Trends of Mycobacterium bovis Isolation and First-Line Anti-tuberculosis Drug Susceptibility Profile: A Fifteen-Year Laboratory-Based Surveillance (original) (raw)
Abstract
Background
Mycobacterium tuberculosis causes the majority of tuberculosis (TB) cases in humans; however, in developing countries, human TB caused by M. bovis may be frequent but undetected. Human TB caused by M. bovis is considered a zoonosis; transmission is mainly through consumption of unpasteurized dairy products, and it is less frequently attributed to animal-to-human or human-to-human contact. We describe the trends of M. bovis isolation from human samples and first-line drug susceptibility during a 15-year period in a referral laboratory located in a tertiary care hospital in Mexico City.
Methodology/Principal Findings
Data on mycobacterial isolates from human clinical samples were retrieved from the laboratory’s database for the 2000–2014 period. Susceptibility to first-line drugs: rifampin, isoniazid, streptomycin (STR) and ethambutol was determined. We identified 1,165 isolates, 73.7% were M. tuberculosis and 26.2%, M. bovis. Among pulmonary samples, 16.6% were M. bovis. The proportion of M. bovis isolates significantly increased from 7.8% in 2000 to 28.4% in 2014 (X 2 trend, p<0.001). Primary STR resistance was higher among M. bovis compared with M. tuberculosis isolates (10.9% vs.3.4%, p<0.001). Secondary multidrug resistance (MDR) rates were 38.5% and 34.4% for M. bovis and M. tuberculosis, respectively (p = 0.637). A rising trend of primary STR monoresistance was observed for both species (3.4% in 2000–2004 vs. 7.6% in 2010–2014; p = 0.02).
Conclusions/Significance
There is a high prevalence and a rising trend of M. bovis isolates in our region. The proportion of pulmonary M. bovis isolates is higher than in previous reports. Additionally, we report high rates of primary anti-tuberculosis resistance and secondary MDR in both M. tuberculosis and M. bovis. This is one of the largest reports on drug susceptibility of M. bovis from human samples and shows a significant proportion of first-line anti-tuberculosis drug resistance.
Author Summary
Human tuberculosis caused by Mycobacterium bovis (HTBMb) is a lesser-known form of the disease. The main route of transmission of HTBMb is the consumption of unpasteurized dairy products, causing mostly extrapulmonary disease. M. bovis is naturally resistant to pyrazinamide, a drug that allows for a shorter treatment course. Therefore, if M. bovis is not properly identified or if there is resistance to other drugs, proper treatment may be hindered. Most laboratories in developing countries do not routinely perform mycobacterial cultures, and only a few laboratories can identify M. bovis. Therefore, HTBMb cases are believed to be underestimated. We report a large proportion of M. bovis isolates and an increasing isolation trend across time. We report a large proportion of M. bovis isolates from pulmonary samples, suggesting the possibility of human-to-human airborne transmission. Also, we showed that M. bovis isolates were more frequently resistant to streptomycin, perhaps as a result of antibiotic usage in cattle. This work underscores the need for identification to the species level, proper susceptibility testing, as well as a stricter control of bovine tuberculosis.
Introduction
Tuberculosis (TB) remains an important health problem in several regions of the world, especially in countries with a high prevalence of HIV infection. Mycobacterium tuberculosis complex (MTBC) includes closely related species among which M. tuberculosis, M. bovis, and M. africanum are the most frequently associated with human disease. [1] Unlike M. tuberculosis, M. bovis can infect a broad range of mammals, including cattle and, therefore, it is considered a zoonosis. The main mechanism of contagion in humans is the consumption of unpasteurized dairy products and, less frequently, animal-to-human and human-to-human contact. [2–4]
Historically, the burden of human TB caused by M. bovis (HTBMb) has been closely related to that of bovine TB (BTB) in the same region.[5] Unfortunately, data from most developing countries, where there is still inappropriate BTB control, is scarce.[6,7] There are several factors that explain the underreporting of HTBMb in these regions. First, control programs rely on acid-fast bacilli (AFB) smears as the primary diagnostic method in suspected TB cases; and mycobacterial culture is performed only when drug resistance is suspected, or treatment is failing. Second, most laboratories use culture medium containing glycerol, and this reduces the probability of M. bovis isolation. Lastly, only a few laboratories can identify MTBC at the species level.[8,9] In Mexico, one national reference laboratory (Instituto Nacional de Diagnóstico y Referencia Epidemiológicos) and 32 public health laboratories are capable of performing mycobacterial cultures. However, species-level identification is not routinely performed. Consequently, data regarding first-line anti-tuberculosis drug susceptibility for MBTC are scarce. Therefore, describing the burden of HTBMb and first-line drug susceptibility pattern has important implications for treatment, referral, and public health policies in developing countries. We describe the trends of M. bovis isolation and first-line drug susceptibility for a referral laboratory in a tertiary care hospital during a 15-year period.
