THE VITAMIN D HORMONE: A MULTITUDE OF ACTIONS POTENTIALLY INFLUENCING THE PHYSICAL FUNCTION DECLINE IN OLDER PERSONS (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 3.
Abstract
Vitamin D, a secosteroid (pro)-hormone, has been traditionally considered as a key regulator of bone metabolism, and calcium and phosphorous homeostasis through a negative feedback with the parathyroid hormone. However, during the last twenty years, the role played by vitamin D has been largely revised by recognizing it a pleiotropic action on a wide spectrum of systems, apparati, and tissues. Thus, vitamin D has growingly been involved as a primary determinant of biological modifications and specific clinical conditions. The effect of vitamin D on skeletal muscle and related outcomes (including physical function decline and disability) is surely one of the most relevant to study in the context of global aging. In the present review, the subclinical and clinical consequences of vitamin D deficiency/insufficiency, extremely frequent conditions in older age, are described. Special focus is given to skeletal muscle and physical function. Limitations of available scientific evidence on the topic are also discussed.
Keywords: Vitamin D, physical function, skeletal muscle, sarcopenia, aging
INTRODUCTION
Vitamin D has been traditionally considered as a key regulator of bone metabolism, and calcium and phosphorous homeostasis through a negative feedback with the parathyroid hormone(1, 2). It is also well-established that vitamin D deficiency causes rickets in children and osteomalacia and osteoporosis in adults.
The history of vitamin D has began since ancient times. Bone deformities among infants due to malnourishment were firstly described by Soranus of Ephesus and Galen of Pergamum, two physicians practicing medicine at Ancient Rome in the 2nd century. However, the first clear descriptions of rickets were provided by Daniel Whistler in 1645, and by Francis Glisson in 1650 in England, where this condition was endemic at the time; it was even called morbus anglicus, or "the English disease". At the beginning of the 20th century, with the development of experimental research and the discovery of vitamins by Sir Patrick Gowland Hopkins and Christiaan Eijkman (who shared the 1929 Nobel prize in Physiology for this), the study of rickets and vitamin D received a considerable boost, till the introduction in 1924 of irradiated milk and bread in United States which nearly eradicated rickets(3, 4).
Today, about one billion persons, mostly elders, worldwide present vitamin D deficiency(1). The prevalence of low vitamin D concentrations in subjects older than 65 years of age has been estimated around 50%(5–8), but this figure is highly variable because influenced by sociodemographic, clinical, therapeutical, and environmental factors.
A large and growing body of evidence suggests that vitamin D is not only critical for bone tissue and calcium metabolism, but may also represent a crucial determinant for the development of major (sub)clinical conditions and health-related events(1, 9, 10). In particular, the hypothesis that vitamin D may represent a relevant factor influencing the disabling process has been proposed by several studies(11, 12). In this review, we discuss current knowledge about the multidimensional actions of vitamin D and its supplementation in the organism, having a special focus on its effects on skeletal muscle and physical function.
Physiology and metabolism
