High prevalence of hypovitaminosis D of patients with autoimmune rheumatic diseases in China (original) (raw)
Abstract
We aimed to determine the prevalence of hypovitaminosis D in patients with autoimmune rheumatic diseases (ARDs) in China and its association with demographic characteristics of the patients. We recruited 384 patients in this cross-sectional study including 121 cases of systemic lupus erythematosus (SLE), 131 rheumatoid arthritis (RA), 102 spondyloarthritis (SpA) and 30 other ARDs. For each patient, demographic information was collected and serum concentration of 25OHD3 was measured by electrochemiluminescence immunoassay (ECLIA). The multivariate logistic regression model was used to investigate the association between vitamin D deficiency and patient characteristics. The mean serum vitamin D level of the 384 patients was 18.91 (8.12) ng/mL, and the median age was 37.33 (12.01) yrs. Among these patients, 222 (57.81%) and 127 (33.07%) were found to be vitamin D deficiency and insufficiency, respectively. From the disease perspective, the percentages of insufficiency and deficiency were as follow: 97.52% and 84.30% in SLE, 87.02% and 48.85% in RA, 88.24% and 40.20% in SpA, 90.89% and 57.81% in other ARDs patients. The causative factors for vitamin D deficiency included SLE per se (OR 12.54, P < 0.001) and high body mass index (BMI) (OR 1.88, P < 0.001). However, the seniors were less likely to have vitamin D deficiency (OR 0.95, P = 0.005). No correlation was disclosed between vitamin D deficiency and gender or disease duration. Hypovitaminosis D is highly prevalent among autoimmune rheumatic diseases population in China. The SLE per se and the obesity are the risk factors for vitamin D deficiency. Clinicians are advised to supplement vitamin D in these patients.
Keywords: Autoimmune rheumatic diseases, hypovitaminosis D, prevalence, vitamin D, 25OHD3
Introduction
Autoimmune rheumatic diseases (ARDs) form a heterogeneous group of disorders characterized by dysregulation of the immune system causing multiple organ injury. To date, more than one hundred kinds of ARDs have been identified including rheumatoid arthritis (RA), systematic lupus erythematosus (SLE), spondyloarthritis (SpA), Sjogren’s syndrome (SS), vasculitis, idiopathic inflammatory myositis, scleroderma, Behcet’s disease, etc. It is estimated that 355 millions of patients all over the world suffer from different ARDs. Complicate interplay between hereditary and environmental factors contributes to the pathogenesis of ARDs. The morbidity is high in patients with ARDs. In China, the morbidity of RA is 0.3%-0.5%, SpA is 0.2%-0.4%, SLE is 30-70/100,000. The risk of muscle weakness, osteopenia, osteoporosis and fractures is quite high in patients with ARDs, which affect their quality of life seriously.
In the past decades,vitamin D has raised interest due to its physiological functions in maintaining calcium and phosphorus homeostasis, and bone health. Recent years have witnessed a gradual recognition of the role of vitamin D in the homeostasis of the immune system along with the discovery of vitamin D receptors in almost all major immune cells, including monocytes, macrophages, dendritic cells, natural killer cells, and B and T lymphocytes [1]. It has been estimated that one billion people worldwide have vitamin D deficiency or insufficiency [2], and it has already become a largely unrecognized global epidemic. Vitamin D deficiency can affect patients of all ages and are associated with factors such as time of a day, season of the year, ethnicity, region and latitude [3,4]. Several studies showed that 40-100% of U.S. and European seniors living in the community (not nursing homes) are deficient in Vitamin D [5-7]. A large-scale study in India found that vitamin D deficiency prevailed all over the country, with a high prevalence of 70%-100% in the general population [8].
The fact that vitamin D has been implicated as a factor in several different autoimmune diseases, including SLE and RA, suggests that vitamin D might be one of the environmental factors that among others normally participates in the control of self tolerance [9]. It has been suggested that patients with SLE have decreased vitamin D level. A number of recent studies in SLE patients have reported the prevalence of vitamin D insufficiency and deficiency to be between 38-96% [10-15], 8-30% respectively [10-16]. The prevalence of hypovitaminosis D of patients from different regions and ethnicities was significantly different. This phenomenon was similar in RA patients [17-19]. Henan Province is the largest province in China with a population of about 100 millions, and naturally, there are a large number of ARDs in our province. Unfortunately, clinicians and scientists in Henan have not taken full use of such advantages and resources to obtain epidemiological data on the status of vitamin D deficiency. Therefore, the study of vitamin D levels in patients with ARDs in Henan province is of great significance and may impact our clinical practice.
