Pistachio supplementation attenuates motor and cognition impairments induced by cisplatin or vincristine in rats (original) (raw)
Abstract
Background:
A large number of natural products and dietary components have been evaluated as potential chemoprotective agents. In the present investigation we report the effects of treatment with the dietary antioxidant, pistachio, on cisplatin- or vincristine-induced neurotoxicity in male Wistar rats.
Materials and Methods:
Dietary pistachio (10%) was assessed for its neuroprotective effects through the alteration in performance of hippocampus- and cerebellum-related behaviors following chronic cisplatin (5 mg/kg) or vincristine (0.2 mg/kg) treatment in male rats. We also evaluated the effects of cisplatin, vincristine, and pistachio administration on nociception. Six behavioral tasks were used: open field, rotarod, grasping, Morris water maze (MWM), hot plate, and motor nerve conductive velocity (MNCV).
Results:
We showed that the exposure of adolescent rats to cisplatin or vincristine resulted in a significant decrease in explorative behaviors and memory retention. Pistachio consumption somewhat improved memory and motor abilities in cisplatin- or vincristine-treated rats, while pistachio alone did not show any significant changes in these abilities compared to saline. Cisplatin and vincristine increased the latency of response to nociception, and pistachio did not reverse this effect.
Conclusion:
We conclude that pistachio in the diet following anticancer drugs such as cisplatin and vincristine might have a protective effect against anticancer drug-induced disruptions in motor and cognitive function. However, further studies are needed to elucidate the exact mechanisms of this protective effect of pistachio.
Keywords: Anticancer, cisplatin, neurotoxicity, pistachio, vincristine
INTRODUCTION
The toxic effects of some anticancer drugs have been reported on the peripheral and central nervous systems.[1] Cisplatin and vincristine are two effective antitumor agents with a wide spectrum of activity against various solid tumors. However, their clinical application is limited due to their physiological adverse effects such as nephrotoxicity, neurotoxicity, ototoxicity, and vomiting.[2,3,4,5,6,7] A promising way for the prevention of these physiological side effects is through dietary intervention. Relative to other nuts, pistachios are a rich source of antioxidants[8,9] and phenolic compounds.[10,11] These nuts are consumed largely for their nutritional and health management properties.[12] They are the only nut that contains anthocyanin, the pigments responsible for the color of several fruits and vegetables.[11] It has been shown that anthocyanins possess some antioxidant, antiinflammatory, and anticarcinogenic properties. Moreover, among all the nuts pistachios are one of the most diverse sources of antioxidants including lutein, β-carotene, vitamin E (g-tocopherol and α-tocopherol), and selenium.[8,9] In addition, pistachios are a good source of flavonoids, and the protective effects of flavonoids and tocopherol consumption on cisplatin-induced neurotoxictiy (without changing its antitumor activity) have been reported previously.[13,14,15,16,17]
It has been reported that the treatment of patients who received cisplatin with vitamin E could decrease the incidence and intensity of peripheral neurotoxicity.[18] Furthermore, lutein can improve antioxidant capacity and inhibits DNA and chromosome damage induced by cisplatin.[19] Pistachio possesses arginine which has been reported to significantly increase the histopathological responses in vincristine chemotherapy in patients with initial breast tumors (less than 6 cm in diameter).[20]
In view of the above reported therapeutic value of pistachio nuts, the present study was designed to investigate the effects of this dietary antioxidant on cisplatin- and vincristine-induced neurotoxicity in male Wistar rats and also to evaluate the effects of cisplatin, vincristine, and pistachio administration on nociception.
MATERIALS AND METHODS
Animals
In the present study we investigated the protective effects of treatment with pistachio on cisplatin- or vincristine-induced neurotoxicity in male Wistar rats on postnatal day 23. When we evaluated the weights of the rats on the 23rd day after birth, there were no significant differences in weight (45 ± 5.7) between the saline and treatment groups (48 ± 4.6) on the 1st day of the experimental procedure. All experimental protocols and treatments were approved by the Ethical Committee of the Kerman Neuroscience Research Center (EC/KNRC/90/47). Animals were kept in standard conditions: Constant temperature (23 ± 1°C), with a 12 h light-dark cycle (lights on at 07:00 h).
