in some cells and localized to the basal membrane in others and MUC1 showed intracellular accumulation in many cells. We additionally observed multi-layering of the colonic epithelial cells in some of the non-adenomatous areas, consistent with studies demonstrating that in histologically normal colonic tissues from FAP patients, E-cadherin is significantly down-regulated (Clarke, 2005; Radulescu et al., 2010). These data indicate that heterozygous mutation of APC in mouse and human is sufficient to compromise epithelial polarity and normal tissue architecture, a feature that may represent a key step in tumor initiation.
Figure 6. Heterozygous APC mutation is associated with disrupted epithelial tissue architecture in vivo.
A) In Apc+/+ colon, β-catenin (green) and E-cadherin (red) were analyzed by immunofluorescence and are basolaterally localized. Their localization is more disorganized, diffuse and often basally located in ApcMin/+ tissues. Nuclear β-catenin is only observed in microadenomas (white oval). Insets are higher magnification areas of interest. Scale bars, 20 µm. B) Immunohistochemistry for MUC1 (brown) shows that it is mislocalized and not restricted to the apical membrane in kidney tissues from ApcMin/+ mice compared Apc+/+ tissues. Tissues from ApcMin/+ are characterized by gaps between tubules and multi-layering of epithelial cells (black arrows). Scale bars, 20 µm. C) In non-adenomatous FAP colon tissues, β-catenin (green) is basolateral located, and MUC1 (red) is apical, in some areas (top), but other areas (middle/bottom panels) show cyosolic and basal β-catenin (black arrows) and intracellular MUC1 (white arrows). Multi-layering is evident (small white arrows). Insets are higher magnification areas of interest. Scale bars, 20 µm.
3. Discussion
3.1 Summary
The data presented here underscore the importance of APC in controlling key components of the normal epithelial morphogenesis program, including polarity and cell-cell and cell-matrix communication. Down-regulation of APC in HC11 and MDCK epithelial cell lines disrupted monolayer formation and caused multi-layering when the cells were plated on permeable supports and significantly perturbed acinar/cyst formation in 3D culture conditions. The 3D dysmorphogenesis phenotype of APC-knockdown cells, namely large, non-spherical structures with abnormal polarity, was rescued by introduction of exogenous human APC and its c-terminal end but not a central segment containing the β-catenin binding and down-regulation domain. These data, combined with the observations that β-catenin transcriptional activity was not induced by APC knockdown and overexpression of a stabilized β-catenin mutant does not phenocopy APC depletion in these cells, support a model in which the control of polarity and morphogenesis by APC is independent of canonical Wnt pathway regulation. We identified an APC-knockdown gene signature characterized by several genes involved in cell-cell and cell-matrix interactions and tumor initiation. Importantly, this may be a generalizable and physiological important role for APC since kidney and intestinal tissues from _APc_-mutant mice and FAP patients showed defects in epithelial architecture.
It was striking that APC-knockdown epithelial cells did not have any overt morphological defects or alterations in growth properties when plated on solid substrata (e.g glass or tissue culture plastic) but had very dramatic phenotypes when cultured on permeable supports or in 3D ECM, respectively. A similar phenotype in epithelial morphogenesis has recently been observed with loss of the tumor suppressor gene, ductal epithelium-associated ring chromosome 1 (DEAR1). In human mammary epithelial cells, loss of DEAR1 causes no change in 2D growth; however, these cells exhibit irregular acini morphogenesis and loss of polarity in 3D culture (Chen et al., 2014). These differences presumably occur because on plastic or glass, the cells are not receiving asymmetric polarization cues that are necessary for orienting their axis of polarity in 3D (Bryant and Mostov, 2008). APC may be required for receiving or integrating these cues, such as those derived from cell-matrix interactions.