Our current study aimed to determine the prevalence of vitamin D deficiency and the potential relationship between 25OHD3 levels and demographic characteristics of different ARDs.
Materials and methods
Study design
This study was cross-sectional in nature and performed at the Department of Rheumatology, The First Affiliated Hospital of Zhengzhou University after the approval of the study protocol by the ethics committee. All the participants provided the informed consent forms. Serum samples were collected for the measurement of 25OHD3.
Patients
Three hundred eighty-four patients aged 18-75 years were enrolled in this study from June to September of 2015. The patient enrollment was based on the following guidelines: the 1997 American College of Rheumatology (ACR) classification criteria for SLE [20], 1987 ACR classification criteria for RA [21], 1984 New York criteria [22] or 2009 Assessment of SpondyloArthritis international Society (ASAS) criteria [23] for the diagnosis of SpA. Patients were excluded if they met any of the following criteria: 1) malnutrition; 2) coexistence with other chronic diseases such as hypertension, diabetes mellitus, hypo- or hyperparathyroidism, medical or surgical disorders affecting vitamin D metabolism (gastric surgery, chronic kidney disease, chronic liver disease, intestinal malabsorption, systemic infection, cancers etc.). 3) pregnancy or lactation; 4) vitamin D supplementation of more than 800 IU every day during past 3 months.
All patients were from Henan Province in central China between 31°23’-36°22’ of Northern Latitude, where the average duration of sunlight in this region is about 8-10 h per day. The temperatures range from 20 to 38°C between June and September, which attenuates the seasonal variation in 25OHD3.
Variables studied
Baseline characteristics included age, gender, ethnicity, the kind of disease and the duration (months) since the diagnosis, and the use of calcium and vitamin D supplements through direct patient interview. Height and weight were measured to calculate body mass index (BMI), and categorized as < 18.4, 18.5 to 24.99, 25 to 29.99, and ≥ 30 kg/m2, which are the cut-off points for underweight, normal, overweight, and obesity. All the information was recorded in a structured case record form.
Measurement of serum 25OHD3 levels
After an overnight fasting, a blood sample was taken. Serum 25OHD3 levels of all patients were measured by electrochemiluminescence immunoassay (ECLIA) on an automated analyzer (ELECSYS-2010), using kits supplied by Roche Diagnostics (Germany). This technique provides a broad measuring range and high precision at the low end of detection to aid the assessment of deficient patients. All the blood samples were collected between 8:00 AM and 9:00 AM to avoid any circadian variation.
Definition of vitamin D levels
Vitamin D deficiency was defined based on the levels 25OHD3: deficiency < 20 ng/mL; insufficiency: 20 to 30 ng/mL, and normal level as above 30 ng/mL. Further, severity of vitamin D deficiency was grouped as follows: ≤ 4 ng/mL profound deficiency; 5-8 ng/mL severe deficiency; 9-12 ng/mL moderately severe deficiency; 13-16 ng/mL moderate deficiency; and 17-20 ng/mL marginal deficiency [24-26].
Statistical analysis
Descriptive data for all variables included in the study were reported as mean and standard deviation (SD), numbers and percentage (%), and median and interquartile range (IQR). The association between vitamin D insufficiency [defined as a serum level of 25OHD3 < 30 ng/mL] and predictors was assessed by fitting binary logistic regression univariate and multivariate models. The confidence interval (CI) for the prevalence of vitamin D insufficiency was calculated using the binomial test. All analyses were two-tailed, and _p_-values were considered significant if < 0.05. All statistical tests were performed by using SPSS 17.00 version (SPSS, Inc., Chicago, IL).
Results
Baseline characteristics
Three hundred eighty-four patients were requested to enter the study. All the patients were Han nationality from Henan Province. These participants included 121 SLE, 131 RA, 102 SpA and 30 other ARDs (such as mixed connective tissue disease, Sjogren’s syndrome, adult onset still’s disease, vasculitis, myositis). Mean (SD) age at the time of the study was 37.33 (12.01) yrs (range, 18-71 yrs), with a median disease duration of 27 (8-52) months. Baseline characteristics including age, gender, BMI, disease duration were shown in Table 1.