Experimental design
In the beginning of the experiment, animals were weight-matched and randomly assigned into six groups: Saline, pistachio, cisplatin, cisplatin plus pistachio, vincristine, and vincristine plus pistachio. Each group consisted of 10 animals (n = 10). Control animals (group I) were subjected to intraperitoneal (i.p.) injection of 0.2 ml normal saline for 5 weeks. Group II received pistachio with no other medication for 5 weeks. Cisplatin (5 mg/kg, i.p.) was administered for 5 weeks in group III. Group IV (cisplatin plus pistachio) animals were administered cisplatin plus 10% pistachio for 5 weeks. Group V received vincristine (0.2 mg/kg, i.p.) for 5 weeks. The group VI rats were administered vincristine plus 10% pistachio for 5 weeks. Rats in groups I, III, and V were given the standard rodent diet, while in groups II, IV, and VI, rats were fed with pistachio (10%). Fresh pistachio suspension was prepared daily. The body weight and food intake of all the rats were monitored during the experiment. Cerebellum- and hippocampus-related behavioral dysfunctions in cisplatin- and vincristine-treated rats were analyzed using cerebellum-dependent behavioral, motor learning, and hippocampus functional tasks. We also evaluated the effects of cisplatin, vincristine, and pistachio administration on nociception. Measurements were taken during daylight (between 8:00 and 16:00 h). Six behavioral tasks were used: Open field, rotarod, grasping, Morris water maze (MWM), hot plate, and motor nerve conductive velocity (MNCV). The first set of behavioral tests was performed 24 h after the last injection of anticancer drugs. Open field, rotarod, and grasping tests were performed to screen the muscle coordination activity and explorative behaviors of rats, before subjecting them to water maze evaluation. Hot plate test and MNCV were tested to screen nociceptive activity. Rats showing abnormal swimming patterns in the water maze, coupled with low muscle coordination activity in the rotarod test, were excluded from data analysis in MWM.
Wire grip (grasping) test
This test was performed according to the method described by Wijk et al. to determine the muscle strength and balance of the animals.[21] Each rat was suspended with both forepaws on a horizontal steel wire of length 80 cm and diameter 7 mm. The animal was held in a vertical position when its front paws were placed in contact with the wire. When the rat grasped the wire, it was released, and the latency to fall was recorded with a stopwatch. Rats were randomly tested and each animal was given three trials with a 30-min intertrial rest interval.[22,23]
Rotarod test
The motor performances of the rats were evaluated by an accelerating rotating rod (Hugo Sachs Electronik, Germany). The rotarod accelerated from a minimum speed of 10 rpm to a maximum speed of 60 rpm. Rats were given three trials using maximum time of 300 s with a 30 min intertrial rest interval. The length of time each animal was able to maintain its balance walking on top of the moving rod was recorded.[24,25]
Open field test
The horizontal and vertical movements of rats were recorded for a period of 5 min and then analyzed using Ethovision software (Noldus Information Technology, the Netherlands), a video tracking system for the automation of behavioral experiments. The apparatus consisted of a square arena (90 cm [l] ×90 cm [w] ×30 cm [h]) made of Plexiglas. At the beginning of the session, each rat was placed in the center of the arena and its activity was recorded for 5 min. The following behavioral parameters were scored: Total distance moved (TDM, cm), speed (cm/s), total duration of mobility (s), total duration of immobility (s), and number of rearing (as a measure of vertical activity). At the end of each session, the rats were removed from the open field, and the experimental chamber was thoroughly cleaned with a damp cloth and dried.[26]
Morris water maze task
The testing procedure was basically the same as that described by Frick _et al._[27] The experimental apparatus consisted of a circular water tank 140 cm wide and 45 cm high surrounded by extra-maze cues. A platform 15 cm wide and 35 cm high was placed 1.5 cm above or below the surface of the water. To assess for gross physical, sensory, motor, or motivational impairments, eight rats in anticancer treated groups and five rats in saline group were first trained in a task with a visible escape platform. The water temperature was 21–23°C. Data collection was automated by a video image motion analyzer (Ethovision, Noldus Information Technology, the Netherlands). In a single training protocol each rat completed three blocks (each block consisted of four successive trials with four different releasing points) separated by a 30-min resting period. All of the experimental groups were tested during the lights-on period between 8:00 and 12:00 h. For each trial, rats were randomly released into the water from one of the four quadrants with their face toward the wall of the maze. During acquisition, the location of the platform remained constant and rats were allowed to swim for a duration of 60 s to find the hidden platform. If the rat failed to find the platform within 60 s, it was guided toward the platform. When an animal found the platform, it was allowed to remain there for 20-30 s. After that, it was moved to an animal cage to rest 20-30 s before starting the next trial. The time and distance needed to find the hidden platform were noted and analyzed later. A single probe trial was given 2 h after the last training trial to test the spatial memory of animals in the water maze. In this trial the platform was removed and the rat was allowed to swim for 60 s. The time and distances spent in the target quadrant were analyzed as a measure of spatial memory retention.[28,29]
Hot plate test
Using the hot plate test, pain sensitivity in the rats was assayed by using an apparatus (LSI Letica, LE710, Spain) that included a plate with a diameter of 19 cm and a Plexiglas wall 30 cm high. The plate temperature was adjusted to 52 ± 2°C. Response time to thermal pain was considered as the time between test onset and licking of the front paw or jumping. The cut-off time was set to 60 s.[22]
Motor nerve conductive velocity
Five weeks after cisplatin and vincristine exposure, the animals were anesthetized with i.p. injection of ketamine (50 mg/kg) and xylazine (20 mg/kg) to prevent discomfort, and then their backs were shaved. In a temperature-controlled environment (25 ± 2°C), small incisions were made in the right sciatic notch and ankle. Sciatic-tibial motor MNCV was measured by stimulating proximally at the sciatic notch and distally at the knee via bipolar needle electrodes (PowerLab/ML856; AD Instruments, Sydney, NSW, Australia) with the following specs: Frequency 20 Hz, duration 0.1 ms, amplitude 1.5 V, and sampling 20 k/s.[18] After single stimulus the compound muscle action potential was recorded from the first interosseous muscle of the hind paw by unipolar pin electrodes. The recording was a typical biphasic response with an initial motor (M)-wave, which is a direct motor response due to stimulation of motor fibers. The MNCV was calculated as the ratio of the distance (in mm) between both sites of stimulation divided by the difference between proximal and distal latencies measured in ms.[30] Latency was measured from initial onset to maximum negative peak.