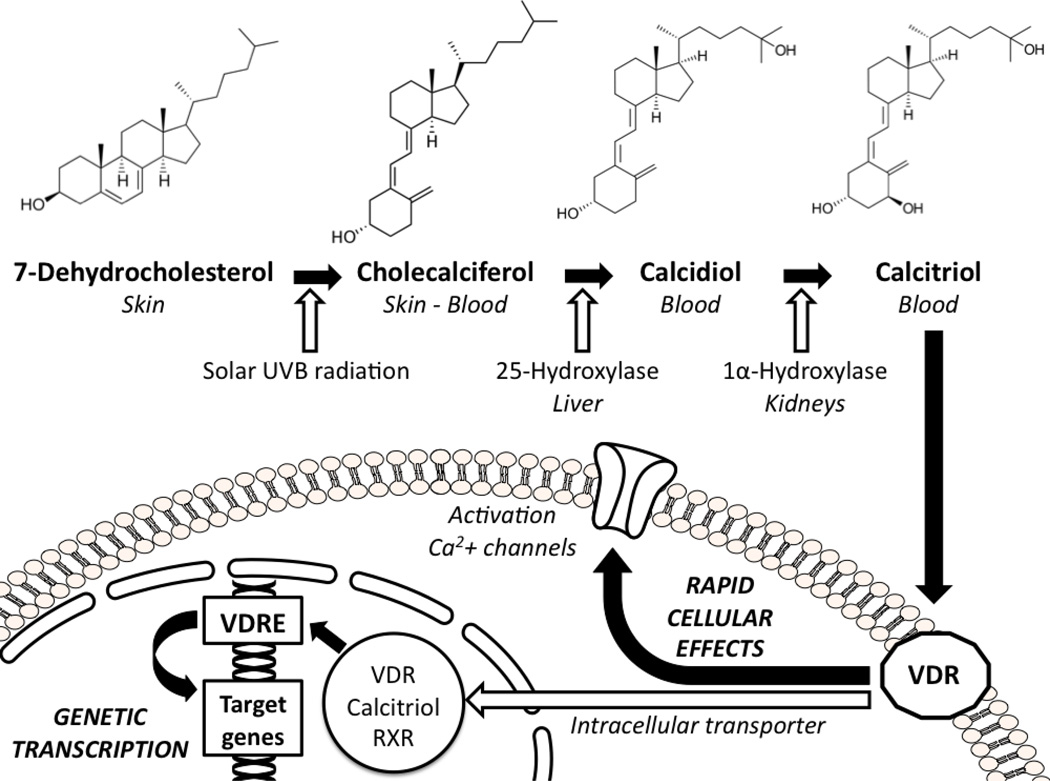
The primary source of vitamin D (up to 95%) is constituted by ultraviolet B radiation, those with wavelength ranging between 290 and 315 nm, from sunlight. In fact, it has been estimated that the exposure of arms and legs to sunlight for 5–10 minutes at mid-day during the first summer months may provide about 3,000 IU of vitamin D2 to a subject with light pigmentation of the skin(1). Sunlight radiations penetrate into the skin and convert the precursor 7-dehydrocholesterol into cholecalciferol or vitamin D3. There is also another common form of vitamin D that is vitamin D2 or ergocalciferol, which has the same metabolic meaning of the former, but it has vegetal origins. Vitamin D is then firstly metabolized in the liver into 25-hydroxy-vitamin D or calcidiol, then in the kidneys by the 25-hydroxy-vitamin D-1α-hydroxylase, a mitochondrial enzyme closely regulated by the parathyroid hormone(13). The result of this metabolic pathway is the production of the active form 1,25-dihydroxy-vitamin D or calcitriol (Figure 1). Calcitriol is about 500–1,000-fold more active than its precursor 25-hydroxy-vitamin D, but this latter is usually measured to estimate the systemic vitamin D status for several reasons. First of all, circulating concentrations of calcitriol are extremely low, about one thousand-fold less than calcidiol. Moreover, 25-hydroxy-vitamin D is stabler and characterized by a longer half-life (about 2–3 weeks) compared to the 1,25-dihydroxy-vitamin D metabolite (about 4–6 hours). When circulating vitamin D concentrations are low, intestinal calcium and phosphorus absorption decreases and parathyroid hormone levels increase. This latter, besides of promoting calcium resorption in the kidneys, also stimulates the immediate production of 1,25-dihydroxy-vitamin D. Thus, with the onset of vitamin D insufficiency, the consequent increase of the parathyroid hormone artificially inflates 1,25-dihydroxy-vitamin D concentrations, potentially providing misleading results about the real vitamin D status(14).
Figure 1.
The vitamin D hormone modifications and its cellular effects.
RXR: retinoic receptor; UVB: ultraviolet B; VDR: vitamin D receptor; VDRE: vitamin D receptor elements.
As mentioned above, the amount of vitamin D mainly derives from sunlight exposure. In fact, the vitamin D dietary intake is usually inadequate and well below the daily requirements of the organism (Table 1). Among foods, only some types of fish are able to provide a reasonable amount of vitamin D3 (about 100–1,000 IU for 100 grams)(1, 15–17). If not artificially fortified, other foods/beverages (including milk, cheeses, and fruit juices) provide very low, if any, amount of vitamin D(1, 15–17). Consequently, the recommended daily dietary intake of vitamin D3 (i.e., a minimum of 800 IU(1)), it is very rarely reached by common diets. Cholecalciferol is not stored in the muscle and fat tissues until its serum concentrations are above 20–50 ng/mL, and only above this threshold the organism is able to use its vitamin D reserves to work independently of dietary intakes or sunlight exposure. Therefore, since this 25-hydroxy-vitamin D concentrations are difficult to be reached especially in older persons, the organism usually works in a reserve status(18, 19). From all this, it becomes clear why the maintenance of adequate vitamin D concentrations necessarily goes through a reasonable exposure to sunlight and vitamin D supplementation(1, 10, 20).
TABLE 1.
