Effects of oral vitamin E on treatment of atopic dermatitis: A randomized controlled trial (original) (raw)
Abstract
Background:
The pathogenesis of atopic dermatitis (AD) remains to be determined; recently a possible change in the immune system with production of immunoglobulins is proposed. As vitamin E is a potent antioxidant, with the ability to decrease the serum levels of immunoglobulin E (IgE) in atopic patients, we aimed to evaluate the effect of oral vitamin E on treatment of AD.
Materials and Methods:
This randomized, double-blind, placebo-controlled trial comprised seventy participants with mild-to-moderate AD, based on the Hanifin and Rajka diagnostic criteria. The patients were randomly selected from teaching skin clinics in Isfahan, Iran. They were randomly assigned to two groups of equal number, receiving vitamin E (400 IU/day) and placebo for four 4 months. Each month, the extent, severity, and subjective symptoms including itch and sleeplessness were measured by SCORAD index. Three months after the end of intervention, the recurrence rate was assessed.
Results:
The improvement in all symptoms, except sleeplessness, was significantly higher in the group receiving vitamin E than in controls (–1.5 vs. 0.218 in itching, –10.85 vs. –3.54 in extent of lesion, and –11.12 vs. –3.89 in SCORAD index, respectively, P < 0.05). Three months after the end of intervention, the recurrence rate of AD was evaluated. Recurrence rate between all 42 individuals, who remained in the study, was 18.6%. Recurrence ratio of the group receiving vitamin E compared to the placebo group was 1.17, without significant differences between the two groups (_P_ > 0.05).
Conclusion:
This study suggests that vitamin E can improve the symptoms and the quality of life in patients with AD. As vitamin E has no side effects with a dosage of 400 IU/day, it can be recommended for the treatment of AD.
Keywords: Atopic dermatitis (AD), SCORAD index, treatment, vitamin E
INTRODUCTION
The pathogenesis of atopic dermatitis (AD) remains to be studied but recently a possible change in the immune system with production of immunoglobulin E (IgE) and immunoglobulin G (IgG) is proposed.[1,2] This disease is known with pruritus, eczematous lesions, dry skin, skin thickening, and prominent ridges of the skin. AD may be related to other diseases such as asthma, hay fever, and hives. The disease epidemic is growing and, 15-30% of the children in the developed countries and 10-20% of adults suffer from AD.[3,4]
The prevalence of the disease is high in immigrant people from developed to developing countries.[5] There are two hypotheses regarding the pathophysiology of this disease. In healthy people, there is a balance between T helper 1 and T helper 2. In patients with AD, the T helper 2, increases and the result is high levels of IgE and interferon-gamma, which are low.[6] The second hypothesis is the stratum corneum skin barrier dysfunction associated with AD. In this case, it makes the entry of antigen and the production of inflammatory cytokine.[7]
AD is often triggered by following stressful life events.[8,9] Compared with healthy controls, adults with AD are more anxious and depressed. Children with AD have more behavioral and psychological problems than the healthy children.[10,11] Living with AD has a profound effect on the quality of life for these children and their families. In turn, improper quality of life in these children is reported equally or more frequently compared to the people with asthma and diabetes.[12] Persistent itching and the absence from work in adults cause a large economic burden,[13] and due to their resistance to conventional therapies, AD patients face recurrence of clinical and social problems.[5]
Most people are discouraged after trying various treatments, including antihistamines, steroids, and cyclosporine A. Vitamin E is known for its antioxidant properties and is found to have a protective effect on common health problems as heart disease, cataracts, cancer, and stroke. Recent studies have also shown that vitamin E can reduce the level of IgE antibodies in patients with AD.[14,15] Given that the treatment with vitamin E is safe and cost-effective, the aim of the current study was to investigate the effect of vitamin E on the treatment of AD.
MATERIALS AND METHODS
This study was conducted as a double-blind, randomized controlled trial (RCT) (trial registry code: IRCT201105016350N1). A total number of 70 known cases of AD were randomly selected from those patients were referred to the dermatology clinics of two teaching hospitals (Alzahra and Noor) affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. The trial was approved by the Research and Ethics Committee, Skin and Leishmaniasis Research Center, Isfahan University of Medical Sciences. Written informed consent was obtained from all the participants.
Those cases were selected that were aged 10-50 years, and their disease was confirmed based on Hanifin and Rajka diagnostic criteria.[3,4] The patients who matched the following criteria were not recruited to the trial: Severe disease with a SCORAD score of more than 50, requiring hospitalization, pregnant women, nursing mothers, coagulopathies, using anticoagulant medications, systemic corticosteroids or immunosuppressant, as well as having a history of allergy to vitamin E, and living in long distances from Isfahan city. Those patients who showed severe allergic symptoms to vitamin E and those who became pregnant during the therapy were excluded from this trial, and they received standard treatment.
