Clinicopathological and prognostic significance of sialyl Lewis X overexpression in patients with cancer: a meta-analysis (original) (raw)
Abstract
Many studies have shown that sialyl Lewis X (sLeX) is related to cancer prognosis and clinicopathology, but failed to provide conclusive results. We conducted the present meta-analysis to identify the association between sLeX overexpression and cancer prognosis. We searched studies in PubMed and Embase databases. Relative risk or hazard ratio with 95% confidence intervals were estimated with the Mantel–Haenszel random-effect method and 29 studies were included. Our meta-analysis showed that sLeX overexpression is significantly related to lymphatic invasion, venous invasion, T stage, N stage, M stage, tumor stage, recurrence, and overall survival. In subgroup analysis, we found that cancer type and ethnicity might be two major contributing factors to the possible presence of heterogeneity among the studies. In conclusion, sLeX overexpression is associated with tumor metastasis, recurrence, and overall survival in cancer patients, it plays an important role in cancer prognosis.
Keywords: sialyl Lewis X, cancer, prognosis, meta-analysis
Introduction
As is known to all, cancer is a common life-threatening disease. According to recent studies, the incidence of cancer increases 1% per year in Europe.1 Among the adult population, a rising trend is reported for soft tissue sarcoma.2 Breast, colorectal, prostate, and lung cancers are the most common oncological cause for death among the European population.3 Cancer cannot be cured, as expected, due to the limited knowledge of iatrotechnique. So, exploration of more precise bio-indicators is valuable for early diagnosis of cancer and improving prognosis of patients.
Cell surface carbohydrates are involved in various biological processes such as cellular differentiation, maturation, proliferation, and malignant transformation.4 Dramatic changes of cell surface carbohydrates are associated with cancer occurrence, tumor invasiveness, and metastatic behavior.5 Sialyl Lewis X (sLeX) (NeuNAcα2,3Galβ1,4[Fucα1,3] GlcNAc), a carbohydrate antigen, is related to cell adhesion and our previous study showed that inhibition of sLeX synthesis leads to decreased adhesion of trophoblast cells to endometrial epithelial cells.6 Also, sLeX is frequently expressed in human cancer cells and primary tumors.7,8 As a ligand for E-selectin and L-selectin, sLeX is related to cell adhesion.9 It has been demonstrated that sLeX was involved in the adhesion of tumor cells to vascular endothelium.10 The potential role of sLeX in the tumor metastatic process has been supported by several clinical studies.11–14
Many studies have identified the relationship between sLeX and cancer prognosis, but individual studies of the influence of sLeX expression in cancer have failed to provide conclusive results. The present meta-analysis was conducted to further explore the relationship between sLeX expression and cancer prognosis and clinicopathology.
Materials and methods
Publication search
We searched published studies in the PubMed and Embase databases up to May 2014 with the following search terms: (slex OR sialyl lewis x) AND (cancer OR neoplasms OR carcinoma OR tumor) AND prognosis. Furthermore, reference lists of main reports and review articles were also reviewed to identify additional relevant publications. The study was conducted and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Selection criteria
Two authors (YL and JXL) reviewed the retrieved titles and abstracts to discriminate the eligible studies for inclusion in our meta-analysis independently. Published studies were included based on the following criteria: 1) written and published in English; 2) patients with cancer diagnosis by pathology; 3) studies about sLeX expression in cancer tissues; 4) sLeX expression was measured by immunohistochemistry (IHC) method; 5) full length paper with sufficient data on sLeX expression and prognosis and prognosis-related factors; 6) we could find the full text. We excluded studies with the following criteria: 1) written and published in a language other than English; 2) studies about cell lines and animals; 3) studies about sLeX expression in serum; 4) review articles without original data; 5) a commentary, letter to the editor, or monograph.
Data extraction
Two authors (YL and WG) performed the data evaluation independently. The following data were extracted from each study: the first author’s last name; publication year; country; cancer source; number of patients; number of sLeX expressions (positive/negative); clinicopathological factors (age, sex, tumor size, histological differentiation, lymphatic invasion, venous invasion, T/N/M stage, tumor stage, and recurrence); survival analysis.
Data synthesis and statistical analysis
Expression of sLeX was analyzed as dichotomous variables, as positive expression versus negative expression. The clinicopathological factors were also conducted as dichotomous variables, as older age versus younger age for age; male versus female for sex; large versus small for tumor size; high versus low for histological differentiation; I and II versus III and IV for tumor stage; pT2 versus more than pT3 for depth of invasion (T stage); with versus without for lymphatic invasion, venous invasion, lymph node metastasis (N stage), distant metastasis (M stage), recurrence. Survival of sLeX expression was analyzed by Cox’s regression analysis conducted as hazard ratio (HR) and 95% confidence interval (95% CI). The data of expression of sLeX and clinicopathological factors or survival rate were extracted and calculated by initial data of studies. These data were analyzed with random-effect method, and were measured in relative risk (RR) with 95% CI. Statistical heterogeneity was estimated by means of Cochran’s Q test and _I_2 test. The _I_2 test represents the percentage of variation to heterogeneity, which is categorized as low (0%–40%), moderate (40%–60%), high (60%–90%), very high (>90%). Subgroup analyses were carried out based on cancer or country of the included studies if a significant heterogeneity was found in overall meta-analysis. To identify any potential publication bias, we used Begg’s test. All statistical analyses were performed with Review Manager 5.2 and STATA 12.0.
