Esophageal replacement in children: Challenges and long-term outcomes (original) (raw)
Abstract
Replacement of a nonexistent or damaged esophagus continues to pose a significant challenge to pediatric surgeons. Various esophageal replacement grafts and techniques have not produced consistently good outcomes to emulate normal esophagus. Therefore, many techniques are still being practiced and recommended with no clear consensus. We present a concise literature review of the currently used techniques and with discussions on the advantages and anticipated morbidity. There are no randomized controlled pediatric studies to compare different types of esophageal replacements. Management and graft choice are based on geographical and personal predilections rather than on any discernible objective data. The biggest series with long-term outcome are reported for gastric transposition and colonic replacement. Comparison of different studies shows no significant difference in early (graft necrosis and anastomotic leaks) or late complications (strictures, poor feeding, gastro-esophageal reflux, tortuosity of the graft, and Barrett's esophagus). The biggest series seem to have lower complications than small series reflecting the decennials experience in their respective centers. Long-term follow-up is recommended following esophageal replacement for the development of late strictures, excessive tortuosity, and Barrett's changes within the graft. Once child overcomes initial morbidity and establishes oral feeding, long-term consequences and complications of pediatric esophageal replacement should be monitored and managed in adult life.
Keywords: Caustics, esophageal atresia, esophageal replacement, esophagus
INTRODUCTION
Functioning esophagus is a gift of nature that can rarely or never be imitated by its replacement. In rare instances, esophageal replacement to restore the anatomical continuity between mouth and stomach is required in children. The common indications in children include long gap esophageal atresia (LGEA), severe peptic, caustic or anastomotic strictures, and some rare esophageal disorders [Table 1].
Table 1.
Indications of oesophageal replacement in children
Many surgeons believe patients are served best by their own esophagus. They direct all the surgical strategies toward preserving the native esophagus. The esophageal replacement is a surgical challenge and technically demanding operation. The factors influencing the outcome are related to infrequent requirement of the esophageal replacement, variable expertise among the surgeons, and lack of ideal conduit. The characteristics of ideal esophageal conduit are described in Table 2. The variability of esophageal interposition grafts and historical lack of evidence-based outcome studies have led to the practice variability. Furthermore, at least in the developed countries, public awareness and industry collaboration have led to significant reduction in the incidence of caustic esophageal stricture.[1,2,3]
Table 2.
Requirements for the ideal oesophageal conduit

Finally, it is difficult to deduct and transfer technique and outcome from the adult esophageal replacement studies due to pathological differences, sturdier esophageal grafts, and the existence of different associated comorbidities than children.
MATERIALS AND METHODS
We performed a MEDLINE search for the most up-to-date data on the commonly used esophageal replacements in children. The advantages and disadvantages of each type of conduit are considered and the published results discussed.
PRESERVATION OF NATIVE ESOPHAGUS IN LONG GAP ESOPHAGEAL ATRESIA: DELAYED PRIMARY ANASTO-MOSIS
The difficulties in managing patients with LGEA patients are well known as are the controversies surrounding the surgical strategy. Surgical expertise and experience and multidisciplinary management are the key factors determining the success rate of preserving the native esophagus.
Initial gastrostomy with delayed primary esophageal anastomosis (within first 2-3 months of life) is overall the most common and simple approach.[4] In a recent series, children considered not suitable for primary anastomosis underwent a delayed primary repair, which was feasible in 92% of cases.[5] The postoperative morbidity in this study was high: 58% patients developed strictures requiring multiple dilatations, 75% patients had clinically significant gastroesophageal reflux of which 50% underwent anti-reflux surgery. However, the survival rate was 100%, and esophageal replacement surgery was avoided in over 90% of cases. Lee et al. reported similar experiences in children undergoing delayed primary anastomosis or esophageal replacement.[1] Esophageal replacement patients had longer gaps (mean 5.5 vertebrae, range: 4–9) compared to delayed primary anastomosis (mean 3.9, range: 2–6), but there were no difference in perioperative complications. However, esophageal replacement had more long-term complications (86%) compared to delayed primary anastomosis (30%).
The other disadvantages of the delayed repair include the requirement of avoiding aspirations and infections by continuous upper pouch suction with Replogle tube without causing mucosal injury and bleeding, maintaining the Replogle tube in the upper pouch, replacement of saliva loss so as to avoid sodium and chloride imbalance. Furthermore, these babies are not fed orally for weeks and therefore develop oro-version once the esophageal continuity is established.
