The Effect of Ginger (Zingiber officinale) on Platelet Aggregation: A Systematic Literature Review (original) (raw)
Abstract
Background
The potential effect of ginger on platelet aggregation is a widely-cited concern both within the published literature and to clinicians; however, there has been no systematic appraisal of the evidence to date.
Methods
Using the PRISMA guidelines, we systematically reviewed the results of clinical and observational trials regarding the effect of ginger on platelet aggregation in adults compared to either placebo or baseline data. Studies included in this review stipulated the independent variable was a ginger preparation or isolated ginger compound, and used measures of platelet aggregation as the primary outcome.
Results
Ten studies were included, comprising eight clinical trials and two observational studies. Of the eight clinical trials, four reported that ginger reduced platelet aggregation, while the remaining four reported no effect. The two observational studies also reported mixed findings.
Discussion
Many of the studies appraised for this review had moderate risks of bias. Methodology varied considerably between studies, notably the timeframe studied, dose of ginger used, and the characteristics of subjects recruited (e.g. healthy vs. patients with chronic diseases).
Conclusion
The evidence that ginger affects platelet aggregation and coagulation is equivocal and further study is needed to definitively address this question.
Introduction
There is increasing evidence that ginger and its constituents might exert meaningful anti-nausea effects during cancer chemotherapy. Our recent systematic review of the literature found preliminary evidence that supported its use as an adjuvant anti-nausea drug to standard anti-emetics in the chemotherapy setting.[1] Concerns over potential “off target” antiplatelet effects, however, could limit the application of ginger in oncology patients, who frequently experience thrombocytopenia due to myelosuppression.
The ginger rhizome has been used in traditional systems of medicine for centuries and more recently, its potentially medicinal properties have been empirically studied.[2] Current research suggests that the active constituents of ginger, namely the gingerol and shogaol classes of compounds, might exert several beneficial effects including anti-inflammatory, antioxidant, and cholesterol lowering properties.[2] In addition, ginger is a promising treatment for nausea associated with a variety of stimuli including post-operative nausea and vomiting, motion sickness, morning sickness, and chemotherapy-induced nausea and vomiting.[1, 3–5]
While the safety profile of ginger supplementation requires further investigation, previous clinical trials report few side-effects, mostly minor in nature (e.g. mild nausea, heartburn).[1] Of these reported side effects, potentially the most significant is an antiplatelet effect. Two published case-studies reported adverse symptoms and abnormal platelet aggregation that was temporally related to recent ingestion of ginger products.[6, 7] In addition, several animal and in vitro studies have reported ginger as well as individual ginger compounds to have an effect on platelet aggregation.[8–10] While this action could be beneficial in vascular diseases, it could potentiate bleeding risk in conditions such as thrombocytopenia or pre-existing platelet dysfunction. This is particularly relevant in the chemotherapy setting, where therapy-induced thrombocytopenia is associated with treatment delays, dose reductions, and bleeding events.[11]
To the authors knowledge, Srivastava et al.[8] were the first group to investigate the effect of ginger on platelet aggregation by using four ginger extracts, produced using different solvents (aqueous, n-hexane, chloroform, and ethyl acetate). They reported that ginger inhibited platelet aggregation using arachidonic acid (AA), epinephrine, adenosine diphosphate (ADP), and collagen as agonists. Others have corroborated this, reporting that certain ginger compounds inhibit in vitro platelet aggregation when using a variety of agonists (AA, collagen, platelet activating factor, and thrombin).[12, 13] This reduction in platelet aggregation was most potent when AA was used as the agonist, requiring lower concentrations to cause inhibition when compared to the other agonists.[9, 12]
While few studies investigating the effect of ginger and its compounds on the clotting cascade have been undertaken, a considerable amount of in vitro research suggests that ginger compounds interact with AA-derived eicosanoid and thromboxane synthesis.[14–18] The AA cascade can produce the eicosanoids involved in inflammation (i.e. prostaglandin E2) as well as thromboxane, which is amongst the many agonists of platelet aggregation. Numerous studies indicate that ginger extract and particular ginger compounds inhibit products specific to the cyclooxygenase pathway, including a reduction in thromboxane B2 (TxB2) production,[19] prostaglandin formation (PGF2a, PGE2, and PGD2),[8, 15] and cyclooxygenase enzyme activity.[16, 18] These same compounds also interact with the lipoxygenase pathway, including reductions in 5-lipoxygenase enzyme activity.[14] Finally, ginger compounds might also inhibit the activity of phospholipase A2, which suggests that ginger exerts its anti-platelet aggregating as well as its potential anti-inflammatory actions through interaction with one of the initial steps in this pathway.[20]
Due to the observed in vitro effects of ginger on the AA cascade, excessive bleeding and interactions with platelet therapy during cancer chemotherapy are of clinical concern. While the results of in vitro studies are consistent, these results are not always translatable to the complex human system. Clinical and observational data, however, provide a reasonable indication of the potential human response. There is a growing body of clinical and epidemiological literature in this area, although no systematic appraisal of the relevant literature has been undertaken to date. In this paper, we summarise and discuss the findings of clinical and observational studies regarding the effect of ginger, compared to placebo or baseline, on platelet aggregation in multiple participant populations.