Table 1.
Demographic characteristics of patients with different autoimmune rheumatic diseases (n = 384)
| Characteristics | SLE | RA | SpA | Others | Total |
|---|---|---|---|---|---|
| N (%) | 121 (31.51) | 131 (34.12) | 102 (26.56) | 30 (7.81) | 384 (100) |
| Age (Yr) | |||||
| Mean (SD) | 32.64 (11.17) | 46.20 (9.79) | 32.11 (8.87) | 35.33 (11.71) | 37.33 (12.01) |
| Median (IQR) | 29 (24-40) | 47 (41-52) | 31.5 (25-40) | 33.5 (25-44.25) | 37 (27-46) |
| Gender (F/M) | 111/10 | 96/35 | 33/69 | 25/5 | 265/119 |
| BMI (Kg/m2) | 22.21 (2.59) | 24.20 (1.62) | 22.62 (1.38) | 23.41 (1.83) | 22.99 (2.14) |
| Disease duration (months) | |||||
| Mean (SD) | 47.19 (49.54) | 37.88 (42.08) | 42.47 (35.26) | 20.17 (19.20) | 38.95 (41.92) |
| Median (IQR) | 25 (3-63) | 27(8-50) | 33.5 (20.75-56.5) | 13 (6-28.5) | 27 (8-52) |
Vitamin D levels at presentation
Mean serum vitamin D level of these patients was 18.91 (8.12) ng/mL. Among the study cohort only 35 (9.11%) patients had normal vitamin D levels, 349 (90.89%) patients had lower than normal serum level of vitamin. Among these, 222 (57.81%) were deficient and 127 (33.07%) insufficient. Among the 222 patients with vitamin D deficiency, 85 (22.14%) patients had marginal deficiency, 71 (18.49%) moderate, 29 (7.55%) moderately severe, 22 (5.73%) severe, and 15 (3.91%) profound deficiency. Of note, 15 cases of profound deficiency were all SLE patients. Further, among 22 cases of moderately severe deficiency, 18 with SLE patients, 4 with RA. The distribution of serum 25OHD3 level quartiles in different ARDs was shown in Figure 1. The mean levels of serum 25OHD3 of these patients were as follows: SLE 13.48 (7.76), RA 20.96 (7.70), SpA 21.92 (6.24), and Others 21.55 (6.19). Prevalence and severity of vitamin D deficiency in different ARDs was shown in Table 2.
Figure 1.
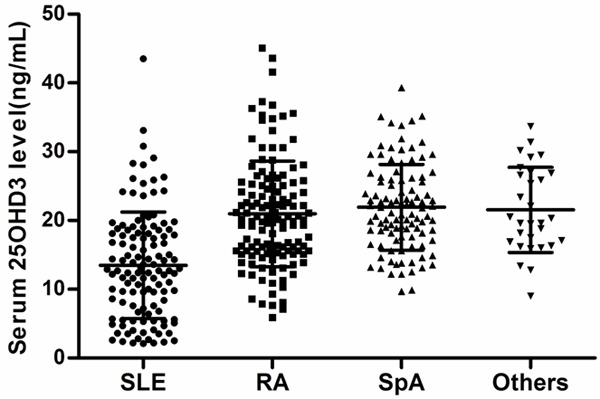
Distribution of serum 25OHD3 level quartiles in relation to different rheumatic diseases. Data are presented as Scatter plot (SLE, n = 121; RA, n = 131; SpA, n = 102; Others, n = 30).
Table 2.
