Dural Arteriovenous Fistula-Associated Reversible Parkinsonism with Presynaptic Dopaminergic Loss (original) (raw)
Dear Sir,
Although the association between reversible parkinsonism with dementia and dural arteriovenous fistula (dAVF) is well documented, its pathophysiological mechanism has not been fully described. Moreover, its relationship to the presynaptic dopaminergic system has not been reported, aside from one case report of hemi-parkinsonism due to midbrain arteriovenous malformation. In this report, we describe a patient presenting as parkinsonism who was shown to have dAVF and presynaptic dopaminergic nerve terminal loss based on dopamine transporter imaging. Interestingly, the parkinsonism in this patient improved following the occlusion of the dAVF without anti-parkinsonian drug therapy.
A 75-year-old male with a four-month history of progressive gait difficulty visited our clinic. Upon admission, he exhibited severe bradykinesia and rigidity involving the axial muscles and all four limbs. Gait was short step with decreased bilateral arm swing and mild postural instability. Both hands exhibited 4–5 Hz rest tremors. The subject’s mini-mental status examination score was 18/30 (disorientation in time, memory recall impairment, reduced attention span, and impaired calculation). He had constipation and a previous history of dream enactment behaviors that suggested rapid eye movement sleep behavior disorder (RBD). Visual hallucinations began approximately one month prior to the clinic visit. In addition, he complained of right occipital headache that was aggravated by daily activities. At the time of admission, his unified Parkinson disease rating scale motor score was 49. Brain magnetic resonance imaging (MRI) revealed hyperintensity signals in the thalamus, globus pallidus, and cerebellum, with numerous distended deep cerebral and cortical venous structures (Figure 1A). These observations were highly suggestive of dAVF, which was confirmed by conventional angiography (Figure 1A). Because our initial clinical assessment was probable dementia with Lewy bodies, we performed N-(3-[18F]fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane ([18F]FP-CIT) positron emission tomography to determine whether his parkinsonian symptoms were related to nigrostriatal dopaminergic degeneration. An early phase scan image, which was obtained during first 10 min following [18F]FP-CIT injection, showed perfusion decreases in the left hemisphere including in the basal ganglia, thalamus and cerebellum (Figure 1B). A late phase scan image, which was obtained after 150 min, showed symmetric bilateral decreases in uptake in the basal ganglia with an anteroposterior gradient (Figure 1B), which was not related to hemodynamic changes induced by dAVF (Figure 1C). Considering the risk of future intracranial hemorrhage and venous sinus thrombosis, an embolization of the dAVF was performed. Follow-up angiography showed complete occlusion of the feeding arteries and normalized filling of the straight sinus. There was an improvement in the T2 signal changes on a follow-up brain MRI following the dAVF occlusion. The subject showed gradual improvements in gait disturbance and parkinsonian motor symptoms, and he became able to perform daily activities without difficulty and without the use of anti-parkinson medication. At two months after embolization and without any drug treatment during that period, his parkinsonian speech, cognitive symptoms and hallucinations had spontaneously improved, but his RBD symptoms remained unchanged.
Figure 1.