Kimura and Soper, Foker et al. developed esophageal elongation methods to solve these problems by creating esophagostomy and permitting sham feeding.[6,7] Kimura's technique lengthens the upper pouch of the esophagus by the sequential advancement of the esophagostomy by mobilizing it over the chest wall. The success of the Kimura's technique with delayed primary anastomosis are fraught with many complications, namely the stricture and anastomotic leaks (30% and 80%, respectively) and requirement of a mean of 3.6 thoracotomies.[8] In contrast, traditional Foker's method is designed to induce rapid growth of the esophagus by external axial tension on the blind ending segments enabling delayed primary anastomosis within 1–2 weeks. Although this external traction method can elongate the blind ends of esophagus and successfully achieves a delayed primary anastomosis in more than 90% of cases, associated risks remain high. This technique has a high risk of infection, disruption of the esophageal ends, risk of anastomotic stricture (70%), anastomotic leak (50%), severe gastroesophageal reflux (60%), and requires multiple thoracotomies with its associated risks.[8,9,10]
The relative novel thoracoscopic Focker's approach in the management of LGEA may add an advantage by achieving maximal mobilization of esophageal ends under improved thoracoscopic vision.[10] Furthermore, the thoracoscopic approach can reduce the risk of infection and disruption of the esophageal ends compared with the traditional open Foker's technique. In addition, if the primary anastomosis is not achievable after application of first tension sutures, a second internal traction sutures can be applied after further mobilization of esophageal ends. This method avoids risk factors of repeated thoracotomies and allows under vision application of tension sutures. In most instances, the esophagus is ready for the delayed primary anastomosis in 3–4 weeks.[11]
MANAGEMENT OF RECALCITRANT ESOPHAGEAL STRICTURES: PRESERVATION TECHNIQUES
Anastomotic stricture and corrosive strictures are generally managed initially by dilations to maintain esophageal continuity and oral feeding. Most LGEA with delayed primary anastomosis develops anastomotic strictures ranging from 50% to 80%.[12] The initial management of esophageal strictures by balloon dilatation has become popular since its first use described by Ball et al. in children in mid-1980s.[13] Today, balloon radial dilatation under endoscopic and/or fluoroscopic control is recognized to be superior, safer, and more effective alternative to the traditional longitudinal shearing bouginage.
Thyoka et al. recently reported their experience with fluoroscopic balloon dilatation of anastomotic strictures in 103 children with EA. In this study, dilatation provided relief of symptoms in 90% of their patients with just 1% risk of perforation. However, only 47% achieved cure with a single dilatation: 53% of the patients required multiple dilatations (median-two, range: 1–40 dilations per patient). The study suggested that surgical replacement might be required in cases that did not respond after ten dilatations.[14] In the same study, only 10% of the strictures were resistant to balloon dilatation and required further interventions, such as stent placement and/or esophageal replacement.
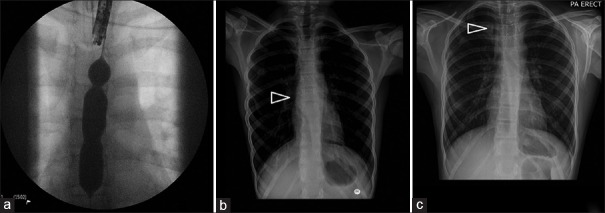
Manfredi et al. reported the use of 41 removable stents (plastic or metal) for treating recalcitrant esophageal strictures and perforation in 24 children after EA repair.[15] The success rate was 80% for closure of esophageal perforations with stent therapy after dilation and 25% for perforations associated with surgical repair. However, the stricture recurrence rate after stent removal was high: 60% at 1 month and nearly 70% at 3 months. This may limit the usefulness of stents in treating recalcitrant esophageal anastomotic strictures. Furthermore, the rate of adverse events of stent placement was high, particularly with metal stents: Migration (21% of plastic stents and 7% of metal stents), granulation tissue (37% of metal stents), and deep ulcerations (22% of metal stents) [Figure 1]. Technically, it is also important to choose the size of the stent carefully because of the risk of compressing the typically malacic trachea in children with EA.
Figure 1.
(a) Fluoroscopic balloon dilatation of a caustics stricture. (b) Stent placed across the stricture. (c) Cephalic migration of the stent
To avoid the disadvantages of multiple dilatations or of an esophageal stent, van der Zee and Hulsker described the use of an indwelling balloon catheter kept at the stricture level and insufflating it three times daily at home.[16] He described this technique in the management of refractory strictures in 19 children with EA, LGEA, peptic, and caustic injuries. The home insufflation of the indwelling catheter to stretch the stricture reduced the total number of endoscopies, the number of hospital admissions, and general anesthesia for the dilatations. This daily insufflation was well tolerated by the children, was easily taught to the parents, prevented recurrent stricture formation, and avoided complex reconstructive surgery in all of their cases.