3.2 Potential molecular mechanisms downstream of APC in regulating polarity and morphogenesis
A multi-layering phenotype specifically and loss of contact inhibition has been observed in MDCK cells in which a kinase-dead or constitutively activated Rac1 effector p21-activated kinase (PAK1) was introduced (Zegers et al., 2003b). Consistent with these data, the expression of a constitutively active PAK1 in MDCK cells misorients the apical surface and induces a multi-lumen phenotype, identical to the effect of β1 integrin inhibition (deLeon et al., 2012). In fact, Yu et al. (Yu et al., 2005) showed that β1 integrin orients epithelial polarity in the 3D MDCK model through Rac1 and laminin signaling, an effect that is likely mediated by PAK1. In addition, APC loss has been shown to regulate directionality of cell extrusion (Marshall et al., 2011) or apical constriction through RhoI and Myosin II in Drosophila (Zimmerman et al., 2010). We have previously shown that Apc mutation in PyMT-driven mammary tumor cells results in hyperactivation of focal adhesion kinase signaling (Prosperi, J. R. et al., 2011). Consistently, enhanced FAK phosphorylation and activation is also observed in intestinal tumor models from _Apc_-knockout mice, and FAK activity is required for tumorigenesis in these animals (Ashton et al., 2010). It is possible that APC regulates the signaling pathways downstream of cell-ECM interactions and its loss uncouples integrin/ECM-mediated polarization and morphogenesis signaling pathways. Further support for this hypothesis is provided by the gene expression changes observed in APC-knockdown cells grown in 3D culture conditions. EMP2 is an intriguing candidate given its role in mediating β1 integrin signaling (Morales et al., 2009b; Fu et al., 2011), previously described as a critical component of MDCK cell polarity (Ojakian and Schwimmer, 1994; Yu et al., 2005), and the APC/FAK crosstalk identified in _Apc_-mutant tumors by our laboratory (Prosperi, J. R. et al., 2011) and others (Ashton et al., 2010). Furthermore, EMP2 and MUC16 are both early markers of tumor development (Fu et al., 2011; Haridas et al., 2011), consistent with the model of APC loss resulting in dysregulation of epithelial polarity preceding tumorigenesis. The specific molecular mechanism by which APC controls these pathways is the focus of current study but may involve interactions of APC with actin itself or actin remodeling proteins such as the Rho GTPase effector IQGAP (Watanabe et al., 2004) or the Rac and Cdc42 guanine-exchange factors Asef1 and 2 (Kawasaki et al., 2000; Kawasaki et al., 2003; Kawasaki et al., 2007).
Another attractive possibility is that APC elicits many of its control of epithelial polarization and morphogenesis through its direct and indirect interactions with the plus-ends of MTs, particularly during mitosis. APC localizes to the kinetochores and centrosomes in mitotic cells, and its mutation is associated with defects in chromosome segregation/cytokinesis, and genomic instability (Fodde et al., 2001; Kaplan et al., 2001). Mitotic spindle orientation is disrupted in intestinal crypts heterozygous for an Apc mutation and even more dramatically in tumors from _Apc_-mutant mice that demonstrate loss of heterozygosity (LOH) (Fleming et al., 2009). Recent work has shown that APC is necessary for proper spindle orientation perpendicular to the apical surface in the stem cell compartment of human and mouse gastrointestinal epithelium, and that this alignment is required for asymmetric stem cell division (Quyn et al., 2010). Studies by den Elzen et al. (den Elzen et al., 2009) demonstrated that adherens junctions provide a necessary cue for proper planar spindle orientation in MDCK monolayers, and that E-cadherin disruption mislocalizes APC. Furthermore, APC is required for spindle alignment during symmetric cell division in this model (den Elzen et al., 2009). The APC partner IQGAP also has been implicated in cytokinesis (Shannon, 2012). Additionally, the kinesin KIF17 helps to localize APC to the plus ends of a subset of MTs, and KIF17 depletion results in aberrant 3D epithelial cysts that lack both a central lumen and polarized apical markers (Jaulin and Kreitzer, 2010). A recently characterized integrin-linked kinase (ILK)-MT pathway to regulate the delivery of apical cargo to the correct membrane domain (Akhtar and Streuli, 2013) raises the attractive possibility that APC provides a link between integrin signaling and MTs in epithelia.