Prevalence of vitamin D deficiency in different ARDs according to various categories of deficiency
| 25OHD3 (ng/mL) | Number of patients | |||||
|---|---|---|---|---|---|---|
| SLE (121) | RA (131) | SpA (102) | Others (30) | Total (384) | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Normal | ≥ 30 | 3 (2.48) | 17 (12.98) | 12 (11.76) | 3 (10) | 35 (9.11) |
| Below Normal | < 30 | 118 (97.52) | 114 (87.02) | 90 (88.24) | 27 (90) | 349 (90.89) |
| Insufficient | 20-30 | 16 (13.22) | 50 (38.17) | 49 (48.04) | 12 (40) | 127 (33.07) |
| Deficient | < 20 | 102 (84.30) | 64 (48.85) | 41 (40.20) | 15 (50) | 222 (57.81) |
| Marginal Deficiency | > 16-20 | 25 (20.66) | 27 (20.61) | 23 (22.55) | 10 (33.33) | 85 (22.14) |
| Moderate Deficiency | > 12-16 | 25 (20.66) | 26 (19.85) | 16 (15.69) | 4 (13.33) | 71 (18.49) |
| Moderately Severe Deficiency | > 8-12 | 19 (15.70) | 7 (5.34) | 2 (1.96) | 1 (3.33) | 29 (7.55) |
| Severe Deficiency | > 4-8 | 18 (14.88) | 4 (3.05) | 0 (0) | 0 (0) | 22 (5.73) |
| Profound Deficiency | ≤ 4 | 15 (12.40) | 0 (0) | 0 (0) | 0 (0) | 15 (3.91) |
Variables associated with vitamin D deficiency
In this study, 34.64% of population aged between 18-30 years, whose prevalence of vitamin D deficiency was 66.92%, while the prevalence of vitamin D deficiency of the group whose age above 60 years was 80%. We also found that the deficiency was more prevalent in female (64.91%) than male (42.02%). However, the insufficiency was more prevalent in the male population (46.22%) than in female (27.17%). Female tended to have a higher risk of vitamin D deficiency. We also found that the deficiency was more prevalent in the obesity population (86.11%) and SLE patients (84.30%) compared to RA (48.85%) or SpA patients (40.20%) (Table 3).
Table 3.
Serum 25OHD3 levels and deficiency status according to selected patients demographic characteristics
| Characteristics | Category | N (%) | 25OHD3 level | Vitamin D deficiency | Vitamin D insufficiency | Normal vitamin D |
|---|---|---|---|---|---|---|
| Mean (SD) | n (%) | n (%) | n (%) | |||
| Age (Yr) | 18-30 | 133 (34.64) | 16.93 (7.82) | 89 (66.92) | 35 (26.32) | 9 (6.76) |
| 31-40 | 91 (23.70) | 19.81 (8.14) | 43 (47.25) | 41 (45.06) | 7 (7.69) | |
| 41-50 | 101 (26.30) | 21.37 (8.51) | 53 (52.48) | 31 (30.69) | 17 (16.83) | |
| 51-60 | 44 (11.46) | 18.73 (7.08) | 25 (56.82) | 17 (38.64) | 2 (4.54) | |
| > 60 | 15 (3.91) | 14.81 (4.89) | 12 (80) | 3 (20) | 0 (0) | |
| Gender | Male | 119 (30.99) | 20.97 (7.04) | 50 (42.02) | 55 (46.22) | 14 (11.76) |
| Female | 265 (69.01) | 17.98 (8.41) | 172 (64.91) | 72 (27.17) | 21 (7.92) | |
| BMI | < 18.49 | 9 (2.34) | 13.56 (7.36) | 8 (88.89) | 1 (11.11) | 0 (0) |
| 18.5-24.99 | 303 (78.91) | 20.12 (8.13) | 152 (50.17) | 117 (38.61) | 34 (11.22) | |
| 25-29.99 | 72 (18.75) | 14.46 (6.16) | 62 (86.11) | 9 (12.5) | 1 (1.39) | |
| Disease | SLE | 121 (31.51) | 13.48 (7.76) | 102 (84.30) | 16 (13.22) | 3 (2.48) |
| RA | 131 (34.15) | 20.96 (7.70) | 64 (48.85) | 50 (38.17) | 17 (12.98) | |
| SpA | 102 (26.56) | 21.92 (6.24) | 41 (40.20) | 49 (48.04) | 12 (11.76) | |
| Others | 30 (7.81) | 21.55 (6.19) | 15 (50) | 12 (40) | 3 (10) | |
| Total | 384 (100) | 18.91 (8.12) | 222 (57.81) | 127 (33.07) | 35 (9.11) |
In the logistic regression analysis, the strongest predictor of vitamin D deficiency was SLE pre se, with an adjusted OR of 12.54 (95% CI 3.10~50.76, P < 0.001). Another significant predictor of vitamin D deficiency was high BMI, with an adjusted OR of 1.88 (95% CI 1.44~2.46, P < 0.001). Interestingly, our data suggest that aging prevents vitamin D deficiency as evidenced by lower prevalence in the seniors with an adjusted OR of 0.95 (95% CI 0.91~0.98, P = 0.005).