Intralesional injection of steroids or mitomycin application is another alternative to decrease the frequency of endoscopic dilatations and recurrent stricture formation. This has shown to augment the effects of dilation in patients with benign esophageal strictures.[17]
ESOPHAGEAL REPLACEMENT
Historical perspective
The first attempt to esophageal reconstruction was in the late 19th century by using a subcutaneous skin tube.[18] Not surprisingly this was an unsatisfactory replacement for several reasons including esthetics, lack of functionality, and possible risk of malignancy.
Gavriliu from Romania is acknowledged as the pioneer of achieving the first successful esophageal substitution by the tubularization of the greater gastric curvature: He reported 52 adult cases by 1951.[19] In subsequent two decades, several reports of successful application of gastric tube interposition technique in children were published in North America and Australia.[20]
The gastric transposition or stomach pull-up was first published by Kummel in 1922.[21] Swenson and Sweet successfully used this method in children in the late 1940s, and since then it has been popularized for its simplicity and reproducibility by many including the Great Ormond Street in London.[22,23]
Although Roux successfully reported first in 1907 jejunum as an interposition graft in a child, this replacement method did not become popular in children.[24] The popularity of jejunum as a replacement graft in children is fraught with complications such as graft necrosis and leakage. Free grafts and microvascular anastomosis are rarely feasible in children due to the fragility of graft vasculature. However, the size of jejunum is appealing in a small chest, therefore, jejunum on his pedicle has been popularized sacrificing the distal jejunum to achieve length. Many groups have reported good outcomes in children with this technique.[25]
Lundblad performed the first colonic interposition in a 3-year-old child with a caustic stricture in 1921. The procedure was successful, and the patient lived and swallowed normally until he died in a car accident 37 years later.[26] Sandblom was the first to report the use of the colon for replacement in EA.[27]
THE PRESENT
Not much has changed since the last century apart from the applications of minimally invasive techniques in some of these cases. The four most commonly used methods of esophageal replacement still in practice are:
- Gastric interposition
- Gastric tube interposition
- Jejunal interposition
- Colonic interposition.
All types of esophageal replacements share common steps: Selection and preparation of graft, resection of rudimentary or strictured esophagus and establishing the continuity between esophagus and stomach. The intra-abdominal phase can be achieved either via median or transverse laparotomy. Minimal invasive approaches to perform the esophagectomy and the replacement have also been implemented in recent years mainly for gastric interposition.[23]
In the case of EA, the distal esophageal stump can be used only if of reasonable length and good quality. In cases of long caustic or peptic esophageal strictures, subtotal esophagectomy should be performed before or at the time of the interposition. Esophagectomy is recommended in these cases because of the risk of metaplasia and dysplasia in unused segments of esophagus.
The interposing graft should ideally be pulled up through a posterior mediastinal route. A proximal esophageal-graft anastomosis can be achieved through a neck incision. If the anastomosis is high within the neck, the thoracic inlet can act as constriction to the passage of the esophageal graft and or anastomosis. In this event, the thoracic inlet can be widened surgically by either resecting the left half of the manubrium or the medial end of the first rib and the sternal head of the left clavicle. Mediastinal esophageal-graft anastomosis can either be performed through left or right thoracotomy depending on the length of proximal esophagus.
Various esophageal substitutes have their advantages, technical difficulties, challenges, and specific complications. Overall, morbidity is reasonably high, the most common being anastomotic leakage, and stricture varying from 10% to 20% regardless of the replacement technique.[23] Strictures, stump esophagitis, and ulcerations of the small or large bowel conduits are all consequences of the acid reflux into the replacement conduit. There should be regular monitoring of the esophageal replacement as chronic exposure to acid reflux may predispose to metaplasia and adenocarcinoma.[28]
Swallowing difficulties and food aversion are common problems despite a successful and adequate replacement. Dysphagia among esophagus-replaced children can be due to dis-coordinated peristalsis, antiperistaltic layout, tortuous esophageal conduit, or significant acid reflux into the graft. Oral aversion is particularly common in infants with LGEA due to delay in establishing oral feeding either due to lack of sham feeds prior to establishing the continuity or delay in replacement. It is imperative to monitor nutrition, growth and development, and lung functions in children after replacing the esophagus as they are known to fall below centiles for both height and weight and may develop recurrent chest infections related to aspirations.[29]
Finally, the overall replacement procedure-related mortality rate could be estimated at about 2%. However, this is a difficult due to lack of up-to-date, large pediatric studies and technical variation.[23]
SURGICAL TECHNICAL POINTS
Gastric interposition
Stomach has rich submucosal arterial and venous plexus and therefore can tolerate extensive mobilization on pedicles of greater and lesser curvature arcades. Through an epigastric midline (or transverse) incision, the stomach is mobilized by taking down most of its superior vessels, the previous gastrostomy, and the distal esophagus. The gastroepiploic and the right gastric vessels are preserved. To achieve more length, the second portion of the duodenum is also mobilized. Pyloroplasty is advocated to provide better drainage as mobilized stomach gets denervated and interposed in the thoracic cavity.
