Self-discrimination in the tendrils of the vine Cayratia japonica is mediated by physiological connection (original) (raw)
Abstract
Although self-discrimination has been well documented, especially in animals, self-discrimination in plants has been identified in only a few cases, such as self-incompatibility in flowers and root discrimination. Here, we report a new form of self-discrimination in plants: discrimination by vine tendrils. We found that tendrils of the perennial vine Cayratia japonica were more likely to coil around neighbouring non-self plants than neighbouring self plants in both experimental and natural settings. The higher level of coiling around a physiologically severed self plant compared with that around a physiologically connected self plant suggested that self-discrimination was mediated by physiological coordination between the tendril and the touched plant as reported for self-discrimination in roots. The results highlight the importance of self-discrimination for plant competition not only underground, but also above-ground.
Keywords: Cayratia japonica, competition, conspecific interactions, self-discrimination, climbing plant
1. Introduction
Self- and non-self-discrimination is taxonomically widespread and involved in various biological functions. For example, self-incompatibility is a widespread self-discrimination mechanism in flowering plants [1,2] and ascidians [3] that distinguishes between self and non-self pollen and sperm, respectively, to prevent self-fertilization. In the vertebrate immune system, self-discrimination plays a central role in identification and destruction of non-self antigens [4]. The mechanisms of self-discrimination are most often based on genotype-specific differences (allogeneic recognition) between self and non-self, such as major histocompatibility complex in the vertebrate immune system and S-locus in plant self-incompatibility [1,2,4].
Another mechanism of discrimination has been reported in roots [5]. Root discrimination allows plants to change their root allocation and morphology in response to either self or non-self neighbours [6–12]. Root discrimination is thought to be mediated by environmentally based physiological coordination rather than by genotype-specific differences as in self-incompatibility and immune systems [7–9] (however, see [10]). For example, Gruntman & Novoplansky [9] have demonstrated that clones derived from the same plant become related to each other as genetic aliens after temporal separation.
Self-discrimination in roots may be an adaptation to minimize wasteful below-ground competition with self [13] by changing root growth direction and spatial pattern of below-ground parts [14]. Therefore, self-discrimination in plants might have a major impact on a variety of ecological interactions. Although growing evidence supports the self-discrimination ability in roots in below-ground competition, little effort has been made to examine self- and non-self-discrimination at the above-ground level [15]. This is somewhat surprising given the fact that neighbour detection and response in vegetation strongly affect competition for resources such as sunlight [16–19]. Here, we report discrimination by vine tendrils as a new form of plant self-discrimination.
Tendrils are specific organs of vine species in families such as the Vitaceae and Cucurbitaceae, which allow vines to climb host plants and other objects [20]. Tendrils are thigmotropically sensitive and can quickly coil around objects in response to mechanical stimuli. For example, pea tendrils start coiling 2 min after touch and reach a maximum curvature by 32 min [21]. Because vines grow rapidly in both vertical and horizontal directions [22], their tendrils can contact their own leaves and stems. Vegetatively reproducing vines also often touch genetically identical individuals growing beside the parent plant. Climbing plants block access to light and lead to strangulation and collapse of the host under the weight of the climbing plant [22,23]. Therefore, the ability of tendrils to distinguish self from non-self might be an adaptive response to avoid the harmful effects of coiling around and climbing itself. However, self-discrimination has not been reported in climbing plants, even though their growth habits have been studied since Darwin's day [24–26].
To examine self-discrimination ability of climbing plants, we focused on Cayratia japonica, a common perennial vine. This species has many tendrils that enable it to grasp and climb its host plants. Because C. japonica grows rapidly in both vertical and horizontal directions, its tendrils are likely to contact stems and leaves of the same plant. In addition, they are likely to meet vegetatively propagated descendants that are connected via the rhizomes and are genetically identical but physiologically separated from ancestral rhizomes [27]. Whereas coiling around a non-self neighbouring plant would be an adaptive response because it would provide an advantage in competition for light and space, coiling around itself would be harmful because of the self-competition. To detect self-discrimination in vine tendrils, we conducted a coiling experiment using transplanted and naturally grown C. japonica. We compared coiling responses to the following neighbours: physiologically connected self plants, physiologically severed self plants and non-self plants. If self-discrimination in the tendril is based on allogeneic recognition, we would expect the tendrils to be more likely to coil around non-self plants than self plants regardless of physiological connection. If self-discrimination is mediated by physiological coordination, we would expect the tendrils to be more likely to coil around physiologically severed self plants than around physiologically connected self plants. Finally, if both allogeneic recognition and physiological coordination mediate self-discrimination, we would expect the coiling response to decrease in the following order: non-self plants, physiologically severed self plants and physiologically connected self plants. This is because, whereas allogeneic recognition will result in a stronger coiling response to non-self plants than to self plants regardless of physiological connection, physiological coordination mediating self-discrimination will result in a stronger coiling response to physiologically severed self plants than to connected self plants.