No correlation was demonstrated between vitamin D deficiency and gender or disease duration. Indeed, neither of these two variables was statistically significant predictors of vitamin D levels in the multiple regression model. Accordantly, no difference in gender or disease duration was seen whether patients had critically low vitamin D levels or not.
Discussion
This is the first study to investigate the prevalence of hypovitaminosis D in patients with ARDs residing in Central China, more specifically, Henan Province. Our study demonstrated a large number of patients (90.89%) with lower levels of serum vitamin D. Furthermore, the prevalence of vitamin D insufficiency and deficiency were 84.30% and 13.22% in SLE, 48.85% and 38.17% in R, 40.20% and 48.04% in SpA, respectively. The result of SLE patients was higher than many other studies conducted in other countries or regions. A lower prevalence of low 25(OH)D levels 73.30% [60% insufficient (< 30 ng/ml), 13.30% deficient (< 10 ng/ml)] was found in an Egyptian study [16]. A study conducted in Chinese lupus reported that 96% patients had vitamin D insufficiency [25(OH)D3 < 30 ng/ml] and 27% patients had vitamin D deficiency (< 15 ng/ml) [14]. The prevalence of low 25(OH)D status [98.8% (9.1% insufficient, 89.7% deficient)] detected in a recent study by Damanhouri [13] done at King Abdul Aziz University Hospital in Jedah, was similar to ours. Several studies have indicated that vitamin D deficiency in patients with RA was also common. Kerr G S et al. [19] reported that in a predominantly elderly, 850 male RA population, the prevalence of 25OHD insufficiency and deficiency were 84% and 43%, respectively. Interestingly, eight case-control studies that compared vitamin D levels in a total of 555 AS patients and 557 healthy controls showed that an average 25-hydroxyvitamin D level was (22.8±14.1) ng/mL in AS patients and (26.6±12.5) ng/mL in healthy controls [27].
The only significant predictors of vitamin D deficiency that we found were the SLE per se and the obesity. Previous studies showed that patients with SLE had lower levels of vitamin D than patients with RA or other ARDs, Sheng and colleagues [28] revealed a significantly lower level of 25(OH)D in SLE patients compared to RA patients and normal controls in a study performed in Shanghai. Consistently, a study done in Copenhagen, Denmark demonstrated a significantly lower 25(OH)D levels in SLE patients compared to osteoarthritis and normal controls. Low 25(OH)D levels among SLE patients may be due to the fact that patients with SLE are frequently photosensitive and use very high ultraviolet photoprotection [12,13,29]. The association between the obesity and vitamin D deficiency in SLE patients has been reported as well [10] . Somewhat surprisingly, we first reported a lower prevalence of vitamin D deficiency in the elderly, which was consistent with a study of SLE patients reported by Ruiz-Irastorza et al. [12]. This phenomenon might be due to the younger age of SLE patients, and their lower levels of 25OHD3 in our study. However, we did not find any statistical association between vitamin D insufficiency or deficiency and gender or disease duration.
Vitamin D is a hormone, consisting of fat soluble compounds of vegetable origin (vitamin D2 or ergocalciferol) or animal origin (vitamin D3 or cholecalciferol). The major source of vitamin D is endogenous production through the exposure to UV radiation, whereas only a small amount is obtained from the diet. Vitamin D supplementation is being considered as an adjuvant therapy for relieving musculoskeletal pain, improving muscle strength [30], reducing falls [30] and fractures [31]. The supplementation of Vitamin D could also reduce the synthesis of inflammatory cytokines and increase the synthesis of anti-inflammatory cytokines. For patients with SLE, vitamin D supplementation on disease activity was studied and the results revealed a statistically significant decrease in the mean level of the physician global assessment score of 0.04, in the mean SELENA-SLEDAI of 0.22, and a 2% decrease in urine protein: creatinine ratio with a 20 ng/mL increase in vitamin D levels [11]. Recent studies showed that increased intake of vitamin D lowered the risk of RA, while decreased level of vitamin D was associated with higher disease activity [17,32].
