Hematopoietic Stem-Cell Transplantation for Advanced Systemic Mastocytosis (original) (raw)
Abstract
Purpose
Advanced systemic mastocytosis (SM), a fatal hematopoietic malignancy characterized by drug resistance, has no standard therapy. The effectiveness of allogeneic hematopoietic stem-cell transplantation (alloHCT) in SM remains unknown.
Patients and Methods
In a global effort to define the value of HCT in SM, 57 patients with the following subtypes of SM were evaluated: SM associated with clonal hematologic non–mast cell disorders (SM-AHNMD; n = 38), mast cell leukemia (MCL; n = 12), and aggressive SM (ASM; n = 7). Median age of patients was 46 years (range, 11 to 67 years). Donors were HLA-identical (n = 34), unrelated (n = 17), umbilical cord blood (n = 2), HLA-haploidentical (n = 1), or unknown (n = 3). Thirty-six patients received myeloablative conditioning (MAC), and 21 patients received reduced-intensity conditioning (RIC).
Results
Responses in SM were observed in 40 patients (70%), with complete remission in 16 patients (28%). Twelve patients (21%) had stable disease, and five patients (9%) had primary refractory disease. Overall survival (OS) at 3 years was 57% for all patients, 74% for patients with SM-AHNMD, 43% for those with ASM, and 17% for those with MCL. The strongest risk factor for poor OS was MCL. Survival was also lower in patients receiving RIC compared with MAC and in patients having progression compared with patients having stable disease or response.
Conclusion
AlloHCT was associated with long-term survival in patients with advanced SM. Although alloHCT may be considered as a viable and potentially curative therapeutic option for advanced SM in the meantime, given that this is a retrospective analysis with no control group, the definitive role of alloHCT will need to be determined by a prospective trial.
INTRODUCTION
Systemic mastocytosis (SM) is a hematopoietic stem-cell disease defined by clonal expansion of mast cells in various organs. SM comprises a pathologically and clinically heterogeneous group of disease variants with variable prognoses.1–5 The clinically indolent forms do not shorten life expectancy and require cytoreductive therapy, whereas advanced SM variants, including mast cell leukemia (MCL), SM with associated clonal hematologic non–mast cell lineage disease (SM-AHNMD), and aggressive systemic mastocytosis (ASM), are associated with survival rates ranging from months to a few years despite cytoreductive therapy.1,4,6,7
No standard treatment exists for advanced SM. Cytoreductive therapy with cladribine or interferon alfa has been used, but no long-lasting responses have been reported.8–10 A majority of patients with SM (> 80%) have a gain of function mutation in the gene encoding the tyrosine kinase KIT, usually KITD816V.11–17 Although various tyrosine kinase inhibitors have been tested in advanced SM,18–26 their clinical benefit seems to be limited.1,18,19,23 Allogeneic hematopoietic stem-cell transplantation (alloHCT) has curative potential for hematologic malignancies such as acute myelogenous leukemia (AML).27 Although alloHCT has been used to treat life-threatening SM and hematologic malignancies associated with SM,28–40 the outcome of alloHCT in patients with advanced SM has never been systematically studied. Indeed, the largest published case series consisted of only three patients.37
The purpose of this retrospective study was to evaluate the clinical outcomes of alloHCT in advanced SM and to assess potential benefits and hazards of alloHCT in all identifiable cases in the United States and Europe.
PATIENTS AND METHODS
Patients were included in the study if they underwent alloHCT for the treatment of SM-AHNMD, MCL, or ASM and if their data on mast cells—and AHNMD in case of SM-AHNMD—were available at both time points (before and after alloHCT). In case of SM-AHNMD, only patients whose SM component was known at the time of AHNMD diagnosis or before alloHCT were included, whereas patients whose SM component was discovered later (ie, hidden and/or occult mastocytosis) in a study were excluded. All these patients were indeed suffering from overt advanced mastocytosis requiring therapy. Note that in all of our patients, overt SM was documented and that any type of myelodysplastic syndrome (MDS) or AML that occurred in the context of SM was regarded as secondary and part of the entire clonal disease process (SM-AHNMD). Patients were identified at individual major transplantation or SM centers in the United States and Europe (the European Competence Network on Mastocytosis was the main source), the Center for International Blood and Marrow Transplant Research, and published reports. After approval by the individual center's institutional research board, information on patients was collected anonymously by using a data collection form. The diagnosis of SM was confirmed by a local investigator before reporting data to the data collection center at the University of Minnesota. Fifty-seven patients were identified: the data on 52 patients (43 never published and nine published) were obtained from 33 individual institutions (Appendix Tables A1, A2, and A3, online only).28,29,33,37–40 The remaining five patients were identified from the literature, but no contact with the respective centers for participation was secured.30,32,34–36 Data on surviving patients were updated during the study period of more than 2 years.
Response Evaluation in SM
Given the limitations of obtaining detailed source data for all patients, we could not use the SM response criteria developed by Valent et al41 or the consensus statement developed by the International Working Group-Myeloproliferative Neoplasms Research and Treatment and the European Competence Network on Mastocytosis.42 Therefore, three parameters were used to assess response in SM: the percentage of bone marrow mast cells, serum tryptase levels, and organ involvement. Organ involvement in SM at the time of diagnosis and changes over time (ie, improved, stable, or progressing) were reported and were included in the criteria used to determine responses (ie, laboratory measures, physical examination, imaging, or a combination thereof). Response was defined as ≥ 50% decrease in both serum tryptase levels and bone marrow mast cell percentage from biopsies (not aspirates) when both tests were available or, if the results of only one test were available, ≥ 50% improvement in one test if the results were corroborated by clinical determination of improvement and the absence of worsening of any other organ involvement. The lowest level of response was recorded if there was inconsistency among these three parameters. Complete response (CR) was defined as resolution of SM in all organs along with normalization of serum tryptase levels. Progression was defined as ≥ 50% increase in any of the three parameters, and stable disease was defined as less than 50% change in all three parameters. Although quantitative assessment was desirable for response evaluation, qualitative assessment that clearly indicated outcome (eg, worsened, increased, no evidence of mast cells in biopsies) was used in few patients. Because this is a retrospective analysis, there were no predetermined uniform time points for response evaluations. However, a vast majority of patients were evaluated at day 100, at relapse of hematologic malignancy or SM, or at progression of SM. Duration of response was analyzed in 36 patients (nonresponders are excluded as well as four responders with missing time of response). Response assessments were made independently by C.U., C.A., and P.V., and each investigator who provided patient information confirmed the final assigned response. In some patients, the local hematopathologist re-evaluated the original bone marrow specifically for mast cell burden. Response was accepted as reported to the French Registry by the individual institutions (n = 5; patients 42 to 46, and as previously published (n = 5; patients 1, 6, 12, 14, and 16). Treatment history in these patients was focused on SM-directed cytoreductive therapy that consisted primarily of interferon, steroids, tyrosine kinase inhibitors, and cladribine, but included other agents such as hydroxyurea, thalidomide, gemtuzumab ozogamicin, arsenic trioxide, cyclosporine, fludarabine, and cytarabine.
Statistical Analysis
Patient and disease characteristics were summarized by disease type. Cumulative incidence of treatment-related mortality was calculated with relapse as a competing risk. Kaplan-Meier estimations and the log-rank test were used to estimate and compare overall survival (OS) and progression-free survival (PFS) from time of transplantation. At the beginning of the study, the poor prognosis of patients with MCL was apparent and, therefore, we excluded these patients from univariable analysis of OS and PFS to eliminate possible confounding of MCL with other effects. We defined a P value of ≤ .05 as significant in the univariable analysis of risk factors for survival. Analysis was performed by using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient, Donor, and AlloHCT Characteristics
Fifty-seven patients with advanced SM underwent alloHCT between 1990 and May 2013 (Table 1). The majority of patients (n = 38, including one patient with MCL-AML and one patient with myelomastocytic leukemia) were diagnosed with SM-AHNMD, with the most common AHNMD being AML (n = 20, including one patient with myelomastocytic leukemia). Most patients had SM involvement in at least one extramedullary organ or tissue, primarily in the spleen (n = 33), liver (n = 26), skin (n = 12), and lymph nodes (n = 11). The most frequent recurrent cytogenetic abnormality was t(8;21)(q22,q22) (n = 5), and the most frequent molecular abnormality was KITD816V (n = 21). Of 20 patients with AML, 11 had abnormal cytogenetics, and nine had a KIT mutation, 17 received anthracycline plus cytarabine for induction, and six had persisting leukemia at the time of alloHCT. Patient and disease characteristics for each patient are detailed in Appendix Table A1.
Table 1.