Methods
This study was conducted at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, one of the National Institutes of Health in Mexico. This tertiary care referral center accepts adult patients from all over the country. The patient population involves complex medical and surgical cases including rheumatologic patients, bone marrow and solid organ transplant recipients, and HIV patients. The Laboratory of Clinical Microbiology receives local and referred clinical samples from other National Institutes of Health as well as other hospitals in Mexico City and nearby states for mycobacterial culture. Since 1992, as a regular practice, samples from patients suspected to have TB undergo mycobacterial culture, species-level identification and first-line drug susceptibility testing in all instances. We selected the 2000–2014 period because laboratory practices for mycobacterial isolation, identification, and susceptibility testing became more uniform then.
Ethics statement
Institutional approval for this study was obtained from the Comité Institucional de Investigación Biomédica en Humanos. All data analyzed were anonymized.
Data collection and definitions
A search was performed using the Laboratory of Clinical Microbiology database to investigate all MTBC isolates from January 2000 to December 2014. This database includes both local and referred samples. Only the first isolate was considered for the analysis when multiple samples within a 6-month period were available for a single patient. Whenever there were additional isolates from the same patient ≥ 6 months apart, they were considered as separate episodes and included for analysis.
Data on mycobacterial species, drug susceptibility, sample source, treatment, and referral status were obtained. HIV status was obtained for local samples only. Primary resistance (new cases) was defined as those samples from patients without previous anti-tuberculosis treatment. Secondary resistance (treated cases) was defined as those samples from patients who had previously received any anti-tuberculosis treatment. Resistance to both isoniazid (INH) and rifampin (RIF) was defined as multidrug resistance (MDR). Polydrug resistance was defined as resistance to two or more drugs but excluding those classified as MDR. Samples from sputum, bronchoalveolar lavage, endotracheal aspirate, gastric aspirate, lung and pleural biopsy, and pleural fluid were classified as pulmonary. Liver, spleen, and gastrointestinal biopsies, as well as ascites fluid, and fecal samples were classified as abdominal. Additionally, if samples from the same patient were obtained from a pulmonary and an extrapulmonary source, the case was classified as pulmonary.
Microbiology procedures
As a laboratory standard procedure, all samples obtained from bronchoalveolar lavage, cerebrospinal fluid, and biopsies from any tissue or abscess were cultured in mycobacteria-specific culture medium, regardless of TB suspicion. Sputum and urine samples were cultured for mycobacteria at the request of the treating physician.
Samples were digested and decontaminated by the NALC-NaOH method as previously described. [10,11] After digestion, samples were inoculated in both Löwenstein-Jensen medium and MGIT tubes (Becton-Dickinson, Sparks, MA, USA) according to the manufacturer’s specifications. Biopsy samples were additionally inoculated in Stonebrink culture medium. Additionally, smears from all samples were prepared for Ziehl-Neelsen and Auramine-rhodamine stain. Isolates obtained from MGIT tubes were sub-cultured in Stonebrink and Löwenstein-Jensen medium. All positive cultures were further identified by DNA probe (Accuprobe, GEN-PROBE, San Diego, CA). Biochemical tests (niacin production, nitrate reduction, thiophen-2-carboxylic acid anhydride susceptibility, and pyrazinamidase deamidation) for the identification of M. bovis were performed in those positive cultures with dysgonic growth. [12] Spoligotyping was performed for local isolates as previously described, and data was entered into an international database (www.mbovis.org).[13] Susceptibility testing for anti-tuberculosis drugs was performed for INH, RIF, streptomycin (STR) and ethambutol (EMB). For this purpose, and according to the manufacturer’s specifications, the radiometric BACTEC 460 TB culture system (Becton-Dickinson, Sparks, MA, USA) was used from the years 2000 to 2010 with the following drug concentrations: INH (0.1 μg/mL), RIF (2.0 μg/mL), EMB (7.5 μg/mL), and STR (6.0 μg/mL); then, from 2010 on, the BACTEC MGIT 960 culture system (Becton-Dickinson, Sparks, MA, USA) was used with the following concentrations: INH (0.1 μg/mL), RIF (1.0 μg/mL), EMB (5.0 μg/mL), STR (2.0 μg/mL). This laboratory is subjected to regular quality control evaluations by the Centers for Disease Control and Prevention, and the College of American Pathologists for identification and susceptibility testing of mycobacteria species.
Statistical analysis
Statistical analysis was performed using STATA 11.0 software (StataCorp, College Station, TX, USA). Categorical data was summarized using frequency tables, and the X2 test was used for comparison between groups. The M. bovis case proportion by year was analyzed obtaining a X2 for trend by the Armitage test (regression). A p-value <0.05 was determined as statistically significant for all tests.
Results
Mycobacterial species and patient characteristics
During the study period, 81,521 samples were processed for mycobacterial culture (Fig 1). Among these, 1,165 MTBC isolates were identified, 583 (50.0%) as local samples and 582 (49.9%) as referrals after eliminating duplicate cultures. Of these, 73.7% (859/1,165) were identified as M. tuberculosis, and 26.2% (306/1,165) as M. bovis. Sixty-two percent of the M. tuberculosis isolates and 35.2% of the M. bovis isolates were obtained from pulmonary samples (p<0.001; Table 1). Data on AFB stain were available for 954 isolates; the proportion of positive AFB stain was 75.9% (378/680) for M. tuberculosis, and 24.1% (120/274) for M. bovis (p = 0.001). Among the AFB-positive M. bovis isolates, 45.8% (55/120) were pulmonary, and 54.1% (65/120) were extrapulmonary (p = 0.018). Conversely, among the AFB-positive M. tuberculosis isolates, 79.3% (300/378) were pulmonary and 20.6% (78/378) were extrapulmonary (p<0.001).