Histological study
Under deep anesthesia, the animals were sacrificed, and their brains removed and immersed in 10% formaldehyde at 4°C overnight. The brains were processed by standard method for light microscopy study. Coronal sections 1.6-2.8 mm posterior to the bregma were cut at a thickness of 5 μm using a microtome. Neuronal damage in the cerebellum and hippocampus were assessed by Nissl staining with cresyl fast violet.
Statistical analysis method
The results obtained are expressed as means ± SEM. Repeated measures analysis of variance (ANOVA) was calculated for rotarod and for the learning phase of the MWM. Individual comparisons were calculated using Tukey's comparison test where appropriate. The grip test, motor activity, probe data, MNCV, and reaction time were analyzed by one-way ANOVA. All computations were made using the SPSS software package version 16.0 (IBM, Armonk, NY, USA) and difference with P values less than 0.05 were considered statistically significant.
RESULTS
Since no significant differences were found between saline and pistachio groups, data are presented as saline group.
Effects of pistachio, vincristine, and cisplatin on muscle coordination and balancing
Cisplatin (P < 0.05) and vincristine (P < 0.01) induced a significant reduction in the latency-to-fall parameter of the grasping test compared to the saline group. Animals in the cisplatin plus pistachio group showed an increased latency to fall in grasping test compared to the cisplatin group (P < 0.01) [Figure 1a]. Moreover, in the vincristine plus pistachio group, animals showed an increased latency to fall compared to the vincristine group (P <0.01) [Figure 1a].
Figure 1.
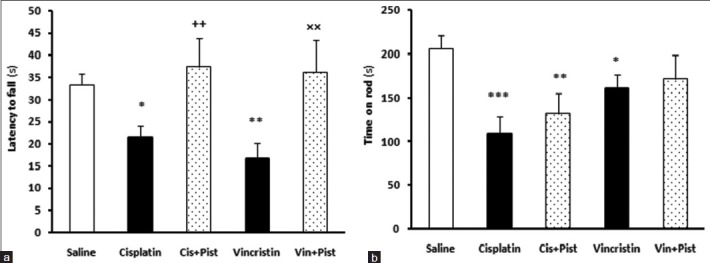
Effects of cisplatin, vincristine, cisplatin plus pistachio (Cis plus Pist), and vincristine plus pistachio (Vin plus Pist) on muscle strength and coordination in grasping test (a) and balance in Rotarod test (b). *P < 0.05, **P < 0.01 and ***P < 0.001 as compared to saline group. ++P < 0.01as compared to cisplatin and ×× P <0.01 compared to vincristine group. Values are expressed as mean ± SEM (_n_= 10)
Cisplatin (P < 0.001) and vincristine (P < 0.05) induced a significant reduction in the time spent on the accelerating rotarod, compared to saline. Cisplatin plus pistachio group did show a decrease in time spent on the rod compared to saline (P < 0.01), although a lesser decrease in time was observed compared to the cisplatin group indicating a better motor balance compared to cisplatin-treated rats [Figure 1b]. The vincristine plus pistachio group showed no significant differences from the saline group: In fact, in this group, pistachio reversed the effect of vincristine [Figure 1b].
Effects of pistachio, vincristine, and cisplatin on anxiety-related behaviors
The open field test was given to examine locomotor activity and anxiety-related behaviors. Our results showed that cisplatin and vincristine did alter some of the parameters evaluated in this test. In addition, pistachio showed a moderating effect on changes induced by cisplatin and vincristine. The rearing number (cisplatin, P < 0.01; vincristine, P < 0.001) and TDM were decreased in the cisplatin and vincristine groups compared to saline (P < 0.01) [Figure 2a and b]. Cisplatin plus pistachio rats did also differ from saline (P < 0.01) in the rearing number and in TDM. The vincristine plus pistachio group showed no significant differences compared to the saline group in rearing number and TDM, however, these parameters were increased compared to the vincristine group (rearing; P < 0.001, TDM; P < 0.05). Cisplatin and vincristine significantly reduced speed compared to saline group (P < 0.01), but there was no significant difference between saline, cisplatin plus pistachio-, and vincristine plus pistachio-treated rats [Figure 2c]. Cisplatin plus pistachio and vincristine plus pistachio treatment significantly increased velocity compared to both cisplatin and vincristine alone (P < 0.05). There was no significant difference in mobility, immobility, and the time spent in center and periphery among the five groups [Figure 2d-g].