Patient and Donor Characteristics in Different SM Subtypes
| Characteristic | ASM | MCL | SM-AHNMD | Total | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 7 | 12 | 38 | 57 | ||||
| Age, years | ||||||||
| Median | 50 | 43 | 45 | 46 | ||||
| Range | 31-62 | 13-60 | 11-67 | 11-67 | ||||
| Male sex | 4 | 57 | 5 | 42 | 21 | 55 | 30 | 53 |
| KIT mutations | ||||||||
| Positive | 1 | 14 | 4 | 33 | 19 | 50 | 24 | 42 |
| Negative | 0 | 0 | 4 | 33 | 5 | 13 | 9 | 16 |
| Not reported | 6 | 86 | 4 | 33 | 14 | 37 | 24 | 42 |
| Cytogenetics | ||||||||
| Normal | 3 | 43 | 6 | 50 | 16 | 42 | 25 | 44 |
| t(8;21) or its variants | 0 | 0 | 0 | 0 | 5 | 13 | 5 | 9 |
| Abnormal (other than t(8;21)) | 0 | 0 | 4 | 33 | 10 | 26 | 14 | 25 |
| Not reported | 4 | 57 | 2 | 17 | 7 | 18 | 13 | 23 |
| No. of involved organs (in addition to bone marrow) | ||||||||
| 0 | 3 | 43 | 1 | 8 | 8 | 21 | 12 | 21 |
| 1-2 | 3 | 43 | 5 | 42 | 15 | 39 | 23 | 40 |
| ≥ 3 | 0 | 0 | 6 | 50 | 12 | 32 | 18 | 32 |
| Not reported | 1 | 14 | 0 | 0 | 3 | 8 | 4 | 7 |
| No. of SM treatment categories | ||||||||
| 0 | 0 | 0 | 2 | 17 | 21 | 55 | 23 | 40 |
| 1-2 | 3 | 43 | 7 | 58 | 9 | 23 | 19 | 33 |
| ≥ 3 | 2 | 29 | 3 | 25 | 7 | 18 | 12 | 21 |
| Not reported | 2 | 29 | 0 | 0 | 1 | 3 | 3 | 5 |
| Time from SM diagnosis to alloHCT | ||||||||
| Median | 19 | 9 | 9 | 9 | ||||
| Range | 7-192 | 3-216 | 2-96 | 2-216 | ||||
| Karnofsky performance score | ||||||||
| ≥ 90 | 0 | 0 | 5 | 42 | 16 | 42 | 21 | 37 |
| < 90 | 3 | 43 | 4 | 33 | 9 | 24 | 16 | 28 |
| Not reported | 4 | 57 | 3 | 25 | 13 | 34 | 20 | 35 |
| Donor | ||||||||
| Sibling | 4 | 57 | 5 | 42 | 25 | 66 | 34 | 60 |
| Unrelated | 2 | 29 | 5 | 42 | 10 | 26 | 17 | 30 |
| UCB or haploidentical | 0 | 0 | 2 | 17 | 1 | 3 | 3 | 5 |
| Not reported | 1 | 13 | 0 | 0 | 2 | 5 | 3 | 5 |
| Donor age, years | ||||||||
| Median | 32 | 39 | 44 | 42 | ||||
| Range | 22-54 | 21-52 | 23-65 | 21-65 | ||||
| Recipient-donor sex mismatched | ||||||||
| Matched | 2 | 29 | 6 | 50 | 23 | 61 | 31 | 54 |
| Mismatched | 4 | 57 | 5 | 42 | 13 | 34 | 22 | 39 |
| Not reported | 1 | 14 | 1 | 8 | 2 | 5 | 4 | 7 |
Most patients received either sibling or unrelated alloHCT, although two patients received alloHCT from umbilical cord blood (UCB) and one received alloHCT from an HLA-haploidentical relative (Table 2). All patients received HLA-matched alloHCT except three patients whose unrelated donors were 9/10 HLA-locus and allele matched, two patients whose UCB was 5/6 HLA matched, and one patient whose HLA-haploidentical donor was 4/8 matched. Although the majority of patients (63%) received myeloablative conditioning (MAC), more than a third of patients received reduced-intensity conditioning (RIC). Detailed information on donor characteristics and alloHCT for individual patients is provided in Appendix Table A2.
Table 2.
AlloHCT Treatment, Complications, and Outcomes in Different SM Subtypes
| Variable | ASM | MCL | SM-AHNMD | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | 90% CI | No. | % | 90% CI | No. | % | 90% CI | No. | % | 90% CI |
| No. of patients | 7 | 12 | 38 | 57 | |||||||
| Conditioning | |||||||||||
| Reduced intensity | 4 | 57 | 3 | 25 | 14 | 37 | 21 | 37 | |||
| Myeloablative | 3 | 43 | 9 | 75 | 24 | 63 | 36 | 63 | |||
| With TBI | 1 | 14 | 3 | 25 | 12 | 32 | 16 | 28 | |||
| Transplantation year | |||||||||||
| 1990-2004 | 1 | 14 | 6 | 50 | 14 | 37 | 21 | 37 | |||
| 2005-2013 | 6 | 86 | 6 | 50 | 24 | 63 | 36 | 63 | |||
| GVHD prophylaxis | |||||||||||
| Methotrexate | 3 | 43 | 6 | 50 | 19 | 50 | 28 | 49 | |||
| No methotrexate | 2 | 29 | 5 | 42 | 16 | 42 | 23 | 40 | |||
| Not reported | 2 | 29 | 1 | 8 | 3 | 8 | 6 | 11 | |||
| Tacrolimus | 0 | 0 | 3 | 25 | 9 | 24 | 12 | 21 | |||
| Cyclosporine | 5 | 71 | 4 | 33 | 24 | 63 | 33 | 58 | |||
| Not reported | 2 | 29 | 5 | 42 | 5 | 13 | 12 | 21 | |||
| Response in SM | |||||||||||
| Primary resistance | 1 | 14 | 3 | 25 | 1 | 3 | 5 | 9 | |||
| Stable disease | 1 | 14 | 0 | 0 | 11 | 30 | 12 | 21 | |||
| Response | 5 | 72 | 9 | 75 | 26 | 68 | 40 | 70 | |||
| Complete remission | 3 | 43 | 3 | 25 | 10 | 26 | 16 | 28 | |||
| Acute GVHD* | |||||||||||
| None or grade 1 | 5 | 71 | 6 | 50 | 19 | 50 | 30 | 53 | |||
| Grade 2 to 4 | 2 | 29 | 5 | 42 | 16 | 42 | 23 | 40 | |||
| Not reported | 0 | 0 | 1 | 8 | 3 | 8 | 4 | 7 | |||
| Chronic GVHD* | |||||||||||
| None | 3 | 43 | 8 | 67 | 12 | 32 | 23 | 40 | |||
| Limited or extensive | 3 | 43 | 0 | 0 | 21 | 55 | 24 | 42 | |||
| Extensive | 1 | 14 | 0 | 0 | 12 | 32 | 13 | 23 | |||
| Not reported | 1 | 14 | 4 | 33 | 5 | 13 | 10 | 18 | |||
| Transplantation-related mortality | |||||||||||
| 6 months | 14 | 1 to 30 | 25 | 6 to 44 | 5 | 1 to 11 | 11 | 4 to 17 | |||
| 1 year | 14 | 1 to 30 | 33 | 12 to 54 | 17 | 7 to 27 | 20 | 11 to 28 | |||
| Overall survival | |||||||||||
| 1 year | 43 | 14 to 70 | 25 | 8 to 46 | 78 | 64 to 87 | 62 | 50 to 72 | |||
| 3 years | 43 | 14 to 70 | 17 | 4 to 37 | 74 | 60 to 84 | 57 | 46 to 68 | |||
| Progression-free survival | |||||||||||
| 1 year | 43 | 14 to 70 | 17 | 4 to 37 | 70 | 55 to 80 | 55 | 44 to 65 | |||
| 3 year | 43 | 14 to 70 | 17 | 4 to 37 | 63 | 47 to 75 | 51 | 39 to 61 |
Disease Responses After AlloHCT
Responses were assigned to 12 patients with one criterion, 30 patients with two criteria, and 10 patients with all three criteria. Overall, SM responded to alloHCT in 40 patients (70%; Tables 2 and 3). The median bone marrow biopsy number after transplantation was 2.2. The median bone marrow mast cell percentage in biopsies (21% [range, 2% to 90%] before alloHCT v 1.8% [range, 0% to 90%] after alloHCT) and serum tryptase levels (130 ng/mL [range, 11 to 889 ng/mL] before alloHCT and 16 ng/mL [range, 2 to 404 ng/mL] after alloHCT) decreased significantly after alloHCT in patients with data available for before and after alloHCT (Figs 1A to 1D). In this comparison, the best values after alloHCT were used, and the median time to reach the best responses for both variables was 3 months (range, 1 to 36 months) after alloHCT. Of the 40 responding patients, 16 (28%) achieved CR (KIT mutations were negative in two of two patients with CR when tested after alloHCT). Twelve patients (21%) had stable disease. Patients with MCL had more primary resistance (three of five patients) and progression after initial response (three of 10 patients). The median time of response duration was 20 months. All 38 patients with SM-AHNMD achieved CR regarding the AHNMD component, but 10 subsequently relapsed with AHNMD, and five of these died. Details of treatment and outcome are given in Appendix Table A3.
Table 3.