Examples of food sources of vitamin D(15). One microgram of dietary vitamin D is equivalent to 40 IU.
| Amount of vitamin D (IU) | |
|---|---|
| Sunlight exposure of arms and legs for 5–10 minutes at mid-day during the first summer months | 3,000 |
| Cod liver oil (one tablespoon, 13.6 g) | 1,360 |
| Wild salmon, cooked dry heat (100 g) | 451 |
| Mackerel, canned (100 g) | 292 |
| Tuna, light, canned in oil (100 g) | 269 |
| Sardines, canned in oil (100 g) | 193 |
| Whole milk with added vitamin D (one cup, 244 g) | 124 |
| Cod, cooked, dry heat (100 g) | 46 |
| Cereals, corn flakes, low sodium (one cup, 25 g) | 36 |
| Egg (medium size, whole, raw, 44 g) | 22 |
| Mozzarella cheese, whole milk (100 g) | 16 |
| Chicken tenders, cooked in conventional oven (100 g) | 10 |
| Beef steak, top sirloin, cooked, broiled (100 g) | 7 |
| Whole milk without added vitamin D (one cup, 244 g) | 5 |
| Cabbage, boiled (100 g) | 0 |
| Italian bread (100 g) | 0 |
| Lettuce, green leaf (100 g) | 0 |
| Orange (one medium fruit, 131 g) | 0 |
| Olive oil (one tablespoon, 13.5 g) | 0 |
| Potato, boiled without skin (medium size, 167 g) | 0 |
| White rice, long-grain, parboiled, (un)enriched, cooked (100 g) | 0 |
During the last twenty years, the role played by vitamin D has been largely revised by recognizing it a pleiotropic action on wide spectrum of systems, apparati, and tissues. Moreover, 1,25-dihydroxy-vitamin D is not only produced by kidneys with the endocrine function of regulating calcium and phosphorous homeostasis. It has been demonstrated that the active form of vitamin D is also produced by several other tissues with autocrine pattern and local effects(21). These findings obviously led at thinking of vitamin D as potentially involved in a number of extra-bone subclinical and clinical conditions, and as a possible determinant of major clinical outcomes. Thus, it is not surprising that vitamin D is today considered as a hormone rather than as a vitamin in the true meaning of the word(1, 22). Differently from most of the other vitamins, the active form of vitamin D (i.e., 1,25-dihydroxy-vitamin D) is not a cofactor of enzymatic reactions or an antioxidant, but a fat-soluble secosteroid hormone. The only "vitamin" property left for this micronutrient is maybe the capacity to determine the onset of clinical conditions for insufficient dietary intake and consequent deficiency(23).
Vitamin D receptor
The 1,25-dihydroxy-vitamin D exhibits a wide spectrum of actions through the interaction with a specific receptor (i.e., vitamin D receptor, VDR), member of a superfamily of nuclear receptors(24). Vitamin D easily passes through biological membranes. After being transferred into the nucleus by an intracellular transporter protein, the 1,25-dihydroxy-vitamin D:VDR complex combined with the retinoic receptor constitutes a heterodimer able to bind the vitamin D responsive elements (VDRE) located on specific promoter regions of the target genes, modulating their expressions(25). Recently, it has been hypothesized the existence of a second vitamin D mechanism of action. In fact, the slow genetic transcription pathway, which requires hours to days, cannot explain alone the evidence of rapid onset cellular responses induced by vitamin D. It is likely that an alternative receptor for vitamin D located on the cellular membrane(26, 27) is able to promote the activation of second messengers (such as cyclic AMP or mitogen-activated protein kinase [MAPK]) influencing calcium channels and determining immediate cellular effects (Figure 1)(21). This may simply be the VDR itself after migration from the nucleus to the cellular membrane(28).
The VDR gene is located on the chromosome 12 (12q13,11). It has been demonstrated the existence of several VDR gene polymorphisms able to modify its expression and determine different phenotypes and biological responses. Discussing about physical function, two VDR polymorphisms are of special interest because not only able to affect bone mineral density, but also body composition, muscle strength, and the response to physical exercise(29–31): the FokI involving a T/C substitution on the exon 2 of the VDR gene, and the BsmI due to the modification of the final part 3' of the VDR gene. The identification of these polymorphism and the demonstration of their effects on muscle and function comes to support the hypothesis of a direct role played by vitamin D in determining sarcopenia, age-related physical decline, and disabling process.
Risk factors for vitamin D deficiency
Numerous endogenous and exogenous risk factors have shown to affect the serum concentrations of vitamin D. The most relevant are:
- Gender - It has been reported that women are more likely to develop hypovitaminosis D compared to men(32);
- Sunlight exposure, latitude, and seasonal variations - Vitamin D concentrations are directly associated with the exposure to solar ultraviolet B photons. Therefore, vitamin D concentrations tend to be lower during the winter season(7, 33, 34) and for higher degrees of latitude (e.g. above 35° North latitude, little or no vitamin D can be produced from November to February(1, 9, 35));
- Dark skin pigmentation - African Americans present an increased risk of low vitamin D concentrations compared to Caucasians, independently of age(36);
- Diet(1);
- Obesity - The sequestration of vitamin D in body fat reduces its availability(1). Moreover, the existence of a relationship between vitamin D, inflammation, and adipose tissue has been hypothesized(37);
- Impaired renal function(38).