3.3 Region of APC required for morphogenesis and polarity
Our observation that the c-terminus of APC is sufficient to rescue the morphogenesis defects in APC-knockdown epithelial cells was surprising given how large the full-length protein is and suggests that the interaction of APC with actin or tubulin through the basic region, or EB1 via its adjacent binding region, might mediate these effects. However, it is possible that the c-terminal PDZ-binding domain is also critical through its association with Scrib and Dlg tumor suppressors, components of the Lgl/Scrib/Dlg basolateral polarity and domain identity complex. In fact, depletion of Scrib in MCF-10A breast epithelial cells causes 3D dysmorphogenesis with luminal filling and decreased apoptosis (Zhan et al., 2008). Dlg is required for APC-mediated front-rear polarity in astrocytes (Etienne-Manneville et al., 2005) and apical-basal polarity in mouse embryonic development and Drosophila (Ma et al., 2005; Van Campenhout et al., 2011; Roberts et al., 2012). In addition, loss of hDlg has been shown to result in resistance to anoikis (Massimi et al., 2012), another mechanism by which loss of APC may regulate the morphological changes observed herein. Additional structure-function and mutagenesis studies are required to further map the domains of APC necessary to control epithelial polarity and morphogenesis.
3.4 Conclusion
Because APC is commonly mutated or down-regulated in epithelial cancers, it is important to consider how loss of APC-mediated control of morphogenesis and polarization may contribute to tumor initiation. Our previous (Prosperi et al., 2009) and current work demonstrate that heterozygous APC-mutant tissues recapitulate many of the architectural abnormalities modeled by APC-knockdown in epithelial cell lines. These data are consistent with other studies illustrating that _Apc_-knockout colonic mucosa and tumors have defective barrier function (Jamora et al., 2003; Segditsas and Tomlinson, 2006) and that intestinal epithelial cells from histologically normal heterozygous Apc transgenic and mutant tissues had altered migration and patterns of gene expression along the crypt-villus axis (Wong et al., 1996; Homma et al., 2002). While kidney tumors have not been identified in the ApcMin/+ mice or FAP patients, there is a connection between Gardner’s syndrome (the non-intestinal tumors of FAP patients) and cilia (Gomez Garcia and Knoers, 2009). Further, homozygous deletion of Apc in the kidney predisposes to tumorigenesis (Sansom et al., 2005), suggesting that APC plays a critical tumor suppressive role in the kidney. These findings collectively support a model in which mutation of one APC allele, or decreased APC expression by gene methylation, is sufficient to perturb epithelial polarization and architecture so as to uncouple growth and survival cues and promote genomic instability to promote carcinogenesis.
4. Materials and Methods
4.1 Cell culture
HC11 mammary epithelial cells were obtained from J. Rosen (Baylor University, Houston, TX, USA) and cultured in RPMI (Mediatech, Manassas, VA, USA) supplemented with 10% FBS (Hyclone, Fisher Scientific, Pittsburgh, PA, USA), 10 ng/ml EGF (Fisher Scientific) and 5 µg/ml insulin (Sigma, St. Louis, MO, USA). Madin Darby Canine Kidney (MDCK) cells were obtained from K. Matlin (University of Chicago, Chicago, IL, USA) and were cultured in DMEM (Mediatech) with 5% FBS, 2 mM L-glutamine (Mediatech), and 10 mM HEPES (Fisher Scientific). MDCK cells that inducibly express stabilized β-catenin were generated by stable transfection of T23 cells (from M. Zegers at the Radboud University Nijmegen Medical Centre, The Netherlands; (Barth et al., 1997)) with pTRE2-S37Aβ-catenin and maintained in growth medium supplemented with 5 µg/ml puromycin (Sigma), 6 µg/ml blasticidin (Life Technologies, Grand Island, NY, USA), and 20 ng/ml doxycycline (DOX, Sigma). To generate that construct, S37A β-catenin (from S. Beyers at Georgetown University, Washington, DC, USA) was excised with BamHI (New England Biolabs, Ipswich, MA, USA) and cloned into the BamHI site of pTRE2 (Clontech, Mountain View, CA, USA). Expression was verified by GFP fluorescence and Western blotting with a HA antibody (Santa Cruz, Dallas, TX, USA). For monolayers, 5 × 105 cells were plated on 6-well 0.4 µm Transwells (Fisher Scientific) and were fixed for immunofluorescence at day 5. For 3D culture, 1.25 × 104 cells were plated on top of 50 µl growth factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA) in an 8-chamber slide in 2% Matrigel assay medium as described (Debnath et al., 2003). Acini were photographed or fixed for IF at the indicated times.