Factors Associated With OS and SM PFS in Patients With no MCL Only (n = 45)
| Factor | No. | % | OS | PFS | ||||
|---|---|---|---|---|---|---|---|---|
| 1-Year | 90% CI | P* | 1-Year | 90% CI | P* | |||
| Diagnosis | ||||||||
| ASM | 7 | 16 | 43 | 14 to 70 | .05 | 43 | 14 to 70 | .07 |
| SM-AHNMD | 38 | 84 | 78 | 64 to 87 | 70 | 55 to 80 | ||
| AML, ALL, MML | 21 | 47 | 75 | 55 to 87 | .75 | 65 | 45 to 80 | .53 |
| MDS, MPN, MM | 17 | 38 | 81 | 57 to 92 | 75 | 52 to 88 | ||
| Age, years | ||||||||
| < 40 | 16 | 36 | 80 | 56 to 92 | .39 | 68 | 44 to 83 | .85 |
| ≥ 40 | 29 | 64 | 67 | 50 to 80 | 65 | 48 to 77 | ||
| Sex | ||||||||
| Female | 20 | 44 | 79 | 58 to 90 | .34 | 74 | 54 to 87 | .32 |
| Male | 25 | 56 | 65 | 46 to 79 | 59 | 40 to 73 | ||
| KIT mutations | ||||||||
| Negative | 5 | 11 | 60 | 19 to 85 | .27 | 40 | 9 to 71 | .40 |
| Positive | 20 | 44 | 82 | 60 to 93 | 74 | 53 to 87 | ||
| Unknown | 20 | 44 | 65 | 40 to 81 | 65 | 40 to 82 | ||
| Cytogenetics | ||||||||
| Normal | 19 | 42 | 68 | 47 to 82 | .61 | 68 | 47 to 82 | .92 |
| t(8;21) | 5 | 11 | 100 | 60 | 19 to 85 | |||
| Other | 10 | 22 | 67 | 35 to 86 | 67 | 35 to 86 | ||
| Unknown | 11 | 24 | 69 | 38 to 87 | 62 | 34 to 82 | ||
| Treatment for SM | ||||||||
| None | 21 | 47 | 79 | 59 to 90 | .11 | 70 | 49 to 83 | .27 |
| One to two agents | 12 | 27 | 81 | 51 to 94 | 74 | 46 to 89 | ||
| Three or more agents | 9 | 20 | 44 | 18 to 68 | 44 | 18 to 68 | ||
| Karnofsky performance score | ||||||||
| < 90 | 12 | 27 | 48 | 23 to 69 | .15 | 49 | 24 to 70 | .28 |
| ≥ 90 | 16 | 36 | 80 | 56 to 92 | 80 | 56 to 92 | ||
| Unknown | 17 | 38 | 82 | 60 to 93 | 64 | 42 to 80 | ||
| AML CR status | ||||||||
| In CR | 14 | 31 | 79 | 54 to 91 | .63 | 71 | 46 to 86 | .37 |
| Not in CR | 6 | 13 | 60 | 19 to 85 | 42 | 9 to 72 | ||
| Percentage of BM mast cells before transplantation | ||||||||
| < 20 | 14 | 31 | 68 | 42 to 85 | .87 | 53 | 29 to 73 | .33 |
| ≥ 20 | 15 | 33 | 73 | 49 to 87 | 73 | 49 to 87 | ||
| Time from diagnosis to alloHCT, months | ||||||||
| ≤ 13 | 29 | 64 | 78 | 62 to 88 | .23 | 71 | 54 to 83 | .27 |
| > 13 | 16 | 36 | 60 | 36 to 77 | 56 | 34 to 74 | ||
| Conditioning | ||||||||
| Reduced intensity | 18 | 40 | 57 | 34 to 74 | .10 | 48 | 27 to 66 | .03 |
| Myeloablative | 27 | 60 | 81 | 65 to 91 | 77 | 61 to 88 | ||
| Myeloablative with TBI | 13 | 29 | 85 | 59 to 95 | .98 | 85 | 59 to 95 | .59 |
| Myeloablative without TBI | 13 | 29 | 84 | 57 to 95 | 76 | 49 to 90 | ||
| Graft source | ||||||||
| BM | 16 | 36 | 75 | 52 to 88 | .86 | 75 | 52 to 88 | .32 |
| PBSCs | 27 | 60 | 72 | 54 to 84 | 62 | 45 to 75 | ||
| Donor age, years | ||||||||
| < 40 | 11 | 24 | 64 | 35 to 82 | .31 | 64 | 35 to 82 | .43 |
| ≥ 40 | 23 | 51 | 76 | 55 to 87 | 76 | 57 to 88 | ||
| Donor sex match | ||||||||
| Match | 25 | 56 | 80 | 62 to 90 | .21 | 72 | 54 to 84 | .18 |
| Female donor, male recipient | 10 | 22 | 44 | 18 to 68 | 34 | 11 to 59 | ||
| Male donor, female recipient | 7 | 16 | 71 | 34 to 90 | 71 | 34 to 90 | ||
| Donor relation | ||||||||
| Sibling | 29 | 64 | 74 | 56 to 85 | .50 | 64 | 47 to 77 | .95 |
| Unrelated | 12 | 27 | 67 | 40 to 84 | 67 | 40 to 84 | ||
| Other (UCB) | 1 | 2 | 0 | 0 | ||||
| aGVHD | ||||||||
| None or grade 1 | 24 | 53 | 73 | 49 to 87 | .49 | 69 | 46 to 84 | .55 |
| Grade 2 to 4 | 18 | 40 | 66 | 44 to 81 | 61 | 35 to 79 |
Fig 1.
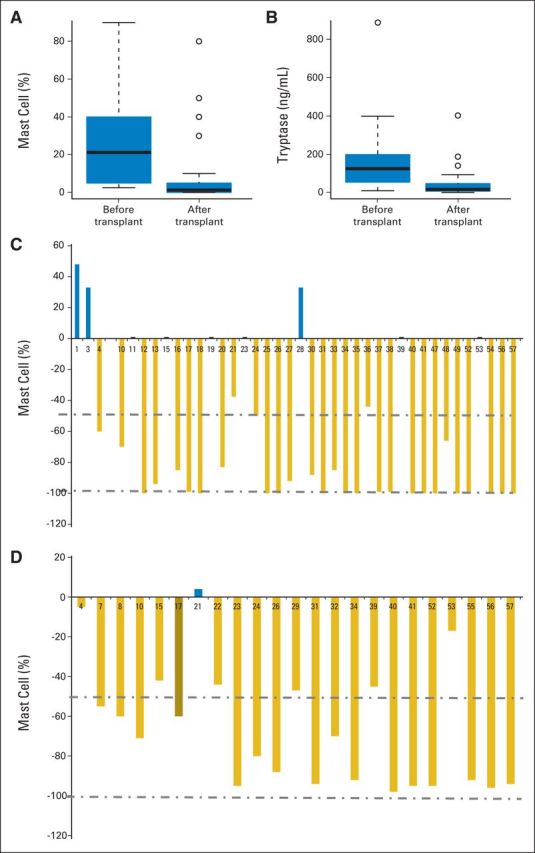
Allogeneic hematopoietic stem-cell transplantation (alloHCT) improves responses in patients with advanced systemic mastocytosis. Changes in (A) bone marrow cell percentage (n = 39; P < .01) and (B) serum tryptase levels (n = 23; P < .01) in patients with before and after alloHCT data available. The post-transplantation data represent the best (lowest) values of both mast cell percentage and serum tryptase, both of which were observed a median of 3 months (range, 1 to 36 months) after alloHCT. The median and interquartile range are indicated by a solid line and rectangle, respectively. Observations outside the interquartile range are indicated by dashed lines or dots. Percentage change in mast cells in (C) bone marrow and (D) in serum tryptase levels in each patient with available before and after alloHCT data.
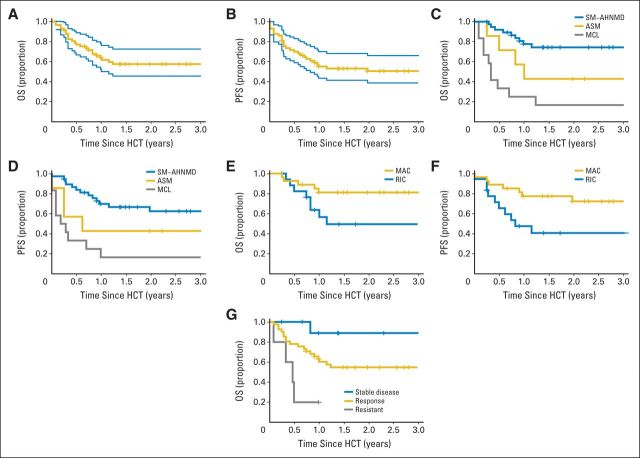
OS and PFS for all patients were 62% (90% CI, 50% to 72%) and 57% (90% CI, 44% to 65%) at 1 year, and 55% (90% CI, 46% to 68%) and 51% (90% CI, 39% to 61%) at 3 years, respectively (Figs 2A and 2B; Tables 2 and 3). OS and PFS were significantly higher in patients with SM-AHNMD and were lowest in patients with MCL (Figs 2C and 2D; P < .01). No deaths or relapses were observed after 15 and 24 months, respectively. The median follow-up among survivors was 32 months (range, 3 to 202 months).
Fig 2.
Allogeneic hematopoietic stem-cell transplantation (alloHCT or HCT) outcomes in patients with advanced systemic mastocytosis (SM). (A) Overall survival (OS) and (B) progression-free survival (PFS) for all patients with advanced SM. Blue lines represent 95% CIs. (C) OS and (D) SM PFS by type of systemic mastocytosis. (E) OS and (F) SM PFS by conditioning regimen intensity. (G) OS by initial response in SM. ASM, aggressive systemic mastocytosis; MAC, myeloablative conditioning; MCL, mast cell leukemia; RIC, reduced-intensity conditioning; SM-AHNMD, SM with an associated clonal hematologic non–mast cell lineage disease.
Factors Affecting PFS and OS
Univariable analysis of relevant variables and outcomes are shown in Table 3. The strongest risk factor for worse OS was a diagnosis of MCL. Other risk factors were evaluated after excluding patients with MCL (n = 12). In the 45 remaining patients, factors that had an effect on survival included a diagnosis of ASM and RIC (Figs 2E and 2F). The median age for patients receiving MAC regimens was 38 years (range, 11 to 62 years) compared with 50 years (range, 17 to 67 years) for patients receiving RIC. The superior outcome with MAC was not fully accounted for by younger patient age; for patients older than age 40 years, 1-year OS was 85% after MAC (n = 13) and 51% after RIC (n = 16). RIC was used as frequently in the entire study cohort as in patients with Karnofsky performance status (KPSs) ≤ 80%, (21 [37%] of 57 and four [25%] of 16, respectively). A lower KPS before alloHCT seemed to lower survival, and six of seven patients with KPSs ≤ 70% died. OS at 1 year was shorter for patients with progression (20%; 90% CI, 2% to 52%) compared with patients with stable disease (89%; 90% CI, 54% to 98%) and with responders (63%; 90% CI, 49% to 74%; Fig 2G).