Methodology
2.1 Data Sources and Searches
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,[21] a systematic search of the literature was conducted using the following databases: MEDLINE, CiNAHL, Embase, and Cochrane Library. The reference lists of retrieved papers were also searched for additional manuscripts. Search terms were not limited by a specific timeframe; rather, all search queries were from the date of the journal’s inception to May 2014. Search terms were broad so as to ensure all relevant manuscripts were captured.
2.2 Study Selection
The search terms used were “ginger AND (platelet OR thrombo* OR clot* OR bleed OR “adverse effects” OR “side effects” OR haemorrhage)”. Studies included in this review 1) were written in English 2) stipulated the independent variable was a ginger preparation or isolated ginger compound, and 3) used measures of platelet aggregation as the primary outcome.
2.3 Data Extraction and Quality Assessment
Extracted data included: participant demographic (e.g age, gender, reported comorbidities), type of ginger intervention (e.g dosage, timing, form of ginger), study design characteristics (e.g. sample size, risk of bias, type of study, study length), and reported outcomes (e.g measures of platelet aggregation, adverse events, dropout rates).
All clinical studies were individually rated for evidence level by author WM using the National Health and Medical Research Council Hierarchy of Evidence guidelines (IV-I, with I being the strongest level of evidence).[22] They were also independently assessed for bias, by two authors (WM and DM) using the Cochrane Handbook for Systematic Reviews of Interventions checklist.[23] Where insufficient information was included in the manuscript to assess particular forms of bias, further information was sought via correspondence with the study authors. Blinding is unlikely to affect the results of the clinical biomarkers measured in these studies, hence trials that were not blinded were rated in the review as low-risk for detection and performance included bias. In addition, due to the small number of trials in this area, no study was excluded based on its risk of bias.
2.4 Data Synthesis and Analysis
A statistically significant (P≤0.05) result was considered evidence of an effect. Relevant study details were retrieved from their respective manuscript using a standardised form. Forest plot and meta-analysis was intended; however, due to the heterogeneity of the studies included in this review, these analyses were found to be unfeasible.
Results
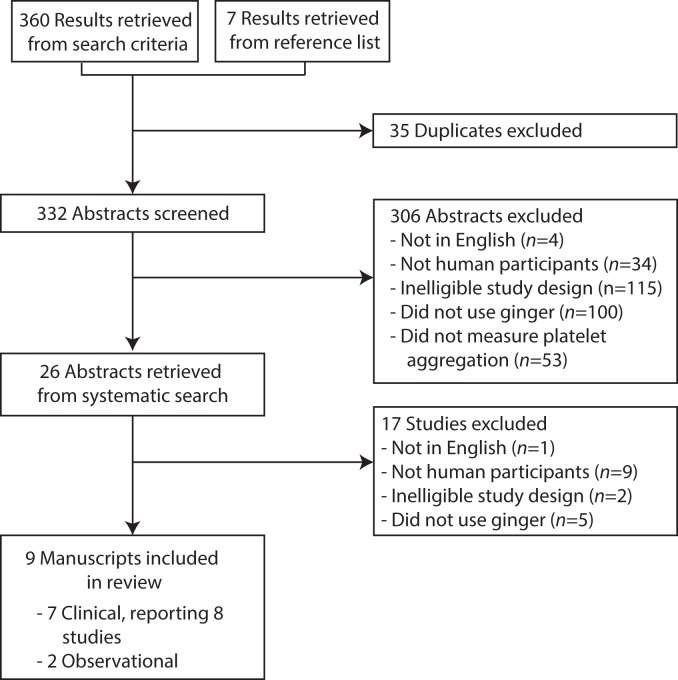
A total of 367 papers were identified (Fig 1). After assessment of study abstracts and the removal of duplicates 26 abstracts were retrieved for further examination. Seventeen were subsequently excluded, resulting in 9 manuscripts included in the final review.