Fig 1. Samples processed for mycobacterial culture during the study period.
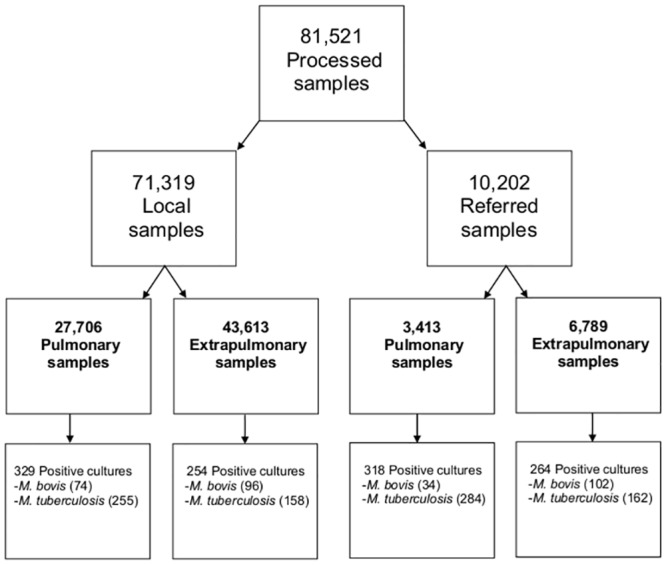
Table 1. Anatomical Site of Isolation.
| Site | Total (N = 1,165) | M. tuberculosis (n = 859) | M. bovis (n = 306) | p* |
|---|---|---|---|---|
| N (%) | n (%) | n (%) | ||
| Pulmonary 1 | 647(55.5) | 539 (62.7) | 108 (35.3) | <0.001 |
| Bone, joint, skin, and soft-tissue | 51 (4.4) | 35 (4.1) | 16 (5.2) | 0.397 |
| Genitourinary | 73 (6.3) | 51 (5.9) | 22 (7.2) | 0.438 |
| Blood or bone marrow | 39 (3.3) | 34 (4) | 5 (1.6) | 0.052 |
| Cerebrospinal fluid | 95 (8.2) | 58 (6.8) | 37 (12.1) | 0.003 |
| Abdominal 2 | 55 (4.7) | 26 (3) | 29 (9.5) | <0.001 |
| Lymph node | 138 (11.8) | 78 (9.1) | 60 (19.6) | <0.001 |
| Other | 67 (5.8) | 38 (4.4) | 29 (9.5) | 0.001 |
| Total extrapulmonary | 518 (44.4) | 320 (37.2) | 198 (64.7) | <0.001 |
One hundred and twelve (19.2%) local isolates were from HIV-infected patients; 63.3% (71/112) were M. tuberculosis, and 36.6% (41/112) were M. bovis (p = 0.054). Among the samples from HIV-infected patients, 52.6% (59/112) were pulmonary samples. Of these, 76.2% (45/59) were identified as M. tuberculosis, and 23.7% (14/59) were M. bovis.
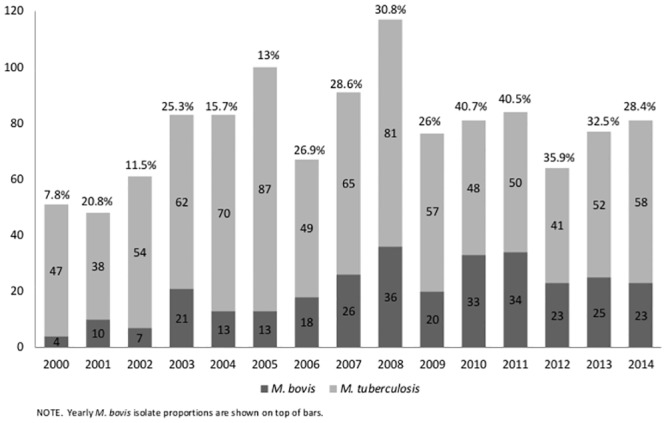
The overall proportion of M. bovis isolation significantly increased from 7.8% in 2000 to 28.4% in 2014 (X 2 trend, p<0.001; Fig 2). Spoligotype pattern was available for 63.5% (108/170) of the local samples (S1 Table).
Fig 2. Mycobacterium tuberculosis complex Isolates per year and M. bovis proportions.
Susceptibility to first-line anti-tuberculosis drugs
Data on first-line anti-tuberculosis drugs susceptibility were available for 1,139 (97.7%) isolates (Table 2). When considering monoresistance among all isolates, 10.9% of M. bovis and 3.2% of M. tuberculosis were resistant to STR (p<0.001). This association remained after stratifying by new and treated cases (p<0.001 and p = 0.032, respectively). Total MDR among all cases was 11.9% for M. tuberculosis and 7.6% for M. bovis (p = 0.038). This same association was observed among new cases (6.8% vs. 3%, p = 0.026). However, among treated cases no difference was observed.