Factors without any identifiable impact on OS and PFS were patient age, donor age, donor type (sibling or unrelated donor), graft source (bone marrow or peripheral blood stem cells), bone marrow mast cell percentage at alloHCT, KIT mutation status, cytogenetic groupings, total body irradiation used in MAC regimens, and CR status at alloHCT (Table 3). OS and PFS at 1 year were not affected by whether or not the patient received prior SM-directed cytoreductive therapy (Table 3). When they were specifically evaluated in patients with SM-AHNMD only, OS and PFS were again similar (73% and 68% for patients with history of prior SM-directed therapy [41%] compared with 79% and 70% for those who did not receive therapy [55%], respectively).
All patients who received alternative donor transplants (two UCB and one HLA-haploidentical relative) and all patients with MCL who received RIC (n = 3) died. In contrast, all five patients with t(8;21) or its variant survived.
Donor Lymphocyte Infusions and Second AlloHCT
Six of 10 patients who received donor lymphocyte infusions (DLIs) for mixed chimerism and/or stable SM disease responded to this treatment (three achieved CR, of which two were durable). However, three patients who received DLIs for SM progression (n = 2) or graft failure with AML relapse (n = 1) had no response and died (Appendix Table A3).
A second alloHCT (two RIC and one MAC regimen) was performed for relapse of myelomastocytic leukemia (n = 1), relapse of AML (n = 1), and progression of both MDS and SM (n = 1) at 4, 5, and 44 months after the first alloHCT, respectively. All three patients were alive in CR of SM or hematologic malignancy after the second alloHCT (Appendix Table A3).
AlloHCT Complications
Treatment-related mortality at 6 months and 1 year was 11% and 20%, respectively, and was highest in MCL (25% and 33%; Table 2). Primary and secondary engraftment failure each occurred in one patient. In the first 100 days after initiation of alloHCT conditioning, symptoms possibly related to mast cell degranulation occurred in five patients and included hot flashs (n = 3), skin rash (n = 1), and abdominal cramps and nausea (n = 1). Acute graft-versus-host disease (GVHD) grades 2 to 4 and chronic extensive GVHD occurred in 40% and 23% of patients, respectively (Table 2). Two patients died from complications related to severe acute GVHD (Appendix Table A3).
DISCUSSION
This study has found that alloHCT can confer long-term OS in patients with advanced SM. The greatest survival benefit was observed in patients with SM-AHNMD who had a 3-year survival probability of 74%. The reported median OS of patients with SM-AHNMD without alloHCT was 2 years.6 Favorable OS in our group is especially significant, given that approximately one third of the patients with AML had active disease at transplantation—an indisputably poor prognostic factor for outcomes after alloHCT.27,43–49 Although the t(8;21)(q22,q22) translocation is considered a favorable prognostic factor in patients with AML without SM,50–52 patients with AML with the same cytogenetic abnormality have poor prognosis in the presence of SM, even hidden and/or occult SM.31,53,54 The presence of KIT mutations in patients with AML with t(8;21) is associated with poor prognosis regardless of the presence of SM.55–60 Most of our patients with SM-AML had KIT mutations. It is possible that not all patients had aggressive SM at the diagnosis of AHNMD, given that C findings (clinical findings related to organs involved in SM, eg, pancytopenia) can be caused by the AHNMD component as well. However, regarding survival, there was no difference between patients who received SM-specific cytoreductive therapy before alloHCT (indicating aggressive SM) and those who did not. One can argue that AML diagnosed in the context of SM (or in the context of _KIT_D816V in which an occult SM is often present) should always be judged as secondary AML and thus as high-risk (poor prognosis) disease regardless of the aggressiveness of SM. Together, our data suggest that alloHCT may overcome the unfavorable prognosis in patients with SM-AML. AlloHCT also provided long-term survival in approximately 40% of patients with ASM, which seems superior to reported survival rates in patients who did not have transplantations (median survival, 3.5 years without any evidence of a plateau).6 Patients with MCL had the poorest outcome among all of the SM subtypes in the study. However, given that the reported median survival of patients with MCL without alloHCT is short (< 12 months), our results suggest that alloHCT may be beneficial for patients with MCL as well.6,61 Evidence of treatment failure among the majority of patients with MCL was shown by early treatment–related mortality and rapid disease progression after initial responses. However, those responses, albeit transient, suggest that immunotherapy has an impact on MCL. The fact that the only long-term survivors were among patients prepared for alloHCT with high-intensity MAC regimens may indicate that the actual tumor burden that has to be overcome by donor cells is a critical determinant influencing transplantation outcome. However, when compared with results in patients with SM-AHNMD (many of whom had AML that was not in remission at the time of alloHCT) or those with ASM, this pattern suggests that not only disease burden but also the intrinsic resistance of MCL cells was an important factor. In general, patients conditioned with myeloablative regimens fared better than patients prepared with reduced-intensity regimens; the advantage of lower regimen-related mortality was canceled out by a higher progression incidence.62,63 The observation that patients with a lower KPS (≤ 70%) experienced higher mortality is consistent with results in many reports on various diseases showing the impact of performance status and comorbidities in particular on the outcome of alloHCT.64,65
Responses in SM were observed in 70% of the patients examined. However, because this is a retrospective analysis and thus there were no predetermined evaluation time points after alloHCT, the duration of response and PFS should be viewed with caution. Given that there is a decrease in serum tryptase levels after alloHCT that may reflect responses in the AHNMD component (eg, AML or MDS)66–68 itself, response evaluation mainly depended on bone marrow mast cell percentage. Responses (observed after RIC and MAC regimens and DLI) were often durable and sometimes developed gradually over years, suggesting that donor-derived cells induced a potent graft-versus-mastocytosis effect. Mast cells have been shown to express HLA class I and, at least under certain conditions such as in vitro stimulation of mast cells by interferon gamma, class II antigens.69–72 These antigens are critical for eliciting a graft-versus-tumor effect through alloreactive T cells and natural-killer cells.73 The apparent plateau of both OS and PFS suggests that alloHCT can be curative in some patients with SM. AlloHCT conferred long-term survival not only for the responders but also for the patients having stable disease. Most of the patients with stable disease had AHNMD as well; therefore, benefiting from alloHCT necessitates curing AHNMD but not the SM component. Another explanation could be that perhaps a longer time is needed to observe improvements in the SM component compared with AHNMD in some patients with SM-AHNMD.
SM-specific complications, including anaphylactoid or severe mediator-related reactions74 due to rapid lysis of mast cells and graft failure due to marrow fibrosis, were rare. Low incidence of graft failure might result from the role of mast cells in increasing allograft tolerance. Healthy mast cells are crucial for skin allograft tolerance, probably through regulatory T-cell–dependent peripheral tolerance in mice.75 The frequency of acute and chronic GVHD was similar to that seen in the overall alloHCT population.76,77
Although this study has several limitations because of its retrospective nature, it summarizes transplantation results from the largest cohort of patients with this rare and fatal disease: alloHCT is reasonably safe, and overall outcome is promising. These data support a prospective study in which before-and-after-alloHCT means should be used to further improve outcomes in patients with ASM and MCL.
Acknowledgment
The authors thank Deepti Radia, Lawrence B. Afrin, Koen van Besien, Andy Artz, Russel Bryness, Aaron T. Gerds, and Irene Cavattoni for their invaluable assistance with identifying patients, and Michael J. Franklin and K. Frank Austen for critical review of the manuscript.
Appendix
Table A1.