2. Methods
(a). Touch experiment with transplanted plants
We collected 18 C. japonica rhizomes (70–200 cm in length and 0.8–4.0 cm in diameter) from three sites at the Fuchu campus of Tokyo University of Agriculture and Technology (Tokyo population) and 24 rhizomes of the same size from three sites at the Hakozaki campus of Kyushu University (Fukuoka population) in May 2014. The sites in each population were located at least 50 m from each other and separated from other sites by asphalt roads. The collected rhizomes were cut into two to four pieces (30–40 cm long) and transplanted individually into a pot with commercial soil mixture (‘Golden’ vermiculite; Iris Ohyama Co. Ltd., Sendai, Japan). All individuals were grown under natural conditions on cultivation shelves in an experimental field at the Fuchu campus for three months. The plants were watered every day. Plants of at least 20 cm in height with multiple tendrils were used.
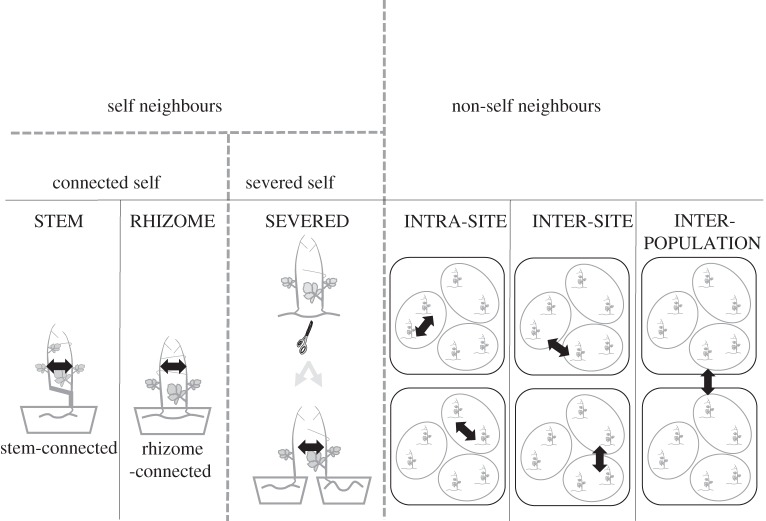
We performed touch experiments (a commonly used approach to evaluate tendril coiling [26,28]). The touch experiment with transplanted plants was started on 2 August and finished on 23 August 2014. Every morning, at 07.00, we chose 20–30 target plants (with fully expanded tendrils) and moved them from cultivation shelves to experimental shelves, which were surrounded by large plastic sheets to prevent wind disturbance (shelf tops were not covered). Target plant tendrils were touched with the stem of a neighbouring plant (figure 1) by fixing stems of the target plants to bamboo sticks with plastic ties. The lower parts of the sticks were inserted into clay. Neighouring plants provided six connection types: STEM, via stems; RHIZOME, via rhizomes; SEVERED, the two plants originated from the same rhizome but were physiologically separated; INTRA-SITE, the two plants originated from different rhizomes at the same sampling site; INTER-SITE, the two plants originated at different sampling sites in the same population; INTER-POPULATION, the two plants originated from different populations. We also touched tendrils with bamboo sticks (0.8–1.2 cm in diameter); the lower parts of the sticks were inserted into clay. We recorded the degree of coiling as uncoiled, slightly coiled (tendrils rotated partially around the stem but did not reach 360°), coiled (at least 360° but not tight), or completely coiled (at least 720° and tight) at 1, 2, 3, 4, 5 and 23 h after contact. Each tendril was used only once and measurements were conducted on 1–4 tendrils per target plant. Plants were randomly assigned to the experiment and IDs of the plants were considered as a random effect in the statistical analysis.
Figure 1.
Diagram of the six types of neighbouring plants (STEM, RHIZOME, SEVERED, INTRA-SITE, INTER-SITE and INTER-POPULATION) in the touch experiment with transplanted C. japonica.