4.2 APC knockdown
Viral supernatant from four unique APC-specific shRNAs in the pLKO.1 lentiviral vector (Sigma) was used to infect epithelial cells at an MOI of 5. Negative control (pLKO.1 empty vector or SHC-002 scrambled shRNA) lentiviral vectors (Sigma) were expressed in HEK293FT cells, and viral supernatant was used for infection into epithelial cells at an MOI of 5. Infected pools were selected in 1.5 µg/ml (HC11) or 4 µg/ml (MDCK) puromycin. Knockdown was verified by Western blotting of the protein lystates as described below. For rescue studies, APC-knockdown and control cells were stably transfected with either empty vector, full-length hAPC, c-terminal APC fragment, or a middle fragment of APC and selected in 2.0 mg/ml (HC11) or 0.6 mg/ml (MDCK) G418. The construct encoding the middle fragment of APC (bp 678–6513) was generated by EcoRI digestion of pBS(SK)APC (Groden et al., 1995) and subcloning into the EcoRI site of pEGFP-C3 (Clontech), and the c-terminal fragment (bp 6513–8500) was cloned by EcoRI/BamHI digestion of pEGFP APC (Heinen et al., 2002) and subcloning into EcoRI/BamHI-digested pEGFP-C3. Expression was verified by transient transfection of the constructs and GFP fluorescence.
4.3 Immunofluorescence
Cells on Transwells were fixed with 4% paraformaldehyde (PFA, Fisher), quenched with 50 mM NH4Cl, and permeabilized with 0.1% Triton X-100 prior to blocking in 0.2% fish skin gelatin. Staining was performed with the following primary antibodies diluted in 0.1% goat serum (except for Scrib, which was diluted in 0.1% donkey serum): anti-APC rabbit polyclonal (1:200; a gift from Inke Näthke, University of Dundee, Dundee, Scotland (Nathke et al., 1996)); anti-β-catenin monoclonal (1:200; BD Biosciences); anti-E-cadherin monoclonal (1:200; BD Biosciences); anti-MUC1 monoclonal (1:100; Abcam, Cambridge, MA, USA), anti-Dlg monoclonal (1:100; BD Biosciences), anti-Scribble goat polyclonal (1:100; Santa Cruz). Expression was detected using Alexa-conjugated secondary antibodies (1:1,000 each; Life Technologies). For visualization of F-actin, cells were co-stained with Alexa-conjugated phalloidin (1:200; Life Technologies). All slides were mounted with Fluoromount G with Hoechst dye. Cells in 2D were fixed with 3.7% formaldehyde, permeabilized with 0.3% Triton X-100, and incubated with the primary antibody anti-green fluorescence protein (GFP; 1:400; Life Technologies) for 1 hour. Expression was detected using Alexa-fluor conjugated secondary antibodies (1:1000; Life Technologies) and cells were counterstained with Hoescht dye and mounted with Fluoromount G. For 3D cultures, cells were fixed in 4% PFA at the indicated time points and permeabilized with 0.5% Triton X-100. Blocking was performed for 1 hr with 0.1% goat serum, followed by overnight staining with the following primary antibodies diluted in blocking buffer: anti-β-catenin (1:200; BD Biosciences); anti-E-cadherin (1:200; BD Biosciences); anti-gp135 culture supernatant (1:2; provided by K. Matlin at the University of Chicago and originally from G. Ojakian, SUNY Downstate, Brooklyn, NY, USA; (Ojakian and Schwimmer, 1988)). Expression was detected using Alexa-fluor conjugated secondary antibodies (1:1,000; Life Technologies). To demarcate the actin cytoskeleton, cysts were stained with Alexa-conjugated phalloidin (1:200; Life Technologies) and counterstained with Hoescht. For tissues, slides were deparaffinized, hydrated, and blocked for 1 hr with 2% BSA and 0.2% NFDM. Tissues were stained for 1 hr with the following primary antibodies diluted in blocking buffer: anti-β-catenin (1:100; BD Biosciences); anti-E-cadherin (1:100; BD Biosciences); and anti-MUC1 (1:100; Abcam). Expression was detected using Alexa-conugated secondary antibodies (1:1,000 in blocking buffer; Life Technologies). All slides were mounted with Fluoromount G with Hoescht dye.