Fig 1. PRISMA study flow diagram.
3.1 Clinical Trials
Seven manuscripts reporting the effect of ginger on platelet aggregation in human participants using a clinical trial design were retrieved (Table 1). Of the seven manuscripts, one described two separate trials, resulting in a total of eight clinical trials included in this review.[24]
Table 1. Extraction table of reviewed clinical trials.
| Author/Date | Study design | Time points | Population | Intervention | Outcome | Results | Country | Level of evidence | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Bordia et al. 1997 | Placebo-controlled trial | Total study period: 3 months. | Patients with confirmed myocardial infarction N = 60 | Dose: 4g per day Unstandardized capsules | Platelet aggregation—Agonist(s): ADP and Epi | Ginger had no significant effect on both measures of aggregation | India | III-1* | Ginger had no significant effect on blood lipids or blood sugar. |
| Outcomes measured at: baseline, 1.5 months and 3 months. | - Method (Device, if reported): Turbidimetric | Results relating to fenugreek excluded from table. | |||||||
| Fibrinogen | No mention of randomisation | ||||||||
| Fibrinolytic activity | P value not reported | ||||||||
| Bordia et al. 1997 | Placebo-controlled trial | Total study period: One day | Patients with confirmed myocardial infarction | Dose: 10g single dose | Platelet aggregation | Reduction of both measures of platelet aggregation when compared to placebo (p<0.05). | India | III-1 | This study was detailed in same manuscript as above. |
| Outcomes measured at: baseline, 4 hours post-consumption | N = 20 | Unstandardized capsules | - Agonist(s): ADP and Epi | ||||||
| - Method (Device, if reported): Turbidimetric | |||||||||
| Janssen et al. 1996 | Randomised, placebo-controlled cross-over trial | Total study period: 6 weeks (3x2 weeks) | Healthy volunteers | Dose: 15g raw & 40g cooked ginger placebo, once per day. | Thromboxane B2 production (Payton Aggregation Module) | Both types of ginger had no significant effect on maximum thromboxane B2 production (p = 0.616) | Netherlands | II | |
| Outcomes measured at day 12 and 14 of each study period. | Age: 22±3 | Contained within 125g custard | |||||||
| N = 18 | |||||||||
| Jiang et al. 2004 | Randomized, open label, three-way cross-over trial | Total study period: 3x13 days, 14 days washout period between each study period. | Healthy male volunteers | Dose: 3.6g (3x 0.4g, thrice per day) | Platelet aggregation | No significant changes in any outcome | Australia | III-1 | No placebo group was included in study |
| Outcomes measured at multiple time points, starting 2 days pre-warfarin consumption to 7 days post-consumption | Age: 20–36 | Unstandardized capsules | - Agonist(s): AA | Results relating to participants receiving ginkgo supplementation were excluded from table. | |||||
| N = 12 | - Method (Device, if reported): Turbidimetric (Chrono-log) | P value not reported | |||||||
| Consumed with 25 mg dose of rac-warfarin, consumed once per study period. | INR | ||||||||
| Plasma warfarin enantiomer protein binding & warfarin enantiomer concentrations | |||||||||
| Urinary S-7-hydroxywarfarin | |||||||||
| Lumb. 1994 | Randomised, double-blinded placebo-controlled cross-over trial | Total study period: 2x1 day, at least 14 days washout period. | Healthy male volunteers | Dose: 2g (4x500mg) dried ginger per day | Platelet aggregation | No significant changes in any outcome at any time point. | UK | II | |
| Outcomes measured immediately before, 3h, and 24h post consumption of ginger | N = 8 | Unstandardized capsules | - Agonist(s): AA, ADP, | ||||||
| collagen, ristocetin, ADP | |||||||||
| - Method (Device, if reported): Electrical impedance (Chrono-log) | |||||||||
| Bleeding time | |||||||||
| Platelet count | |||||||||
| Thromboelastography | |||||||||