Results
Systematic review
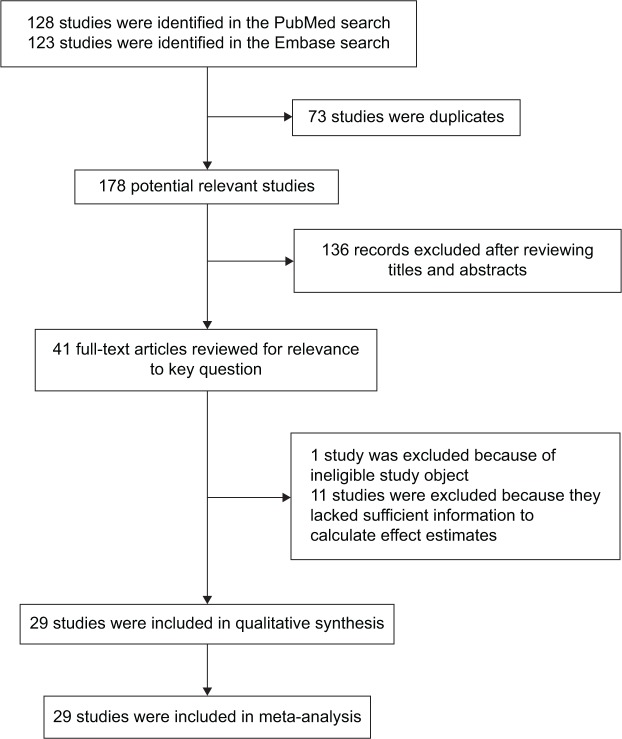
We identified 178 studies that fit our search strategy, 41 studies were identified in our primary search (Figure 1). Finally, 29 studies published between 1993 and 2013 were included in our meta-analysis.11,12,14–40 Detailed characteristics of these studies are provided in Table 1.
Figure 1.
The flow diagram of included/excluded studies.
Table 1.
Characteristics of the included studies
| Study ID | Country | Cancer source | Number of patients | sLeX expression (positive/negative) | Clinicopathological factors | Survival analysis |
|---|---|---|---|---|---|---|
| Nakamori et al18 (1993) | Japan | Colorectal cancer | 132 | 50/82 | Sex, differentiation, T stage, N stage, lymphatic invasion, venous invasion, tumor stage, recurrence | NA |
| Yamaguchi et al19 (1994) | Japan | Colorectal cancer | 170 | 56/114 | Differentiation, T stage, N stage, lymphatic invasion, venous invasion, tumor stage, recurrence | NA |
| Idikio20 (1997) | Canada | Prostate cancer | 38 | 30/8 | Differentiation | NA |
| Nakamori et al21 (1997) | Japan | Colorectal cancer | 159 | 58/101 | Age, sex, differentiation, T stage, N stage, lymphatic invasion, venous invasion, tumor stage | NA |
| Shimodaira et al22 (1997) | Japan | Colorectal cancer | 43 | 28/15 | Tumor size, differentiation, T stage, N stage, lymphatic invasion, venous invasion, tumor stage | NA |
| Ura et al12 (1997) | Japan | Gastric cancer | 110 | 91/19 | T stage, N stage | NA |
| Baldus et al17 (1998) | Germany | Gastric cancer | 127 | 85/42 | Sex, tumor stage | NA |
| Farmer et al23 (1998) | United States | HNSCC | 82 | 51/31 | Age, sex, M stage, tumor stage | NA |
| Fukuoka et al11 (1998) | Japan | Lung cancer | 52 | 34/18 | N stage, M stage | NA |
| Tatsumi et al24 (1998) | Japan | Gastric cancer | 87 | 41/46 | Differentiation, T stage, N stage, M stage, lymphatic invasion, venous invasion | NA |
| Yamaguchi et al25 (1998) | Japan | Breast cancer | 102 | 61/41 | Age, tumor size, N stage | NA |
| Kurahara et al14 (1999) | Japan | OSCC | 70 | 24/46 | M stage | NA |
| Takao et al26 (1999) | Japan | EBDC | 73 | 45/28 | Age, sex, differentiation, T stage, N stage, M stage, lymphatic invasion, venous invasion, tumor stage | NA |
| Futamura et al27 (2000) | Japan | Gastric cancer | 245 | 135/110 | Age, sex, differentiation, T stage, N stage, M stage, venous invasion, tumor stage | NA |
| Grabowski et al28 (2000) | Germany | Colorectal cancer | 182 | 103/79 | Sex, differentiation, T stage, N stage, M stage, tumor stage | Multi |
| Nakagoe et al16 (2000) | Japan | Colorectal cancer | 101 | 76/25 | Tumor stage | Uni |
| Machida et al29 (2001) | Japan | Lung cancer | 25 | 19/6 | Tumor size, N stage, M stage, lymphatic invasion, venous invasion | NA |
| Takahashi et al30 (2001) | Japan | PDAC | 23 | 15/8 | NA | Multi |
| Baldus et al31 (2002) | Germany | Colorectal cancer | 243 | 165/78 | Differentiation, N stage, M stage, tumor stage | NA |