Interestingly, all these risk factors tend to become more and more frequent with increasing age, easily explaining why hypovitaminosis D is a typical condition in the elderly. Moreover, the aging process itself predisposes to vitamin D deficiency, especially because of the age-related skin structure modifications. In fact, a progressive decline in the cutaneous capacity to synthesize vitamin D from ultraviolet B radiations(35, 39) (at least partly due to the reduction of 7-dehydro-cholesterol in the skin) and an increased resistance of target organs to the vitamin D action (probably due to the reduction of VDRs(40) or post-receptorial modifications(41))(42) have been described.
The pleiotropic action of vitamin D
About 50 years ago, Elkeles(43) hypothesized a shift of the calcium from bones, representing the primary deposit of this element in the organism, to soft tissues occurring with aging. This hypothesis also called "theory of calcium mobilization" was proposed to explain some calcium-related conditions typical of older age, such as osteoporosis, atherosclerosis, and hypertension. Although this theory may seems today too simplistic, it still has some value if the multitude of actions played by calcium and, parallelly, by vitamin D in the organism are taken into account. The systemic hormonal role of vitamin D is today largely supported by the evidence of VDR and 25-hydroxy-vitamin D-1α-hydroxylase enzyme in numerous tissues and cells (e.g., bone, brain, prostatic, intestinal, muscular tissues, and immunitary cells)(1, 44). Moreover, it has recently been estimated that the 1,25-dihydroxy-vitamin D is able to modulate the expression of more than 200 genes involved in a wide spectrum of mechanisms, from cell proliferation to cellular differentiation, from apoptosis to angiogenesis(1, 45).
Since vitamin D is characterized by such a multidirectional action in the organism, its effects need to be systemic rather than local or tissue specific. This obvious conclusion is supported by reports showing that vitamin D is predictive of a wide spectrum of major clinical outcomes. For example, Autier et al.(46) recently reported a 7% decreased risk of mortality from vitamin D supplementation in a meta-analysis of 18 randomized clinicals trials. Vitamin D has also been indicated as a critical determinant of cardiovascular health status(47). This statement finds support by evidence from studies on knock-out animals without VDR, which are consequently not influenced by vitamin D, showing development of hypertension and cardiac hypertrophy(48). Moreover, heart failure is a well-recognized consequence of rickets(49), and several studies have shown that low serum concentrations of 25-hydroxy-vitamin D are associated with increased risk of cardiovascular death(50–52). Vitamin D has also been involved in the development and function of the central nervous system. Studies in animal models have demonstrated that vitamin D treatment in rats is able to increase neurons density in the hippocampus(53). Consistently, a low expression of VDR has been reported in hippocampal cells of Alzheimer's disease patients(54). Recently, Wilkins et al.(55, 56) have shown reduced cognitive function in older persons with low vitamin D concentrations. Moreover, a large (and growing) body of evidence on vitamin D is currently devoted to demonstrate a strong link with multiple sclerosis(57–59). Interestingly, the reduction of nervous conduction velocity due to low vitamin D concentrations and the restoration of the former by vitamin D supplementation have been reported(60, 61). The 1,25-dihydroxy-vitamin D is also a potent immunomodulator(62, 63), as demonstrated by its capacity to stimulate the production of catecalcidin, a peptide able to destroy several infective agents including Mycobacterium tuberculosis(64). It is interesting, especially for the potential future implications on humans, a recent study demonstrating vestibular dysfunction in mice without VDR(65). Finally, vitamin D has been associated with psychiatric conditions(66, 67), metabolic syndrome(68), and cancer (although, in this latter case, with some uncertainties)(69). If considered as a whole with the geriatrician's eyes, all this evidence proposes an exceptional number of possible explanations to the link between vitamin D and physical function. In fact, the pleiotropic action of vitamin D in our organism may critically determine the proper physical function which, ultimately, is the resulting of a multidimensional interaction among systems, apparati, and organs.