| Srivastava 1989 | Open-label single-arm trial | Total study period: 7 days | Health female volunteers | Dose: 5g raw ginger per day | Platelet thromboxane B2 production | Ginger consumption resulted in a 37% inhibition of thromboxane B2 production (p<0.01). | Denmark | III-3 | Results relating to onion group excluded from table. |
| Outcomes measured at baseline and 7 days post-consumption | N = 7 | ||||||||
| Verma et al. 1993 | Randomised placebo-controlled trial | Total study period: 14 days, high-calorie diet for first 7 days, high-calorie diet and ginger/placebo consumed for next 7 days. | Health male volunteers | Dose: 5g (4x625mg, twice per day) dry ginger powder | Platelet aggregation | Ginger significantly reduced platelet aggregation using both agonists when compared to placebo group (p<0.001). | India | II | Platelet aggregation reduced close to baseline but did not decrease further. |
| Outcomes measured at baseline, 7, and 14 days | N = 20 | Unstandardized capsules | - Agonist(s): ADP and Epi | ||||||
| Consumed with 100g (2x50g) butter, 2 cups of milk, 8 slices of bread. | - Method (Device, if reported): turbidimetric (ELVI-840) | ||||||||
| Young et al. 2006 | Cross-over trial | Total study period: 72 days, 4x washout period of 7–10 days, 5x7 days intervention consumed | Healthy & Hypertensive volunteers | Dose: 1g dried ginger per day | Platelet aggregation | Ginger combined with nifedipine resulted in a significant decrease in platelet aggregation (p<0.001). Ginger alone had no significant effect. | Taiwan | III-2 | No placebo group |
| Outcomes measured at baseline and 7 days post-consumption for each intervention | N = 10 for each group | Either alone or in combination with 10mg nifedipine | - Agonist(s): ADP, Epi, collagen | Unclear if participants were blinded | |||||
| - Method (Device, if reported): Turbidimetric (Chronolog 560) |
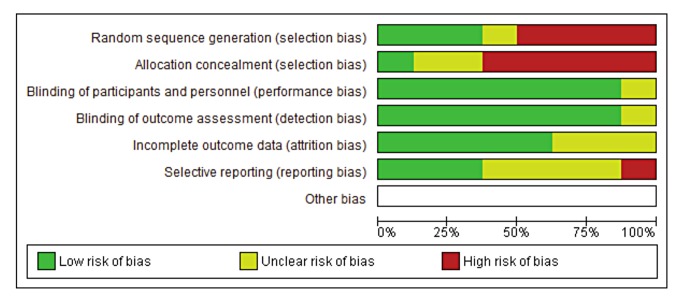
The methodology varied considerably between trials. Half of the studies used a cross-over design[25–28] while three used a parallel design[19, 24] and one was a single arm study.[19] Most of the studies (7/8) had elements of robust study design such as placebo controls, randomisation and double-blinding. However, few studies incorporated all of these elements, with only two studies featuring both randomisation and double-blinding procedures. For example, Jiang et al.[26] used a randomised cross-over design that was also open-label (Table 1). Despite this, the assessment of bias determined the majority of studies were relatively low-risk in terms of performance, detection, and attrition bias while a high risk of random sequence generation and allocation concealment bias was detected (Fig 2).
Fig 2. Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
The average sample size was small. Seven of the eight studies ranged from 7–36 participants[19, 25–29] with one study comprising 60 participants.[24] The duration of each study varied considerably, ranging from one day to three months. Six of the eight studies included healthy participants,[19, 25–27, 29] two studies included patients with confirmed myocardial infarction and one study included hypertensive patients as well as healthy participants.[24, 28] Most studies required participants to consume only ginger, either as a supplement or as a food preparation, while three studies measured the effect of ginger in combination with various medications and food products including nifedipine,[28] warfarin,[26] custard,[30] and a high-calorie diet.[29]
In terms of the ginger preparation used, seven of the eight studies tested a dose of 3.6g to 5g, while one cross-over study investigated larger doses of ginger with each participant receiving either 10g or 40g per day.[30] Most studies delivered ginger at either one time point or once per day, depending on the trial timeframe; however, Jiang et al.[26] and Verma et al.[29] delivered ginger thrice and twice per day, respectively. All studies used an unstandardized ginger preparation, either dried, cooked or raw ginger, delivered in an unprocessed form, within capsules, or mixed into a medium (i.e. custard).