| Konno et al32 (2002) | Japan | Colorectal cancer | 134 | 47/87 | N stage, M stage, venous invasion | Multi |
| Nakagoe et al34 (2002) | Japan | Breast cancer | 87 | 37/50 | Age, differentiation, T stage, N stage, M stage, tumor stage | Multi |
| Nakagoe et al33,34 (2002) | Japan | Gastric cancer | 101 | 31/70 | Age, sex, tumor size, differentiation, T stage, N stage, lymphatic invasion, venous invasion | Multi |
| Kashiwagi et al35 (2004) | Japan | Gallbladder cancer | 54 | 28/26 | T stage, N stage, lymphatic invasion, venous invasion | NA |
| Yu et al36 (2005) | People’s Republic of China | Lung cancer | 61 | 40/21 | Age, sex, T stage, N stage, recurrence | Uni |
| Faried et al37 (2007) | Japan | ESCC | 130 | 40/90 | Sex, differentiation, T stage, N stage, M stage, lymphatic invasion, venous invasion, tumor stage | Multi |
| Croce et al38 (2008) | Argentina | HNSCC | 125 | 29/96 | Age, sex, differentiation, T stage, N stage, tumor stage | NA |
| Sozzani et al39 (2008) | Italy | Breast cancer | 127 | 37/90 | Differentiation, T stage, N stage, venous invasion | NA |
| Portela et al40 (2011) | Spain | Colorectal cancer | 155 | 67/88 | Age, sex, tumor size, differentiation, T stage, N stage, M stage, tumor stage | NA |
| Schiffmann et al15 (2012) | Germany | Colorectal cancer | 215 | 102/113 | Sex, differentiation, T stage, N stage, M stage | NA |
Association of sLeX expression with cancer prognosis and clinicopathology
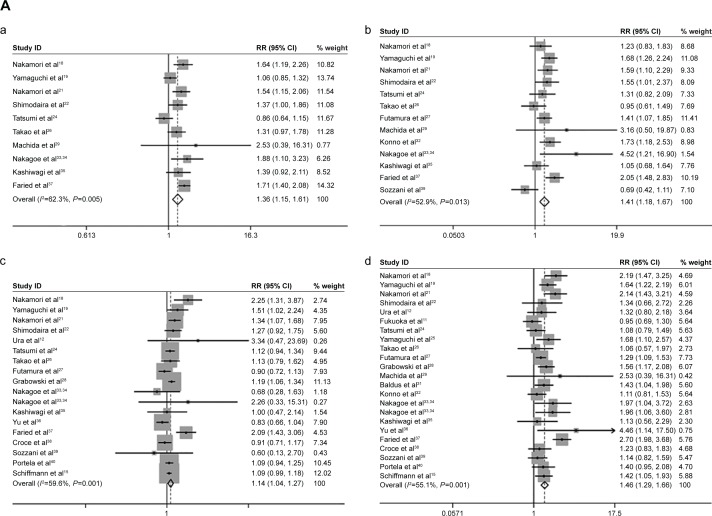
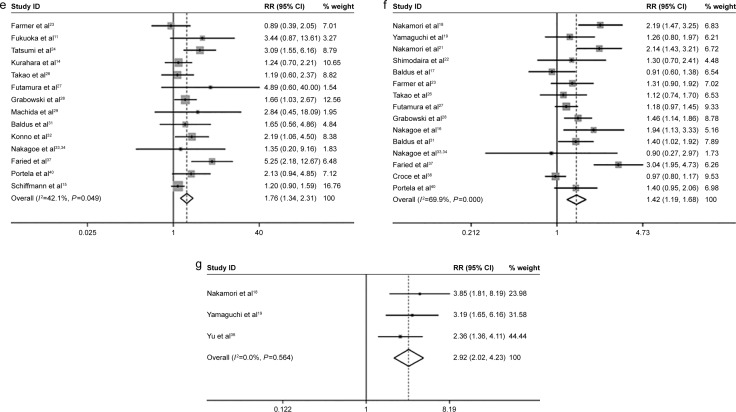
sLeX expression correlated with prognostic factors, including lymphatic invasion (lymphatic invasion versus non-lymphatic invasion) (pooled RR =1.36, 95% CI: 1.15–1.61, _I_2=62.3%), venous invasion (venous invasion versus non-venous invasion) (pooled RR =1.41, 95% CI: 1.18–1.67, _I_2=52.9%), T stage (pT3–4 stage versus pT2 stage) (pooled RR =1.14, 95% CI: 1.04–1.27, _I_2=59.6%), N stage (lymph node metastasis versus non-lymph node metastasis) (pooled RR =1.46, 95% CI: 1.29–1.66, _I_2=55.1%), M stage (distant metastasis versus non-distant metastasis) (pooled RR =1.76, 95% CI: 1.34–2.31, _I_2=42.1%), tumor stage (stage III/IV versus stage I/II) (pooled RR =1.42, 95% CI: 1.19–1.68, _I_2=69.9%), tumor recurrence (recurrence versus non-recurrence) (pooled RR =2.92, 95% CI: 2.02–4.23, _I_2=0.0%) (Figure 2A).
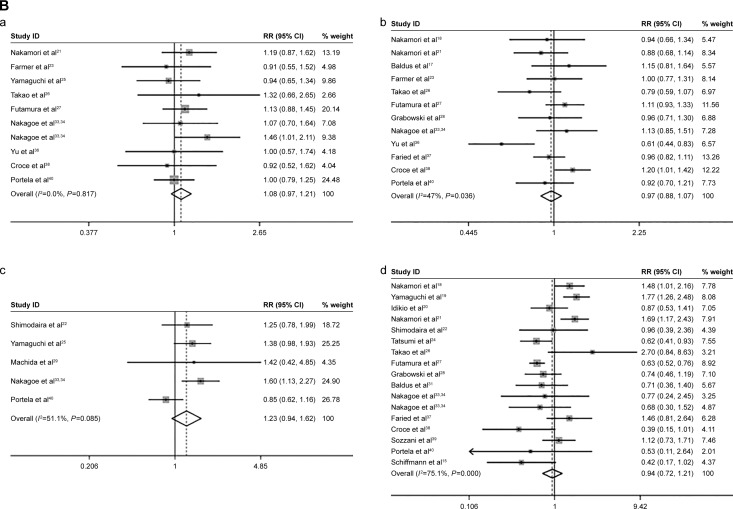
Figure 2.
The association between sLeX and cancer prognostic factors.
Notes: (A) The cancer prognostic factors which were significantly related to sLeX overexpression. (a) Lymphatic invasion; (b) venous invasion; (c) T stage; (d) N stage; (e) M stage; (f) tumor stage; (g) recurrence. (B) The cancer prognostic factors which were not significantly related to sLeX overexpression. (a) Age; (b) sex; (c) tumor size; (d) differentiation. Weights are from random effects analysis.
Abbreviations: RR, relative risk; CI, confidence interval; sLeX, sialyl Lewis X.