Next, the proximal esophagus or the esophagostomy is mobilized through a neck incision. Thoracotomy is generally required to complete the resection of the esophagus particularly in the case of extensive adhesions from previous surgery or caustic injuries.
A transhiatal posterior mediastinal tunnel is created. A large hemostat is placed from the neck and the stay sutures in the fundus are pulled. Particular attention must be taken not to twist the stomach during the pull-up maneuver.
The gastroesophageal anastomosis is finally performed in the neck using the apex of the stomach [Figure 2]. A large nasogastric tube is left in the stomach through the anastomosis and a feeding jejunostomy can be created before closing the abdominal wound.
Figure 2.
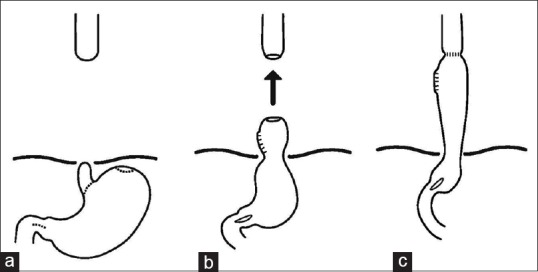
Esophageal replacement with gastric pull-up. The esophageal stump is excised, pyloroplasty is performed (a), the stomach is pulled up (b), and the cervical esophagus (c)
Stomach pull-up has several advantages: It is relatively simple, involves a single anastomosis in the neck, and it has a good length and blood supply; hence, theoretical reduced risk of necrosis, anastomotic leak, and stricture.
Critics of this approach claim that the stomach occupies a large space in the chest has a potential for causing compression of intrathoracic organs and does not empty effectively.
Gastroesophageal reflux can lead to recurrent aspirations, heartburn, regurgitation, and bad breath. In addition, vagotomy, which is inevitable during gastric mobilization, may lead to delayed gastric emptying and dumping.
Gastric tube interposition
The gastric tube can be created from the greater or lesser gastric curvature, in a reversed (antiperistaltic) or isoperistaltic fashion [Figure 3].
Figure 3.
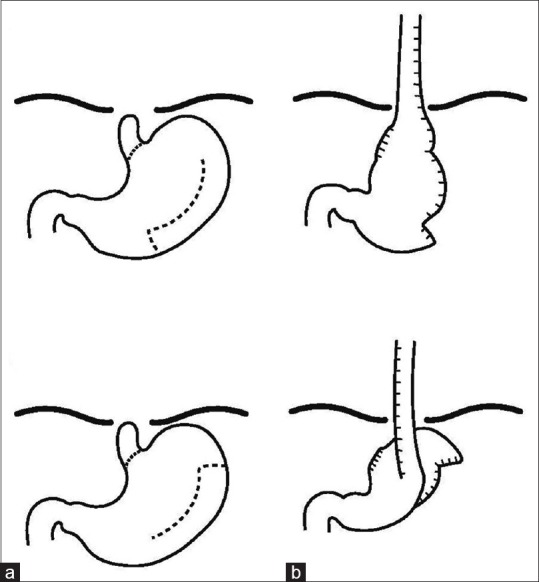
Different types of gastric tubes: (a) Reverse gastric tube created from the greater curvature and (b) isoperistaltic gastric tube created from the greater curvature
After excising the distal esophagus the gastrocolic omentum, the right gastroepiploic artery and the short gastric vessels are divided.
A gastric tube is then constructed usually from the greater curve either using a gastrointestinal anastomosis stapler or with a hand-sewn anastomosis around a catheter 18-24 Fr tube.
The esophageal hiatus is enlarged and the gastric tube is brought into the chest, taking care to avoid kinking of the tube.