Table 2. First-line drug resistance profile.
| All cases n (%) | Primary resistance (New cases) n (%) | Secondary resistance (Treated cases) n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance profile | Total N = 1,139 | M. tuberculosis n = 835 | M. bovis n = 304 | p* | Total n = 946 | M. tuberculosis n = 681 | M. bovis n = 265 | P | Total n = 193 | M. tuberculosis n = 154 | M. bovis n = 39 | p |
| Any drug resistance | ||||||||||||
| INH | 228 (20) | 179 (21.4) | 49 (16.1) | 0.047 | 135 (14.3) | 104 (15.3) | 31 (11.7) | 0.158 | 93 (48.2) | 75 (48.7) | 18 (46.2) | 0.776 |
| RIF | 143 (12.6) | 114 (13.7) | 29 (9.5) | 0.064 | 72 (7.6) | 58 (8.5) | 14 (5.3) | 0.092 | 71 (36.8) | 56 (36.4) | 15 (38.5) | 0.808 |
| EMB | 27 (2.4) | 25 (3) | 2 (0.7) | 0.022 | 14 (1.5) | 13 (1.9) | 1 (0.4) | 0.080 | 13 (6.7) | 12 (7.8) | 1 (2.6) | 0.245 |
| STR | 120 (10.5) | 84 (10.1) | 36 (11.8) | 0.386 | 90 (9.5) | 59 (8.7) | 31 (11.7) | 0.153 | 30 (15.5) | 25 (16.2) | 5 (12.8) | 0.599 |
| Total any drug resistance | 309 (27.1) | 221 (26.5) | 88 (28.9) | 0.405 | 205 (21.7) | 139 (20.4) | 66 (24.9) | 0.132 | 104 (53.9) | 82 (53.2) | 22 (56.4) | 0.723 |
| Monoresistance | ||||||||||||
| INH | 77 (6.8) | 54 (6.5) | 23 (7.6) | 0.514 | 57 (6) | 36 (5.3) | 21 (7.9) | 0.126 | 20 (10.4) | 18 (11.7) | 2 (5.1) | 0.230 |
| RIF | 19 (1.7) | 13 (1.6) | 6 (2) | 0.627 | 16 (1.7) | 10 (1.5) | 6 (2.3) | 0.394 | 3 (1.6) | 3 (1.9) | 0 (0) | -- |
| EMB | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| STR | 60 (5.3) | 27 (3.2) | 33 (10.9) | <0.001 | 52 (5.5) | 23 (3.4) | 29 (10.9) | <0.001 | 8 (4.1) | 4 (2.6) | 4 (10.3) | 0.032 |
| Total monoresistance | 156 (13.7) | 94 (11.3) | 62 (20.4) | <0.001 | 125 (13.2) | 69 (10.1) | 56 (21.1) | <0.001 | 31 (16.1) | 25 (16.2) | 6 (15.4) | 0.897 |
| Multidrug resistance | ||||||||||||
| INH+RIF | 76 (6.6) | 55 (6.5) | 21 (6.9) | 0.848 | 34 (3.5) | 27 (3.9) | 7 (2.6) | 0.326 | 42 (21.7) | 28 (18.1) | 14 (35.9) | 0.017 |
| INH+RIF+EMB | 15 (1.3) | 13 (1.6) | 2 (0.7) | 0.239 | 6 (0.6) | 5 (0.7) | 1 (0.4) | 0.535 | 9 (4.7) | 8 (5.2) | 1 (2.6) | 0.486 |
| INH+RIF+STR | 24 (2.1) | 24 (2.9) | 0 (0) | -- | 10 (1.1) | 10 (1.5) | 0 (0) | -- | 14 (7.3) | 14 (9.1) | 0 (0) | -- |
| INH+RIF+EMB+STR | 7 (0.6) | 7 (0.8) | 0 (0) | -- | 4 (0.4) | 4 (0.6) | 0 (0) | -- | 3 (1.6) | 3 (1.9) | 0 (0) | -- |
| Total multidrug resistance | 122 (10.7) | 99 (11.9) | 23 (7.6) | 0.038 | 54 (5.7) | 46 (6.8) | 8 (3) | 0.026 | 68 (35.2) | 53 (34.4) | 15 (38.5) | 0.637 |
| Polydrug resistance | ||||||||||||
| INH+EMB | 2 (0.2) | 2 (0.2) | 0 (0) | -- | 2 (0.2) | 2 (0.3) | 0 (0) | -- | 0 (0) | 0 (0) | 0 (0) | -- |
| INH+STR | 25 (2.2) | 22 (2.6) | 3 (1) | 0.093 | 21 (2.2) | 19 (2.8) | 2 (0.8) | 0.056 | 4 (2.1) | 3 (1.9) | 1 (2.6) | 0.809 |
| RIF+STR | 1 (0.1) | 1 (0.1) | 0 (0) | -- | 1 (0.1) | 1 (0.1) | 0 (0) | -- | 0 (0) | 0 (0) | 0 (0) | -- |
| RIF+EMB+STR | 1 (0.1) | 1 (0.1) | 0 (0) | -- | 1 (0.1) | 1 (0.1) | 0 (0) | -- | 0 (0) | 0 (0) | 0 (0) | -- |
| Total polydrug resistance | 31 (2.7) | 28 (3.4) | 3 (1) | 0.03 | 26 (2.7) | 24 (3.5) | 2 (0.8) | 0.019 | 5 (2.6) | 4 (2.6) | 1 (2.6) | 0.991 |
An increasing trend of STR monoresistance among new cases was found when considering both species (3.4% in 2000–2004 vs. 7.6% in 2010–2014, p = 0.02) (Table 3).