(b). Touch experiment with field-grown plants
We performed touch experiments with field-grown plants using a similar method to the first experiment with transplanted plants [26,28]. Cayratia japonica plants were grown in a field at the Hakozaki campus of Kyushu University. We chose 109 individuals with one or more fully extended uncoiled tendrils (a total of 149 tendrils) as target plants. The experiment was started on 5 July and finished on 14 July 2013. At 08.00, we collected three types of leaves (‘self’, leaves from the parent plant; ‘non-self’, leaves of the same species obtained at least 300 m from the target plant; and ‘other’, leaves of Solidago canadensis). We selected S. canadensis because this species is the dominant weed in this field. Each leaf petiole was covered with wet cotton and placed in a 15 ml plastic tube filled with water. We touched the tendril tips with petioles by wiring the plastic tubes beside the tendrils. We recorded the degree of coiling 2, 4 and 8 h after contact with the leaf as uncoiled, moderately coiled (tendrils had rotated partially around the leaf but did not reach 360°) or completely coiled (at least 360°). The data from tendrils that moved away from the leaves due to wind disturbance (a total of 41, 51 and 40 tendrils for 2, 4 and 8 h after contact, respectively) were excluded from statistical analysis.
(c). Excavation experiment
We also conducted an excavation experiment to examine the effect of rhizome connection between neighbouring plants on the coiling response in the field. We selected pairs of C. japonica plants located within 20 cm of each other and growing together in the same direction from an area of approximately 30 000 m2 at the Hakozaki campus of Kyushu University (a total of 94 plants with 294 tendrils). Selected plants had one to seven tendrils and their above-ground parts were 50–200 cm in length. The experiment was started on 20 July and finished on 28 July 2013. We counted the total number of tendrils and the number of tendrils that coiled around the conspecific neighbouring plant. After measuring the distance between the plants, we excavated all pairs to detect rhizome connections. When the rhizomes of the two neighbouring plants were connected, we defined the plants as a connected pair. If not, we defined the two plants as an unconnected pair. Finally, we calculated the proportions of the tendrils that coiled around connected and unconnected neighbours.
(d). Statistical analysis
(i). Touch experiment with transplanted plants
Ordinal logistic regression with a random effect was used to examine the effects of the six types of neighbouring plants and a bamboo stick on tendril coiling around the stem because the levels of the response variable (the degree of coiling response) are defined on an ordinal scale [29]. The models were fitted by using the clmm function in the ‘ordinal’ package in R [30]. We used the level of coiling at 1, 2, 3, 4, 5 and 23 h after contact as a response variable, the type of the neighbouring plant as an explanatory variable and the target plant ID as a random effect. We analysed the model for each time point separately. To examine the effect of physical connection on coiling, we tested the differences in the levels of coiling between physiologically connected self plants (pooled STEM and RHIZOME data) and physiologically severed self plants (SEVERED). To examine the effect of genetic identity on coiling, we tested the differences between SEVERED and non-self plants (pooled INTRA-SITE, INTER-SITE and INTER-POPULATION data). To examine the effect of the connected organ in physiologically connected plants, we tested the differences between the STEM and RHIZOME data. To examine the effect of genetic differences among the types of non-self plants, we tested the differences between the INTRA-SITE, INTER-SITE and INTER-POPULATION data.
(ii). Touch experiment with field-grown plants
Similar to the touch experiment with transplanted plants, ordinal logistic regression was used to examine the effects of each leaf type presented to the tendril (self, non-self or another species) on tendril coiling around the leaf. We analysed the model for each time point separately. We used the leaf type and tendril order (counted from the uppermost tendril) as explanatory variables, and the level of coiling as a response variable. Whenever we found a significant effect of the leaf type, we used multiple-comparison tests to detect significant differences between conspecific leaves (self versus non-self) and between heterospecific leaves (non-self versus another species) with a sequential Bonferroni procedure to correct _p_-values for multiple tests [31].
(iii). Excavation experiment
We examined the effects of rhizome connection to the neighbour on the probability of coiling around the neighbouring plant. We used generalized linear mixed models with connection to the neighbour (connected versus unconnected), distance to the neighbouring plant and interaction with the neighbouring plant as explanatory variables, and plant ID as a random effect [32]. This analysis used the lmer function of the R software. When the interaction term was not significant, it was excluded from the models. The binomial distribution and logit link function were used in these analyses because the response data could be categorized into two events [33].
For all statistical analyses, we used the likelihood ratio test to evaluate the significance of the explanatory variables.