Measures of platelet aggregation varied between studies. The majority (6/8) used light transmittance aggregometry or impedance aggregometry,[24, 26–29] while two studies assessed thromboxane B2 production.[19, 25] Three studies also recorded multiple additional outcomes including INR,[26] fibrinogen and fibrinolytic activity,[24] bleeding time, thromboelastography and platelet count.[27] Of the six that used aggregometry, there was a mix of agonists used with ADP (5/6) and epinephrine (4/6) being the most common. Three studies also used one or more of the following agonists: collagen, AA, or ristocetin.[26–28]
The reported effect of ginger on platelet function were equivocal. Two studies reported inhibition of platelet aggregation.[24, 29] The first study found that ginger significantly inhibited platelet aggregation in healthy males after consumption of a high-calorie diet.[29] The second study reported that ginger the co-administration of 1g of ginger with nifedipine resulted in an inhibition of platelet aggregation in normo- and hypertensive subjects.[28] However, in this study, when ginger was administered alone, there was no significant effect.
In contrast, two studies reported that 2–3.6g of ginger had no effect on measures of platelet aggregation in health adults.[26, 27] Moreover, Jiang et al.[26] found that the co-administration of 3.6g of ginger with 25mg of warfarin had no effect on the international normalized ratio (INR) or the pharmacokinetics and pharmacodynamics of warfarin in healthy male participants. Lumb et al.[27] also reported no significant effect on bleeding time, platelet count, and thromboelastography in a similar population. Bordia et al.[24] reported that 4g/day of ginger for three months did not affect platelet aggregation, fibrinogen, or fibrinolytic activity in patients with coronary artery disease; however, when participants were given a bolus dose of 10g ginger, there was a significant inhibition of platelet aggregation in patients with coronary artery disease.
The two studies that investigated the effect of ginger on thromboxane B2 generation in healthy adults reported conflicting results. Srivastava et al.[19] reported that 5g of ginger over 7 days resulted in a 37% inhibition of thromboxane B2 production (p<0.01), while Janssen et al.[25] found that 15g and 40g of raw and cooked ginger, respectively, had no effect when each were consumed for two weeks (p = 0.616).
3.2 Observational Data
Two observational studies investigated the association of ginger use and platelet-related adverse effects. Shalansky et al.[31] conducted a 16-week longitudinal study of 171 participants prescribed warfarin. During this period, participants were asked to record bleeding events as well as factors that the investigators hypothesised could influence INR and bleeding risk, including a selection of complementary therapies. Of the 171 participants, 87 reported bleeding events with excessive bruising (41%) and nosebleeds (15%) being the two most commonly-reported events. The study reported a significant association between self-reported bleeding events and ginger (OR 6.63, 95% CI 3.49–12.61), as well as cayenne (OR 8.0, 95% CI 3.57–17.92), willow bark (OR 9.00, 95% CI 6.42–12.62), St. John’s wort (OR 4.70, 95% CI 1.49–14.79), and coenzyme Q10 (OR 3.91, 95% CI 2.09–7.31). Upon further analysis, ginger (OR 3.20, 95% CI 2.42–4.24) and coenzyme Q10 (OR 3.69, 95% CI 1.88–7.24) were independently associated with self-reported bleeding events in a fully adjusted multivariate model. No complementary therapies were associated with a risk of abnormal INR.
In contrast, Leung et al.[32] surveyed 314 patients prescribed warfarin therapy, in which they retrospectively assessed self-reported bleeding events and exposure to factors that could influence bleeding risk and INR in the previous month. While only two patients reported using ginger during this period, the study authors determined that ginger, along with all other assessed complementary therapies, was not associated with bleeding risk or abnormal INR.
Discussion
Despite consistent in vitro data demonstrating that ginger compounds interact with several steps involved in platelet aggregation, the results of human studies are inconsistent. It is difficult to draw conclusions from these studies as a whole, due to the limited number of studies and their heterogeneous methods. These inconsistencies include the dose, dosing regimen, and formulation of ginger used, the timeframe studied, and the characteristics of subjects recruited (e.g. healthy vs. patients with chronic diseases).
Of the eight clinical trials analysed for this review, three found ginger affected measures of platelet aggregation[24, 28, 29] and one study found ginger reduced thromboxane B2 production.[19] When the included studies were separated by patient medical background (e.g. healthy, hypertensive), no consistent treatment effect could be elucidated. However, there are several limitations that could limit the real-world applicability of these results.
First, Young et al.[28] reported that ginger had an effect only when it was combined with nifedipine, but not when it was ingested by itself. While not fully elucidated, it is thought that the anti-aggregation effect of nifedipine results from the inhibition of intracellular Ca2+, which attenuates platelet hyperactivity. [33] Other anti-platelet medications are not reported to possess this mechanism of action and therefore, these results might only be applicable to this combination.