All the patients received the usual treatment for AD, including Children Vaseline (Firooz Company, Iran), Glycerin Soap (Ejhe Company, Iran), hydroxyzine tablets (Daroopakhsh Company, Iran) and topical hydrocortisone cream 1% (Tehran Daroo Company, Iran). The selected 70 patients were assigned in two groups of equal number (simple randomization): One group received vitamin E 400 units (Nature Company) and the other group received placebo. The placebo had no active ingredient and was without smell; it was packed in the same package as vitamin E.
Clinical evaluation was conducted by the SCORAD index[16], which is a combined index consisting of the observable objective criteria, the extent and severity of injuries, as well as subjective criteria as itching and loss of sleep.
The lesion extent was composed of six measures as follows: Redness (erythema) edema/papules (solid lesion, leading to less than 5 mm in size), rash symptoms (excoriation), prominent ridges and thickening of skin horny layer of epidermis lichenification), serous fluid secreted from the epidermis (oozing), or curd made (crusts), which were scored from 0 to 3. SCORAD index formula is defined as: A/5 + 7B/2 + C, in which: A is the lesion extent of the area and considered from 0 to 10, B is the intensity of the lesion and considered from 0 to 18, and C is the subjective symptoms counted from 0 to 20. The total score is 103.The extent of the lesion is ranked 0-100 and for assessment, in the area of measurement we draw the line and after that, the size of the involved surface area is calculated with the help of the Rule No. 9 and then the results are collected. For assessing the severity of the lesion, the surface that is the main cause and the reason of referring patients, is considered and to measure the subjective symptoms, a ruler scaled from 0 to 10 is used in order to determine the severity of the symptoms.[17] Types of eczema, are defined as follows:
- Mild eczema score on the SCORAD index is less than 25; for example, mild skin dryness and itching.
- Median eczema score on the SCORAD index is more than 25 and less than 50; for example, the average skin redness, itching, sleep disturbance, and the secondary infection of skin (mild to median).
- Severe eczema score on the SCORAD index is more than 50; for example, severe skin redness, severe itching, insomnia, raised ridges of skin, thickening of the horny layer of the epidermis secondary severe skin infection.[18] Patients with SCORAD index of more than 50 were not enrolled in this trial.
Each month, the severity, extent of lesions, and the overall subjective appearance of the patients (insomnia and pruritus) were measured by using the SCORAD index, and recorded daily. Also every two weeks, the patients telephone were followed up to check out any unusual symptoms, side effects, worsening of symptoms, and how to use oral vitamin E, were performed and recorded. Three months after the treatment, rates of relapse (return of at least 50% of the eczematous lesions on the SCORAD index) was evaluated. During the treatment period, two people from the group receiving vitamin E and three from the placebo group quitted the trial because of personal reasons.
Statistical analysis
Data analysis was conducted by Statistical Package for the Social Sciences (SPSS) version 12 for Windows (SPSS Inc., Chicago, IL) by using linear regression and chi-square tests. The significance level was set at P < 0.05.
RESULTS
The male-to-female ratio was similar 43% vs. 57% in both groups [Table 1]. As presented in Table 2, the mean score for sleep disturbance or sleeplessness and itching lesions in the group receiving vitamin E and the mean total score of the SCORAD index were negative in both groups.
Table 1.
Distribution of study population study according to gender
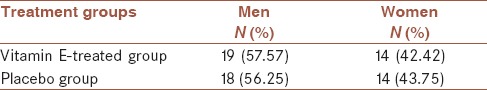
Table 2.
Mean scores of different variables in vitamin E-treated and placebo groups
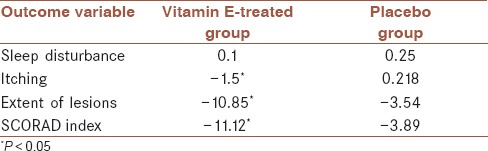
Itching, extent of lesions, and SCORAD index improvement was significantly higher in vitamin E treated group compared to placebo group (−1.5 vs. 0.218 in itching, −10.85 vs. −3.54 in extent of lesion, and −11.12 vs. −3.89 in SCORAD index, respectively, P < 0.05). The highest reduction in total score of SCORAD index, and lowest reduction of sleep disturbance or sleeplessness score was observed in the placebo group. In the group receiving vitamin E, the total average differences in all measured variables were negative, which shows favorable response to vitamin E therapy.
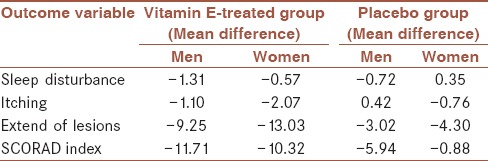
As presented in Table 3, in both groups, the mean score of pruritus and the extent of lesions showed a greater reduction in women, and the differences in the mean total SCORAD index decreased more in men than in women.