4.4 Western blotting
Total cell lysates were extracted with 50 mM Tris pH7.5, 0.1% IGEPAL, 100 mM NaCl, 1 mM MgCl2, 5 mM EDTA and protease inhibitors and sonicated. 50 µg of lysates from each cell line was separated on a 7% SDS-PAGE gel and transferred to Immobilon P membrane. Blots were probed with 1:3000 anti-APC polyclonal antibody (Wang et al., 2009) obtained from K. Neufeld (University of Kansas, Lawrence, KS, USA) and 1:1000 anti-actin monoclonal antibody (Sigma) in blocking buffer (5% nonfat dried milk in TBST). HCT116 and SW480 lysates were used as a control for full-length and mutant APC, respectively. Densitometry was performed using Image J software (NIH).
4.5 TCF reporter assay
Cells were plated in a 24-well plate at a density of 1×104 cells/well and transfected using Optifect (Life Technologies) with 2.6 µg of pTOPflash or pFOPflash (obtained from H. Clevers at the Hubrecht Institute, The Netherlands) along with pRL-TK (Promega) for determination of transfection efficiency. Lysates were harvested after 48 hrs and analyzed using the Dual Luciferase Assay System kit (Promega, Madison, WI, USA). Luciferase activity was normalized for transfection efficiency and graphed as ratio of TOPflash/FOPflash activity. The SW480 human colon cancer cell line was used as a positive control. Cells were treated with 30mM LiCl as a positive control for Wnt activation in MDCK cells.
4.6 Morphological analyses
Acini/cysts were visualized under phase-contrast microscopy using an inverted Zeiss Axioscope microscope and images captured with Axiovision software. At 8 d post-plating, the morphology was categorized as spherical or non-spherical. For quantification, 10 fields with at least 50 structures were counted per cell line, and the percentage of each category were plotted. Statistical analyses were performed using Fisher’s exact test. ImageJ software (NIH) was used for analysis of cyst size, and a one-way ANOVA determined significance.
4.7 Microarray and gene expression analysis of 3D cultures
RNA was isolated from 3D cultures of MDCK cells (at day 5) and subjected to microarray analysis using the Functional Genomics Core facility at the University of Chicago and three biological replicates. RNA quality was confirmed using the Agilent Bioanalyzer 2100 prior to hybridization to Affymetrix Canine Genome 2.0 arrays. Data analysis was performed using dChip software (Harvard), and the threshold for selecting significant genes was set for a relative difference of >1.2-fold with p<0.05. For validation of altered gene expression, cDNA was synthesized using iScript Reverse Transcriptase (Bio-Rad, Hercules, CA, USA), and real-time PCR was performed with Advanced Sybr Green (Life Technologies). Primer sequences used are listed in Table 1. Gene expression changes were quantified using the ΔΔC(t) method.
Table 1.
Primers used for gene expression analysis of APC-knockdown MDCK cells.
| Gene | Forward Primer (5’-3’) | Reverse Primer( 5’-3’) |
|---|---|---|
| CXCR7 | TTG CTA CCT GCA TGG GATATG | AGA GGT TCC GCT TCG TTT C |
| ADAMTS6 | CAC TGG TAG TGG CAG ACAAA | CTA GGC TGG AAT CAC GGTAAA G |
| LCN2 | GAG CCA TGA GAC CCT TCTTAC | CCA GGT GGC ATG TGT TTATTT AG |
| COL4A1 | CAG AGA TGG TCT GGA AGGATT G | GTC GGG CGT CGA AGT AAA T |
| MUC16 | CTT CTG GGC CAT CAT CCTTAT C | CCT CGT ATT CTC CCT CCTTCT |
| EMP2 | TGG TGG GTA GGA GAA GAGTT | GCC TGC ATC GTG GAG TAA T |
Supplementary Material
1
2
3
4
Highlights.
- Adenomatous Polyposis Coli (APC) knockdown alters 3D morphogenesis.
- APC knockdown results in mis-localization of the apical marker gp135.