Figure 2.
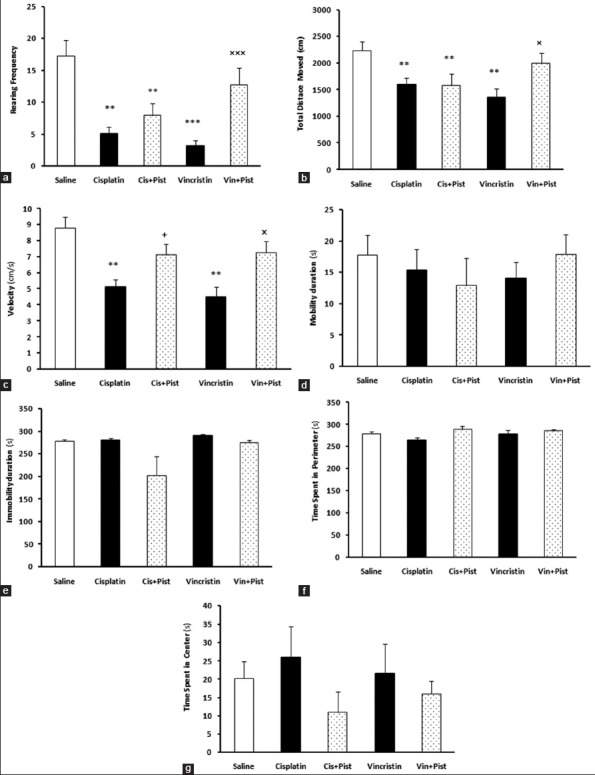
Cisplatin and vincristine significantly reduced rearing frequency (a), TDM (b), and velocity (c). Vincristine plus pistachio (Vin plus Pist)-treated rats showed significant difference in rearing frequency, TDM, and velocity compared to vincristine-treated rats. Cisplatin plus pistachio (Cis plus Pist) had significant difference with cisplatin rats in velocity. There was no significant difference in mobility (d), immobility (e), time spent in perimeter (f), and time spent in the center (g) among three groups. **P< 0.01, ***P< 0.001 as compared to the saline group. +P< 0.05 as compared to cisplatin group. ×P< 0.05 and ×××P< 0.001 as compared to the vincristine group
Pistachio prevents memory alterations induced by cisplatin and vincristine
All groups showed a significant reduction in swimming distance in the second and third blocks of the MWM task, showing memory acquisition (cisplatin, vincristine [P < 0.001], vincristine plus pistachio, and cisplatin plus pistachio [P < 0.05]). The cisplatin plus pistachio and vincristine plus pistachio groups did not show any significant difference in the distance swam to reach the platform, compared to the saline group in the second block of MWM, while a significant increase was observed in the third block (P < 0.05). Moreover, cisplatin (P < 0.01) and vincristine (P < 0.05) increased the escape latency parameter compared to the saline group. The vincristine plus pistachio group did not differ from the saline group in time latency in MWM [Figure 3a and b]. The vincristine plus pistachio group did not differ from saline group in time latency in MWM [Figure 3b]. The cisplatin plus pistachio group (P < 0.05) showed increased time latency in comparison with saline (P < 0.01) in the second block [Figure 3b].
Figure 3.

Hidden platform training in the Morris water maze acquisition after 5 weeks treatment with saline, cisplatin, vincristine, cisplatin plus pistachio, and vincristine plus pistachio. Distance traveled (a), time spent (b), and velocity (c) of the animals during training trial sessions. The path length traveled (d), percentage of swimming time (e), and crossing numbers in the correct quadrant (f) of the animals in the probe trial. *P< 0.05, **P< 0.01 and ***P< 0.001 as compared to the saline group. ×P< 0.05 as compared to vincristine group
In the probe test, the percentage of path length traveled in the correct quadrant was significantly decreased in the cisplatin, vincristine, and cisplatin plus pistachio rats compared to saline (P < 0.05), while the vincristine plus pistachio group did not show any differences from the saline group [Figure 3d]. Results from the probe test measured as mean percentage time spent in the correct quadrant showed that cisplatin, cisplatin plus pistachio (P < 0.01), and vincristine (P < 0.05), significantly decreased the percentage of time spent in the correct quadrant compared to saline (P < 0.01). The vincristine plus pistachio group did not show any difference in percentage of time spent in the correct quadrant compared to the saline group [Figure 3e]. There was no difference in swim speed and the number of crossings to the correct quadrant [Figure 3c and f].