The anastomosis with the proximal esophagus is performed in a single layer either in the neck or in the chest (via thoracotomy) depending on the length of the proximal normal esophagus. A transanastomotic nasogastric tube is left in place and a feeding gastrostomy is also performed.
Tube necrosis is rare because the gastric tube has an excellent and reliable blood supply offered mainly by the submucosal plexus and in part by the spared gastroepiploic vessels. Furthermore, the gastric tube can bridge relatively long gaps and retains its tubular shape without developing dilatation and tortuosity even though it acts purely as a passive conduit. The main disadvantages of the gastric tube are a long suture line, a reduced stomach capacity, and reflux of acid produced by the tube. Esophagitis and metaplasia have been described in children following gastric tube grafting.[30] In adults, a comparative study of over two hundred patients concluded that transposition of the whole stomach had more advantages over the gastric tube because there was no loss of stomach volume, the blood supply was better, dysphagia was less severe, and the stricture rate was smaller.[31]
A gastric tube can be formed from the lesser gastric curve with preservation of the lower native esophagus and the esophagogastric junction. However, strictures occur frequently, possibly as a consequence of the degree of devascularization following division of the left gastric artery.
Jejunal interposition
The jejunum can be used either as pedicled or as a free graft. Best practice is for a staged pedicled jejunum interposition. The jejunum can be prepared early, for instance at the time of the initial gastrostomy or at a later laparotomy by ligating the vessel at the base of the jejunal loop in continuity. This promotes hypertrophy of the vascular arcade and therefore improves venous drainage once pulled up in the neck. The last stage involves creating the pedicle graft: The first mesenteric vessels are divided close to the main mesenteric route [Figure 4]. The jejunum is divided close to Treitz ligament and again opposite the level of the third mesenteric branch. The isolated jejunal segment is then skeletonized upward leaving the uppermost part in place for interposition. The conduit with vascular pedicle is passed (without twisting and redundancy) through the mesentery of the transverse colon and posterior to the stomach. The jejunum is anastomosed to the stomach and finally, introduced through the esophageal hiatus into the mediastinum where the anastomosis with the proximal esophagus is completed. Bowel continuity is restored where the graft was taken.
Figure 4.
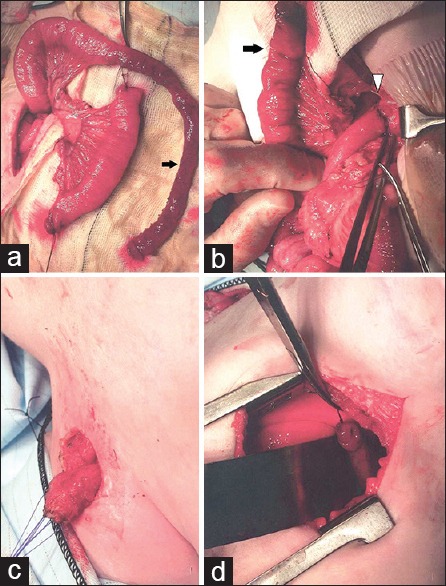
Jejunal interposition in a child with long gap esophageal atresia. (a) The pedicled graft of jejunum is prepared. The vascular arcade is preserved and extravascular length is gained by excising a segment of bowel (arrow). (b) The jejunal graft (arrow) is prepared while jejuno-jejunum anastomosis (arrowhead) is performed in the abdomen. (c) The esophagostomy is mobilized in the neck. (d) The jejunal graft is pulled up into the chest and the esophago-jejunostomy is performed in the chest
A free jejunal graft involves a microsurgical anastomosis between the vascular pedicle of the graft and suitable vessels in the chest. However, this technique has a high rate of failure, morbidity, and mortality. A recent meta-analysis of adult experience reported up to 10% mortality, 36% anastomotic leak rate, and 5–11% graft loss frequency.[32] The main advantages of jejunal conduit are the caliber appropriateness of the interposed jejunal segment and the retained peristaltic activity [Figure 5]. Thus, there is no compression of the mediastinal structures and the peristalsis keeps the conduit clear from acid reflux. The precarious blood supply of the jejunum is responsible for the most serious complication seen with this technique. Complete graft necrosis which can lead to perforation of the graft and mediastinitis with high morbidity and if unrecognized, mortality.
Figure 5.