Table 3. Primary resistance trends of Mycobacterium tuberculosis complex across time.
| Total % | M. tuberculosis % | M. bovis % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2000–2004 | 2005–2009 | 2010–2014 | p* | 2000–2004 | 2005–2009 | 2010–2014 | p | 2000–2004 | 2005–2009 | 2010–2014 | p |
| No. isolates tested | 258 | 335 | 353 | 213 | 238 | 230 | 45 | 97 | 123 | |||
| Drug resistance pattern | ||||||||||||
| Any drug resistance | ||||||||||||
| Any resistance to INH | 10.1 | 16.2 | 10.4 | 0.874 | 10.9 | 17.1 | 11.2 | 0.963 | 6.3 | 14.2 | 8.9 | 0.977 |
| Any resistance to RIF | 4.1 | 10.7 | 5.6 | 0.690 | 4.5 | 12.2 | 6.1 | 0.573 | 2.2 | 6.7 | 4.7 | 0.755 |
| Any resistance to EMB | 0.8 | 2.0 | 1.4 | 0.595 | 0.9 | 2.9 | 1.7 | 0.567 | 0 | 0 | 0.8 | 0.341 |
| Any resistance to STR | 6.2 | 8.0 | 11.1 | 0.023 | 6.2 | 7.4 | 10.2 | 0.103 | 6.3 | 9.3 | 12.8 | 0.174 |
| Total any drug resistance | 13.4 | 20.2 | 18.5 | 0.119 | 13.4 | 19.9 | 17.0 | 0.308 | 13.5 | 21.1 | 21.2 | 0.320 |
| Monoresistance | ||||||||||||
| Monoresistance to INH | 4.8 | 6.4 | 5.6 | 0.713 | 4.5 | 5.6 | 5.0 | 0.824 | 6.3 | 8.5 | 6.8 | 0.959 |
| Monoresistance to RIF | 0.8 | 1.8 | 2.2 | 0.172 | 0.5 | 2.1 | 1.7 | 0.283 | 2.2 | 1.0 | 3.1 | 0.501 |
| Monoresistance to STR | 3.4 | 4.0 | 7.6 | 0.012 | 2.7 | 2.1 | 5.0 | 0.169 | 6.3 | 8.5 | 12.1 | 0.191 |
| Total monoresistance | 8.5 | 11.4 | 14.1 | 0.023 | 7.4 | 9.2 | 10.9 | 0.187 | 13.5 | 16.4 | 19.6 | 0.281 |
| Multidrug resistance | ||||||||||||
| INH+RIF | 2.3 | 6.9 | 1.4 | 0.333 | 2.8 | 7.1 | 1.7 | 0.514 | 0 | 6.2 | 0.8 | 0.583 |
| INH+RIF+EMB | 0.4 | 0.9 | 0.6 | 0.839 | 0.5 | 1.2 | 0.4 | 0.945 | 0 | 0 | 0.8 | 0.341 |
| INH+RIF+STR | 0.8 | 1.5 | 0.8 | 0.999 | 0.9 | 2.1 | 1.3 | 0.770 | 0 | 0 | 0 | -- |
| INH+RIF+EMB+STR | 0 | 0.9 | 0.3 | 0.708 | 0 | 1.2 | 0.4 | 0.577 | 0 | 0 | 0 | -- |
| Total Multidrug resistance | 3.4 | 9.2 | 3.0 | 0.566 | 4.1 | 10.5 | 3.8 | 0.837 | 0 | 5.8 | 1.6 | 0.866 |
Discussion
This report demonstrates a high prevalence and a rising trend in the proportion of M. bovis isolation in our laboratory. This report is one of the largest on first-line anti-tuberculosis drug profile of M. bovis and shows a noteworthy proportion of first-line anti-tuberculosis drug resistance and secondary MDR isolates.