Effect of pistachio consumption on response to thermal pain and MNCV
An increased reaction time in the hot plate test was observed in the cisplatin, vincristine (P < 0.001), cisplatin plus pistachio, and vincristine plus pistachio (P < 0.01) groups compared to saline. However, no significant difference was observed between these treatment groups [Figure 4a]. On the other hand, the administration of anticancer drugs alone or along with pistachio did not change the MNCV [Figure 4b].
Figure 4.
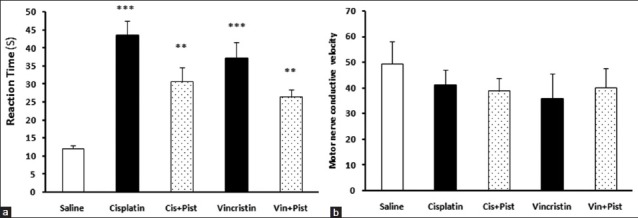
Effects of cisplatin, vincristine, cisplatin plus pistachio, and vincristine plus pistachio on the pain threshold values of rats subjected to the hot plate test (a). Administration of anticancer drugs alone or along with pistachio did not change MNCV (b). *P < 0.01 and *P < 0.001 as compared to the control group
Histological evaluation
Light microscopic study of cerebellum and hippocampi [Figure 5] sections showed normal morphology in the saline and pistachio groups (10×). The hippocampi of rats that had been treated with cisplatin or vincristine showed severe injury, and degenerative changes including shrunken nuclei and dark cytoplasm were observed in these cells [Figure 5]. Meanwhile, degenerative changes in the cell population of the hippocampi sections were still evident in cisplatin plus pistachio or vincristine plus pistachio, but these changes were blunted in these groups compared to cisplatin or vincristine [Figure 5].
Figure 5.
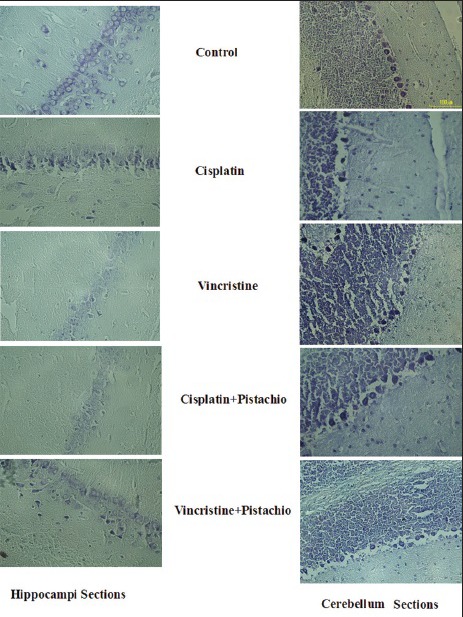
Neurons from the cerebellar cortex and hippocampus 5 weeks after exposure to anticancer drugs alone or along with pistachio. Most neurons from the cerebellum and hippocampus in saline-treated rats have normal morphology, but for the cisplatin-, vincristine-, cisplatin plus pistachio-and vincristine plus pistachio-treated groups, several degenerated cells can be seen with shrunken, aged nucleolus and dark cytoplasm
DISCUSSION
The findings of this research showed that exposure to the anticancer compounds cisplatin and vincristine could induce strong alterations in the motor and exploratory activities of the rats. These compounds could also cause impairments in the spatial learning and memory of the animals.
Anticancer drugs are potent neurotoxins that could produce widespread lesions within the cortex, thalamus, hippocampal dentate gyrus, and caudate nucleus in a dose-dependent manner.[31] It has been shown that vincristine could destroy the dentate granule cells, and damage other neurons, including hippocampal pyramidal cells.[32] On the other hand, the negative effects of cisplatin on cerebellar cortex neurons, especially the Purkinje cells, which are well established as being essential to movement control.[33,34,35]
Our obtained results showed that pistachios could moderate these chemotherapeutics-induced changes in the central nervous system (CNS). Pistachios improved the motor function in cisplatin- and vincristine-treated rats. Muscle weakness in cisplatin- and vincristine-treated rats was confirmed by the observed reduction in the latency-to-fall parameter of the grasping test and in the time on the rod parameter of the rotarod test in comparison with the control animals. Pistachios significantly increased these factors, implicating the protective effects of these nuts on cisplatin- and vincristine- induced muscle weakness.
Consistent with our previous findings,[24,32] cisplatin and vincristine alone or with the pistachios did not alter the anxiety level in our studied rats, and all groups spent equivalent time in the central and peripheral areas of the open field apparatus. In contrast, locomotor activity was affected by cisplatin and vincristine, and a decreased level of TDM and velocity parameters of the open field test was observed in drug-treated rats. This observed reduction might be due to the fact that drug-treated rats were weaker and their movement was impaired by the neurotoxic agents.[24,36] Motor centers in the brain are the possible sites of anticancer action, and the pistachio might induce its protective effect on the neurons of these areas; further research focusing on the nut sites of action in the CNS would clarify the exact mechanism of pistachio neuroprotection. Pistachio administration modified these alterations to some extent. Phenolic compounds,[10,11] such as anthocyanins, proanthocyanidins, and phenolic acids,[11] might participate in these modulatory effects.