The limitations of this study include patients are representative of ARDs in Central China presenting in The first affiliated hospital of Zhengzhou university, mainly those with SLE, RA, SpA, which limits its global interpretation. We have not selected patients according to the incidence of the disease, and the distribution of diseases in different age groups is not uniform. Therefore, our data only reflect the vitamin D status of these three kinds of ARDs. It was not possible to assess good quality data on sun exposure and vitamin D dietary intake diet in our study population. Anyway, this is real world population based data reflecting the reality of clinical practice.
In conclusion, low vitamin D status is prevalent in Chinese autoimmune rheumatic diseases patients.SLE patients and the obesity tended to have a lower status. Future studies looking at a potential role of vitamin D in the pathophysiology and treatment of ARDs are necessary and further intervention studies are required to confirm a direct relationship between vitamin D status and SLE, RA or SpA disease activity. One such study to evaluate vitamin D status and disease activity in SLE patients is in progress by us.
Acknowledgements
This study was supported by grants from Medical Science & Technology Project of Henan Province 201204019, and key projects of Medical Science & Technology Project of Henan Province 201502008.
Disclosure of conflict of interest
None.
References
- 1.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitaminD3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 6.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. The prevalence of vitamin D inadequacy among women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 7.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 8.Vaishya R, Vijay V, Agarwal AK, Jahangir J. Resurgence of vitamin D: Old wine in new bottle. J Clin Orthop Trauma. 2015;6:173–83. doi: 10.1016/j.jcot.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new etiological and therapeutical considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz-Ortego J, Torrente-Segarra V, Prieto-Alhambra D, Salman-Monte TC, Carbonell-Abello J. Prevalence and predictors of vitamin D deficiency in non-supplemented women with systemic lupus erythematosus in the Mediterranean region: a cohort study. Scand J Rheumatol. 2012;41:472–5. doi: 10.3109/03009742.2012.697189. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Bello KJ, Fang H, Magder LS. Vitamin D in systemic lupus erythematosus: modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis Rheum. 2013;65:1865–71. doi: 10.1002/art.37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology. 2008;47:920–3. doi: 10.1093/rheumatology/ken121. [DOI] [PubMed] [Google Scholar]
- 13.Damanhouri L. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi Med J. 2009;30:1291–5. [PubMed] [Google Scholar]
- 14.Mok CC, Birmingham DJ, Leung HW, Hebert LA, Song H, Rovin BH. Vitamin D levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology. 2012;51:644–52. doi: 10.1093/rheumatology/ker212. [DOI] [PubMed] [Google Scholar]
- 15.Souto M, Coelho A, Guo C, Mendonça L, Argolo S, Papi J, Farias M. Vitamin D insufficiency in Brazilian patients with SLE: prevalence, associated factors, and relationship with activity. Lupus. 2011;20:1019–26. doi: 10.1177/0961203311401457. [DOI] [PubMed] [Google Scholar]
- 16.Hamza RT, Awwad KS, Ali MK, Hamed AI. Reduced serum concentrations of 25-hydroxy vitamin D in Egyptian patients with systemic lupus erythematosus:relation to disease activity. Med Sci Monit. 2011;17:CR711–8. doi: 10.12659/MSM.882131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 18.Leventis P, Patel S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology. 2008;47:1617–21. doi: 10.1093/rheumatology/ken296. [DOI] [PubMed] [Google Scholar]
- 19.Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, Thiele GM, Johnson D, Mikuls TR. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38:53–9. doi: 10.3899/jrheum.100516. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 23.Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, Dougados M, Hermann KG, Landewé R, Maksymowych W, van der Heijde D. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68:ii1–44. doi: 10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 24.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 25.Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D deficient subjects. N Engl J Med. 1982;306:722–5. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 26.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 27.Pokhai GG, Bandagi S, Abrudescu A. Vitamin D levels in ankylosing spondylitis: Does deficiency correspond to disease activity? Rev Bras Reumatol. 2014;54:330–4. doi: 10.1016/j.rbr.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxy vitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 29.Müller K, Kriegbaum NJ, Baslund B, Sørensen OH, Thymann M, Bentzen K. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D in patients with systemic lupus erythematosus. Clin Rheumatol. 1995;14:397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 31.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 32.Oelzner P, Müller A, Deschner F, Hüller M, Abendroth K, Hein G, Stein G. Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int. 1998;62:193–8. doi: 10.1007/s002239900416. [DOI] [PubMed] [Google Scholar]