The proportion of M. bovis isolates in this study (26.2%) is much higher than that reported by other hospital-based studies in Latin America (0.4%) and by other hospitals in Mexico (<1%). [14,15] This may be explained by the larger study period of the present report and the high proportion of samples from immunosuppressed patients who are at a greater risk for M. bovis infection, as documented in previous studies.[16] In fact, isolates obtained from HIV-infected patients accounted for 19.2% of the local samples.[17]
We also identified a rising trend in the proportion of cases caused by M. bovis across time. HMBTb is considered a reflection of the BTB burden in the region. In fact, we recently identified a high burden of bovine and human TB in a dairy production facility in rural Mexico. [2] This also correlates with previous reports of M. bovis among artisanal dairy products, which have been linked to HMBTb cases in Mexico and along the south border cities of the United States.[18,19] Mexico is considered a country of “sporadic occurrence” of BTB by the World Organization for Animal Health. However, like in many countries in the region, the test-and-slaughter strategy for bovine tuberculosis control is not universally implemented. [6] The main obstacles for BTB control in Mexico are financial and cultural. Official government data reports an overall prevalence of BTB of 2.05% in 2015, but it reaches 16.5% among dairy farms.[20] The reason for this difference may be explained by the fact that meat producing regions require to be certified as BTB free for cattle export. On the contrary, in dairy production farms, BTB only mildly affects production and pasteurization eliminates M. bovis. [21] Unfortunately, about 30% of the milk production in Mexico is sold without pasteurization, mostly to small retailers and artisan cheese producers.[22]
The respiratory route of contagion is considered less efficient for M. bovis than for M. tuberculosis. However, recent data detailing outbreaks in the community and hospitals demonstrated that human-to-human contagion is not as unlikely as previously believed.[3,23,24] Interestingly, we observed an important proportion (16.6%) of M. bovis isolates recovered from pulmonary samples from a mainly urban population. Therefore, it may be hypothesized that airborne human-to-human transmission of M. bovis may occur in the community, but remains undetected in our region given that mycobacterial culture is not routinely performed. Unfortunately, as an important limitation of this study, the lack of clinical and epidemiologic data precludes us from reaching a definite conclusion.
We also report a high rate of first-line anti-tuberculosis resistance for MBTC. The proportion of INH (14%), RIF (7.6%), and STR (5.5%) primary resistance found among new cases is considerably higher for INH and RIF than in previous reports from our group in 1995 (INH 6%, RIF 2%, STR 6%).[25] These proportions are also higher than those from other reports in Mexico (1995 to 2006) among new cases (INH 9%, RIF 3%, and MDR 4.5%), and are also higher than recent data from the National Survey on TB Drug Resistance in Mexico (INH 3.5%, RIF 0.1%, STR 4% and MDR 2.3%).[26,27] This discrepancy may be explained by the different periods from which data was collected and the dissimilar patient population (ours being hospital-based and including more HIV-infected, and immunosuppressed patients).
When comparing the resistance profiles of M. bovis and M. tuberculosis, we observed a considerably higher primary MDR M. tuberculosis proportion, and a significantly higher STR monoresistance among M. bovis isolates. Data regarding drug susceptibility for M. bovis TB in humans and animals is limited. A study from San Diego reported 7% resistance for INH and 1% for RIF among 167 M. bovis TB cases.[28] Drug susceptibility has also been reported from outbreaks caused by MDR strains; however, most other case series of HTBMb report full susceptibility to all first-line anti-tuberculosis drugs.[14,15,29–32] A report from the National TB Genotyping Service of the United States informed of 17% of STR resistance among 165 M. bovis isolates; however, no explanation for this was proposed. [33] Drug susceptibility from M. bovis isolates collected from farm animals or wildlife is almost uniformly reported as fully susceptible to anti-tuberculosis drugs.[34–36]. We believe that a high proportion of primary resistance for STR among M. bovis isolates, may be explained by the use of aminoglycosides for treating other diseases in cattle.[37] Some have suggested that primary resistance to INH and RIF in M. bovis may indicate human-to-human transmission.[32] However, we surveyed BTB in a dairy farm, where samples from cows were obtained during necropsy. We recovered 150 M. bovis isolates; among these, we observed an even higher rate of STR resistance (15.6%) and a similar rate of INH (9.2%) and RIF (3.4%) resistance. (M. Bobadilla, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, personal communication).
It has been suggested that HTBMb cases may be at a higher risk for developing MDR strains if natural resistance to pyrazinamide is not considered and monoresistance to INH or RIF is present. [38,39] We identified a similar proportion of secondary MDR M. bovis and M. tuberculosis isolates. Unfortunately, only a few cases were analyzed and no data on previous treatments or outcomes were available, therefore we are unable to conclude if this assumption is true. Nevertheless, it should be recognized that TB cases caused by MDR M. bovis may result in disease that is harder to treat on a second- line drug regimen. This highlights the need for performing species-level identification and drug susceptibility testing whenever M. bovis is suspected.
In conclusion, we believe that data contained in this study is relevant in terms of public health and highlights the need for more stringent control of BTB in our country. It also underscores the importance of proper identification of M. bovis given that the considerable rate of primary resistance to INH and RIF along with the natural pyrazinamide resistance may result in treatment failures and select for MDR strains.
Supporting Information
S1 Table. Spoligotypes of Mycobacterium bovis isolates from Human Samples in a Tertiary Care Hospital in Mexico City: 2000–2014.
(DOCX)
S1 Checklist. STROBE Checklist.