Patient and SM Characteristics
| Author* | Country | Patient No. | Sex | Patient Age at AlloHCT (years) | Diagnosis | Year of Diagnosis of Primary SM | Cytogenetic Abnormality | KIT Mutation |
|---|---|---|---|---|---|---|---|---|
| Chen et al36 | Taiwan | 1 | M | 18 | MCL-AML | 2001 | t(8;21)(q22;q22) | No |
| Devine | United States | 2 | M | 36 | SM-AHNMD (MPN-MDS with Eos) | 1997 | Normal | NA |
| Födinger et al29 | Austria | 3 | F | 11 | SM-AHNMD (AML) progressed from MDS-RAEB-II) | 1991 | t(8;1)(q22,q21), de1(5)(q13,q23) | NA |
| Hsu | United States | 4 | M | 62 | ASM | 2005 | Normal | NA |
| Doubek | Czech Republic | 5 | M | 57 | ASM | 2010 | Normal | D816V |
| Nagai et al35 | Japan | 6 | F | 32 | SM-AHNMD (AML) | 2006 | 46XX, t(8;21) (q22;q22), del9 (q22;q34) | D816Y |
| Nakamura et al37 | United States | 7 | M | 49 | SM-AHNMD (MPN with Eos) | 1999 | NA | D816V |
| Nakamura et al37 | United States | 8 | F | 50 | SM-AHNMD (MDS-RA) | 1998 | NA | D816V |
| Nakamura et al37 | United States | 9 | M | 47 | MCL (progressed from SM) | 1996 | NA | D816G |
| Valent | Austria | 10 | F | 36 | MCL | 2005 | Normal | No D816V found |
| Valent | Austria | 11 | F | 44 | SM-AHNMD (AML) | 1995 | NA | NA |
| Przepiorka et al30 | United States | 12 | M | 32 | ASM | Approximately 1990 | Normal | NA |
| Pullarkat et al33 | United States | 13 | F | 51 | SM-AHNMD (AML) | 2003 | t(8;21) (q22;22) and del(9)(q12;q22) | D816V |
| Rønnov-Jessen et al32 | Denmark | 14 | F | 38 | SM-AHNMD (AML) | 1991 | NA | NA |
| Sperr et al28 | Austria | 15 | M | 17 | MML | 2001 | Complex: t(8;10;21)(q22;q21;q22), t(11;19)(q13;13), del(9)(q22) | No D816V found |
| Spyridonidis et al34 | Germany | 16 | M | 31 | MCL | Approximately 2000 | t(13;14) (q10;q10) | No codon 816 and 560 mutation found |
| Stuart | United States | 17 | M | 56 | SM-AHNMD (AML progressed from MDS) | 2009 | Normal | D816V |
| Popat | United States | 18 | M | 46 | MCL | 2001 | Diploidy | NA |
| Hogan | United States | 19 | M | 61 | SM-AHNMD (AML) | 2011 | Monosomy 7 | D816V |
| Hogan | United States | 20 | F | 26 | SM-AHNMD (AML) | 2010 | Normal | D816V |
| Kreil | Germany | 21 | F | 50 | ASM | 1991 | NA | NA |
| Reiter | Germany | 22 | F | 27 | SM-AHNMD (AML) | 2005 | Normal | D816V |
| Gruhn | Germany | 23 | M | 17 | SM-AHNMD (MDS-RCMD) | 2007 | Normal | D816V |
| Reiter | Germany | 24 | M | 65 | SM-AHNMD (AML) | 2008 | Trisomy 8 | D816V |
| Hermine | France | 25 | M | 61 | SM-AHNMD (AML) | 2007 | Trisomy 8 | D816V |
| Gromke et al38 | Germany | 26 | M | 53 | SM-AHNMD (AML) | 2002 | Normal | D816V |
| Tholouli | England | 27 | F | 47 | MCL, progressed from SM | 2011 | Complex: 43,XX,-1,add(2)(p?13),-4,add(5)(p15),-9,del(10)(q2?4q2?6),-11,-17,der(17)? t(11;17) (q13;q25), 20, +2mar,+r[4]/88,idemx2[1]/46,XX[6] | D816Y |
| Chantom et al40 | United States | 28 | F | 23 | MCL, progressed from solidary mastocytoma | 2004 | Normal | NA |
| Schmid | Germany | 29 | F | 51 | SM-AHNMD (AML progressed from MDS-MPN) | 2009 | Normal | No D816V found |
| Valentini et al39 | Italy | 30 | F | 45 | MCL | Normal | D816V | |
| Baurmann | Germany | 31 | F | 44 | SM-AHNMD (MDS-MPN with Eos) | 2003 | Normal | D816V |
| Baurmann | Germany | 32 | F | 49 | SM-AHNMD (MDS-RCMD) | 2008 | Normal | D816V |
| Shore | 33 | M | 59 | MCL | 2008 | Normal | D816V | |
| Shore | 34 | M | 57 | SM-AHNMD (MDS) | 2011 | del7q | D816V | |
| Scott | United States | 35 | M | 35 | SM-AHNMD (MDS-MPN) | 2004 | Normal | NA |
| Scott | United States | 36 | M | 31 | MCL | 2004 | Complex:43-44,XY,?del(3)(p?),-8,?add(9)(q34), −10,?der(11)t(8;11)(q?11.2;q?13), add(16)(q24),?del(17)(q11.2) add(19)(q13.3),add(20)(q13.3)[cp13]/46,XY[7] | No D816V found |
| Scott | United States | 37 | M | 21 | SM-AHNMD (AML) in CR2 | 2006 | t(8;21)(q22;q22) | NA |
| Scott | United States | 38 | M | 46 | SM-AHNMD (AML) secondary to MDS | 2008 | NA | No D816V found |
| Scott | United States | 39 | F | 43 | SM-AHNMD (MDS) | 2010 | 46,XX[30], abnormal IFISH (25.6%) for additional copy of EVI1 (3q26) | D816V |
| Scott | United States | 40 | F | 31 | SM-AHNMD (MDS) | 2008 | Normal | No D816V found |
| Legrand | France | 41 | M | 62 | SM-AHNMD | 2008 | −Y | D816V |
| Damaj/Yakoub-agha | France | 42 | F | 31 | ASM | 2001 | NA | NA |
| Damaj/Yakoub-agha | France | 43 | M | 57 | SM-AHNMD (MM) | 1998 | NA | NA |
| Damaj/Yakoub-agha | France | 44 | M | 42 | SM-AHNMD (ALL) | 2003 | NA | NA |
| Damaj/Yakoub-agha | France | 45 | M | 47 | ASM | 2007 | NA | NA |
| Damaj/Yakoub-agha | France | 46 | F | 50 | ASM | 2002 | NA | NA |
| Godley | United States | 47 | F | 50 | MCL | 2005 | del (1p), add (6q), −9, add (16p) | NA |
| Van Lint | Italy | 48 | F | 36 | SM-AHNMD (AML) | 2002 | inv16 | No D816V found |
| Perales | United States | 49 | F | 40 | MCL | 1992 | NA | NA |
| Perales | United States | 50 | M | 50 | SM-AHNMD (MPN-MDS), SM secondary to UP | 2000 | Normal | NA |
| Perales | United States | 51 | F | 57 | SM-AHNMD (MDS RAEB), SM secondary to UP | 2011 | Normal | NA |
| Gilman | United States | 52 | F | 13 | MCL | 2012 | Normal | No D816V found |
| Vercellotti | United States | 53 | M | 60 | SM-AHNMD (MPN-MDS RAEB-I, 8% blasts) | 2010 | +8 | D816V |
| Ustun | United States | 54 | F | 64 | SM-AHNMD (AML) (therapy induced) | 2009 | −7 | NA |
| Nakamura | United States | 55 | F | 36 | SM-AHNMD (MPN-CMML) | 2011 | Normal | NA |
| Nakamura | United States | 56 | M | 67 | SM-AHNDM (MDS-MPN) | 2011 | Normal | D816V |
| Nakamura | United States | 57 | M | 41 | SM-AHNMD (AML, secondary to MDS) | 2003 | Normal | NA |
Table A2.
Transplantation Characteristics
| Patient No. | Diagnosis | Date of First AlloHCT | Time From Primary SM Diagnosis to alloHCT (months) | CR Status of AML or MML at AlloHCT | KPS at AlloHCT (%) | Graft Type | Conditioning | Donor | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Regimen | Type | Age (years) | Sex | |||||||
| 1 | MCL-AML | December 2001 | 4 | CR | NA | PBSC | MAC | Busulfan-cyclophosphamide-etoposide | NA | ||
| 2 | SM-MPN-MDS | March 2001 | 48 | NA | 70 | BM | MAC | Busulfan-cyclophosphamide | URD | 34 | F |
| 3 | SM-AML | 1992 | 10 | CR | NA | BM | MAC | Busulfan-cyclophosphamide-etoposide | NA | ||
| 4 | ASM | February 2007 | 18 | NA | 70 | PBSC | MAC | TBI-cyclophosphamide-cytarabine | URD | 34 | M |
| 5 | ASM | April 2012 | 21 | NA | 80 | PBSC | RIC | FLAMSA-RIC and splenic XRT | RD | 54 | F |
| 6 | SM-AML | 2006 | 4.5 | Active AML | NA | PBSC | MAC | TBI-cyclophosphamide | RD | F | |
| 7 | SM-MPN | 2002 | 36 | NA | NA | PBSC | RIC | Fludarabine-cyclophosphamide | RD | 43 | M |
| 8 | SM-MDS | 2001 | 36 | NA | NA | PBSC | RIC | Fludarabine-cyclophosphamide | RD | 54 | F |
| 9 | MCL | 2001 | 60 | NA | NA | PBSC | RIC | Fludarabine-cyclophosphamide | RD | 52 | F |
| 10 | MCL | March 2007 | 21 | NA | 90 | PBSC | MAC | Busulfan-cyclophosphamide-antithymocyte globulin | URD | M | |
| 11 | SM-AML | December 1996 | 8 | CR | 100 | BM | MAC | TBI-etoposide | RD | 42 | F |
| 12 | ASM | 1996 | 9 | NA | NA | BM | MAC | Busulfan-cyclophosphamide-thiotepa | NA | ||
| 13 | SM-AML | 2004 | 9 | CR | 90 | PBSC | MAC | FTBI-etoposide | RD | 48 | F |
| 14 | SM-AML | 1990 | 2 | Active AML | NA | BM | MAC | TBI-cyclophosphamide | RD | F | |
| 15 | MML | July 2001 | 4 | CR regarding myeloblasts | NA | PBSC | RIC | Fludarabine-TBI | RD | F | |
| 16 | MCL | 2000 | Approximately 3 | NA | NA | BM | MAC | Busulfan-cyclophosphamide | URD | F | |
| 17 | SM-AML | March 2010 | 11 | Active AML | 80 | PBSC | MAC | Busulfan-cyclophosphamide | RD | 49 | M |
| 18* | MCL | May 2002 | 9 | NA | 90 | BM | RIC | Fludarabine-melphalan-antithymocyte globulin | URD | ||
| 19 | SM-AML | November 2011 | 6 | CR | 90 | PBSC | RIC | Fludarabine-melphalan | RD | 63 | M |
| 20 | SM-AML | September 2011 | 9 | CR | 90 | PBSC. | MAC | TBI-cyclophosphamide | URD | 30 | M |
| 21 | ASM | July 2006 | 60 | NA | 50 | PBSC | RIC | Fludarabine-melphalan-thiotepa | URD | 22 | M |
| 22* | SM-AML | August 2012 | 84 | CR | 80 | PBSC | RIC | FLAMSA-RIC | URD | 23 | F |
| 23 | SM-MDS | September 2008 | 13 | NA | 80 | BM | MAC | Busulfan-cyclophosphamide-antithymocyte globulin | URD | 48 | M |
| 24 | SM-AML | April 2011 | 7 | Relapse/active AML | 90 | PBSC | RIC | FLAMSA-RIC | RD | 63 | F |