3. Results
(a). Touch experiment with transplanted plants
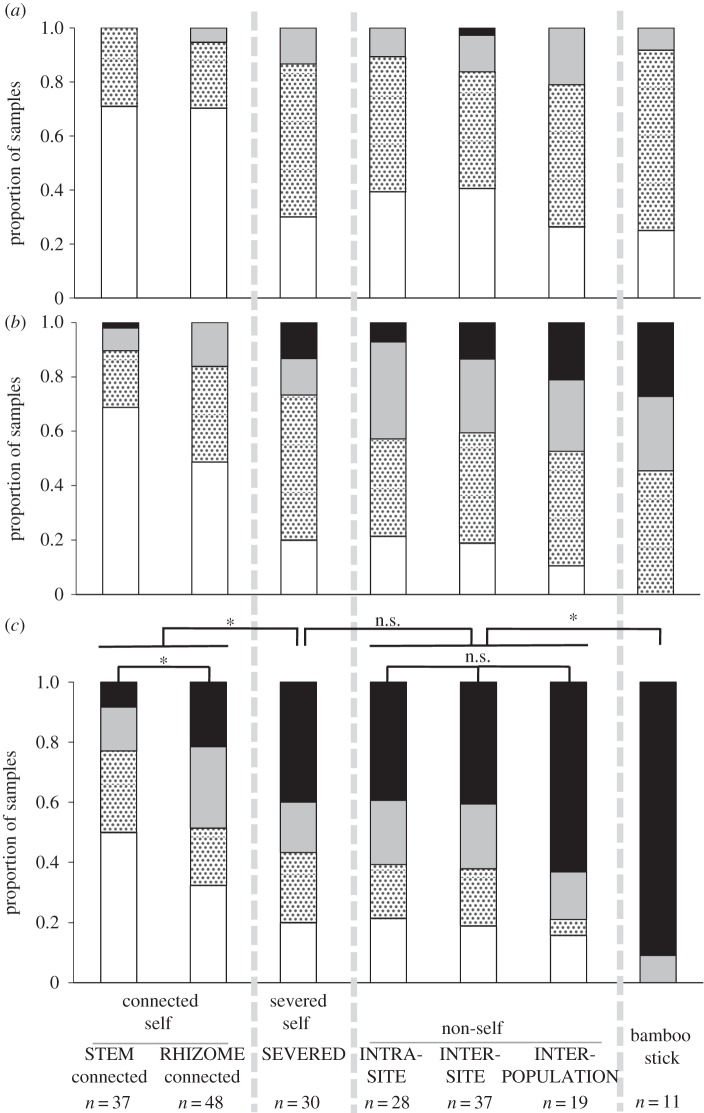
The physiological connection between two plants affected the tendril coiling response (figure 2; electronic supplementary material, table S1). Tendrils were significantly more likely to coil around physically severed self plants (SEVERED) than around physically connected self plants (pooled STEM and RHIZOME data) by 23 h after the start of the experiment. Among the connected plants, tendrils were significantly more likely to coil around plants connected via rhizomes (RHIZOME) than around plants connected via stems (STEM). No significant difference was detected between physiologically severed self (SEVERED) and non-self plants (pooled INTRA-SITE, INTER-SITE and INTER-POPULATION data) or among the types of non-self plants (INTRA-SITE, INTER-SITE and INTER-POPULATION) (figure 2; electronic supplementary material, table S1). Tendrils were significantly more likely to coil around the bamboo stick than around non-self plants (pooled INTRA-SITE, INTER-SITE and INTER-POPULATION data).
Figure 2.
Degree of tendril coiling in transplanted C. japonica that touched self plants (connected via stems or roots, or separated), non-self plants collected from the same site (INTRA-SITE), different sites within the same population (INTER-SITE) or different populations (INTER-POPULATION), or a bamboo stick after (a) 1 h, (b) 5 h and (c) 23 h from the start of the experiment_._ Proportions of completely coiled (black), coiled (grey), slightly coiled (dotted) and uncoiled (white) tendrils are shown. Asterisks indicate statistically significant differences (p < 0.05; see electronic supplementary material, table S1).
(b). Touch experiment with field-grown plants
Tendril coiling responses differed among the leaf types (figure 3; electronic supplementary material, table S2): tendrils were significantly more likely to coil around non-self leaves than around self leaves by 8 h after the start of the experiment, and significantly more likely to coil around S. canadensis leaves than around non-self conspecific leaves 2, 4 and 8 h after the start of the experiment (figure 3; electronic supplementary material, table S2). Tendril order (which reflects their age) also affected the coiling response: old tendrils were significantly more sensitive to the leaf type than young tendrils at 2, 4 and 8 h (electronic supplementary material, table S2).
Figure 3.