(DOC)
Acknowledgments
The authors would like to express great appreciation to Beatriz E. Remus MD and Guillermo M. Ruíz-Palacios MD, for their valuable and constructive suggestions during the preparation of the final manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Cosivi O, Meslin FX, Daborn CJ, Grange JM (1995) Epidemiology of Mycobacterium bovis infection in animals and humans, with particular reference to Africa. Rev Sci Tech 14: 733–746. [DOI] [PubMed] [Google Scholar]
- 2.Torres-Gonzalez P, Soberanis-Ramos O, Martinez-Gamboa A, Chavez-Mazari B, Barrios-Herrera MT, et al. (2013) Prevalence of latent and active tuberculosis among dairy farm workers exposed to cattle infected by Mycobacterium bovis. PLoS Negl Trop Dis 7: e2177 10.1371/journal.pntd.0002177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, et al. (2007) Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet 369: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 4.LoBue PA, Betacourt W, Peter C, Moser KS (2003) Epidemiology of Mycobacterium bovis disease in San Diego County, 1994–2000. Int J Tuberc Lung Dis 7: 180–185. [PubMed] [Google Scholar]
- 5.Grange JM (2001) Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 81: 71–77. [DOI] [PubMed] [Google Scholar]
- 6.Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, et al. (1998) Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis 4: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller B, Durr S, Alonso S, Hattendorf J, Laisse CJ, et al. (2013) Zoonotic _Mycobacterium bovis_-induced tuberculosis in humans. Emerg Infect Dis 19: 899–908. 10.3201/eid1906.120543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kantor IN, Ambroggi M, Poggi S, Morcillo N, Da Silva Telles MA, et al. (2008) Human Mycobacterium bovis infection in ten Latin American countries. Tuberculosis (Edinb) 88: 358–365. [DOI] [PubMed] [Google Scholar]
- 9.de Kantor IN, LoBue PA, Thoen CO (2010) Human tuberculosis caused by Mycobacterium bovis in the United States, Latin America and the Caribbean. Int J Tuberc Lung Dis 14: 1369–1373. [PubMed] [Google Scholar]
- 10.Vestal AL, United States. National Communicable Disease Center. Laboratory Consultation and Development Section. (1969) Procedures for the isolation and identification of mycobacteria. Atlanta,: National Communicable Disease Center, Laboratory Consultation and Development Section; for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington. vii, 118 p. p.
- 11.Lennette EH, Spaulding EH, Truant JP (1974) Manual of clinical microbiology. Washington: American Society for Microbiology; xxi, 970 p. p. [Google Scholar]
- 12.Balows A, American Society for Microbiology. (1991) Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; xix, 1364 p. p. [Google Scholar]
- 13.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, et al. (1997) Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordova E, Gonzalo X, Boschi A, Lossa M, Robles M, et al. (2012) Human Mycobacterium bovis infection in Buenos Aires: epidemiology, microbiology and clinical presentation. Int J Tuberc Lung Dis 16: 415–417. 10.5588/ijtld.10.0605 [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Alvarez R, Badillo-Lopez C, Cerna-Cortes JF, Castillo-Ramirez I, Rivera-Gutierrez S, et al. (2010) First insights into the genetic diversity of Mycobacterium tuberculosis isolates from HIV-infected Mexican patients and mutations causing multidrug resistance. BMC Microbiol 10: 82 10.1186/1471-2180-10-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Qin H, Jain S, Preziosi M, Minuto JJ, et al. (2010) Tuberculosis due to Mycobacterium bovis in patients coinfected with human immunodeficiency virus. Clin Infect Dis 51: 1343–1346. 10.1086/657118 [DOI] [PubMed] [Google Scholar]
- 17.Cicero R, Olivera H, Hernandez-Solis A, Ramirez-Casanova E, Escobar-Gutierrez A (2009) Frequency of Mycobacterium bovis as an etiologic agent in extrapulmonary tuberculosis in HIV-positive and-negative Mexican patients. Eur J Clin Microbiol Infect Dis 28: 455–460. 10.1007/s10096-008-0649-5 [DOI] [PubMed] [Google Scholar]
- 18.Rodwell TC, Kapasi AJ, Moore M, Milian-Suazo F, Harris B, et al. (2010) Tracing the origins of Mycobacterium bovis tuberculosis in humans in the USA to cattle in Mexico using spoligotyping. Int J Infect Dis 14 Suppl 3: e129–135. 10.1016/j.ijid.2009.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira-Suarez AL, Estrada-Chavez Y, Zuniga-Estrada A, Lopez-Rincon G, Hernandez DU, et al. (2014) Detection of Mycobacterium tuberculosis complex by PCR in fresh cheese from local markets in Hidalgo, Mexico. J Food Prot 77: 849–852. [DOI] [PubMed] [Google Scholar]
- 20.SENASICA. Situacion actual. Campaña Nacional contra la tuberculosis bovina. Available: http://www.senasica.gob.mx/?id=4369