Table 3.
Gender differences in mean scores of variables on in vitamin E-treated and placebo groups
Relapse rate, according to the SCORAD index, was determined 3 months after the intervention. From the total of 55 patients who remained in the study, 23.6% reported relapse. The relapse rate was 25% (7/28) in the treatment group vs. 22.2% in the placebo group (6/21) than the placebo group with no significant differences between groups. No side effect was reported in either group.
DISCUSSION
This was a RCT of low dose vitamin E single therapy for patients with AD. The results of this study suggest efficacy of vitamin E supplementation and improvement of some clinical symptoms in patients with AD.
Topical corticosteroids are usually a main component of treatment protocol for acute phase of AD. The most common complications of these medications are burning, itching, and dryness, which are due to a steroid carrier molecule. Topical corticosteroids are associated with local and systemic side effects. Telangiectasia, purpura, stretch mark, and skin atrophy are some of their local complications. Atrophy may improve with discontinuation, but sometimes irreversible damage happens.[19,20] Other local side effects include rosacea, acne, folliculitis, and perioral dermatitis. Increased intraocular pressure, cataract, and glaucoma may result from long-term use of topical corticosteroids around the eyes. Topical corticosteroids may be systemically absorbed and systemic side effects including suppression of hypothalamus-pituitary-adrenal axis are major concerns.[21,22] Vitamin E is an essential nutrient with antioxidant activity. Human body cannot produce this vitamin and the skin levels of vitamin E depend on its oral or topical use.
Natural sources of vitamin E are vegetables, vegetable oils, and nuts. A nutritional survey conducted on 10,000 people in US showed that most women and men do not get the recommended amount of vitamin E.[23] Isomeric form of alpha-tocopherol that is naturally found in foods, also called natural alpha-tocopherol (_RRR_-alpha-tocopherol), is biologically active.[24] The most important action of alpha-tocopherol in the body is its antioxidant property.
Alpha-tocopherol prevents oxidative stress of free radicals on cell membrane. Furthermore, vitamin E is involved in activation of some molecules and enzymes in immune and inflammatory cells.[25] Vitamin E protects the macrophage membrane against oxidative damage and reduces production of prostaglandins through affecting the immune system.
Oxidative stress and altered antioxidant activity may be involved in acute exacerbations of AD in children.[26] Gueck et al. reported significant decrease of prostaglandins and histamine release from mast cells.[27]
Noh et al. found that serum IgE levels could be a predictive factor for prognosis of dermatologic diseases and demonstrated that serum IgE levels has declined significantly in patients treated with gamma interferon.[28] Tsouri-Nikita et al. studied 96 patients with AD and found a significant association between vitamin E and serum IgE levels; moreover, the clinical symptoms improved after treatment with vitamin E.[6,14]
Few side effects have been reported in adults taking <2,000 mg/day of alpha-tocopherol; however, long-term side effects have not been studied. Patients receiving anticoagulant or those with vitamin K deficiency have an increased risk for bleeding and should be under direct supervision of a physician for receiving vitamin E.[29]
Although contact dermatitis, burning, and itching have been reported on topical use of vitamin E,[14] none of them occurred in patients receiving vitamin D (400 IU/day) of our study.
CONCLUSION
Low dose of vitamin E (400 IU/day) can be effective in the treatment of AD patients with no side effects. However, larger well-designed double blind RCTs are required before its integration in clinical guideline for treatment of AD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
FJ contributed in the conception of the work, analysis and interpretation of data for the work, drafting and critical revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. GF contributed in the conception and design of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AM contributed in the conception of the work, acquisition of data, drafting the work, approval of the final version of the manuscript, and agreed for all aspects of the work. SMH contributed in the design of the work, analysis of data, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This project is funded by Isfahan University of Medical Sciences (research project number 388213), We would like to thank all Participants of this study and SDLRC staff for their assistance in running the project.