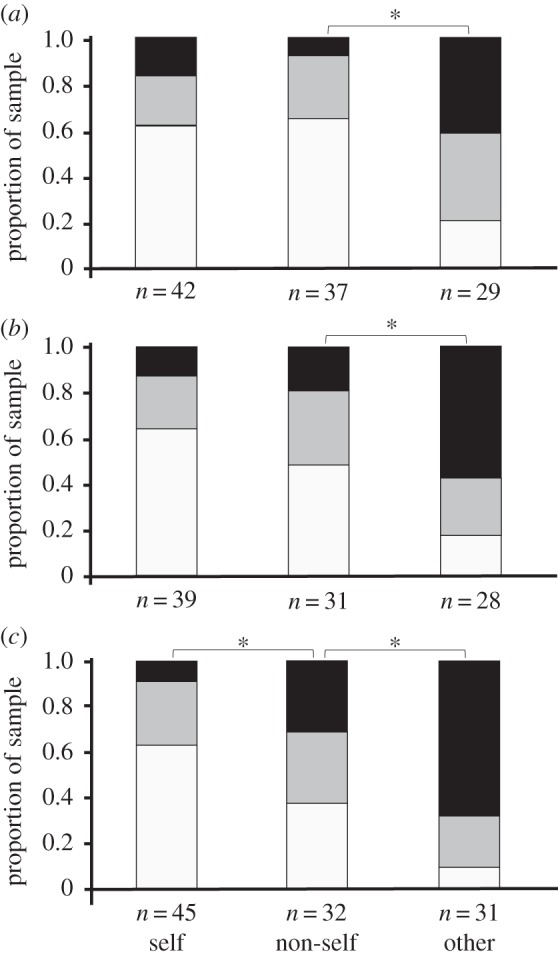
Degree of tendril coiling in field-grown C. japonica that touched leaves collected from the same plant (self), conspecific plants (non-self) or S. canadensis (other) after (a) 2 h, (b) 4 h and (c) 8 h from the start of the experiment. Proportions of uncoiled (white), moderately coiled (grey) and completely coiled (black) tendrils are shown. Asterisks indicate statistically significant differences (p < 0.05; see electronic supplementary material, table S2).
(c). Excavation experiment
The rate of coiling around the neighbouring plant was significantly affected by the type of neighbour (d.f. = 1, 90; deviance = 18.12, p < 0.001) but not by the distance from the neighbour (d.f. = 1, 90; deviance = 1.78, p = 0.182) or by the neighbour type × distance interaction (d.f. = 3, 89; deviance = 0.10, p = 0.75). Tendrils were nearly five times as likely to coil around unconnected neighbours (46.93%) as around connected neighbours (9.27%).
4. Discussion
Root self-discrimination and its consequences for underground competition have been reported [7,9,10,34]. However, the role of self-discrimination in above-ground interactions has largely been ignored (but see [35] for the effect of kin discrimination on above-ground traits). Our study has demonstrated that the vine C. japonica has a self-discrimination ability via its tendrils. The comparison of the coiling response among connected self plants, severed self plants and non-self plants showed that the response was stronger in severed self plants than in connected self plants, and similar in severed self plants and non-self plants (figure 2). In the field experiment, tendrils were more likely to coil around unconnected neighbours than around neighbours connected via rhizomes. These data suggest that, similar to root self-discrimination [7–9], self-discrimination in C. japonica tendrils is mediated by physiological coordination between the tendril and the touched plant. On the other hand, genotype-specific discrimination, as in the case of self-incompatibility and in the immune response, may not play a major role in the self-discrimination in C. japonica tendrils. The observed differences in the coiling response in the touch experiment with transplanted plants were unlikely to be caused by severance because all target plants were derived from a severed rhizome. It was predicted that physiologically based self-discrimination mechanisms that enable avoidance of harmful interactions with the self are common in plants [9]. The present study calls for further work to test this phenomenon in other taxa.
We found that tendrils were more likely to coil around neighbours connected via rhizomes than around neighbours connected via stems. This suggests that the degree of physiological coordination between the tendril and the touched plant is affected by the physiological organ integration. The degree of physiological integration and resource transportation between connected plants can be affected by the distance between them [36,37]. Therefore, the internal physiological environment may be shared between plants connected via stems to a greater extent than between those connected via rhizomes. Avoidance of coiling around plants that share a similar physiological environment with the tendril might be a proximal mechanism of self-discrimination in C. japonica tendrils, although underlying mechanisms are not known.
Tendrils are highly sensitive to mechanical stimuli [28] and the coiling response appears to be mediated by hormones, including octadecanoids, jasmonates and indole-3-acetic acid [38–40]. In line with earlier studies [7,9], Chen et al. [5] proposed internal synchronized oscillation of phytohormones within two ramets as a potential mechanism for physiologically mediated self-discrimination in roots (oscillation hypothesis). This hypothesis states that, as long as the ramets are physiologically connected, internal oscillation remains intact and the roots within two ramets recognize each other as self plant. Once ramets get disconnected, their internal oscillations do not match and their roots recognize each other as belonging to non-self plants. In the same way, matching of the oscillation patterns of phytohormones involved in the coiling response between the tendril surface and the touched plant may promote or suppress thigmotaxis and might be the potential mechanism for self-discrimination in C. japonica tendrils. Volatile chemicals might also affect self-discrimination in C. japonica tendrils. Volatile compounds play an important role in inter-plant interactions [41–43]. Volatile cues from genetically identical sagebrush Artemisia tridentata cuttings induce resistance to herbivores more efficiently than cues from non-self cuttings [15]. These findings indicate the potential importance of communication mediated by volatile compounds in self-discrimination in vine tendrils.