Meantime, we found that sLeX overexpression was not significantly related to cancer prognosis and clinicopathology factors, including age (older versus younger) (pooled RR =1.08, 95% CI: 0.97–1.21, _I_2=0.0%), sex (male versus female) (pooled RR =0.97, 95% CI: 0.88–1.07, _I_2=47.0%), tumor size (larger versus smaller) (pooled RR =1.23, 95% CI: 0.94–1.62, _I_2=51.1%), tumor differentiation (lower differentiation versus higher differentiation) (pooled RR =0.94, 95% CI: 0.72–1.21, _I_2=75.1%) (Figure 2B).
sLeX overexpression on cancer survival
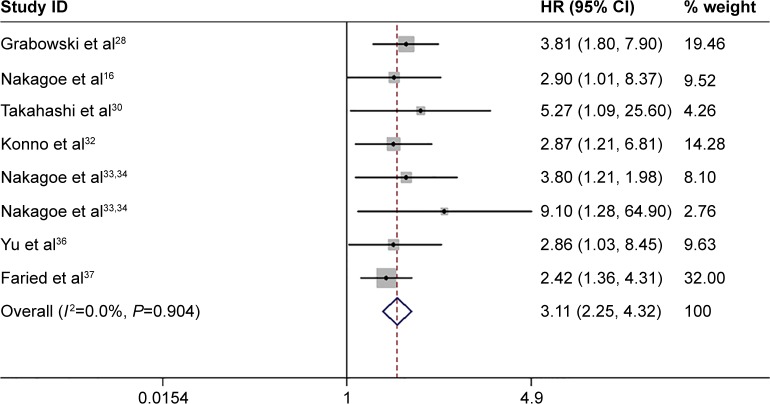
Eight studies analyzed the overall survival (OS) of human cancer with positive/negative sLeX overexpression, the HRs ranged from 2.42 to 9.10.18,30,32,34–36,38,39 The summarized HR of negative versus positive was 3.11 (95% CI: 2.25–4.32) with low heterogeneity (_I_2=0.0%) (Figure 3).
Figure 3.
Meta-analysis with a random-effect model for the association of sLex overexpression with overall survival.
Note: Weights are from random effects analysis.
Abbreviations: HR, hazard ratio; CI, confidence interval; sLeX, sialyl Lewis X.
Subgroup analyses
We chose subgroup analyses in meta-analysis with relative high heterogeneity (_I_2>40%). In subgroup analyses, studies were stratified by cancer category (colorectal cancer, gastric cancer, lung cancer, breast cancer, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, oral squamous cell carcinoma, gallbladder cancer, pancreatic ductal adenocarcinoma, prostate cancer, and extrahepatic bile duct carcinoma) or ethnicity (Asia, America, and Europe). In addition, most of these analyses showed low heterogeneity after stratification (Tables 2 and 3).
Table 2.
Subgroup analyses of country
| Number of studies | Summary RR (95% CIs) | _I_2 value | ph | |
|---|---|---|---|---|
| Sex | ||||
| Overall | 12 | 0.97 (0.88, 1.07) | 47.0% | 0.036 |
| Asia | 7 | 0.92 (0.80, 1.06) | 56.5% | 0.032 |
| Europe | 3 | 0.99 (0.83, 1.18) | 0.0% | 0.593 |
| Americas | 2 | 1.13 (0.95, 1.34) | 24.2% | 0.251 |
| Tumor size | ||||
| Overall | 5 | 1.23 (0.94, 1.62) | 51.1% | 0.085 |
| Asia | 4 | 1.43 (1.16, 1.77) | 0.0% | 0.853 |
| Europe | 1 | 0.85 (0.62, 1.16) | NA | NA |
| Differentiation | ||||
| Overall | 17 | 0.94 (0.72, 1.21) | 75.1% | 0.000 |
| Asia | 11 | 1.11 (0.80, 1.55) | 82.3% | 0.000 |
| Europe | 4 | 0.66 (0.46, 0.93) | 0.0% | 0.715 |
| Americas | 2 | 0.63 (0.25, 1.57) | 67.8% | 0.078 |
| Venous invasion | ||||
| Overall | 13 | 1.41 (1.18, 1.67) | 52.9% | 0.013 |
| Asia | 12 | 1.49 (1.29, 1.72) | 31.0% | 0.143 |
| Europe | 1 | 0.69 (0.42, 1.11) | NA | NA |
| T stage | ||||
| Overall | 18 | 1.14 (1.04, 1.27) | 59.6% | 0.001 |
| Asia | 13 | 1.23 (1.03, 1.47) | 67.5% | 0.000 |
| Europe | 4 | 1.11 (1.05, 1.19) | 0.0% | 0.497 |
| Americas | 1 | 0.91 (0.71, 1.17) | NA | NA |
| N stage | ||||
| Overall | 23 | 1.46 (1.29, 1.66) | 55.1% | 0.001 |
| Asia | 17 | 1.53 (1.28, 1.82) | 65.7% | 0.000 |
| Europe | 5 | 1.40 (1.21, 1.61) | 0.0% | 0.724 |
| Americas | 1 | 1.23 (0.83, 1.83) | NA | NA |
| M stage | ||||
| Overall | 14 | 1.76 (1.34, 2.31) | 42.1% | 0.049 |
| Asia | 9 | 2.20 (1.47, 3.30) | 38.3% | 0.113 |
| Europe | 4 | 1.37 (1.09, 1.72) | 0.0% | 0.410 |
| Americas | 1 | 0.89 (0.39, 2.05) | NA | NA |
| Tumor stage | ||||
| Overall | 15 | 1.42 (1.19, 1.68) | 69.9% | 0.000 |
| Asia | 9 | 1.62 (1.24, 2.10) | 69.4% | 0.001 |
| Europe | 4 | 1.32 (1.10, 1.59) | 22.3% | 0.277 |
| Americas | 2 | 1.08 (0.79, 1.49) | 58.7% | 0.120 |
Table 3.