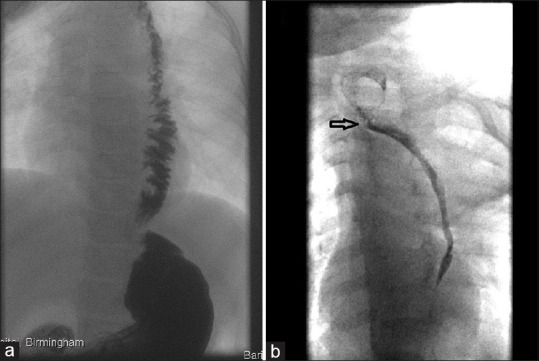
Contrast swallow of a child with pedicled jejunal graft. (a) The jejunal graft has maintained its caliber and peristalsis; (b) the graft is stenosed, featureless, and lacking peristalsis. The arrow indicates the esophago-jejunostomy
Colonic interposition
The left transverse colonic graft, based on the left colic artery, is the most frequently used conduit to replace the esophagus; the ascending or descending colon have been also used. The most widely used procedure in the past was retrosternal esophagocolonic anastomosis. The posterior mediastinal route has been recently been used with better success for the interposing colon.
The colonic graft should be positioned in an isoperistaltic direction. However, the antiperistaltic direction can be used in cases where the available tract has a short vascular pedicle making the isoperistaltic placement of the bypass impossible.
The colon is pulled up into the neck and an end-to-side or end-to-end esophagocolic anastomosis is carried out.
Distally the cologastric anastomosis is done at the level of the cardia with an anti-reflux “Thal type” wrap of the stomach to prevent acid reflux. Another anti-reflux option consists in performing a cologastric anastomosis to the posterior wall of the stomach by creating a curve in the interposed colon. This causes the compression of the colon when the stomach is full.[29]
In addition, a pyloroplasty as a drainage procedure may further facilitate gastric emptying and prevent reflux.
These operations have traditionally been performed through a laparotomy or thoraco-laparotomy. Recently, the laparoscopically assisted esophagectomy and colon interposition have been described in children with promising results.[33]
Recognized specific complication of colonic interposition is the redundancy of the conduit with stasis and dysphagia. This is believed to be due to the negative pressure in the thoracic cavity and the colonic conduit's passive nature, emptying essentially by gravity. This complication can be reduced by avoiding a breach in the pleura and resecting excess colon at the cervical end prior to anastomosis. The better success rates have been achieved by placing colonic conduit in the posterior mediastinum which gives a shorter and straighter course.
THE FUTURE
In the future, tissue engineering may represent a valid therapeutic alternative to treat esophageal tissue loss. Tissue engineering is a multidisciplinary approach including cell biology, material engineering, physiology, and gene therapy aiming to develop suitable replacements of tissues. Studies in vitro and animal models have shown promising results in the creation of rudimentary esophageal tissue using tissue engineering and regenerative medicine technology. Two broad categories of tissues are available. The use of cell-seeded scaffolds and cellular scaffolds which aim to the migration of autologous epithelial and smooth muscle cells to repopulate the new conduit.[34]
However, major questions of vascular supply, cell-cell and cell-scaffold interaction, and replicating peristaltic contractility remain outstanding. Moreover, complications such as leakage, strictures, infections, inflammations, immune rejection, poor re-epithelialization, and poor muscle regeneration in the grafts suggest that several issues still need to be addressed before declaring the bioengineered neoesophagus an effective treatment for complex congenital and acquired esophageal malformations in adults and children.
CONCLUSIONS
Esophageal substitution remains a major challenge in children. Since none of the replacement techniques available fully replicates the features of a normal esophagus, some pediatric surgeons continue to trust the axiom that the best esophageal conduit is the native esophagus. However, excessive, unproductive attempts at preserving the native esophagus may ultimately prove detrimental to the child and family.
Most available retrospective pediatric series compare only two types of esophageal replacement reflecting either selection or a change in practice. Overall, the long-term outcomes in the published retrospective studies of the various available techniques of replacement in children are similar.
It does appear, however, that the best-published outcomes are produced in large volumes centers thus reflecting the experience and dedication of the surgeons involved in perfecting and championing their techniques. This is a clear indication for the centralization of the management of the “difficult” esophageal cases as well as for replacing the esophagus to few specialist centers to improve outcomes, enhance expertise, and progress with the research.