Pistachio is the only nut that contains anthocyanins, a type of bioflavonoid and potent antioxidant responsible for the color of many vegetables and fruits.[11,37] This phenolic compound has antiinflammatory, anticarcinogenic, antioxidative, and neuroprotective properties. These effects have been attributed to the proteasome-inhibitory[38] and radical scavenging properties,[39,40] the interaction with signaling cascades,[40] age-related changes in adrenergic and noradrenergic receptor function,[41] the enhancement of dopamine release[42] and shifts in cerebrovascular permeability.[43] It has been also reported that anthocyanins and anthocyanidins could limit the brain cell damage at protein, membrane lipid, and DNA levels.[38]
Cisplatin and vincristine administration caused significant disruptions in the spatial learning and memory of the animals. In our experiment, pistachios reversed some learning and memory impairments caused by cisplatin and vincristine administration. Pistachio nuts have several amino acids and among them glutamate and glutamine are present in the highest concentration. The role of glutamate as a neurotransmitter in learning and memory is well-established.[44] Moreover, dietary supplementation experiments in humans and animal models have shown that using flavonoid-rich plants and foods could improve memory and synaptic plasticity. Emerging evidence suggests that dietary-derived flavonoids have the potential to improve the human memory and neurocognitive performances via their ability to protect vulnerable neurons, elevate existing neuronal function, and encourage neuronal regeneration.[45,46,47,48,49] These characteristics of pistachio nuts might explain their protective effects on cisplatin- and vincristine-induced memory impairments, although the antioxidant activity[50] of pistachios is another important factor which should not be neglected.
Our data also showed that cisplatin and vincristine improved the reaction time in the hot plate test and that pistachio nuts moderated this effect. In opposition to our finding, Khasabova et al. showed the occurrence of hyperalgesia following cisplatin administration, which could be weakened by administration of the cannabinoid (CB) agonist, arachidonyl-ethanol-amide.[51] They also showed that motor performance was not changed in the rotarod test, while a muscle weakness was observed in our drug-treated rats, and this may be the reason for the observed differences. These differences could also be attributed to the different ages of rats, dosages, and duration of drug administration. The observed cisplatin- and vincristine-induced muscle weakness in our study might be the reason for the increased latency in response to the thermal stimuli. Cisplatin damages peripheral neural tissues, including dorsal root ganglia (DRGs) and sensory fibers,[52] which is thought to be responsible for the neuropathy observed in cancer patients treated with anticancer drugs, although some molecular mechanisms of muscle contraction also are involved. However, further research seems to be needed for elucidating the role of neurotransmitters and their mechanisms in cisplatin- and vincristine-induced neurotoxicity and pistachio's interaction with these compounds.
CONCLUSION
In conclusion, we have shown that pistachio nuts have a protective effect on cisplatin- and vincristine-induced neurotoxicity. Since these nuts are rich in natural antioxidant and phenolic compounds, their protective effects could be attributed to these substances. However, further research investigating the pistachio's exact mechanism of protection, site of action, and its possible interaction with the antitumor properties of these potent anticancer agents must be done before it can be used as a supplement for cancer patients.
ACKNOWLEDGMENTS
The present manuscript is the product of a research project that was approved by the Kerman University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kamisli S, Ciftci O, Kaya K, Cetin A, Kamisli O, Ozcan C. Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol Ind Health. 2013 doi: 10.1177/0748233713483192. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Ali BH, Al-Salam S, Al Husseini IS, Al-Lawati I, Waly M, Yasin J, et al. Abrogation of cisplatin-induced nephrotoxicity by emodin in rats. Fundam Clin Pharmacol. 2013;27:192–200. doi: 10.1111/j.1472-8206.2011.01003.x. [DOI] [PubMed] [Google Scholar]
- 3.Paksoy M, Ayduran E, Sanlý A, Eken M, Aydýn S, Oktay ZA. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med Oncol. 2011;28:615–21. doi: 10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- 4.Screnci D, McKeage MJ. Platinum neurotoxicity: Clinical profiles, experimental models and neuroprotective approaches. J Inorg Biochem. 1999;77:105–10. doi: 10.1016/s0162-0134(99)00135-x. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Zhu J, Sandler AB, Schiller J, Belani CP, Langer C, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: A Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer. 2001;92:2639–47. doi: 10.1002/1097-0142(20011115)92:10<2639::aid-cncr1617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Liu W, Frenz D. Cisplatin ototoxicity to the rat inner ear: A role for HMG1 and iNOS. Neurotoxicology. 2006;27:22–30. doi: 10.1016/j.neuro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–7. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 9.Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr. 2010;140:1093–8. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chemi. 2005;92:521–5. [Google Scholar]