Subgroup analyses of cancer types
| Subgroup | Number of studies | Summary RR (95% CIs) | _I_2 value | ph |
|---|---|---|---|---|
| Sex | ||||
| Overall | 12 | 0.97 (0.88, 1.07) | 47.0% | 0.036 |
| Colorectal cancer | 4 | 0.92 (0.80, 1.06) | 0.0% | 0.978 |
| Gastric cancer | 3 | 1.12 (0.97, 1.29) | 0.0% | 0.981 |
| HNSCC | 2 | 1.13 (0.95, 1.34) | 24.2% | 0.251 |
| EBDC | 1 | 0.79 (0.59, 1.07) | NA | NA |
| Lung cancer | 1 | 0.61 (0.44, 0.83) | NA | NA |
| ESCC | 1 | 0.96 (0.82, 1.11) | NA | NA |
| Tumor size | ||||
| Overall | 5 | 1.23 (0.94, 1.62) | 51.1% | 0.085 |
| Colorectal cancer | 2 | 0.99 (0.68, 1.44) | 46.7% | 0.171 |
| Breast cancer | 1 | 1.38 (0.98, 1.93) | NA | NA |
| Lung cancer | 1 | 1.42 (0.42, 4.85) | NA | NA |
| Gastric cancer | 1 | 1.60 (1.13, 2.27) | NA | NA |
| Differentiation | ||||
| Overall | 17 | 0.94 (0.72, 1.21) | 75.1% | 0.000 |
| Colorectal cancer | 8 | 1.06 (0.74, 1.52) | 69.6% | 0.002 |
| Gastric cancer | 3 | 0.63 (0.53, 0.75) | 0.0% | 0.978 |
| Breast cancer | 2 | 1.07 (0.72, 1.60) | 0.0% | 0.548 |
| Prostate cancer | 1 | 0.87 (0.53, 1.41) | NA | NA |
| EBDC | 1 | 2.70 (0.84, 8.63) | NA | NA |
| ESCC | 1 | 1.46 (0.81, 2.64) | NA | NA |
| HNSCC | 1 | 0.39 (0.15, 1.01) | NA | NA |
| Lymphatic invasion | ||||
| Overall | 10 | 1.36 (1.15, 1.61) | 62.3% | 0.005 |
| Colorectal cancer | 4 | 1.36 (1.09, 1.68) | 56.7% | 0.074 |
| Gastric cancer | 2 | 1.23 (0.55, 2.73) | 85.4% | 0.009 |
| EBDC | 1 | 1.31 (0.97, 1.78) | NA | NA |
| Lung cancer | 1 | 2.53 (0.39, 16.31) | NA | NA |
| Gallbladder cancer | 1 | 1.39 (0.92, 2.11) | NA | NA |
| ESCC | 1 | 1.71 (1.40, 2.08) | NA | NA |
| Venous invasion | ||||
| Overall | 13 | 1.41 (1.18, 1.67) | 52.9% | 0.013 |
| Colorectal cancer | 5 | 1.57 (1.33, 1.84) | 0.0% | 0.746 |
| Gastric cancer | 3 | 1.48 (1.04, 2.12) | 35.6% | 0.212 |
| Breast cancer | 1 | 0.69 (0.42, 1.11) | NA | NA |
| EBDC | 1 | 0.95 (0.61, 1.49) | NA | NA |
| Lung cancer | 1 | 3.16 (0.50, 19.87) | NA | NA |
| Gallbladder cancer | 1 | 1.05 (0.68, 1.64) | NA | NA |
| ESCC | 1 | 2.05 (1.48, 2.83) | NA | NA |
| T stage | ||||
| Overall | 18 | 1.14 (1.04, 1.27) | 59.6% | 0.001 |
| Colorectal cancer | 7 | 1.22 (1.08, 1.38) | 65.6% | 0.008 |
| Gastric cancer | 4 | 1.04 (0.85, 1.28) | 29.7% | 0.234 |
| Breast cancer | 2 | 0.66 (0.31, 1.40) | 0.0% | 0.895 |
| EBDC | 1 | 1.13 (0.79, 1.62) | NA | NA |
| Lung cancer | 1 | 0.83 (0.66, 1.04) | NA | NA |
| Gallbladder cancer | 1 | 1.00 (0.47, 2.14) | NA | NA |
| ESCC | 1 | 2.09 (1.43, 3.06) | NA | NA |
| HNSCC | 1 | 0.91 (0.71, 1.17) | NA | NA |
| N stage | ||||
| Overall | 23 | 1.46 (1.29, 1.66) | 55.1% | 0.001 |
| Colorectal cancer | 9 | 1.54 (1.34, 1.75) | 24.5% | 0.226 |
| Gastric cancer | 4 | 1.28 (1.11, 1.47) | 0.0% | 0.393 |
| Breast cancer | 3 | 1.46 (1.04, 2.04) | 41.6% | 0.180 |
| Lung cancer | 3 | 2.00 (0.44, 8.97) | 80.2% | 0.006 |
| EBDC | 1 | 1.06 (0.57, 1.97) | NA | NA |
| Gallbladder cancer | 1 | 1.13 (0.56, 2.29) | NA | NA |
| ESCC | 1 | 2.70 (1.98, 3.68) | NA | NA |
| HNSCC | 1 | 1.23 (0.83, 1.83) | NA | NA |
| M stage | ||||
| Overall | 14 | 1.76 (1.34, 2.31) | 42.1% | 0.049 |
| Colorectal cancer | 5 | 1.47 (1.15, 1.87) | 9.2% | 0.354 |
| Gastric cancer | 2 | 3.23 (1.67, 6.22) | 0.0% | 0.678 |
| Lung cancer | 2 | 3.21 (1.07, 9.69) | 0.0% | 0.871 |
| Breast cancer | 1 | 1.35 (0.20, 9.16) | NA | NA |
| EBDC | 1 | 1.19 (0.60, 2.37) | NA | NA |
| ESCC | 1 | 5.25 (2.18, 12.67) | NA | NA |
| HNSCC | 1 | 0.89 (0.39, 2.05) | NA | NA |
| OSCC | 1 | 1.24 (0.70, 2.21) | NA | NA |
| Tumor stage | ||||
| Overall | 15 | 1.42 (1.19, 1.68) | 69.9% | 0.000 |
| Colorectal cancer | 8 | 1.58 (1.36, 1.82) | 13.0% | 0.328 |
| Gastric cancer | 2 | 1.11 (0.88, 1.39) | 19.5% | 0.265 |
| HNSCC | 1 | 1.08 (0.79, 1.49) | 58.7% | 0.120 |
| Breast cancer | 1 | 0.90 (0.27, 2.97) | NA | NA |
| EBDC | 1 | 1.12 (0.74, 1.70) | NA | NA |
| ESCC | 1 | 3.04 (1.95, 4.73) | NA | NA |
Publication bias
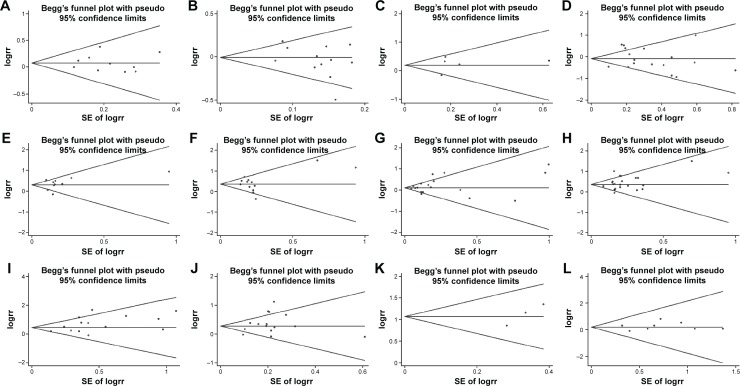
Begg’s test was created for assessment of possible publication bias. It suggested that publication bias had little influence on these meta-analysis results (_P_>0.05) (Figure 4).