Skeletal muscle and physical function
The hypothesis that vitamin D is involved in the prevention of sarcopenia, physical decline, and disability is extremely interesting. The idea that vitamin D might be linked to muscular function was initially proposed several decades ago, in particular during the years between the two World Wars. Several clinical studies, especially conducted in Germany and Soviet Union, were aimed at demonstrating the effects of ultraviolet radiation on physical performance(70, 71). In 1927, a controversy arose in the sports world when the German Swimmers' Association decided to use sunlamps to boost its athletes' performances because considered as a sort of doping(71). Gorkin et al.(70) demonstrated relevant improvements (about 6%) in 100-meter dash speed in four students after ultraviolet radiation compared with matched controls. In 1940, Parade and Otto(71) discussed a series of previous experiments demonstrating that sunlamp irradiation was beneficial on muscle strength, and suggested a systemic effect of ultraviolet irradiation. During the following years, the relationship between muscle and vitamin D was more directly substantiated by case reports describing myopathies in patients with ostheomalacia(72, 73). If the myopathy was initially thought to be due to the osteomalacia condition and the poor health status, subsequent reports describing relevant beneficial effects (up to the complete regression of myopathy) after vitamin D supplementation reversed the scenario(74–76). Therefore, muscular symptoms were started to be considered not as an epiphenomenon of hypovitaminosis, but as a direct consequence of it. The identification of VDR in muscular tissue from biopsies performed in animal models(77), and, more recently, in humans(78, 79) has definitively confirmed the presence of a direct interaction between vitamin D and skeletal muscle.
It is likely that proper muscular functioning is determined by an adequate amount of available vitamin D, as suggested by evidence from animal and human models. An altered muscular development has been described in knock-out mice without VDR(80). Consistently, the histologic exam of muscle tissue from subjects with osteomalacia is characterized by increases interfibrillar spaces, intramuscular adipose tissue infiltrates, and fibrosis(81). Interestingly, muscle biopsies performed before and after vitamin D supplementation have documented an increased number and section area of type II (or fast) muscle fibers(82, 83). It is noteworthy this type of fibers is the one more involved in the fall prevention, thus providing a possible explanation to data showing higher tendency to fall in subjects with low vitamin D concentrations. In this context, it is useful to remember some clinical features of subjects with hypovitaminosis D: weakness and/or (especially proximal) muscle pain, unstable gait, difficulties in climbing up stairs or raising from a sit position on a chair, and generalized loss of muscular mass without relevant sensorial or osteo-tendineal abnormalities(84, 85). A large body of evidence currently demonstrates that low vitamin D concentrations represent an independent risk factor for falls in older persons(86–89). However, when studies have tested the effect of vitamin D supplementation on the fall event outcome, results became more contradictory. In fact, together with studies presenting positive findings(90–92), negative results were reported(93, 94), too. This discrepancy of data can also be confirmed by several recent meta-analyses on the topic(95–97).
Available evidence about the efficacy of vitamin D supplementation is also controversial when, looking at a different outcome, we consider physical function. In fact, although most of the epidemiological studies support such association(8, 55, 98–104) with only few and sometimes partial(105) exceptions(106), intervention studies are currently far from being definitive.
Current clinical and research issues
Surely, several methodological issues at the basis of available evidence are, at least partially, responsible for such impossibility in drawing definitive conclusions(11). First of all, the selection criteria adopted to recruit the study populations may have significantly affected the available evidence. For example, a recent work by Lips et al.(107) described a significant improvement of balance after vitamin D supplementation (8400 IU of vitamin D3 per week for 16 weeks) only in participants with higher medio-lateral postural sway at the baseline. Although this trial results are mainly negative for the vitamin D effects on physical performance, on the other hand suggest that supplementation may be important in patients with clinical signs/symptoms of hypovitaminosis D. Moreover, it is noteworthy that the "severe vitamin D deficiency" exclusion criterion adopted in this trial may have selected a relatively healthier group of participants, thus eliminating from the study sample those subjects more amenable to benefit from the intervention.
A critical issue to consider when evaluating the findings from clinical trials is represented by the adherence to the intervention. Effectively, some of the reported findings were obtained adopting an "intention-to-treat" approach, but several studies were characterized by low levels of adherence to the intervention(95, 96). Moreover, an inadequate length of the follow-up/intervention (sometimes no longer than six months) might have concurred in biasing the available evidence(108). In the attempt to facilitate the adherence to the treatment, some studies have recently tested the effects of annual high-dose (300,000–500,000 IU) vitamin D supplementations on falls(94) and fractures(94, 109), but results were not encouraging and even suggested an increased risk of fractures in the intervention arm of the trials. As discussed by Dawson-Hughes(110), these results do not cancel the large evidence supporting the beneficial effects from vitamin D supplementation, but underline the need of a more cautious approach especially when considering high-dose and/or long term interventions. It should always be taken into account that, although vitamin D supplementation is relatively safe, the risk of adverse drug reactions, in particular hypercalcemia and its consequences (e.g., nephrolithiasis, gastro-intestinal abnormalities, hypertension, arterial stiffness, cognitive impairment, electrocardiographic modifications)(111) is always present(112), especially in persons with impaired renal function and/or treated with thiazide diuretics.