- 11.Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. 2010;92:1115–22. doi: 10.1016/j.biochi.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Dreher ML. Pistachio nuts: Composition and potential health benefits. Nutr Rev. 2012;70:234–40. doi: 10.1111/j.1753-4887.2011.00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Pace A, Giannarelli D, Galiè E, Savarese A, Carpano S, Della Giulia M, et al. Vitamin E neuroprotection for cisplatin neuropathy: A randomized, placebo-controlled trial. Neurology. 2010;74:762–6. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- 14.Saif MW. Oral calcium ameliorating oxaliplatin-induced peripheral neuropathy. J Appl Res. 2004;4:576–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishibashi K, Okada N, Miyazaki T, Sano M, Ishida H. Effect of calcium and magnesium on neurotoxicity and blood platinum concentrations in patients receiving mFOLFOX6 therapy: A prospective randomized study. Int J Clin Oncol. 2010;15:82–7. doi: 10.1007/s10147-009-0015-3. [DOI] [PubMed] [Google Scholar]
- 16.Gamelin L, Boisdron-Celle M, Delva R, Guérin-Meyer V, Ifrah N, Morel A, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10:4055–61. doi: 10.1158/1078-0432.CCR-03-0666. [DOI] [PubMed] [Google Scholar]
- 17.Aksoy N, Aksoy M, Bagci C, Gergerlioglu HS, Celik H, Herken E, et al. Pistachio intake increases high density lipoprotein levels and inhibits low-density lipoprotein oxidation in rats. Tohoku J Exp Med. 2007;212:43–8. doi: 10.1620/tjem.212.43. [DOI] [PubMed] [Google Scholar]
- 18.Navis I, Sriganth P, Premalatha B. Dietary curcumin with cisplatin administration modulates tumour marker indices in experimental fibrosarcoma. Pharmacol Res. 1999;39:175–9. doi: 10.1006/phrs.1998.0425. [DOI] [PubMed] [Google Scholar]
- 19.Serpeloni JM, Grotto D, Mercadante AZ, de Lourdes Pires Bianchi M, Antunes LM. Lutein improves antioxidant defense in vivo and protects against DNA damage and chromosome instability induced by cisplatin. Arch Toxicol. 2010;84:811–22. doi: 10.1007/s00204-010-0576-y. [DOI] [PubMed] [Google Scholar]
- 20.Heys SD, Ogston K, Miller I, Hutcheon AW, Walker LG, Sarker TK, et al. Potentiation of the response to chemotherapy in patients with breast cancer by dietary supplementation with L-arginine: Results of a randomised controlled trial. Int J Oncol. 1998;12:221–5. doi: 10.3892/ijo.12.1.221. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk N, Rijntjes E, van De Heijning BJ. Perinatal and chronic hypothyroidism impair behavioural development in male and female rats. Exp Physiol. 2008;93:1199–209. doi: 10.1113/expphysiol.2008.042416. [DOI] [PubMed] [Google Scholar]
- 22.Shabani M, Nazeri M, Parsania S, Razavinasab M, Zangiabadi N, Esmaeilpour K, et al. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33:1314–21. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Razavinasab M, Moazzami K, Shabani M. Maternal mobile phone exposure alters intrinsic electrophysiological properties of CA1 Pyramidal neurons in rat offspring. Toxicol Ind Health. 2014 doi: 10.1177/0748233714525497. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Shabani M, Larizadeh MH, Parsania S, Hajali V, Shojaei A. Evaluation of destructive effects of exposure to cisplatin during developmental stage: No profound evidence for sex differences in impaired motor and memory performance. Int J Neurosci. 2012;122:439–48. doi: 10.3109/00207454.2012.673515. [DOI] [PubMed] [Google Scholar]
- 25.Haghani M, Shabani M, Moazzami K. Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience. 2013;250:588–98. doi: 10.1016/j.neuroscience.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Razavinasab M, Shamsizadeh A, Shabani M, Nazeri M, Allahtavakoli M, Asadi-Shekaari M, et al. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson's disease. Fundam Clin Pharmacol. 2013;27:632–40. doi: 10.1111/fcp.12015. [DOI] [PubMed] [Google Scholar]
- 27.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: Species differences in a one-day water maze task. Neuroreport. 2000;11:3461–5. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 28.Parsania S, Shabani M, Moazzami K, Razavinasab M, Larizadeh MH, Nazeri M, et al. Gender difference in motor impairments induced by chronic administration of vinblastine. Iran J Basic Med Sci. 2014;17:433–40. [PMC free article] [PubMed] [Google Scholar]