Figure 4.
Begg’s test results of sLex overexpression and prognostic factors.
Notes: (A) Age; (B) sex; (C) tumor size; (D) differentiation; (E) lymphatic invasion; (F) venous invasion; (G) T stage; (H) N stage; (I) M stage; (J) tumor stage; (K) recurrence; (L) overall survival.
Abbreviations: sLex, sialyl Lewis X; SE, standard error.
Discussion
The cancer statistics of the USA, in 2013,41 clearly indicated that the methods of treatment for cancer need to be improved. Exploring new molecular biological prognostic and predictive markers is a hot topic in modern medicine. Nakagoe et al first reported that sLeX was expressed in serum of patients with gastric and colorectal cancer as a tumor-associated carbohydrate antigen, which was also proven by clinicopathological and immunohistochemical studies.42 The relationship between sLeX expression and cancer prognosis was identified by a number of studies, which did not show conformable results. To our knowledge, this is the first meta-analysis that systematically evaluates the relationship between sLeX expression and cancer prognosis and clinicopathology.
In the present study, a combined analysis of 29 articles (3,253 cancer patients) which showed the detection of high sLeX expression in tumor tissues with poor prognosis outcome in cancer patients was conducted. Our results indicated that sLeX expression was significantly correlated with lymphatic invasion, venous invasion, deep invasion (T stage), lymph node metastasis (N stage), distant metastasis (M stage), tumor stage, tumor recurrence, and OS. On the other hand, although a high level of sLeX expression was found in patients like the elderly, females, or patients with large size tumor and high differentiation, these results did not show any significance.
What makes sLeX overexpression account for the poor prognosis in cancer? By chemical analyses, it was shown that sLeX oligosaccharide was the minimal structure binding to E-, L-, and P-selectin,43 which was closely involved in the interaction between the endothelium and cancer cells. sLeX is most commonly found in malignant tumors and plays a key role in cancer stem cell metastasis, hypoxia, and TNF-α, and promotes tumor adhesion, invasion, and metastasis by upregulating the sLeX expression in the tumor microenvironment.44–46 In the present meta-analysis study, we also found that sLeX expression was correlated with tumor recurrence. On the other hand, it is widely accepted that expression of cell surface carbohydrates is altered during malignant transformation and tumor progression, and may influence determination of metastatic behavior of tumor cells.21,47 It has been identified that sLeX was a terminal tetrasaccharide moiety present on numerous membrane glycoproteins and glycolipids of epithelial and lymphatic cells.28 With such characters, a high level of sLeX contributes to cell adhesion, metastasis, and invasion because the cell surface antigens can combine with other cells directly. sLeX in conjunction with mucins, promotes cellular motility, thus contributing to tumor cell spreading and metastasis.11,48 Furthermore, sLeX is expressed on granulocytes and monocytes which mediates inflammatory extravasation.49,50 However, the molecular biological mechanisms of how sLeX overexpression affects the cancer prognosis are complicated and still need further exploration. For the first time, our meta-analysis study revealed that sLeX could be a potential biomarker for poor cancer prognosis.
Due to the differences in nationality and cancer types which could cause heterogeneity among the studies, we conducted a subgroup analysis. In the subgroup analysis, the sLeX overexpression may play different roles caused by differentiation, venous invasion, T stage, M stage, tumor stage, and sex factors among different types of cancers. These factors contribute to the possible presence of heterogeneity between the studies. The difference might be owing to the molecular biological mechanisms of interactions between sLeX overexpression, and the occurrence and development of different types of cancers. Otherwise, ethnicity may be another factor that contributes to heterogeneity in sex, tumor size, differentiation, venous invasion, T stage, and M stage. It might be owing to the differences in genetic backgrounds and the environment among different races. We also found high heterogeneity in some subgroups, because biological behavior of cancer might be affected by many possible factors during the complicated process of tumor development.
Some limitations of this meta-analysis need to be acknowledged. First, all published studies and papers were written in English, some related published or unpublished studies that met the inclusion criteria were missed. Most of the studies reported positive results, while studies of negative results were all rejected. Second, some cancers such as oral squamous cell carcinoma, gallbladder cancer, pancreatic ductal adenocarcinoma, prostate cancer, and extrahepatic bile duct carcinoma were included in only one article respectively, so we could not evaluate pooled data in subgroup analyses. Third, all of the included studies had data of the sLeX expression which was detected by IHC methods. It might have some bias because of different antibodies and different standards of positive/negative sLeX expression. However, it was not available for us to do a subgroup analysis to analyze the underlying bias of IHC on the pooled odds ratios or HRs. Finally, multivariate analyses were not performed on OS data in most included studies, we calculated the pooled HR only from available HRs.