Second, Verma et al.[29] found that ginger reduced a rise in platelet aggregation after a two week high-calorie diet when compared to control (high calorie diet plus placebo). However, it should be noted that this diet exceeded the participants’ normal dietary intake (approximately 1600kcal increase in dietary intake, according to USDA food data[34]), which might make these results difficult to compare to patients who consume a eucaloric diet.
The third study reported a significant reduction in platelet aggregation when a bolus of 10g ginger was administered to patients with a confirmed myocardial infarction.[24] However, the same authors found a lower dose of 4g ginger had no effect in the same population when taken daily over three months.
A primary limitation of the studies reviewed is the lack of quantification or standardisation of bioactive compounds in the ginger preparations used. This could partly explain the inconsistent results. Previous research indicates that the concentration of the principal compounds within ginger, namely gingerol and shogaols, varies greatly depending on the storage and preparation of ginger products.[35, 36] This variation could result in significant differences in bioactive compounds between studies. For example, 6-shogaol is only present in appreciable amounts in dried or heated ginger as it is a degradation product of 6-gingerol.[37] Hence, preparations that used dried ginger are likely to have significantly different effects compared to raw ginger.
A final limitation relates to the clinical significance of ginger’s potential anti-platelet effect. Several studies have reported that ginger is effective for nausea in multiple settings including morning sickness, motion sickness and chemotherapy-induced nausea and vomiting (CINV).[4, 5, 38] However, the majority of these studies used ginger doses that were considerably lower than those used in the studies included in this review. For example, in two recent reviews of the effect of ginger on morning sickness[5] and CINV[1], from a total of 19 studies, no study used a dose above 2g with most studies using a dosage around 1g. In contrast, the majority of studies in this review that found a significant effect on platelet aggregation used doses above 5g.[19, 24, 29] Young et al.[28] were the only exception in reporting 1g in combination with nifedipine to have an effect on platelet aggregation; however, when 1g of ginger was administered alone, there was no significant effect. Hence, further research in this area should investigate the effect of lower doses of ginger on platelet aggregation in order to determine if the potential effect of ginger on platelet aggregation is clinically relevant when used as an adjuvant anti-nausea treatment during chemotherapy at doses shown to be effective in previous studies.
The two observational studies included in this review also reported conflicting results.[31, 32] This could be due to the differences in their study designs. One study undertook a retrospective analysis that could have resulted in recall bias,[32] while the other study undertook a prospective approach.[31] In the retrospective study,[32] only two patients from a cohort of 314 participants reported consuming ginger, both of whom reported experiencing bleeding events. Due to the limited sample of participants who consumed ginger, it is difficult to draw meaningful conclusions. Information regarding the dose of ginger consumed by participants was not reported in either observational study, which might further account for the difference in results.
While there was only one clinical trial investigating the interaction between ginger and warfarin, Jiang et al.[26] found no significant change to patient INR when ginger was administered for seven days. This is partially corroborated by the results of a study of Wistar rats in which a proprietary ginger formulation, in combination with warfarin, had no additive effect on whole blood clotting time, prothrombin time or activated partial thromboplastin time.[10] This is a particularly relevant finding, as ginger is routinely cited as potentially interacting with warfarin therapy.[39, 40] While further studies are required to investigate interaction of ginger and blood thinning medication, current evidence does not support an interaction.
The results of this review indicate that the role of ginger in platelet aggregation is unclear and therefore, future clinical trials are needed to further investigate this area, particularly in at-risk populations such as chemotherapy patients. However, until these trials are undertaken, the effect of ginger on platelet aggregation cannot be confidently dismissed. Previous research has indicated that patient use of dietary supplements is often not reported to treating physicians. For example, a review of surveys that investigated the rate of non-disclosure of complementary and alternative medicines in chemotherapy patients found that between 40–50% of patients did not discuss these therapies with their physician.[41] Hence, where patients are at particular risk of bleeding, clinicians should ascertain patient consumption of dietary supplements and screen for any known potentiator of bleeding risk.
Conclusion
Due to the potential effects of ginger on platelet aggregation, ginger is a commonly-cited example of an herbal supplement that should be avoided in patients with thrombocytopenia, platelet function defects or coagulopathy, such as populations using ginger for its antiemetic effect in cancer chemotherapy. While in vitro data, as well as some clinical studies and epidemiological evidence suggest that ginger inhibits platelet aggregation, the evidence is equivocal with multiple limitations, particularly within the clinical data, which prevents firm recommendations being made. Limitations include the lack of standardisation of ginger preparations used, significant variations in dosage and time frame studied, and the high level of bias in the study designs used. Therefore, further research is needed to clearly define the safety, or otherwise, of ginger in patient population at increased risk of bleeding.