Long-term follow-up of these children is essential because of gradual changes in the function of the graft, strictures at the anastomosis, deteriorating dysphagia, poor nutrition and growth, and the unknown risks of metaplasia and malignancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee HQ, Hawley A, Doak J, Nightingale MG, Hutson JM. Long-gap oesophageal atresia: Comparison of delayed primary anastomosis and oesophageal replacement with gastric tube. J Pediatr Surg. 2014;49:1762–6. doi: 10.1016/j.jpedsurg.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Chirica M, Brette MD, Faron M, Munoz Bongrand N, Halimi B, Laborde C, et al. Upper digestive tract reconstruction for caustic injuries. Ann Surg. 2015;261:894–901. doi: 10.1097/SLA.0000000000000718. [DOI] [PubMed] [Google Scholar]
- 3.Pearson EG, Downey EC, Barnhart DC, Scaife ER, Rollins MD, Black RE, et al. Reflux esophageal stricture – A review of 30 years’ experience in children. J Pediatr Surg. 2010;45:2356–60. doi: 10.1016/j.jpedsurg.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Sri Paran T, Decaluwe D, Corbally M, Puri P. Long-term results of delayed primary anastomosis for pure oesophageal atresia: A 27-year follow up. Pediatr Surg Int. 2007;23:647–51. doi: 10.1007/s00383-007-1925-7. [DOI] [PubMed] [Google Scholar]
- 5.Zani A, Cobellis G, Wolinska J, Chiu PP, Pierro A. Preservation of native esophagus in infants with pure esophageal atresia has good long-term outcomes despite significant postoperative morbidity. Pediatr Surg Int. 2016;32:113–7. doi: 10.1007/s00383-015-3821-x. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Soper RT. Multistaged extrathoracic esophageal elongation for long gap esophageal atresia. J Pediatr Surg. 1994;29:566–8. doi: 10.1016/0022-3468(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Foker JE, Linden BC, Boyle EM, Jr, Marquardt C. Development of a true primary repair for the full spectrum of esophageal atresia. Ann Surg. 1997;226:533–41. doi: 10.1097/00000658-199710000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sroka M, Wachowiak R, Losin M, Szlagatys-Sidorkiewicz A, Landowski P, Czauderna P, et al. The Foker technique (FT) and Kimura advancement (KA) for the treatment of children with long-gap esophageal atresia (LGEA): Lessons learned at two European centers. Eur J Pediatr Surg. 2013;23:3–7. doi: 10.1055/s-0033-1333891. [DOI] [PubMed] [Google Scholar]
- 9.van der Zee DC, Vieirra-Travassos D, Kramer WL, Tytgat SH. Thoracoscopic elongation of the esophagus in long gap esophageal atresia. J Pediatr Surg. 2007;42:1785–8. doi: 10.1016/j.jpedsurg.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg SS, Flake AW. Experience with thoracoscopic repair of long gap esophageal atresia in neonates. J Laparoendosc Adv Surg Tech A. 2015;25:932–5. doi: 10.1089/lap.2015.0124. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Uchida H, Kawashima H, Sato K, Takazawa S, Jimbo T, et al. Successful two-stage thoracoscopic repair of long-gap esophageal atresia using simple internal traction and delayed primary anastomosis in a neonate: Report of a case. Surg Today. 2013;43:906–9. doi: 10.1007/s00595-012-0426-z. [DOI] [PubMed] [Google Scholar]
- 12.Bagolan P, Iacobelli BD, De Angelis P, di Abriola GF, Laviani R, Trucchi A, et al. Long gap esophageal atresia and esophageal replacement: Moving toward a separation? J Pediatr Surg. 2004;39:1084–90. doi: 10.1016/j.jpedsurg.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 13.Ball WS, Strife JL, Rosenkrantz J, Towbin RB, Noseworthy J. Esophageal strictures in children. Treatment by balloon dilatation. Radiology. 1984;150:263–4. doi: 10.1148/radiology.150.1.6689772. [DOI] [PubMed] [Google Scholar]
- 14.Thyoka M, Barnacle A, Chippington S, Eaton S, Drake DP, Cross KM, et al. Fluoroscopic balloon dilation of esophageal atresia anastomotic strictures in children and young adults: Single-center study of 103 consecutive patients from 1999 to 2011. Radiology. 2014;271:596–601. doi: 10.1148/radiol.13122184. [DOI] [PubMed] [Google Scholar]