In conclusion, our meta-analysis showed that a high level of sLeX expression was significantly associated with lymphatic invasion, venous invasion, deep invasion, lymph node metastasis, distant metastasis, tumor stage, tumor recurrence, and OS in cancer. sLeX might be a new prognostic biomarker, and it might become a new diagnostic and therapeutic target for cancer. Further studies are required to explore the molecular biological mechanisms of sLeX and factors that caused significant heterogeneity in the present meta-analysis study.
Acknowledgments
This study was supported by National Natural Science Foundation of China (number 81001113). The authors are most grateful to all the participants in this study.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, Stiller CA, Coebergh JW. Cancer in children and adolescents in Europe: developments over 20 years and future challenges. Eur J Cancer. 2006;42(13):2183–2190. doi: 10.1016/j.ejca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Fucic A, Gamulin M, Ferencic Z, et al. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, and brain. Environ Health. 2012;11(Suppl 1):S8. doi: 10.1186/1476-069X-11-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Sell S. Cancer-associated carbohydrates identified by monoclonal antibodies. Hum Pathol. 1990;21(10):1003–1019. doi: 10.1016/0046-8177(90)90250-9. [DOI] [PubMed] [Google Scholar]
- 5.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179(4):358–369. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Zhang Y, Liu Y, et al. FUT7 antisense sequence inhibits the expression of FUT7/sLeX and adhesion between embryonic and uterine cells. IUBMB Life. 2008;60(7):461–466. doi: 10.1002/iub.62. [DOI] [PubMed] [Google Scholar]
- 7.Kannagi R, Kitahara A, Itai S, et al. Quantitative and qualitative characterization of human cancer-associated serum glycoprotein antigens expressing epitopes consisting of sialyl or sialyl-fucosyl type 1 chain. Cancer Res. 1988;48(13):3856–3863. [PubMed] [Google Scholar]
- 8.Itai S, Nishikata J, Takahashi N, et al. Differentiation-dependent expression of I and sialyl I antigens in the developing lung of human embryos and in lung cancers. Cancer Res. 1990;50(23):7603–7611. [PubMed] [Google Scholar]
- 9.Takada A, Ohmori K, Takahashi N, et al. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochem Biophys Res Commun. 1991;179(2):713–719. doi: 10.1016/0006-291x(91)91875-d. [DOI] [PubMed] [Google Scholar]
- 10.Takada A, Ohmori K, Yoneda T, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53(2):354–361. [PubMed] [Google Scholar]
- 11.Fukuoka K, Narita N, Saijo N. Increased expression of sialyl Lewis(x) antigen is associated with distant metastasis in lung cancer patients: immunohistochemical study on bronchofiberscopic biopsy specimens. Lung Cancer. 1998;20(2):109–116. doi: 10.1016/s0169-5002(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 12.Ura H, Denno R, Hirata K, et al. Close correlation between increased sialyl-Lewisx expression and metastasis in human gastric carcinoma. World J Surg. 1997;21(7):773–776. doi: 10.1007/s002689900304. [DOI] [PubMed] [Google Scholar]
- 13.Davidson B, Gotlieb WH, Ben-Baruch G, et al. Expression of carbohydrate antigens in advanced-stage ovarian carcinomas and their metastases-A clinicopathologic study. Gynecol Oncol. 2000;77(1):35–43. doi: 10.1006/gyno.1999.5708. [DOI] [PubMed] [Google Scholar]
- 14.Kurahara S, Shinohara M, Ikebe T, et al. Immunohistochemical study of sialyl Le(a) and sialyl Le(x) antigen in oral squamous cell carcinoma: the association of sialyl Le(a) expression with the metastatic potential. Head Neck. 1999;21(4):330–337. doi: 10.1002/(sici)1097-0347(199907)21:4<330::aid-hed7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Schiffmann L, Schwarz F, Linnebacher M, et al. A novel sialyl Le(X) expression score as a potential prognostic tool in colorectal cancer. World J Surg Oncol. 2012;10:95. doi: 10.1186/1477-7819-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagoe T, Fukushima K, Nanashima A, et al. Expression of Lewis(a), sialyl Lewis(a), Lewis(x) and sialyl Lewis(x) antigens as prognostic factors in patients with colorectal cancer. Can J Gastroenterol. 2000;14(9):753–760. doi: 10.1155/2000/149851. [DOI] [PubMed] [Google Scholar]
- 17.Baldus SE, Zirbes TK, Monig SP, et al. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), sialosyl-Lewis(x) and sialosyl-Tn. Tumor Biol. 1998;19(6):445–453. doi: 10.1159/000030036. [DOI] [PubMed] [Google Scholar]
- 18.Nakamori S, Kameyama M, Imaoka S, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53(15):3632–3637. [PubMed] [Google Scholar]
- 19.Yamaguchi A, Saitoh M, Goi T, et al. Sialyl-lewis-x antigen immunoreaction of colorectal-cancer and its relationship to hematogenous metastasis. Oncol Rep. 1994;1(4):731–734. doi: 10.3892/or.1.4.731. [DOI] [PubMed] [Google Scholar]
- 20.Idikio HA. Sialyl-Lewis-X, Gleason grade and stage in non-metastatic human prostate cancer. Glycoconj J. 1997;14(7):875–877. doi: 10.1023/a:1018502424487. [DOI] [PubMed] [Google Scholar]
- 21.Nakamori S, Kameyama M, Imaoka S, et al. Involvement of carbohydrate antigen sialyl Lewis(x) in colorectal cancer metastasis. Dis Colon Rectum. 1997;40(4):420–431. doi: 10.1007/BF02258386. [DOI] [PubMed] [Google Scholar]
- 22.Shimodaira K, Nakayama J, Nakamura N, et al. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57(23):5201–5206. [PubMed] [Google Scholar]