Supporting Information
S1 File. PRISMA Guidelines Checklist.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Marx WM, Teleni L, McCarthy AL, Vitetta L, McKavanagh D, Thomson D, et al. Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: a systematic literature review. Nutrition Reviews. 2013;71(4):245–54. 10.1111/nure.12016 [DOI] [PubMed] [Google Scholar]
- 2.Chrubasik S, Pittler M, Roufogalis B. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684–701. 10.1016/j.phymed.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Chaiyakunapruk N, Kitikannakorn N, Nathisuwan S, Leeprakobboon K, Leelasettagool C. The efficacy of ginger for the prevention of postoperative nausea and vomiting: a meta-analysis. Am J Obstet Gynecol. 2006;194(1):95–9. Epub 2006/01/04. 10.1016/j.ajog.2005.06.046 . [DOI] [PubMed] [Google Scholar]
- 4.Thomson M, Corbin R, Leung L. Effects of ginger for nausea and vomiting in early pregnancy: a meta-analysis. Journal of the American Board of Family Medicine: JABFM. 2014;27(1):115–22. Epub 2014/01/07. 10.3122/jabfm.2014.01.130167 . [DOI] [PubMed] [Google Scholar]
- 5.Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G481–9. Epub 2003/02/11. 10.1152/ajpgi.00164.2002 . [DOI] [PubMed] [Google Scholar]
- 6.Lesho EP, Saullo L, Udvari-Nagy S. A 76-year-old woman with erratic anticoagulation. Cleve Clin J Med. 2004;71(8):651–6. Epub 2004/09/29. . [DOI] [PubMed] [Google Scholar]
- 7.Dorso CR, Levin RI, Eldor A, Jaffe EA, Weksler BB. Chinese food and platelets. N Engl J Med. 1980;303(13):756–7. Epub 1980/09/25. . [PubMed] [Google Scholar]
- 8.Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984;43(8–9):S335–46. Epub 1984/01/01. . [PubMed] [Google Scholar]
- 9.Srivastava KC. Isolation and effects of some ginger components of platelet aggregation and eicosanoid biosynthesis. Prostaglandins Leukot Med. 1986;25(2–3):187–98. Epub 1986/12/01. . [DOI] [PubMed] [Google Scholar]
- 10.Weidner MS, Sigwart K. The safety of a ginger extract in the rat. Journal of Ethnopharmacology. 2000;73(3):513–20. [DOI] [PubMed] [Google Scholar]
- 11.Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, Cost, and Outcomes of Bleeding and Chemotherapy Dose Modification Among Solid Tumor Patients With Chemotherapy-Induced Thrombocytopenia. Journal of Clinical Oncology. 2001;19(4):1137–46. [DOI] [PubMed] [Google Scholar]
- 12.Liao YR, Leu YL, Chan YY, Kuo PC, Wu TS. Anti-platelet aggregation and vasorelaxing effects of the constituents of the rhizomes of Zingiber officinale. Molecules. 2012;17(8):8928–37. Epub 2012/07/28. 10.3390/molecules17088928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res. 2001;103(5):387–97. Epub 2001/09/13. . [DOI] [PubMed] [Google Scholar]
- 14.Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med. 1986;24(2–3):195–8. Epub 1986/10/01. . [DOI] [PubMed] [Google Scholar]
- 15.Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. 2002;67(6):475–8. Epub 2002/12/07. . [DOI] [PubMed] [Google Scholar]
- 16.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorganic chemistry. 2001;29(3):156–63. Epub 2001/07/05. 10.1006/bioo.2001.1208 . [DOI] [PubMed] [Google Scholar]
- 17.Nie H, Meng LZ, Zhang H, Zhang JY, Yin Z, Huang XS. Analysis of anti-platelet aggregation components of Rhizoma Zingiberis using chicken thrombocyte extract and high performance liquid chromatography. Chin Med J (Engl). 2008;121(13):1226–9. Epub 2008/08/20. . [PubMed] [Google Scholar]
- 18.Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thrombosis Research. 2003;111(4–5):259–65. 10.1016/j.thromres.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Srivastava KC. Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukot Essent Fatty Acids. 1989;35(3):183–5. Epub 1989/03/01. . [DOI] [PubMed] [Google Scholar]