- 21.de Kantor IN, Ritacco V (2006) An update on bovine tuberculosis programmes in Latin American and Caribbean countries. Vet Microbiol 112: 111–118. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Guerrero L, Milian-Suazo F, Arriaga-Diaz C, Romero-Torres C, Escartin-Chavez M (2008) [Molecular epidemiology of cattle and human tuberculosis in Mexico]. Salud Publica Mex 50: 286–291. [DOI] [PubMed] [Google Scholar]
- 23.LoBue PA, LeClair JJ, Moser KS (2004) Contact investigation for cases of pulmonary Mycobacterium bovis. Int J Tuberc Lung Dis 8: 868–872. [PubMed] [Google Scholar]
- 24.Guerrero A, Cobo J, Fortun J, Navas E, Quereda C, et al. (1997) Nosocomial transmission of Mycobacterium bovis resistant to 11 drugs in people with advanced HIV-1 infection. Lancet 350: 1738–1742. [DOI] [PubMed] [Google Scholar]
- 25.Sifuentes-Osornio J, Ponce-de-Leon LA, Camacho-Mezquita FE, Bobadilla-del-Valle JM, Infante-Suarez ML, et al. (1995) [Resistance of Mycobacterium tuberculosis in Mexican patients. I. Clinical features and risk factors]. Rev Invest Clin 47: 273–281. [PubMed] [Google Scholar]
- 26.Bojorquez-Chapela I, Backer CE, Orejel I, Lopez A, Diaz-Quinonez A, et al. (2013) Drug resistance in Mexico: results from the National Survey on Drug-Resistant Tuberculosis. Int J Tuberc Lung Dis 17: 514–519. 10.5588/ijtld.12.0167 [DOI] [PubMed] [Google Scholar]
- 27.Zazueta-Beltran J, Leon-Sicairos C, Canizalez-Roman A (2009) Drug resistant Mycobacterium tuberculosis in Mexico. J Infect Dev Ctries 3: 162–168. [DOI] [PubMed] [Google Scholar]
- 28.LoBue PA, Moser KS (2005) Treatment of Mycobacterium bovis infected tuberculosis patients: San Diego County, California, United States, 1994–2003. Int J Tuberc Lung Dis 9: 333–338. [PubMed] [Google Scholar]
- 29.Samper S, Martin C, Pinedo A, Rivero A, Blazquez J, et al. (1997) Transmission between HIV-infected patients of multidrug-resistant tuberculosis caused by Mycobacterium bovis. AIDS 11: 1237–1242. [DOI] [PubMed] [Google Scholar]
- 30.Rivero A, Marquez M, Santos J, Pinedo A, Sanchez MA, et al. (2001) High rate of tuberculosis reinfection during a nosocomial outbreak of multidrug-resistant tuberculosis caused by Mycobacterium bovis strain B. Clin Infect Dis 32: 159–161. [DOI] [PubMed] [Google Scholar]
- 31.Laniado-Laborin R, Muniz-Salazar R, Garcia-Ortiz RA, Vargas-Ojeda AC, Villa-Rosas C, et al. (2014) Molecular characterization of Mycobacterium bovis isolates from patients with tuberculosis in Baja California, Mexico. Infect Genet Evol 27: 1–5. 10.1016/j.meegid.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 32.Hughes VM, Skuce R, Doig C, Stevenson K, Sharp JM, et al. (2003) Analysis of multidrug-resistant Mycobacterium bovis from three clinical samples from Scotland. Int J Tuberc Lung Dis 7: 1191–1198. [PubMed] [Google Scholar]
- 33.Hlavsa MC, Moonan PK, Cowan LS, Navin TR, Kammerer JS, et al. (2008) Human tuberculosis due to Mycobacterium bovis in the United States, 1995–2005. Clin Infect Dis 47: 168–175. 10.1086/589240 [DOI] [PubMed] [Google Scholar]
- 34.Parreiras PM, Lobato FC, Alencar AP, Figueiredo T, Gomes HM, et al. (2004) Drug susceptibility of Brazilian strains of Mycobacterium bovis using traditional and molecular techniques. Mem Inst Oswaldo Cruz 99: 749–752. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald SD, Schooley AM, Berry DE, Kaneene JB (2010) Antimicrobial Susceptibility Testing of Mycobacterium bovis Isolates from Michigan White-Tailed Deer during the 2009 Hunting Season. Vet Med Int 2011: 903683 10.4061/2011/903683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly M, Diegel KL, Fitzgerald SD, Schooley A, Berry DE, et al. (2006) Patterns of antimicrobial susceptibility in Michigan wildlife and bovine isolates of Mycobacterium bovis. J Vet Diagn Invest 18: 401–404. [DOI] [PubMed] [Google Scholar]
- 37.Economou V, Gousia P (2015) Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 8: 49–61. 10.2147/IDR.S55778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin AM, Gibbons N, Fitzgibbon M, Power JT, Foley SC, et al. (2012) Primary isoniazid resistance in Mycobacterium bovis disease: a prospect of concern. Am J Respir Crit Care Med 186: 110–111. [DOI] [PubMed] [Google Scholar]
- 39.Kurbatova EV, Cavanaugh JS, Dalton T, E SC, Cegielski JP (2013) Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999–2009. Clin Infect Dis 57: 1081–1093. 10.1093/cid/cit452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Table. Spoligotypes of Mycobacterium bovis isolates from Human Samples in a Tertiary Care Hospital in Mexico City: 2000–2014.
(DOCX)
S1 Checklist. STROBE Checklist.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.