Last but not least, the reading and comparison of vitamin D studies is hindered by the extreme heterogeneity of cut-points defining its status of deficiency. Over the years and still today, vitamin D deficiency and insufficiency have been defined in multiple ways. The first definitions of minimum serum 25-hydroxy-vitamin D concentrations were likely too low and underestimating the importance of this vitamin. Therefore, over time, these cut-points have progressively been raised. With the increase of the minimum concentrations defining the low vitamin D status, there has been a parallel increase of recommended dietary intakes and supplementation dosages over time. In fact, almost every year, normal ranges of vitamin D concentrations have been raised(113, 114). This has not facilitated the methodological homogeneity and, thus, comparability of available clinical trials testing vitamin D supplementation. While, as discussed above, low vitamin D concentrations are quite frequent, vitamin D toxicity, which is characterized by hypercalcemia and hyperphosphotemia, is a very rare condition, mainly caused by a long-term high-dose supplementation(115, 116). Since any excess of cholecalciferol is destroyed by sunlight, a prolonged exposure to sunlight cannot cause vitamin D3 intoxication. The most commonly and currently adopted cut-points to define vitamin D status (more exactly 25-hydroxy-vitamin D)(10, 15) are reported in Table 2. Interestingly, both definition presented in the Table define vitamin D severe deficiency using the same cut-point (i.e., 10 ng/mL or 25 nmol/L). A biological rationale for choosing this cut-point is provided by the identification at this level of an inflection in the negative association existing between vitamin D and parathyroid hormone(117). In other words, when vitamin D is at this concentration or below, parathyroid hormone is suppressed(117–120). Some Authors have also proposed the 30 ng/ml (or 75 nmol/L) cut-point to determine inadequate vitamin D concentrations(10, 21). Below this threshold, it is better evident the parallel and inverse association between parathyroid hormone and vitamin D. Unfortunately, the biological support for the definitions of most cut-points definitions (i.e., insufficiency, normal range, toxicity) is not so strong and/or free of controversies.
TABLE 2.
Serum concentrations of vitamin D (25-hydroxi-vitamin D).
| Status | ng/mL | nmol/L |
|---|---|---|
| Derived by Lee et al.(10) | ||
| Severe deficit | <10 | <25 |
| Deficit | 10–20 | 25–50 |
| Insufficiency | 21–29 | 51–74 |
| Normal values | 30–150 | 75–375 |
| Toxicity | >150 | >375 |
| From the Dietary Supplement Fact Sheet of the NIH-Office of dietary supplements(15) | ||
| Deficiency leading to rickets and osteomalacia | <10 | <25 |
| Inadequate for bone and overall health | 10–14 | 25–37.4 |
| Adequate for bone and overall health | 15–200 | 37.5–500 |
| Potentially toxic | >200 | >500 |
CONCLUSION
Several uncertainties are still currently present about the role that vitamin D plays on physical function, and whether this possible effect may not merely be the indirect manifestation of a poor health status(101). Therefore, it is crucial the design and development of new studies specifically aimed at: 1) defining clinically relevant vitamin D cut-points distinguishing across the different stati of this biomarker; 2) evaluating the effects of interventions aimed at the maintenance/improvement of physical function in the elderly; 3) verifying the preventive effect of such interventions for the main clinical outcomes of geriatric medicine, especially incident disability and institutionalization; 4) calculating the cost-effectiveness of treatments for vitamin D deficiency; 5) clarifying pathophysiological mechanisms at the basis of the relationship between hypovitaminosis D and adverse events in older persons, and 6) determining safety and potential adverse events of vitamin D supplementation in older persons. Although there is apparently a long way still to run, currently available data are overall encouraging. Hopefully, the improvement of our knowledge on vitamin D-related mechanisms will provide a major preventive instrument for older persons.
ACKNOWLEDGEMENTS
Funding
Drs. Cesari and Pahor are supported by the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center (NIH grant 1P30AG028740) and the National Institutes of Health - National Institute on Aging (NIA grant 1R01AG026556-01A2).