- 15.Manfredi MA, Jennings RW, Anjum MW, Hamilton TE, Smithers CJ, Lightdale JR. Externally removable stents in the treatment of benign recalcitrant strictures and esophageal perforations in pediatric patients with esophageal atresia. Gastrointest Endosc. 2014;80:246–52. doi: 10.1016/j.gie.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 16.van der Zee D, Hulsker C. Indwelling esophageal balloon catheter for benign esophageal stenosis in infants and children. Surg Endosc. 2014;28:1126–30. doi: 10.1007/s00464-013-3288-6. [DOI] [PubMed] [Google Scholar]
- 17.Sweed AS, Fawaz SA, Ezzat WF, Sabri SM. A prospective controlled study to assess the use of mitomycin C in improving the results of esophageal dilatation in post corrosive esophageal stricture in children. Int J Pediatr Otorhinolaryngol. 2015;79:23–5. doi: 10.1016/j.ijporl.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Bircher E. Ein Beitrag zur plastischen Bildung eines neuen Oesophagus. Zentralbl Chir. 1907;34:1479–82. [Google Scholar]
- 19.Gavriliu D. Present status of the procedure for reconstruction of the esophagus by gastric tube (138 operated cases) Ann Chir. 1965;19:219–24. [PubMed] [Google Scholar]
- 20.Ein SH, Shandling B, Simpson JS, Stephens CA, Vizas D. Fourteen years of gastric tubes. J Pediatr Surg. 1978;13:638–42. doi: 10.1016/s0022-3468(78)80107-9. [DOI] [PubMed] [Google Scholar]
- 21.Kummel H. Ueber intrathorakale oesophagus plastik. Beitr Klin Chir. 1922;126:264. [Google Scholar]
- 22.Sweet RH. A new method of restoring continuity of the alimentary canal in cases of congenital atresia of the esophagus with tracheo-esophageal fistula not treated by immediate primary anastomosis. Ann Surg. 1948;127:757–68. [PubMed] [Google Scholar]
- 23.Spitz L. Esophageal replacement: Overcoming the need. J Pediatr Surg. 2014;49:849–52. doi: 10.1016/j.jpedsurg.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Roux C. L’esophago-jejuno-gastrome: Nouvelle operation pour retrecessement infranchissable de l’esophage. Semin Med. 1907;27:34–40. [Google Scholar]
- 25.Cauchi JA, Buick RG, Gornall P, Simms MH, Parikh DH. Oesophageal substitution with free and pedicled jejunum: Short- and long-term outcomes. Pediatr Surg Int. 2007;23:11–9. doi: 10.1007/s00383-006-1770-0. [DOI] [PubMed] [Google Scholar]
- 26.Lundblad O. Uber antethorakale Osophagoplastik. Acta Chir Scand. 1921;53:535–41. [Google Scholar]
- 27.Sandblom P. Treatment of congenital atresia of the esophagus from a technical point of view. Acta Chir Scand. 1948;97:25–34. [PubMed] [Google Scholar]
- 28.Arul GS, Parikh D. Oesophageal replacement in children. Ann R Coll Surg Engl. 2008;90:7–12. doi: 10.1308/003588408X242222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima M, Destro F, Cantone N, Maffi M, Ruggeri G, Dòmini R. Long-term follow-up after esophageal replacement in children: 45-Year single-center experience. J Pediatr Surg. 2015;50:1457–61. doi: 10.1016/j.jpedsurg.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 30.Borgnon J, Tounian P, Auber F, Larroquet M, Boeris Clemen F, Girardet JP, et al. Esophageal replacement in children by an isoperistaltic gastric tube: A 12-year experience. Pediatr Surg Int. 2004;20:829–33. doi: 10.1007/s00383-004-1190-y. [DOI] [PubMed] [Google Scholar]
- 31.Collard JM, Tinton N, Malaise J, Romagnoli R, Otte JB, Kestens PJ. Esophageal replacement: Gastric tube or whole stomach? Ann Thorac Surg. 1995;60:261–6. doi: 10.1016/0003-4975(95)00411-d. [DOI] [PubMed] [Google Scholar]
- 32.Gaur P, Blackmon SH. Jejunal graft conduits after esophagectomy. J Thorac Dis. 2014;6(Suppl 3):S333–40. doi: 10.3978/j.issn.2072-1439.2014.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteves E, Sousa-Filho HB, Watanabe S, Silva JF, Neto EC, da Costa AL. Laparoscopically assisted esophagectomy and colon interposition for esophageal replacement in children: Preliminary results of a novel technique. J Pediatr Surg. 2010;45:1053–60. doi: 10.1016/j.jpedsurg.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Maghsoudlou P, Eaton S, De Coppi P. Tissue engineering of the esophagus. Semin Pediatr Surg. 2014;23:127–34. doi: 10.1053/j.sempedsurg.2014.04.003. [DOI] [PubMed] [Google Scholar]