- 20.Nievergelt A, Marazzi J, Schoop R, Altmann KH, Gertsch J. Ginger phenylpropanoids inhibit IL-1beta and prostanoid secretion and disrupt arachidonate-phospholipid remodeling by targeting phospholipases A2. J Immunol. 2011;187(8):4140–50. Epub 2011/09/13. 10.4049/jimmunol.1100880 . [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health and Medical Research Council. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines Commonwealth of Australia: National Health and Medical Research Council; 2009. [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 24.Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1997;56(5):379–84. Epub 1997/05/01. . [DOI] [PubMed] [Google Scholar]
- 25.Janssen PL, Meyboom S, van Staveren WA, de Vegt F, Katan MB. Consumption of ginger (Zingiber officinale roscoe) does not affect ex vivo platelet thromboxane production in humans. Eur J Clin Nutr. 1996;50(11):772–4. Epub 1996/11/01. . [PubMed] [Google Scholar]
- 26.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59(4):425–32. Epub 2005/04/02. 10.1111/j.1365-2125.2005.02322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumb AB. Effect of dried ginger on human platelet function. Thromb Haemost. 1994;71(1):110–1. Epub 1994/01/01. . [PubMed] [Google Scholar]
- 28.Young HY, Liao JC, Chang YS, Luo YL, Lu MC, Peng WH. Synergistic effect of ginger and nifedipine on human platelet aggregation: a study in hypertensive patients and normal volunteers. Am J Chin Med. 2006;34(4):545–51. Epub 2006/08/03. 10.1142/s0192415x06004089 . [DOI] [PubMed] [Google Scholar]
- 29.Verma SK, Singh J, Khamesra R, Bordia A. Effect of ginger on platelet aggregation in man. Indian J Med Res. 1993;98:240–2. Epub 1993/10/01. . [PubMed] [Google Scholar]
- 30.Janssen PLTMK, Meyboom S, Van Staveren WA, De Vegt F, Katan MB. Consumption of ginger (Zingiber Officinale Roscoe) does not affect ex vivo platelet thromboxane production in humans. European Journal of Clinical Nutrition. 1996;50(11):772–4. [PubMed] [Google Scholar]
- 31.Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C. Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy. 2007;27(9):1237–47. Epub 2007/08/29. 10.1592/phco.27.9.1237 . [DOI] [PubMed] [Google Scholar]
- 32.Leung VW, Shalansky SJ, Lo MK, Jadusingh EA. Prevalence of use and the risk of adverse effects associated with complementary and alternative medicine in a cohort of patients receiving warfarin. Ann Pharmacother. 2009;43(5):875–81. Epub 2009/04/30. 10.1345/aph.1L631 . [DOI] [PubMed] [Google Scholar]
- 33.Shih CY, Lin MH, Fan HC, Chen FC, Chou TC. Mechanisms of antiplatelet activity of nifedipine: role of peroxisome proliferator-activated receptor-beta-gamma-dependent processes. Journal of hypertension. 2014;32(1):181–92. Epub 2013/10/16. 10.1097/hjh.0000000000000007 . [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. 2014.
- 35.Schwertner H, Rios D, Pascoe J. Variation in concentration and labeling of ginger root dietary supplements. Obstet Gynecol. 2006;107:1337–43. 10.1097/01.AOG.0000217697.33787.8c [DOI] [PubMed] [Google Scholar]
- 36.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1–2):41–7. Epub 2007/06/15. 10.1016/j.jchromb.2007.05.011 . [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan R, Wong WH, Pichika MR. Impact of extraction processes on the 6-shogaol content in Zingiber officinale (Halia Penang) and its antiproliferative activities on human colorectal cancer cell lines (HT-29). Planta Med. 2011;77(05):112 10.1055/s-0031-1273641 [DOI] [Google Scholar]
- 38.Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20(7):1479–89. Epub 2011/08/06. 10.1007/s00520-011-1236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. American Journal of Health-System Pharmacy. 2000;57(13):1221–30. [PubMed] [Google Scholar]
- 40.Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Archives of internal medicine. 1998;158(20):2200–11. Epub 1998/11/18. . [DOI] [PubMed] [Google Scholar]
- 41.Davis EL, Oh B, Butow PN, Mullan BA, Clarke S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist. 2012;17(11):1475–81. Epub 2012/08/31. 10.1634/theoncologist.2012-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 File. PRISMA Guidelines Checklist.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.