- These effects are mediated independent of the Wnt/β-catenin pathway.
- Epithelial membrane protein 2 (EMP2) is up-regulated with APC loss.
Acknowledgements
The authors thank Jennifer Cole, Colles Price, Erin Smithberger, Michael O’Brien, and Andrew Kam for their technical assistance. We also thank Dr. Karl Matlin (University of Chicago) for his critical insight on the project. These studies were funded by an American Cancer Society New England Division-Spin Odyssey Postdoctoral Fellowship (PF-10-225-01-TBG) to JRP, and grants from the University of Chicago Digestive Disease Research Core Center (NIDDK P30DK42086) and American Cancer Society Lakeshore Division (215889) to KHG.
Abbreviations
ADAMTS6
ADAM metallopeptidase with thrombospondin type 1, motif 6
APC
Adenomatous Polyposis Coli
COL4A1
Alpha subunit of type IV collagen
CXCR7
C-X-C chemokine receptor type 7
DEAR1
Ductal epithelium-associated rind chromosome 1
DOX
Doxycycline
ECM
Extracellular Matrix
EMP2
Epithelial membrane protein 2
FAK
Focal Adhesion Kinase
FAP
Familial Adenomatous Polyposis
GEF
Guanine-Exchange Factor
ILK
Integrin-linked kinase
LCN2
Lipocalin 2
LOH
Loss of heterozygosity
MDCK
Madin Darby Canine Kidney
MT
Microtubule
PAK
P21 activated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
JRP, KHG, and ACL conceived the experiments and wrote the manuscript. JRP, FFY, ACL, and AS performed experiments. JRP, ACL, IH, KO, and KHG analyzed data.
References
- Aikio M, Alahuhta I, Nurmenniemi S, Suojanen J, Palovuori R, Teppo S, Sorsa T, Lopez-Otin C, Pihlajaniemi T, Salo T, et al. Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma. PLoS One. 2012;7:e51044. doi: 10.1371/journal.pone.0051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askham JM, Moncur P, Markham AF, Morrison EE. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- Barth AI, Siemers KA, Nelson WJ. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci. 2002;115:1583–1590. doi: 10.1242/jcs.115.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Bevitt DJ, Li Z, Lindrop JL, Barker MD, Clarke MP, McKie N. Analysis of full length ADAMTS6 transcript reveals alternative splicing and a role for the 5' untranslated region in translational control. Gene. 2005;359:99–110. doi: 10.1016/j.gene.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- Chen N, Balasenthil S, Reuther J, Killary AM. DEAR1, a Novel Tumor Suppressor That Regulates Cell Polarity and Epithelial Plasticity. Cancer Res. 2014;74:5683–5689. doi: 10.1158/0008-5472.CAN-14-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR. Studying the consequences of immediate loss of gene function in the intestine: APC. Biochem Soc Trans. 2005;33:665–666. doi: 10.1042/BST0330665. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- deLeon O, Puglise JM, Liu F, Smits J, ter Beest MB, Zegers MM. Pak1 regulates the orientation of apical polarization and lumen formation by distinct pathways. PLoS One. 2012;7:e41039. doi: 10.1371/journal.pone.0041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell. 2009;20:3740–3750. doi: 10.1091/mbc.E09-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg JA, Datta A, Mostov KE, Hunt CA. MDCK cystogenesis driven by cell stabilization within computational analogues. PLoS Comput Biol. 2011;7:e1002030. doi: 10.1371/journal.pcbi.1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming ES, Temchin M, Wu Q, Maggio-Price L, Tirnauer JS. Spindle misorientation in tumors from APC(min/+) mice. Mol Carcinog. 2009;48:592–598. doi: 10.1002/mc.20506. [DOI] [PubMed] [Google Scholar]
- Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Fu M, Rao R, Sudhakar D, Hogue CP, Rutta Z, Morales S, Gordon LK, Braun J, Goodglick L, Wadehra M. Epithelial membrane protein-2 promotes endometrial tumor formation through activation of FAK and Src. PLoS One. 2011;6:e19945. doi: 10.1371/journal.pone.0019945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gomez Garcia EB, Knoers NV. Gardner's syndrome (familial adenomatous polyposis): a cilia-related disorder. Lancet Oncol. 2009;10:727–735. doi: 10.1016/S1470-2045(09)70167-6. [DOI] [PubMed] [Google Scholar]
- Groden J, Joslyn G, Samowitz W, Jones D, Bhattacharyya N, Spirio L, Thliveris A, Robertson M, Egan S, Meuth M, et al. Response of colon cancer cell lines to the introduction of APC, a colon-specific tumor suppressor gene. Cancer Research. 1995;55:1531–1539. [PubMed] [Google Scholar]
- Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG. Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem. 2005;280:7875–7882. doi: 10.1074/jbc.M413192200. [DOI] [PubMed] [Google Scholar]
- Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen CD, Goss KH, Cornelius JR, Babcock GF, Knudsen ES, Kowalik T, Groden J. The APC tumor suppressor controls entry into S-phase through its ability to regulate the cyclin D/RB pathway. Gastroenterology. 2002;123:751–763. doi: 10.1053/gast.2002.35382. [DOI] [PubMed] [Google Scholar]
- Homma MK, Li D, Krebs EG, Yuasa Y, Homma Y. Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein. Proc Natl Acad Sci U S A. 2002;99:5959–5964. doi: 10.1073/pnas.092143199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou KL, Hao MG, Bo JJ, Wang JH. CXCR7 in tumorigenesis and progression. Chin J Cancer. 2010;29:456–459. doi: 10.5732/cjc.009.10404. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 2010;190:443–460. doi: 10.1083/jcb.201006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol. 2003;5:211–215. doi: 10.1038/ncb937. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Sagara M, Shibata Y, Shirouzu M, Yokoyama S, Akiyama T. Identification and characterization of Asef2, a guanine-nucleotide exchange factor specific for Rac1 and Cdc42. Oncogene. 2007;26:7620–7267. doi: 10.1038/sj.onc.1210574. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–1197. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K, Wittmann T, Nathke IS, Waterman-Storer CM. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17:2331–2345. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn K. Basement membrane (type IV) collagen. Matrix Biol. 1995;14:439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell. 2011;22:3962–3970. doi: 10.1091/mbc.E11-05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi P, Zori P, Roberts S, Banks L. Differential regulation of cell-cell contact, invasion and anoikis by hScrib and hDlg in keratinocytes. PLoS One. 2012;7:e40279. doi: 10.1371/journal.pone.0040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, Kawahara T, Kobayashi S, Okada M, Toyoshima K, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein [see comments] Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- Mogensen MM, Tucker JB, Mackie JB, Prescott AR, Nathke IS. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J Cell Biol. 2002;157:1041–1048. doi: 10.1083/jcb.200203001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales SA, Mareninov S, Coulam P, Wadehra M, Goodglick L, Braun J, Gordon LK. Functional consequences of interactions between FAK and epithelial membrane protein 2 (EMP2) Invest Ophthalmol Vis Sci. 2009a;50:4949–4956. doi: 10.1167/iovs.08-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales SA, Mareninov S, Wadehra M, Zhang L, Goodglick L, Braun J, Gordon LK. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009b;50:462–469. doi: 10.1167/iovs.07-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Bartolini F, Okada K, Wen Y, Gundersen GG, Goode BL. Regulated binding of adenomatous polyposis coli protein to actin. J Biol Chem. 2007;282:12661–12668. doi: 10.1074/jbc.M610615200. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Research. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. Journal of Cell Biology. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Odenwald M, Prosperi JR, Goss KH. APC/β-catenin-rich complexes at membrane protrusions are required for mammary tumor cell migration and invasion. BMC Cancer. 2013:13. doi: 10.1186/1471-2407-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988;107:2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107(Pt 3):561–576. [PubMed] [Google Scholar]
- Okada K, Bartolini F, Deaconescu AM, Moseley JB, Dogic Z, Grigorieff N, Gundersen GG, Goode BL. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J Cell Biol. 2010;189:1087–1096. doi: 10.1083/jcb.201001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperi JR, Goss KH. Nguyen Susan D., editor. Wnt Pathway-Independent Activities of the APC Tumor Suppressor. Tumor Suppressors. 2011 [Google Scholar]
- Prosperi JR, Lue HH, Goss KH. Goss Kahn., editor. Dysregulation of the WNT Pathway in Solid Tumors. Targeting the Wnt Pathway in Cancer. 2011:81–128. [Google Scholar]