It is unclear whether non-self plants in the touch experiments were really genetically different clones, because C. japonica is a perennial species and easily propagates asexually. Some of the non-self plants, especially in INTRA-SITE pairs in the touch experiment with transplanted plants, might be physiologically disconnected self. Nevertheless, there were no significant differences in the coiling responses among INTRA-SITE, INTER-SITE and INTER-POPULATION pairs. This suggests that genetic differences between the tendril and the touched plant have little influence on the coiling response in comparison with physiological integration between them. Further studies are needed to clarify the effect of genetic differences between the tendril and the touched plant on the coiling response of C. japonica. Our interpretation that self-discrimination in this vine depends on physiological connection is not affected by whether or not some of the INTER-SITE pairs may belong to genetically severed self.
Self-discrimination in tendrils may lead to avoidance of coiling around the self plant, which may in turn change the growth direction and architecture of the vine plant. Because vine coiling is harmful for host plants, the avoidance of self-coiling might increase the negative effect on conspecific and heterospecific neighbours. A mathematical model predicts that self-discrimination in clonal plants can considerably affect their growth and spatial pattern by decreasing competition between neighbouring ramets on the same clonal fragment [14]. The spatial architecture and distribution of C. japonica may be affected by the self-discrimination described in our study, because tendril coiling around plants would be directly related to resource availability and growth direction. Therefore, self-discrimination in vine tendrils minimizes competitive interactions between self plants (‘competitive avoidance’ in [13]). Additional studies must be conducted to test the effects of self-discrimination in the above-ground parts of C. japonica on inter- and intra-specific interactions.
Climbing plants show great species diversity, large biomass and important ecosystem functions in tropical rainforests [22,44]. This study implies that physiological integration of the rhizomes of climbing plants affects their coiling patterns and spatial distribution. Therefore, ecological processes that lead to physiological separation of the rhizomes, such as underground disturbances and herbivory, may change the above-ground growth patterns of climbing plants. Direct observation of these patterns before and after rhizome separation treatment in the natural environment may allow evaluation of the ecological effects of physiologically mediated self-discrimination of climbing plants. Furthermore, here we described self-discrimination in one species of tendril-bearing climbing plant. We need to examine the self-discrimination systems in other forms of climbing plants (those that use stem twining, leaf climbers, root climbers and hook climbers [24,26]). Stem-twining vines might have different self/non-self responses from those of tendril-coiling vines because twining vines often show self-twining when they fail to encounter a suitable support.
In conclusion, our data suggest that self-discrimination in C. japonica tendrils is mediated by physiological coordination of the coiling response. Our study implies that self-discrimination at the above-ground level plays an important role in the architecture and interaction of plants, similar to self-discrimination in roots. Vine plants can become model systems to investigate the ecological impact of self-discrimination on spatial architecture and inter- and intra-specific competition because above-ground interactions of tendrils are much easier to observe in real time than underground interactions of roots.
Supplementary Material
Table S1 Neighbouring plant types affect tendril coiling responses in Cayratia japonica (generalized linear mixed models). Table S2 Tendril order and leaf type affect tendril coiling responses in Cayratia japonica (generalized linear model analysis).
Acknowledgements
We thank Dr Satoshi Nakayama and Dr Takahiro Hosokawa for useful comments on the manuscript, and members of the ecology group at Kyushu University for helpful discussions of our data.
Data accessibility
The datasets supporting this article have been uploaded as part of electronic supplementary material.
Authors' contributions
Both authors contributed equally to this work.
Competing interests
We have no competing interests.
Funding
This work was supported in part by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists (no. 15K18611), Grant-in-Aid for JSPS Fellows (no. 26–3225) and Ono Acoustics Research Fund.