- 23.Farmer RW, Richtsmeier WJ, Scher RL. Identification of sialyl Lewis-x in squamous cell carcinoma of the head and neck. Head Neck. 1998;20(8):726–731. doi: 10.1002/(sici)1097-0347(199812)20:8<726::aid-hed11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Tatsumi M, Watanabe A, Sawada H, et al. Immunohistochemical expression of the sialyl Lewis x antigen on gastric cancer cells correlates with the presence of liver metastasis. Clin Exp Metastasis. 1998;16(8):743–750. doi: 10.1023/a:1006584829246. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi A, Ding K, Maehara M, Goi T, Nakagawara G. Expression of nm23-H1 gene and Sialyl Lewis X antigen in breast cancer. Oncology. 1998;55(4):357–362. doi: 10.1159/000011878. [DOI] [PubMed] [Google Scholar]
- 26.Takao S, Uchikura K, Yonezawa S, Shinchi H, Aikou T. Mucin core protein expression in extrahepatic bile duct carcinoma is associated with metastases to the liver and poor prognosis. Cancer. 1999;86(10):1966–1975. doi: 10.1002/(sici)1097-0142(19991115)86:10<1966::aid-cncr13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Futamura N, Nakamura S, Tatematsu M, et al. Clinicopathologic significance of sialyl Le(x) expression in advanced gastric carcinoma. Br J Cancer. 2000;83(12):1681–1687. doi: 10.1054/bjoc.2000.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabowski P, Mann B, Mansmann U, et al. Expression of SIALYL-Le(x) antigen defined by MAb AM-3 is an independent prognostic marker in colorectal carcinoma patients. Int J Cancer. 2000;88(2):281–286. [PubMed] [Google Scholar]
- 29.Machida E, Nakayama J, Amano J, Fukuda M. Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 2001;61(5):2226–2231. [PubMed] [Google Scholar]
- 30.Takahashi S, Oda T, Hasebe T, et al. Overexpression of sialyl Lewis x antigen is associated with formation of extratumoral venous invasion and predicts postoperative development of massive hepatic metastasis in cases with pancreatic ductal adenocarcinoma. Pathobiology. 2001;69(3):127–135. doi: 10.1159/000048767. [DOI] [PubMed] [Google Scholar]
- 31.Baldus SE, Monig SP, Hanisch FG, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewisa and sialyl-lewisx antigens in colorectal adenocarcinoma. Histopathology. 2002;40(5):440–449. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 32.Konno A, Hoshino Y, Terashima S, Motoki R, Kawaguchi T. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin Exp Metastasis. 2002;19(1):61–70. doi: 10.1023/a:1013879702702. [DOI] [PubMed] [Google Scholar]
- 33.Nakagoe T, Fukushima K, Itoyanagi N, et al. Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128(5):257–264. doi: 10.1007/s00432-002-0334-5. [DOI] [PubMed] [Google Scholar]
- 34.Nakagoe T, Fukushima K, Sawai T, et al. Increased expression of sialyl Lewis(x) antigen as a prognostic factor in patients with stage 0, I, and II gastric cancer. Cancer Lett. 2002;175(2):213–221. doi: 10.1016/s0304-3835(01)00705-4. [DOI] [PubMed] [Google Scholar]
- 35.Kashiwagi H, Kijima H, Dowaki S, et al. Clinicopathological significance of sialyl Lex expression in human gallbladder carcinoma. Oncol Rep. 2004;11(6):1139–1143. [PubMed] [Google Scholar]
- 36.Yu CJ, Shih JY, Lee YC, et al. Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer. 2005;47(1):59–67. doi: 10.1016/j.lungcan.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Faried A, Kimura H, Faried LS, et al. Expression of carbohydrate antigens in human esophageal squamous cell carcinoma: Prognostic application and its diagnostic implications. Ann Surg Oncol. 2007;14(2):960–967. doi: 10.1245/s10434-006-9200-z. [DOI] [PubMed] [Google Scholar]
- 38.Croce MV, Rabassa ME, Pereyra A, Segal-Eiras A. Differential expression of MUC1 and carbohydrate antigens in primary and secondary head and neck squamous cell carcinoma. Head Neck. 2008;30(5):647–657. doi: 10.1002/hed.20756. [DOI] [PubMed] [Google Scholar]
- 39.Sozzani P, Arisio R, Porpiglia M, Benedetto C. Is Sialyl Lewis x antigen expression a prognostic factor in patients with breast cancer? Int J Surg Pathol. 2008;16(4):365–374. doi: 10.1177/1066896908324668. [DOI] [PubMed] [Google Scholar]
- 40.Portela SV, Martin CV, Romay LM, et al. sLea and sLex expression in colorectal cancer: implications for tumourigenesis and disease prognosis. Histol Histopathol. 2011;26(10):1305–1316. doi: 10.14670/HH-26.1305. [DOI] [PubMed] [Google Scholar]
- 41.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 42.Nakagoe T, Ishikawa H, Nakao H, et al. Sialyl Lewis(x)-I (SLX) as a tumor-associated carbohydrate antigen in sera in patients with gastric and colorectal cancer – evaluation according to clinico-pathological factors. Gan To Kagaku Ryoho. 1989;16(4 Pt 1):819–825. Japanese. [PubMed] [Google Scholar]
- 43.Foxall C, Watson SR, Dowbenko D, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl-Lex oligosaccharide. J Cell Biol. 1992;117(4):895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike T, Kimura N, Miyazaki K, et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci U S A. 2004;101(21):8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St Hill CA, Krieser K, Farooqui M. Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior. Cancer. 2011;117(19):4493–4505. doi: 10.1002/cncr.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desiderio V, Papagerakis P, Tirino V, et al. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget. 2015;6(1):71–84. doi: 10.18632/oncotarget.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irimura T, Reading CL. Surface properties of metastatic tumor cells. Cancer Bull. 1987;39(3):132–141. [Google Scholar]
- 48.Byrd JC, Bresalier RS. Mucins and mucin-binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 49.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273(19):4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman BJ, Paulson JC, Arrhenius TS, Gaeta FC, Granger DN. Thrombin receptor peptide-mediated leukocyte rolling in rat mesenteric venules: roles of P-selectin and sialyl Lewis X. Am J Physiol. 1994;267(3 Pt 2):H1049–H1053. doi: 10.1152/ajpheart.1994.267.3.H1049. [DOI] [PubMed] [Google Scholar]