| 25 | SM-AML | January 2010 | 26 | Active AML | 80 | BM | MAC | RD | F | ||
| 26 | SM-AML | June 2008 | 72 | CR (CRi) | 90 | PBSC | MAC | Tresulfan-fludarabine | URD | 41 | M |
| 27* | MCL | May 2012 | 11 | NA | 100 | UCB | MAC | Fludarabine-cyclophosphamide-TBI | UCB | M | |
| 28 | MCL | October 2004 | 216 | NA | 90 | PBSC | MAC | Fludarabine-busulfan | RD | 21 | F |
| 29 | SM-AML | March 2011 | 13 | CR (CRi) | 90 | PBSC | RIC | FLAMSA-RIC | RD | 46 | F |
| 30 | MCL | January 2005 | 21 | NA | 70 | PBSC | MAC | Busulfan-cyclophosphamide | RD | 41 | F |
| 31 | SM-MDS-MPN | September 2007 | 17 | NA | 90 | PBSC | MAC | TBI-fludarabine -cyclophosphamide-antithymocyte globulin | RD | 46 | F |
| 32* | SM-MDS | September 2010 | 85 | NA | 100 | BM | MAC | Thiotepa-busulfan-cyclophosphamide-antithymocyte globulin | URD | F | |
| 33 | MCL | March 2009 | 7 | NA | 80 | PBSC | MAC | Fludarabine-busulfan-antithymocyte globulin | URD | M | |
| 34 | SM-MDS | May 2012 | 5 | NA | 90 | BM | MAC | Fludarabine-melphalan | URD | 45 | M |
| 35 | SM-MDS-MPN | October 2000 | 8 | NA | NA | PBSC | MAC | Busulfan-cyclophosphamide | RD | 33 | F |
| 36 | MCL | February 2005 | 8 | NA | NA | PBSC | MAC | Fludarabine-busulfan | URD | 21 | M |
| 37 | SM-AML | February 2005 | 12 | CR2 | NA | PBSC | MAC | TBI-cyclophosphamide | RD | 25 | M |
| 38 | SM-AML | February 2007 | 5 | CR | NA | PBSC | RIC | TBI-fludarabine | URD | 40 | M |
| 39 | SM-MDS | February 2009 | 10 | NA | 80 | PBSC | MAC | Busulfan-cyclophosphamide | URD | 41 | M |
| 40 | SM-MDS | February 2011 | 5 | NA | 90 | BM | MAC | TBI-cyclophosphamide | RD | 26 | F |
| 41 | SM-MPN | January 2009 | 5 | NA | 70 | BM | RIC | Fludarabine-melphalan | RD | 63 | F |
| 42 | ASM | November 2009 | 65 | NA | NA | BM | MAC | Busulfan-cyclophosphamide | RD | 29 | M |
| 43 | SM-MM | February 2003 | 18 | NA | NA | PBSC | RIC | Busulfan-fludarabine-antithymocyte globulin | RD | 39 | F |
| 44 | SM-ALL | January 1999 | 5 | NA | NA | BM | MAC | TBI-cyclophosphamide | RD | 38 | M |
| 45 | ASM | June 2005 | 19 | NA | NA | PBSC | RIC | RD | M | ||
| 46 | ASM | November 2007 | 7 | NA | NA | PBSC | RIC | Fludarabine-busulfan | RD | M | |
| 47 | MCL | August 2002 | 5 | NA | 90 | PBSC | RIC | Fludarabine-melphalan-alemtuzumab | RD | F | |
| 48 | SM-ASM-AML | October 2005 | 8 | Active AML | 60 | BM | MAC | TBI-cyclophosphamide | RD | 44 | M |
| 49 | MCL | June 2003 | 8.5 | NA | 70 | PBSC | MAC | TBI-thiotepa-fludarabine | RD | 37 | M |
| 50 | SM-MPN-MDS | November 2000 | 96 | NA | 80 | TBI-thiotepa-fludarabine | RD | 45 | M | ||
| 51 | SM-MDS | February 2003 | 32 | NA | NA | BM | MAC | Busulfan-melphalan | RD | 53 | F |
| 52 | MCL | July 2011 | 5 | NA | 80 | PBSC | MAC | TBI-thiotepa-fludarabine-antithymocyte globulin-local radiation to vertebrae | Haploidentical (mother) | 42 | F |
| 53 | SM-MPN-MDS | May 2013 | 5 | NA | 90 | PBSC | RIC | TBI-cyclophosphamide-fludarabine | RD | 62 | F |
| 54* | SM-AML | February 2011 | 3 | CR | 90 | UCB | RIC | TBI-cyclophosphamide-fludarabine | UCB | ||
| 55 | SM-CMML | January 2010 | 4 | NA | 90 | PBSC | MAC | Fludarabine-melphalan-TMI | RD | 36 | M |
| 56 | SM-AML (MDS-MPN) | December 2011 | 8 | NA | 90 | PBSC | RIC | Fludarabine-melphalan | RD | 65 | F |
| 57 | SM-AML secondary to MDS?) | December 2011 | 3 | CR | 90 | PBSC | RIC | Fludarabine-melphalan | URD | 44 | M |
Table A3.
Response in SM and Survival After First AlloHCT
| Patient No. | Disease | Reference | Serum Tryptase (ng/mL) | Percentage of Mast Cells in BM* | Other SM-Related Changes After AlloHCT | SM Response to First AlloHCT | Progression of SM After Initial Response | Treatment After AlloHCT and Response | Alive (yes/no) | Time From First AlloHCT (months) | Cause of Death | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Diagnosis | At AlloHCT | After AlloHCT | At Diagnosis | At AlloHCT | After AlloHCT | Yes/No | Time | |||||||
| 1 | MCL-AML | Chen et al36 | 40 | 27.5 | 40 at 1 month | Hepatomegaly, splenomegaly, and pancytopenia persisted | SD | No | Yes | 12 | ||||
| 2 | SM-MPN | ↑ | ↑ ↑ | 0 at 3 months | Eosinophil infiltrate (44%) in BM resolved (5%); dyserythropoiesis resolved | R | No | No | 4 | Infection and MOF | ||||
| 3 | SM-AML | Födinger et al29 | ↑ | 4 | 5 at 3 months | SD | No | Yes | 60 | |||||
| 4 | ASM | > 200 | 189 at 3 months | 75 | 30 at 3 months | R | No | No | 3 | CNS bleeding | ||||
| 5 | ASM | 0 | 0 at 1 month | Improvement in spleen and liver size | R | Yes | At 7 months | Cladribine, steroids, DLI: P | No | 12 | Progression of SM | |||
| 6 | SM-AML | Nagai et al35 | 5 | 7 | ↑ ↑ at 6 months | P | No treatment | Yes | 12 | |||||
| 7 | SM-MPN | Nakamura et al37 | 889 | 404 at 6 months | Eosinophilia decreased (15 × 109/L to 0.6 × 109/L | R | Yes | At 6 months | DLI | Yes | 9 | |||
| 8 | SM-MDS | Nakamura et al37 | 150 | 60 at 10 months | ↑ ↑ | ↑ | Episodic hot flashes and syncope resolved | R | No | DLI† | Yes | 39 | ||
| 9 | MCL | Nakamura et al37 | > 200 | ↑ ↑ | Hepatosplenomegaly and pancytopenia worsened | P | No treatment | No | 5 | Progression of SM | ||||
| 10 | MCL | 333 | 95 at 2 months | 5 | 1.5 at 2 months | R | No | No | 3 | Sepsis | ||||
| 11 | SM-AML | 7 | 5 | 5 at 3 months | SD | No | Yes | 206 | ||||||
| 12 | ASM | Przepiorka et al30 | 30 | 0 at 1 month | Splenomegaly resolved | R (CR) | No | Yes | 24 | |||||
| 13 | SM-AML | Pullarkat et al33 | 17.4 | 90 | 0 at 10 months | R (CR) | No | Yes | 108 | |||||
| 14 | SM-AML | Rønnov-Jessen et al32 | ↑ | ↑ | Liver infiltration persisted | SD | Yes | 8 | ||||||
| 15 | MML | Sperr et al28 | 745 | 126 | 73 at 2 months | 10 | 5 | 5 at 2 months | SD | Yes | At 3 months | Second alloHCT after MAC: CR | Yes | 144 |
| 16 | MCL | Spyridonidis et al34 | 70 by morphology | 14 by flow | 5 at 1 month; 2.4 by flow at 12 months | Urine histamine level was normalized | R | No | DLI† | Yes | 44 | |||
| 17 | SM-AML | 97 | 69 | 27 at 3 months | 5-10 | > 10 | 1 at 3 months | Mediator-related symptoms resolved, skin lesions improved | R | No | Yes | 30 | ||
| 18 | MCL | 90 | 30 | 0 at 1 month | R (CR) | No | No | 2 | Sepsis | |||||
| 19 | SM-AML | 71 | 10 | 10 | 10 at 3 months | SD | No | Yes | 16 | |||||
| 20 | SM-AML | 542 | 291 | 30 | 30 | 5 at 7 months | R | No | Yes | 18 | ||||
| 21 | ASM | 136 | 142 at 1 month | 80 | 50 at 1 month | SD | Yes | At 3 months | Dasatinib: P | No | 10 | Progression of SM | ||
| 22 | SM-AML | 230 | 34 | 19 at 4 months | < 10 | 0 at 4 months | Improvement in liver, skin, and lymph nodes | R (CR) | No | Yes | 11 | |||
| 23 | SM-MDS | 530 | 200 | 9.6 at 3 months | 50 | 5 | 5 at 3 months | Improvement in liver and skin | R | Yes | At 24 months | DLI: SD; second alloHCT with RIC: CR | Yes | 52 |
| 24 | SM-AML | 875 | 114 | 22 at 1 month | 35 | 10 | < 5 at 1 month | Improvement in liver function tests | R | No | Second alloHCT with RIC for AML†:CR | Yes | 21 | |
| 25 | SM-AML | 349 | 15 at 3 months | 60 | 10 | 0 | Improvement in bone, spleen, and liver | R | No | No | 11 | AML relapse | ||
| 26 | SM-AML | Pardanani et al20 | 90 | < 11 at 36 months | 70 | 78 | 0 at 1 month | Improvement in nodes, spleen, and liver | R (CR) | No | Yes | 42 | ||
| 27 | MCL | 496 | 15 | 99 | 40 | 3 at 2 months | Improvement in skin, spleen, and liver; cytogenetic remission | R | Yes | At 4 months | No treatment | No | 4 | Progression of SM |
| 28 | MCL | Chantorn et al40 | > 200 | 5; then 79 (MCL) | 60 | 80 at 1 month | Mediator-related symptoms persisted | P | No treatment | No | 4 | Progression of SM | ||
| 29 | SM-AML | 37 | 38 | 20 at 3 months | 15 | Improvement in skin, splenomegaly persisted | SD | Yes | At 3 months | Cladribine | No | 6 | Graft failure-sepsis | |
| 30 | MCL | Valentini et al39 | > 200 | 35 | 90 | 10 | 1.5 at 1 month | R | No | No | 1 | Traffic accident | ||
| 31 | SM-MDS-MPN | 216 | 145 | 9 at 24 months | 5 | < 5 | 0 at 24 months | Splenomegaly resolved; _KIT_D816V became undetectable in BM cells | R (CR) | No | DLI† | Yes | 72 | |
| 32 | SM-MDS | 61 | 48 | 14 at 34 months | 80 | 35 | 4 at 34 months | Severe myelofibrosis improved | R | No | DLI† | Yes | 34 | |
| 33 | MCL | 535 | 70 | 70 | 10 at 3 months | R | No | No | 8 | Respiratory failure | ||||
| 34 | SM-MDS | > 400 | 25 | 2.1 at 12 months | 80 | 20 | 0 at 12 months | R (CR) | No | TKI†, DLI† | yes | 12 | ||