References
- 1.Nasrallah JB. 2002. Recognition and rejection of self in plant reproduction. Science 296, 305–308. ( 10.1126/science.296.5566.305) [DOI] [PubMed] [Google Scholar]
- 2.Nasrallah JB. 2005. Recognition and rejection of self in plant self-incompatibility: comparisons to animal histocompatibility. Trends Immunol. 26, 412–418. ( 10.1016/j.it.2005.06.005) [DOI] [PubMed] [Google Scholar]
- 3.Harada Y, Sawada H. 2008. Allorecognition mechanisms during ascidian fertilization. Int. J. Dev. Biol. 52, 637–645. ( 10.1387/ijdb.072544yh) [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway CA. 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300. ( 10.1126/science.1068883) [DOI] [PubMed] [Google Scholar]
- 5.Chen BJW, During HJ, Anten NPR. 2012. Detect thy neighbor: identity recognition at the root level in plants. Plant Sci. 195, 157–167. ( 10.1016/j.plantsci.2012.07.006) [DOI] [PubMed] [Google Scholar]
- 6.Mahall B, Callaway R. 1992. Root communication mechanisms and intracommunity distributions of two Mojave Desert shrubs. Ecology 73, 2145–2151. ( 10.2307/1941462) [DOI] [Google Scholar]
- 7.Falik O, Reides P, Gersani M, Novoplansky A. 2003. Self/non-self discrimination in roots. J. Ecol. 91, 525–531. ( 10.1046/j.1365-2745.2003.00795.x) [DOI] [Google Scholar]
- 8.Holzapfel C, Alpert P. 2003. Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia 134, 72–77. ( 10.1007/s00442-002-1062-x) [DOI] [PubMed] [Google Scholar]
- 9.Gruntman M, Novoplansky A. 2004. Physiologically mediated self/non-self discrimination in roots. Proc. Natl Acad. Sci. USA 101, 3863–3867. ( 10.1073/pnas.0306604101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang S, Clark RT, Zheng Y, Iyer-Pascuzzi AS, Weitz JS, Kochian LV, Edelsbrunner H, Liao H, Benfey PN. 2013. Genotypic recognition and spatial responses by rice roots. Proc. Natl Acad. Sci. USA 110, 2670–2675. ( 10.1073/pnas.1222821110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahall BE, Callaway RM. 1991. Root communication among desert shrubs. Proc. Natl Acad. Sci. USA 88, 874–876. ( 10.1073/pnas.88.3.874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahall BE, Callaway RM. 1996. Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am. J. Bot. 83, 93–98. ( 10.2307/2445959) [DOI] [Google Scholar]
- 13.Novoplansky A. 2009. Picking battles wisely: plant behaviour under competition. Plant Cell Environ. 32, 726–741. ( 10.1111/j.1365-3040.2009.01979.x) [DOI] [PubMed] [Google Scholar]
- 14.Herben T, Novoplansky A. 2008. Implications of self/non-self discrimination for spatial patterning of clonal plants. Evol. Ecol. 22, 337–350. ( 10.1007/s10682-007-9214-4) [DOI] [Google Scholar]
- 15.Karban R, Shiojiri K. 2009. Self-recognition affects plant communication and defense. Ecol. Lett. 12, 502–506. ( 10.1111/j.1461-0248.2009.01313.x) [DOI] [PubMed] [Google Scholar]
- 16.Schmitt J, McCormac AC, Smith H. 1995. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. Am. Nat. 146, 937–953. ( 10.1086/285832) [DOI] [Google Scholar]
- 17.Pierik R, Mommer L, Voesenek LA. 2013. Molecular mechanisms of plant competition: neighbour detection and response strategies. Funct. Ecol. 27, 841–853. ( 10.1111/1365-2435.12010) [DOI] [Google Scholar]
- 18.De Wit M, Kegge W, Evers JB, Vergeer-van Eijk MH, Gankema P, Voesenek LACJ, Pierik R. 2012. Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proc. Natl Acad. Sci. USA 109, 14 705–14 710. ( 10.1073/pnas.1205437109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen BJW, During HJ, Vermeulen PJ, Anten NPR. 2014. The presence of a below-ground neighbour alters within-plant seed size distribution in Phaseolus vulgaris. Ann. Bot. 114, 937–943. ( 10.1093/aob/mcu162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn K, Bowling A. 2011. Biology and physiology of vines. Hortic. Rev. (Am. Soc. Hortic. Sci). 38, 1–21. [Google Scholar]
- 21.Jaffe MJ, Galston AW. 1968. The physiology of tendrils. Annu. Rev. Plant Physiol. 19, 417–434. ( 10.1146/annurev.pp.19.060168.002221) [DOI] [Google Scholar]
- 22.Putz F, Mooney H. 1991. The biology of vines. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Paul G, Yavitt J. 2011. Tropical vine growth and the effects on forest succession: a review of the ecology and management of tropical climbing plants. Bot. Rev. 77, 11–30. ( 10.1007/s12229-010-9059-3) [DOI] [Google Scholar]