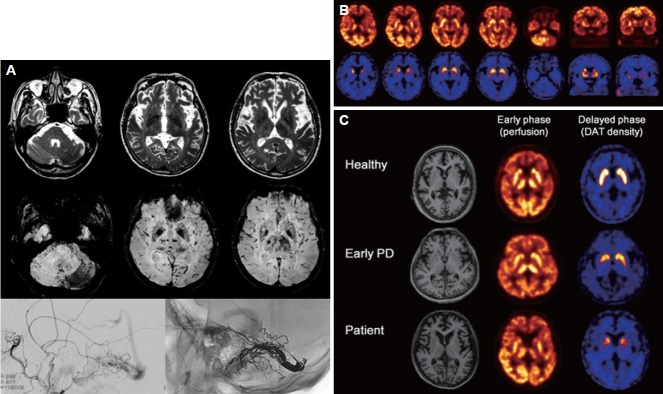
A: Brain magnetic resonance imaging at admission. T2-weighted images (top) show a vasogenic edema in the left thalamocapsular region. Susceptibility-weighted images (middle) show hypointensity signals in the thalamus, cerebellum, and occipital lobe with numerous, distended deep cerebral and cortical venous structures that are prominent on the left side. Bilateral hyposignal intensities in the globus pallidus are also noted. A conventional angiography image obtained before occlusion reveals a dural arteriovenous fistula creating a shunt from the left middle meningeal artery to the left transverse and sigmoid sinuses (bottom right). A post dural arteriovenous fistula occlusion image shows that the feeding arteries have disappeared and an absence of retrograde venous sinus drainage (bottom left). B: Images of N-(3-[18F]fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane positron emission tomography scans. The early phase scan image (upper column) shows decreases in perfusion in the left hemisphere including in the basal ganglia, thalamus, and cerebellum. The late phase scan image (lower column) obtained after 150 min, shows symmetric, bilateral decreases in uptake in the basal ganglia with an anteroposterior gradient. C: Striatal N-(3-[18F]fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane uptake in this patient (bottom) compared to those of a healthy control (top) and a patient with early Parkinson’s disease (middle).
Venous hypertension, which results from increased blood flow via draining veins or from obstruction to drainage, has been associated with the neurological deficits [1]. Reversal of parkinsonism associated with dAVF might be explained by the restoration of cerebral hypoperfusion; however, not all patients with dAVF develop parkinsonism, and there are only a few case reports of reversible parkinsonism in cases of dAVF [2-4].
There is one report of dopamine transporter imaging in supratentorial hemorrahge-associated parkinsonism. In that case, anatomical distortion and compression of the midbrain might have resulted in the decreased 18F-fluorodopa uptake in the side contralateral to the acute supratentorial hematoma. However, in that case, the possible mechanisms such as presynaptic dopaminergic degeneration related to hemodynamic alterations and the possibility of coincident Parkinson’s disease were not elucidated [5]. Our patient is the first reported case to show decreased [18F]FP-CIT uptake in dAVF-associated parkinsonism. He exhibited symptoms suggestive of RBD, which is a premotor sign of synucleinopathy. Thus, we inferred that this patient might have a premanifest parkinsonian disorder, most likely dementia with Lewy bodies or Parkinson’s disease. The early phase scan of our patient showed decreased perfusion, and the amount of dopamine that is released is reported to be dependent on the cerebral blood flow [6]. Thus, these findings suggest that hemodynamic impairment could cause parkinsonism via an accentuation of the underlying dopamine deficiency in subjects with preclinical stage parkinsonism [5].
Because cases with reversible parkinsonism in the presence of dAVF are rare, dopamine transporter imaging studies are warranted in future cases to elucidate the presynaptic dopaminergic alteration–related mechanism of this rare manifestation.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins M, Hussain N, Lee D, Jog MS. Reversible parkinsonism and MRI diffusion abnormalities in cortical venous thrombosis. Neurology. 2001;57:364–366. doi: 10.1212/wnl.57.2.364-a. [DOI] [PubMed] [Google Scholar]
- 3.Lee PH, Lee JS, Shin DH, Kim BM, Huh K. Parkinsonism as an initial manifestation of dural arteriovenous fistula. Eur J Neurol. 2005;12:403–406. doi: 10.1111/j.1468-1331.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda S, Waragai M, Shinotoh H, Takahashi N, Takagi K, Hattori T. Intracranial dural arteriovenous fistula (DAVF) presenting progressive dementia and parkinsonism. J Neurol Sci. 1999;165:43–47. doi: 10.1016/s0022-510x(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 5.Turjanski N, Pentland B, Lees AJ, Brooks DJ. Parkinsonism associated with acute intracranial hematomas: an [18F] dopa positron-emission tomography study. Mov Disord. 1997;12:1035–1038. doi: 10.1002/mds.870120630. [DOI] [PubMed] [Google Scholar]
- 6.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]