- Prosperi JR, Khramtsov AI, Khramtsova GF, Goss KH. Apc mutation enhances PyMT-induced mammary tumorigenesis. PLoS One. 2011;6:e29339. doi: 10.1371/journal.pone.0029339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperi JR, Becher KR, Willson TA, Collins MH, Witte DP, Goss KH. The APC tumor suppressor is required for epithelial integrity in the mouse mammary gland. J Cell Physiol. 2009;220:319–331. doi: 10.1002/jcp.21766. [DOI] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Nathke IS. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Radulescu S, Ridgway RA, Appleton P, Kroboth K, Patel S, Woodgett J, Taylor S, Nathke IS, Sansom OJ. Defining the role of APC in the mitotic spindle checkpoint in vivo: APC-deficient cells are resistant to Taxol. Oncogene. 2010;29:6418–6427. doi: 10.1038/onc.2010.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65:166–176. [PubMed] [Google Scholar]
- Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol. 2005;7:463–473. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinacher-Schick A, Gumbiner BM. Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the beta-catenin destruction complex in polarized epithelial cells. J Cell Biol. 2001;152:491–502. doi: 10.1083/jcb.152.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Delury C, Marsh E. The PDZ protein discs-large (DLG): the 'Jekyll and Hyde' of the epithelial polarity proteins. FEBS J. 2012;279:3549–3558. doi: 10.1111/j.1742-4658.2012.08729.x. [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Ihrke G, Bienz M. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. Embo J. 2001;20:5929–5939. doi: 10.1093/emboj/20.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Griffiths DF, Reed KR, Winton DJ, Clarke AR. Apc deficiency predisposes to renal carcinoma in the mouse. Oncogene. 2005;24:8205–8210. doi: 10.1038/sj.onc.1208956. [DOI] [PubMed] [Google Scholar]
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- Shannon KB. IQGAP Family Members in Yeast, Dictyostelium, and Mammalian Cells. Int J Cell Biol. 2012;2012:894817. doi: 10.1155/2012/894817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Research. 1994;54:3672–3675. [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, Takeuchi T, Kanda T, Huibregtse JM, Akiyama T, et al. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells. 2006;11:453–464. doi: 10.1111/j.1365-2443.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- Van Campenhout CA, Eitelhuber A, Gloeckner CJ, Giallonardo P, Gegg M, Oller H, Grant SG, Krappmann D, Ueffing M, Lickert H. Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4-2 e3 ubiquitin ligases. Dev Cell. 2011;21:479–491. doi: 10.1016/j.devcel.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Azuma Y, Friedman DB, Coffey RJ, Neufeld KL. Novel association of APC with intermediate filaments identified using a new versatile APC antibody. BMC Cell Biol. 2009;10:75. doi: 10.1186/1471-2121-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Wong MH, Hermiston ML, Syder AJ, Gordon JI. Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:9588–9593. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W, Schwertfeger KL, Vargo-Gogola T, Rosen JM. Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J Cell Biol. 2005;171:663–673. doi: 10.1083/jcb.200505098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing PX, Lees C, Lodding J, Prenzoska J, Poulos G, Sandrin M, Gendler S, McKenzie IF. Mouse mucin 1 (MUC1) defined by monoclonal antibodies. Int J Cancer. 1998;76:875–883. doi: 10.1002/(sici)1097-0215(19980610)76:6<875::aid-ijc18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, Anton ES. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923–929. doi: 10.1038/embor.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003a;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Zegers MM, Forget MA, Chernoff J, Mostov KE, ter Beest MB, Hansen SH. Pak1 and PIX regulate contact inhibition during epithelial wound healing. EMBO J. 2003b;22:4155–4165. doi: 10.1093/emboj/cdg398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SG, Thorpe LM, Medrano VR, Mallozzi CA, McCartney BM. Apical constriction and invagination downstream of the canonical Wnt signaling pathway require Rho1 and Myosin II. Dev Biol. 2010;340:54–66. doi: 10.1016/j.ydbio.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4