Footnotes
Declaration of interest
None
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003;112:e132–e135. doi: 10.1542/peds.112.2.e132. [DOI] [PubMed] [Google Scholar]
- 4.A dose of vitamin D history. Nat Struct Biol. 2002;9:77. doi: 10.1038/nsb0202-77. [DOI] [PubMed] [Google Scholar]
- 5.Gloth FMr, Gundberg CM, Hollis BW, Haddad JGJ, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 6.Goldray D, Mizrahi-Sasson E, Merdler C, et al. Vitamin D deficiency in elderly patients in a general hospital. J Am Geriatr Soc. 1989;37:589–592. doi: 10.1111/j.1532-5415.1989.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 7.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 8.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 12.Annweiler C, Bridenbaugh S, Schott AM, Berrut G, Kressig RW, Beauchet O. Vitamin D and muscle function: new prospects?[letter] Biofactors. 2009;35(1):3–4. doi: 10.1002/biof.4. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietary Supplement Fact Sheet: Vitamin D. 2009 [Google Scholar]
- 16.USDA Nutrient Database for Standard Reference. 2009 [Google Scholar]
- 17.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103:631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 21.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Vieth R. Why “Vitamin D” is not a hormone, and not a synonym for 1,25-dihydroxy-vitamin D, its analogs or deltanoids. J Steroid Biochem Mol Biol. 2004;89–90:571–573. doi: 10.1016/j.jsbmb.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Adams JS, Chen H, Chun R, et al. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. J Steroid Biochem Mol Biol. 2004;89–90:461–465. doi: 10.1016/j.jsbmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Peng L, Malloy PJ, Feldman D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol. 2004;18:1109–1119. doi: 10.1210/me.2003-0344. [DOI] [PubMed] [Google Scholar]
- 26.Nemere I. 24,25-dihydroxyvitamin D3 suppresses the rapid actions of 1,25-dihydroxyvitamin D3 and parathyroid hormone on calcium transport in chick intestine. J Bone Miner Res. 1999;14:1543–1549. doi: 10.1359/jbmr.1999.14.9.1543. [DOI] [PubMed] [Google Scholar]
- 27.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29:407–414. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–135. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 29.Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–390. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 30.Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:10–15. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 31.Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004;150:323–328. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 32.Maggio D, Cherubini A, Lauretani F, et al. 25(OH)D Serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:1414–1419. doi: 10.1093/gerona/60.11.1414. [DOI] [PubMed] [Google Scholar]
- 33.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166:1517–1524. [PMC free article] [PubMed] [Google Scholar]
- 34.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51:1075–1081. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 36.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 37.Lee P, Campbell LV. Vitamin D deficiency: the invisible accomplice of metabolic endotoxemia? Curr Pharm Des. 2009;15:2751–2758. doi: 10.2174/138161209788923895. [DOI] [PubMed] [Google Scholar]
- 38.Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes DP. Vitamin D and ageing. Biogerontology. 2010;11:1–16. doi: 10.1007/s10522-009-9252-0. [DOI] [PubMed] [Google Scholar]
- 40.Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 41.Duque G, El Abdaimi K, Macoritto M, Miller MM, Kremer R. Estrogens (E2) regulate expression and response of 1,25-dihydroxyvitamin D3 receptors in bone cells: changes with aging and hormone deprivation. Biochem Biophys Res Commun. 2002;299:446–454. doi: 10.1016/s0006-291x(02)02657-8. [DOI] [PubMed] [Google Scholar]
- 42.Lund B, Sorensen OH, Lund B, Agner E. Serum 1,25-dihydroxyvitamin D in normal subjects and in patients with postmenopausal osteopenia. Influence of age, renal function and oestrogen therapy. Horm Metab Res. 1982;14:271–274. doi: 10.1055/s-2007-1018990. [DOI] [PubMed] [Google Scholar]
- 43.Elkeles A. A comparative radiological study of calcified atheroma in males and females over 50 years of age. Lancet. 1957;273:714–715. doi: 10.1016/s0140-6736(57)92256-0. [DOI] [PubMed] [Google Scholar]
- 44.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 45.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 46.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 47.Norman PE, Powell JT. Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler Thromb Vasc Biol. 2005;25:39–46. doi: 10.1161/01.ATV.0000148450.56697.4a. [DOI] [PubMed] [Google Scholar]
- 48.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 49.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010 doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 50.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 53.Landfield PW, Cadwallader-Neal L. Long-term treatment with calcitriol (1,25(OH)2 vit D3) retards a biomarker of hippocampal aging in rats. Neurobiol Aging. 1998;19:469–477. doi: 10.1016/s0197-4580(98)00079-7. [DOI] [PubMed] [Google Scholar]
- 54.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 55.Wilkins CH, Birge SJ, Sheline YI, Morris JC. Vitamin D deficiency is associated with worse cognitive performance and lower bone density in older African Americans. J Natl Med Assoc. 2009;101:349–354. doi: 10.1016/s0027-9684(15)30883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 57.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 58.Kragt J, van Amerongen B, Killestein J, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15:9–15. doi: 10.1177/1352458508095920. [DOI] [PubMed] [Google Scholar]
- 59.van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–590. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 60.Ekbom K, Hed R, Kirstein L, Astroem KE. Weakness of proximal limb muscles, probably due to myopathy after gastrectomy. Preliminary report. Acta Med Scand. 1964;176:493–496. [PubMed] [Google Scholar]
- 61.Smith R, Stern G. Myopathy, osteomalacia and hyperparathyroidism. Brain. 1967;90:593–602. doi: 10.1093/brain/90.3.593. [DOI] [PubMed] [Google Scholar]