- 29.Hajali V, Sheibani V, Esmaeili-Mahani S, Shabani M. Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behav Brain Res. 2012;228:311–8. doi: 10.1016/j.bbr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Zangiabadi N, Asadi-Shekaari M, Sheibani V, Jafari M, Shabani M, Asadi AR, et al. Date fruit extract is a neuroprotective agent in diabetic peripheral neuropathy in streptozotocin-induced diabetic rats: A multimodal analysis. Oxid Med Cell Longev 2011. 2011 doi: 10.1155/2011/976948. 976948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rzeski W, Pruskil S, Macke A, Felderhoff-Mueser U, Reiher AK, Hoerster F, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004;56:351–60. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- 32.Shabani M, Larizadeh MH, Parsania S, Asadi Shekaari M, Shahrokhi N. Profound destructive effects of adolescent exposure to vincristine accompanied with some sex differences in motor and memory performance. Can J Physiol Pharmacol. 2012;90:379–86. doi: 10.1139/y11-132. [DOI] [PubMed] [Google Scholar]
- 33.Avella D, Pisu MB, Roda E, Gravati M, Bernocchi G. Reorganization of the rat cerebellar cortex during postnatal development following cisplatin treatment. Exp Neurol. 2006;201:131–43. doi: 10.1016/j.expneurol.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Pisu MB, Roda E, Guioli S, Avella D, Bottone MG, Bernocchi G. Proliferation and migration of granule cells in the developing rat cerebellum: Cisplatin effects. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1226–35. doi: 10.1002/ar.a.20249. [DOI] [PubMed] [Google Scholar]
- 35.Wick A, Wick W, Hirrlinger J, Gerhardt E, Dringen R, Dichgans J, et al. Chemotherapy-induced cell death in primary cerebellar granule neurons but not in astrocytes: In vitro paradigm of differential neurotoxicity. J Neurochem. 2004;91:1067–74. doi: 10.1111/j.1471-4159.2004.02774.x. [DOI] [PubMed] [Google Scholar]
- 36.Hill A, Bergin P, Hanning F, Thompson P, Findlay M, Damianovich D, et al. Detecting acute neurotoxicity during platinum chemotherapy by neurophysiological assessment of motor nerve hyperexcitability. BMC Cancer. 2010;10:451. doi: 10.1186/1471-2407-10-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadinova MG, Docheva-Drenska DI, Boyadjieva NI. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009;16:345–9. doi: 10.1097/gme.0b013e3181847619. [DOI] [PubMed] [Google Scholar]
- 38.Dreiseitel A, Schreier P, Oehme A, Locher S, Rogler G, Piberger H, et al. Inhibition of proteasome activity by anthocyanins and anthocyanidins. Biochem Biophys Res Commun. 2008;372:57–61. doi: 10.1016/j.bbrc.2008.04.140. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Alonso M, Rimbach G, Sasai M, Nakahara M, Matsugo S, Uchida Y, et al. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Mol Nutr Food Res. 2005;49:1112–9. doi: 10.1002/mnfr.200500100. [DOI] [PubMed] [Google Scholar]
- 40.Marko D, Puppel N, Tjaden Z, Jakobs S, Pahlke G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol Nutr Food Res. 2004;48:318–25. doi: 10.1002/mnfr.200400034. [DOI] [PubMed] [Google Scholar]
- 41.Bickford PC, Shukitt-Hale B, Joseph J. Effects of aging on cerebellar noradrenergic function and motor learning: Nutritional interventions. Mech Ageing Dev. 1999;111:141–54. doi: 10.1016/s0047-6374(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 42.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saija A, Princi P, D’Amico N, De Pasquale R, Costa G. Effect of Vaccinium myrtillus anthocyanins on triiodothyronine transport into brain in the rat. Pharmacol Res. 1990;22(Suppl 3):59–60. doi: 10.1016/s1043-6618(09)80029-7. [DOI] [PubMed] [Google Scholar]
- 44.McEntee WJ, Crook TH. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 1993;111:391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- 45.Spencer JP. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc. 2008;67:238–52. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 46.Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, et al. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum Exp Toxicol. 2003;22:473–80. doi: 10.1191/0960327103ht388oa. [DOI] [PubMed] [Google Scholar]
- 47.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, et al. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–61. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 48.Viggiano A, Viggiano A, Monda M, Turco I, Incarnato L, Vinno V, et al. Annurca apple-rich diet restores long-term potentiation and induces behavioral modifications in aged rats. Exp Neurol. 2006;199:354–61. doi: 10.1016/j.expneurol.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Wang L, Wu J, Cai J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol. 2006;148:147–53. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajaei A, Barzegar M, Mobarez AM, Sahari MA, Esfahani ZH. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem Toxicol. 2010;48:107–12. doi: 10.1016/j.fct.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci. 2012;32:7091–101. doi: 10.1523/JNEUROSCI.0403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregg RW, Molepo JM, Monpetit VJ, Mikael NZ, Redmond D, Gadia M, et al. Cisplatin neurotoxicity: The relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol. 1992;10:795–803. doi: 10.1200/JCO.1992.10.5.795. [DOI] [PubMed] [Google Scholar]