| 35 | SM-MDS-MPN | 20-25 | 0 at 1 month | Splenomegaly persisted | R | No | No | 7 | Severe GVHD | |||||
| 36 | MCL | 1,500 | 90 | 50 at 1 month | Splenomegaly worsened | P | No | 1 | Progression of SM | |||||
| 37 | SM-AML | 26 | 25 | 1 at 2 months | R | No | Yes | 68 | ||||||
| 38 | SM-AML | 110 | 4 | 0.1 at 2 months | R | No | DLI†,TKI† | No | 9 | AML relapse | ||||
| 39 | SM-MDS | 919 | 11.3 | 6.2 at 3 months | 40 | 2-3 | 2 at 12 months | SD | No | Yes | 28 | |||
| 40 | SM-MDS | 820 | 394 | 4.4 at 12 months | 20 | 0 at 1 month | R (CR) | No | Yes | 19 | ||||
| 41 | SM-AHNMD | 230 | 200 | 9 at 3 months | 30 | 30 | 0 at 3 months | No evidence of mast cells in liver and GI tract biopsies; _KIT_D816V became undetectable in the BM cells; diarrhea resolved | R (CR) | No | No | 30 | Pulmonary fibrosis | |
| 42 | ASM | 230 | R (CR) | No | Yes | 27 | ||||||||
| 43 | SM-MM | SD | No | Yes | 105 | |||||||||
| 44 | SM-ALL | R (CR) | No | Yes | 56 | |||||||||
| 45 | ASM | 250 | P | No | 6 | Progression of SM | ||||||||
| 46 | ASM | R (CR) | No | Yes | 30 | |||||||||
| 47 | MCL | 95 | 90 | 0 at 3 months | R (CR) | Yes | At 12 months | No | 15 | Progression of SM | ||||
| 48 | SM-AML | 15 | 15 | < 5 at 4 months | R | Yes | At 12 months | DLI | No | 12 | Progression of SM and AML | |||
| 49 | MCL | 38 | 10 | 0 at 12 months | Mast cells persisted in liver biopsy | R | No | DLI† | Yes | 82 | ||||
| 50 | SM-MDS-MPN | Present | Some mast cells | < 5 at 36 months | Eosinophils significantly decreased in BM | R | No | DLI† | Yes | 150 | ||||
| 51 | SM-MDS | Rare | Rare-scattered | SD | No | Yes | 104 | |||||||
| 52 | MCL | > 1,200 | 63 | 3 at 1 month | 80 | 10 | 0 at 1 month | R (CR) | Yes | At 1 month | DLI | No | 2 | Progression of SM |
| 53 | SM-MDS-MPN | 49 | 45 | 37 at 1 month | 5 | 5 at 1 month | SD | No | Yes | 3 | ||||
| 54 | SM-AML | 5 | 0 at 3 months | R (CR) | No | No | 5 | AML relapse | ||||||
| 55 | SM-MPN | 69.4 | 67 | 5 at 3 months | 5 | 0 at 3 months | Hepatosplenomegaly resolved | R (CR) | No | Yes | 33 | |||
| 56 | SM-MDS-MPN | > 400 | > 400 | 16 at 3 months | 15-20 | 30 | 0 at 3 months | Ascites continued | R | No | No | 10 | GVHD-sepsis | |
| 57 | SM-AML | 145 | 9 at 1 month | 5 | 50 | 0 at 1 month | Hepatosplenomegaly persisted | R | No | No | 14 | AML relapse |
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Cem Akin, Novartis (C); Peter Valent, Novartis (C) Stock Ownership: None Honoraria: Wolfgang R. Sperr, Novartis Research Funding: Gregory Vercellotti, Sangart, Seattle Genetics Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Celalettin Ustun, Hans Hagglund, Daniel Weisdorf, Cem Akin, Peter Valent
Collection and assembly of data: Celalettin Ustun, Andreas Reiter, Bart L. Scott, Ryotaro Nakamura, Gandhi Damaj, Sebastian Kreil, William J. Hogan, Miguel-Angel Perales, Tsiporah Shore, Herrad Baurmann, Robert Stuart, Bernd Gruhn, Michael Doubek, Jack W. Hsu, Eleni Tholouli, Tanja Gromke, Lucy A. Godley, Livio Pagano, Andrew Gilman, Eva Maria Wagner, Tor Shwayder, Martin Bornhäuser, Esperanza B. Papadopoulos, Alexandra Böhm, Gregory Vercellotti, Maria Teresa Van Lint, Christoph Schmid, Werner Rabitsch, Faezeh Legrand, Ibrahim Yakoub-agha, John Barrett, Olivier Hermine, Wolfgang R. Sperr, Uday Popat, Steven Devine, H. Joachim Deeg, Peter Valent
Data analysis and interpretation: Celalettin Ustun, Andreas Reiter, Bart L. Scott, Ryotaro Nakamura, Gandhi Damaj, Sebastian Kreil, Ryan Shanley, William J. Hogan, Miguel-Angel Perales, Tsiporah Shore, Herrad Baurmann, Jack W. Hsu, Martin Bornhäuser, Vinod Pullarkat, Wael Saber, John Barrett, Edwin P. Alyea, Steven Devine, H. Joachim Deeg, Daniel Weisdorf, Cem Akin, Peter Valent
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Robyn J, Metcalfe DD. Systemic mastocytosis. Adv Immunol. 2006;89:169–243. doi: 10.1016/S0065-2776(05)89005-4. [DOI] [PubMed] [Google Scholar]
- 3.Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, et al. Prognosis in adult indolent systemic mastocytosis: A long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124:514–521. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Horny HP, Metcalfe DD, Bennet JM, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (ed 4) Lyon, France: IARC; 2008. pp. 54–63. [Google Scholar]
- 5.Ustun C, Savage NM, Gotlib J, et al. Systemic mastocytosis with associated clonal hematological non-mast-cell lineage disease: A case review. Am J Hematol. 2012;87:191–193. doi: 10.1002/ajh.22208. [DOI] [PubMed] [Google Scholar]
- 6.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood. 2009;113:5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 7.Pardanani A, Lim KH, Lasho TL, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114:3769–3772. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116:5812–5817. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 9.Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- 10.Kluin-Nelemans HC, Jansen JH, Breukelman H, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326:619–623. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura Y, Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci. 2004;61:2924–2931. doi: 10.1007/s00018-004-4273-y. [DOI] [PubMed] [Google Scholar]
- 12.Akin C. Clonality and molecular pathogenesis of mastocytosis. Acta Haematol. 2005;114:61–69. doi: 10.1159/000085563. [DOI] [PubMed] [Google Scholar]
- 13.Nakata Y, Kimura A, Katoh O, et al. c-kit point mutation of extracellular domain in patients with myeloproliferative disorders. Br J Haematol. 1995;91:661–663. doi: 10.1111/j.1365-2141.1995.tb05364.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 15.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: Establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 17.Longley BJ, Jr, Metcalfe DD, Tharp M, et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci U S A. 1999;96:1609–1614. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35:1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Akin C, Fumo G, Yavuz AS, et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- 20.Pardanani A, Elliott M, Reeder T, et al. Imatinib for systemic mast-cell disease. Lancet. 2003;362:535–536. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- 21.Aichberger KJ, Sperr WR, Gleixner KV, et al. Treatment responses to cladribine and dasatinib in rapidly progressing aggressive mastocytosis. Eur J Clin Invest. 2008;38:869–873. doi: 10.1111/j.1365-2362.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- 22.Ustun C, Corless CL, Savage N, et al. Chemotherapy and dasatinib induce long-term hematologic and molecular remission in systemic mastocytosis with acute myeloid leukemia with KIT D816V. Leuk Res. 2009;33:735–741. doi: 10.1016/j.leukres.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Verstovsek S, Tefferi A, Cortes J, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14:3906–3915. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotlib J, Berubé C, Growney JD, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleixner KV, Mayerhofer M, Sonneck K, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 26.Gleixner KV, Mayerhofer M, Aichberger KJ, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: Comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: Myths, controversies, and unknowns. Blood. 2011;117:2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 28.Sperr WR, Drach J, Hauswirth AW, et al. Myelomastocytic leukemia: Evidence for the origin of mast cells from the leukemic clone and eradication by allogeneic stem cell transplantation. Clin Cancer Res. 2005;11:6787–6792. doi: 10.1158/1078-0432.CCR-05-1064. [DOI] [PubMed] [Google Scholar]
- 29.Födinger M, Fritsch G, Winkler K, et al. Origin of human mast cells: Development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994;84:2954–2959. [PubMed] [Google Scholar]
- 30.Przepiorka D, Giralt S, Khouri I, et al. Allogeneic marrow transplantation for myeloproliferative disorders other than chronic myelogenous leukemia: Review of forty cases. Am J Hematol. 1998;57:24–28. doi: 10.1002/(sici)1096-8652(199801)57:1<24::aid-ajh4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Pullarkat VA, Bueso-Ramos C, Lai R, et al. Systemic mastocytosis with associated clonal hematological non-mast-cell lineage disease: Analysis of clinicopathologic features and activating c-kit mutations. Am J Hematol. 2003;73:12–17. doi: 10.1002/ajh.10322. [DOI] [PubMed] [Google Scholar]