- 62.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56:2143–2149. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 63.Moro JR, Iwata M, von Andriano UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 65.Minasyan A, Keisala T, Zou J, et al. Vestibular dysfunction in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;114:161–166. doi: 10.1016/j.jsbmb.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 66.McGrath J, Selten JP, Chant D. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration--data from Australia and the Netherlands. Schizophr Res. 2002;54:199–212. doi: 10.1016/s0920-9964(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 67.Gloth FMr, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3:5–7. [PubMed] [Google Scholar]
- 68.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 69.Davis CD. Vitamin D and cancer: current dilemmas and future research needs. Am J Clin Nutr. 2008;88:565S–569S. doi: 10.1093/ajcn/88.2.565S. [DOI] [PubMed] [Google Scholar]
- 70.Gorkin Z, Gorkin MJ, Teslenko NE. The effect of ultraviolet irradiation upon training for 100m sprint. Fiziol Zh USSR. 1938;25:695–701. [Google Scholar]
- 71.Parade GW, Otto H. Effect of sunlamp on performance. Zeitschrift fur Klinische Medizin. 1940;137:17–21. [Google Scholar]
- 72.Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D. Myopathy in chronic renal failure. Q J Med. 1974;43:509–524. [PubMed] [Google Scholar]
- 73.Irani PF. Electromyography in nutritional osteomalacic myopathy. J Neurol Neurosurg Psychiatry. 1976;39:686–693. doi: 10.1136/jnnp.39.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med. 2000;160:1199–1203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- 75.Mingrone G, Greco AV, Castagneto M, Gasbarrini G. A woman who left her wheelchair. Lancet. 1999;353:806. doi: 10.1016/s0140-6736(98)10206-4. [DOI] [PubMed] [Google Scholar]
- 76.Russell JA. Osteomalacic myopathy. Muscle Nerve. 1994;17:578–580. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 77.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 78.Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 79.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 80.Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikawa S, Nakamura T, Tanabe H, Imamura T. Osteomalacic myopathy. Endocrinol Jpn. 1979;26:65–72. doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 82.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 83.Sorensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 84.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–629. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 85.Prineas JW, Mason AS, Henson RA. Myopathy in metabolic bone disease. Br Med J. 1965;1:1034–1036. doi: 10.1136/bmj.1.5441.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17:1318–1328. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 87.Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–2985. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 88.Pfeifer M, Begerow B, Minne HW, et al. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109:87–92. doi: 10.1055/s-2001-14831. [DOI] [PubMed] [Google Scholar]
- 89.Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51:1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 90.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 91.Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 92.Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 93.Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–299. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 94.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 95.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 96.Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM. 2007;100:185–192. doi: 10.1093/qjmed/hcm005. [DOI] [PubMed] [Google Scholar]
- 97.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51:1219–1226. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 98.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 99.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 100.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zamboni M, Zoico E, Tosoni P, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57:M7–M11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 102.Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. 2000;72:1529–1534. doi: 10.1093/ajcn/72.6.1529. [DOI] [PubMed] [Google Scholar]
- 103.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 104.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 105.Dam TT, von Muhlen D, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50:912–917. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 107.Lips P, Binkley N, Pfeifer M, et al. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010;91:985–991. doi: 10.3945/ajcn.2009.28113. [DOI] [PubMed] [Google Scholar]
- 108.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 109.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2007;46:1852–1857. doi: 10.1093/rheumatology/kem240. [DOI] [PubMed] [Google Scholar]
- 110.Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation: too much of a good thing? JAMA. 2010;303:1861–1862. doi: 10.1001/jama.2010.598. [DOI] [PubMed] [Google Scholar]
- 111.Kinder BK, Stewart AF. Hypercalcemia. Curr Probl Surg. 2002;39:349–448. doi: 10.1067/msg.2002.122220. [DOI] [PubMed] [Google Scholar]
- 112.Akishita M, Arai H, Arai H, et al. Survey on geriatricians’ experiences of adverse drug reactions caused by potentially inappropriate medications: Commission report of the Japan Geriatrics Society. Geriatr Gerontol Int. 2010 doi: 10.3143/geriatrics.46.271. [DOI] [PubMed] [Google Scholar]
- 113.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 114.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 115.Adams JS, Lee G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann Intern Med. 1997;127:203–206. doi: 10.7326/0003-4819-127-3-199708010-00004. [DOI] [PubMed] [Google Scholar]
- 116.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345:66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 117.Cheng S, Tylavsky F, Kroger H, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78:485–492. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 118.Tylavsky FA, Ryder KA, Lyytikainen A, Cheng S. Vitamin D, parathyroid hormone, and bone mass in adolescents. J Nutr. 2005;135:2735S–2738S. doi: 10.1093/jn/135.11.2735S. [DOI] [PubMed] [Google Scholar]
- 119.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 120.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]