- 24.Isnard S, Silk W. 2009. Moving with climbing plants from Charles Darwin's time into the 21st century. Am. J. Bot. 96, 1205–1221. ( 10.3732/ajb.0900045) [DOI] [PubMed] [Google Scholar]
- 25.Gray A. 1858. Note on the coiling of tendrils of plants. Proc. Am. Acad. Arts Sci. 4, 98–99. [Google Scholar]
- 26.Darwin C. 1865. On the movements and habits of climbing plants. J. Linn. Soc. Lond. Bot. 9, 1–118. ( 10.1111/j.1095-8339.1865.tb00011.x) [DOI] [Google Scholar]
- 27.West A, Lewis D, Richardson R. 2012. Fragment size and planting depth affect the regenerative capacity of bushkiller (Cayratia japonica). Invasive Plant Sci. 5, 397–401. ( 10.1614/IPSM-D-12-00007.1) [DOI] [Google Scholar]
- 28.Braam J. 2005. In touch: plant responses to mechanical stimuli. New Phytol. 165, 373–389. ( 10.1111/j.1469-8137.2004.01263.x) [DOI] [PubMed] [Google Scholar]
- 29.Guisan A, Harrell FE. 2000. Ordinal response regression models in ecology. J. Veg. Sci. 11, 617–626. ( 10.2307/3236568) [DOI] [Google Scholar]
- 30.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Rice WR. 1989. Analyzing tables of statistical tests. Evolution 43, 223–225. ( 10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- 32.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 33.Crawley MJ. 2012. The R book. Chichester, UK: John Wiley and Sons. [Google Scholar]
- 34.Falik O, de Kroon H, Novoplansky A. 2006. Physiologically-mediated self/non-self root discrimination in Trifolium repens has mixed effects on plant performance. Plant Signal. Behav. 1, 116–121. ( 10.4161/psb.1.3.2639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy GP, Dudley SA. 2009. Kin recognition: competition and cooperation in Impatiens (Balsaminaceae). Am. J. Bot. 96, 1990–1996. ( 10.3732/ajb.0900006) [DOI] [PubMed] [Google Scholar]
- 36.D'hertefeldt T, Jónsdóttir I. 1999. Extensive physiological integration in intact clonal systems of Carex arenaria. J. Ecol. 87, 258–264. ( 10.1046/j.1365-2745.1999.00345.x) [DOI] [Google Scholar]
- 37.D'Hertefeldt T, Falkengren-Grerup U. 2002. Extensive physiological integration in Carex arenaria and Carex disticha in relation to potassium and water availability. New Phytol. 156, 469–477. ( 10.1046/j.1469-8137.2002.00529.x) [DOI] [PubMed] [Google Scholar]
- 38.Blechert S, Bockelmann C, Füßlein M, Schrader T, Stelmach B, Niesel U, Weiler E. 1999. Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207, 470–479. ( 10.1007/s004250050506) [DOI] [Google Scholar]
- 39.Stelmach BA, Müller A, Hennig P, Laudert D, Andert L, Weiler EW. 1998. Quantitation of the octadecanoid 12-oxo-phytodienoic acid, a signalling compound in plant mechanotransduction. Phytochemistry 47, 539–546. ( 10.1016/S0031-9422(97)00547-5) [DOI] [PubMed] [Google Scholar]
- 40.Stelmach B, Müller A, Weiler E. 1999. 12-Oxo-phytodienoic acid and indole-3-acetic acid in jasmonic acid-treated tendrils of Bryonia dioica. Phytochemistry 51, 187–192. ( 10.1016/S0031-9422(99)00017-5) [DOI] [Google Scholar]
- 41.Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW. 2000. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125, 66–71. ( 10.1007/PL00008892) [DOI] [PubMed] [Google Scholar]
- 42.Karban R. 2001. Communication between sagebrush and wild tobacco in the field. Biochem. Syst. Ecol. 29, 995–1005. ( 10.1016/S0305-1978(01)00046-1) [DOI] [Google Scholar]
- 43.Runyon J, Mescher M, De Moraes C. 2006. Volatile chemical cues guide host location and host selection by parasitic plants. Science 313, 1964–1967. ( 10.1126/science.1131371) [DOI] [PubMed] [Google Scholar]
- 44.Couvreur TLP, Kissling WD, Condamine FL, Svenning JC, Rowe NP, Baker WJ. 2015. Global diversification of a tropical plant growth form: environmental correlates and historical contingencies in climbing palms. Front. Genet. 5, 1–18. ( 10.3389/fgene.2014.00452) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Neighbouring plant types affect tendril coiling responses in Cayratia japonica (generalized linear mixed models). Table S2 Tendril order and leaf type affect tendril coiling responses in Cayratia japonica (generalized linear model analysis).
Data Availability Statement
The datasets supporting this article have been uploaded as part of electronic supplementary material.