- 32.Rønnov-Jessen D, Løvgreen Nielsen P, Horn T. Persistence of systemic mastocytosis after allogeneic bone marrow transplantation in spite of complete remission of the associated myelodysplastic syndrome. Bone Marrow Transplant. 1991;8:413–415. [PubMed] [Google Scholar]
- 33.Pullarkat V, Bedell V, Kim Y, et al. Neoplastic mast cells in systemic mastocytosis associated with t(8;21) acute myeloid leukemia are derived from the leukemic clone. Leuk Res. 2007;31:261–265. doi: 10.1016/j.leukres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Spyridonidis A, Thomas AK, Bertz H, et al. Evidence for a graft-versus-mast-cell effect after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:515–519. doi: 10.1038/sj.bmt.1704627. [DOI] [PubMed] [Google Scholar]
- 35.Nagai S, Ichikawa M, Takahashi T, et al. The origin of neoplastic mast cells in systemic mastocytosis with AML1/ETO-positive acute myeloid leukemia. Exp Hematol. 2007;35:1747–1752. doi: 10.1016/j.exphem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Chen TY, Chen JS, Huang WT, et al. Rapid engraftment of mast cells of donor origin in a case of acute myeloid leukemia with mast cell leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32:111–114. doi: 10.1038/sj.bmt.1704098. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura R, Chakrabarti S, Akin C, et al. A pilot study of nonmyeloablative allogeneic hematopoietic stem cell transplant for advanced systemic mastocytosis. Bone Marrow Transplant. 2006;37:353–358. doi: 10.1038/sj.bmt.1705245. [DOI] [PubMed] [Google Scholar]
- 38.Gromke T, Elmaagacli AH, Ditschkowski M, et al. Delayed graft-versus-mast-cell effect on systemic mastocytosis with associated clonal haematological non-mast cell lineage disease after allogeneic transplantation. Bone Marrow Transplant. 2013;48:732–733. doi: 10.1038/bmt.2012.198. [DOI] [PubMed] [Google Scholar]
- 39.Valentini CG, Rondoni M, Pogliani EM, et al. Mast cell leukemia: A report of ten cases. Ann Hematol. 2008;87:505–508. doi: 10.1007/s00277-007-0430-3. [DOI] [PubMed] [Google Scholar]
- 40.Chantorn R, Shwayder T. Death from mast cell leukemia: A young patient with longstanding cutaneous mastocytosis evolving into fatal mast cell leukemia. Pediatr Dermatol. 2012;29:605–609. doi: 10.1111/j.1525-1470.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 41.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: Current treatment options and proposed response criteria. Leuk Res. 2003;27:635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 42.Gotlib J, Pardanani A, Akin C, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121:2393–2401. doi: 10.1182/blood-2012-09-458521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oran B, Popat U, Rondon G, et al. Significance of persistent cytogenetic abnormalities on myeloablative allogeneic stem cell transplantation in first complete remission. Biol Blood Marrow Transplant. 2013;19:214–220. doi: 10.1016/j.bbmt.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warlick ED, Cioc A, Defor T, et al. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: Importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sayer HG, Kröger M, Beyer J, et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: Disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31:1089–1095. doi: 10.1038/sj.bmt.1704062. [DOI] [PubMed] [Google Scholar]
- 47.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48:630–641. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 49.Ustun C, Wiseman AC, Defor TE, et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant. 2013;48:1415–1420. doi: 10.1038/bmt.2013.124. [DOI] [PubMed] [Google Scholar]
- 50.Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:89–99. doi: 10.1002/ajh.22246. [DOI] [PubMed] [Google Scholar]
- 51.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 52.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 53.Wang SA, Hutchinson L, Tang G, et al. Systemic mastocytosis with associated clonal hematological non-mast cell lineage disease: Clinical significance and comparison of chromosomal abnormalities in SM and AHNMD components. Am J Hematol. 2013;88:219–224. doi: 10.1002/ajh.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson RC, Savage NM, Chiang T, et al. Hidden mastocytosis in acute myeloid leukemia with t(8;21)(q22;q22) Am J Clin Pathol. 2013;140:525–535. doi: 10.1309/AJCP1Q0YSXEAHNKK. [DOI] [PubMed] [Google Scholar]
- 55.Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): A Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24:3904–3911. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 56.Boissel N, Leroy H, Brethon B, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML) Leukemia. 2006;20:965–970. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 57.Park SH, Chi HS, Min SK, et al. Prognostic impact of c-KIT mutations in core binding factor acute myeloid leukemia. Leuk Res. 2011;35:1376–1383. doi: 10.1016/j.leukres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: An Italian retrospective study. Blood. 2006;107:3463–3468. doi: 10.1182/blood-2005-09-3640. [DOI] [PubMed] [Google Scholar]
- 59.Wang YY, Zhou GB, Yin T, et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: Implication in stepwise leukemogenesis and response to Gleevec. Proc Natl Acad Sci U S A. 2005;102:1104–1109. doi: 10.1073/pnas.0408831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fritsche-Polanz R, Fritz M, Huber A, et al. High frequency of concomitant mastocytosis in patients with acute myeloid leukemia exhibiting the transforming KIT mutation D816V. Mol Oncol. 2010;4:335–346. doi: 10.1016/j.molonc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgin-Lavialle S, Lhermitte L, Dubreuil P, et al. Mast cell leukemia. Blood. 2013;121:1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 62.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: The role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 63.Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 64.Marks DI, Wang T, Pérez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperr WR, Mitterbauer M, Mitterbauer G, et al. Quantitation of minimal residual disease in acute myeloid leukemia by tryptase monitoring identifies a group of patients with a high risk of relapse. Clin Cancer Res. 2005;11:6536–6543. doi: 10.1158/1078-0432.CCR-05-0732. [DOI] [PubMed] [Google Scholar]
- 67.Sperr WR, El-Samahi A, Kundi M, et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: A novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39:914–923. doi: 10.1111/j.1365-2362.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 68.Sperr WR, Jordan JH, Baghestanian M, et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood. 2001;98:2200–2209. doi: 10.1182/blood.v98.7.2200. [DOI] [PubMed] [Google Scholar]
- 69.Dimitriadou V, Mécheri S, Koutsilieris M, et al. Expression of functional major histocompatibility complex class II molecules on HMC-1 human mast cells. J Leukoc Biol. 1998;64:791–799. doi: 10.1002/jlb.64.6.791. [DOI] [PubMed] [Google Scholar]
- 70.Banovac K, Ghandur-Mnaymneh L, Leone J, et al. Intrathyroidal mast cells express major histocompatibility complex class-II antigens. Int Arch Allergy Appl Immunol. 1989;90:43–46. doi: 10.1159/000234998. [DOI] [PubMed] [Google Scholar]
- 71.Malaviya R, Twesten NJ, Ross EA, et al. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996;156:1490–1496. [PubMed] [Google Scholar]
- 72.Kambayashi T, Allenspach EJ, Chang JT, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warren EH, Deeg HJ. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens. 2013;81:183–193. doi: 10.1111/tan.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 76.Weisdorf D. GVHD the nuts and bolts. Hematology Am Soc Hematol Educ Program. 2007:62–67. doi: 10.1182/asheducation-2007.1.62. [DOI] [PubMed] [Google Scholar]
- 77.Devine SM, Adkins DR, Khoury H, et al. Recent advances in allogeneic hematopoietic stem-cell transplantation. J Lab Clin Med. 2003;141:7–32. doi: 10.1067/mlc.2003.5. [DOI] [PubMed] [Google Scholar]