REFERENCES
- 1.Muro Y. Autoantibodies in atopic dermatitis. J Dermatol Sci. 2001;25:171–8. doi: 10.1016/s0923-1811(01)00084-6. [DOI] [PubMed] [Google Scholar]
- 2.Tada J, Toi Y, Yoshioka T, Fujiwara H, Arata J. Antinuclear antibodies in patients with atopic dermatitis and severe facial lesions. Dermatology. 1994;189:38–40. doi: 10.1159/000246756. [DOI] [PubMed] [Google Scholar]
- 3.Hanifin GM, Rajka G. Diagnostic Features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–7. [Google Scholar]
- 4.Kang KF, Tian RM. Atopic dermatitis. An evaluation of clinical and laboratory findings. Int J Dermatol. 1987;26:27–32. doi: 10.1111/j.1365-4362.1987.tb04572.x. [DOI] [PubMed] [Google Scholar]
- 5.Callard RE, Harper JI. The skin barrier, atopic dermatitis and allergy: A role for Langerhans cells? Trends Immunol. 2007;28:294–8. doi: 10.1016/j.it.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty A, Lewis, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopic. Lancet. 2000;356:1573–4. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 7.Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: A glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–4. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- 8.Bockelbrink A, Heinrich J, Schäfer I, Zutavern A, Borte M, Herbarth O, et al. LISA Study Group. Atopic eczema in children: Another harmful sequel of divorce. Allergy. 2006;61:1397–402. doi: 10.1111/j.1398-9995.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 9.Picardi A, Abeni D. Stressful life events and skin diseases: Disentangling evidence from myth. Psychother Psychosomatic. 2001;70:118–36. doi: 10.1159/000056237. [DOI] [PubMed] [Google Scholar]
- 10.Hashizume H, Horibe T, Ohshima A, Ito T, Yagi H, Takigawa M. Anxiety accelerates T-helper 2-tilted immune responses in patients with atopic dermatitis. Br J Dermatol. 2005;152:1161–4. doi: 10.1111/j.1365-2133.2005.06449.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta MA, Gupta AK. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: Epidemiology and management. Am J Clin Dermatol. 2003;4:833–42. doi: 10.2165/00128071-200304120-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lewis-Jones S. Quality of life and childhood atopic dermatitis: The misery of living with childhood eczema. Int J Clin Pract. 2006;60:984–92. doi: 10.1111/j.1742-1241.2006.01047.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernice RK. Atopic Dermatitis. [Last accessed on 2015 Oct 13]. Available from: http://emedicine.medscape.com/article/1049085-overview .
- 14.Tsouri-Nikita E, Hercogova J, Lotti T, Menchini G. Evaluation of dietary intake of vitamin E in the treatment of atopic dermatitis: A study of the clinical course and evaluation of the immunoglobulin E serum levels. Int J Dermatol. 2002;41:146–50. doi: 10.1046/j.1365-4362.2002.01423.x. [DOI] [PubMed] [Google Scholar]
- 15.Jansén CT, Haapalahti J, Hopsu-Havu VK. Immunoglobulin E in the human atopic skin. Arch Dermatol Forsch. 1973;246:209–302. [PubMed] [Google Scholar]
- 16.Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: Consensus report of the European Task Force in Atopic Dermatitis. Dermatology. 1997;195:10–9. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 17.Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 18.Pucci N, Lombardi E, Novembre E, Farina S, Bernardini R, Rossi ME, et al. Urinary eosinophil protein X and serum eosinophil cationic protein in infants and young children with atopic dermatitis: Correlation with disease activity. J Allergy Clin Immunol. 2000;105:353–7. doi: 10.1016/s0091-6749(00)90087-3. [DOI] [PubMed] [Google Scholar]
- 19.Gates T. Atopic dermatitis: Diagnosis, treatment, and aeromedical implications. Aviat Space Environ Med. 2007;78:29–37. [PubMed] [Google Scholar]
- 20.Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352:2314–24. doi: 10.1056/NEJMcp042803. [DOI] [PubMed] [Google Scholar]
- 21.Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. European Tacrolimus Ointment Study Group. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005;152:1282–9. doi: 10.1111/j.1365-2133.2005.06592.x. [DOI] [PubMed] [Google Scholar]
- 22.Wolff K, Stuetz A. Pimecrolimus for the treatment of inflammatory skin disease. Expert Opin Pharmacother. 2004;5:643–55. doi: 10.1517/14656566.5.3.643. [DOI] [PubMed] [Google Scholar]
- 23.Thiele JJ, Ekanayake-Mudiyanaselage S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646–67. doi: 10.1016/j.mam.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. Philadelphia: Lippincott Williams & Wilkiins; 2006. pp. 396–411. [Google Scholar]
- 25.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–62. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 26.Tsukahara H, Shibata R, Oshima Y, Todoroki Y, Sato S, Ohta N, et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003;72:2509–16. doi: 10.1016/s0024-3205(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 27.Gueck T, Rudolph J, Fuhrmann H. Influence of vitamin E on mast cell mediator release. Vet Dermatol. 2002;13:301–5. doi: 10.1046/j.1365-3164.2002.00307.x. [DOI] [PubMed] [Google Scholar]
- 28.Noh GW, Lee KY. Blood eosinophils and serum IgE as predictors for prognosis of interferon-gamma therapy in atopic dermatitis. Allergy. 1998;53:1202–7. doi: 10.1111/j.1398-9995.1998.tb03842.x. [DOI] [PubMed] [Google Scholar]
- 29.Washington DC: National Academy Press; 2000. Food and Nutrition Board, Institute of Medicine. Vitamin E. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; pp. 186–283. [Google Scholar]