Long-Term Neurotoxic Effects of Early Life Exposure to Tetrachloroethylene-contaminated Drinking Water (original) (raw)
. Author manuscript; available in PMC: 2017 Jan 1.
Published in final edited form as: Ann Glob Health. 2016 Jan-Feb;82(1):169–179. doi: 10.1016/j.aogh.2016.01.013
Abstract
Background
Tetrachloroethene (PCE) is a common environmental and occupational contaminant and an acknowledged neurotoxicant. From 1968 through 1983 widespread contamination of public drinking water supplies with PCE occurred in the Cape Cod region of Massachusetts. The source of the contamination was a vinyl liner applied to the inner surface of water distribution pipes.
Objectives
A retrospective cohort study (“the Cape Cod Health Study”) was undertaken to examine possible health consequences of early life exposure to PCE-contaminated drinking water. This review describes the study methods and findings regarding the impact of prenatal and childhood exposure on neurological outcomes during early adulthood, including vision, neuropsychological functioning, brain structure, risky behaviors, and mental illness. The review also describes the strengths and challenges of conducting population-based epidemiological research in this unique setting.
Methods
Subjects were identified by cross-matching birth certificate and water system data. Information on health outcomes and confounding variables was collected from self-administered surveys (N= 1,689), neuropsychological tests (N=63), vision exam (N=63), and magnetic resonance imaging (N=42). Early life exposure to PCE was estimated using a leaching and transport model. The data analysis compared the occurrence of each health outcome among subjects with prenatal and early childhood PCE exposure to unexposed subjects while considering the impact of confounding variables.
Results
The study found evidence that early life exposure to PCE-contaminated drinking water has long-term neurotoxic effects. The strongest associations were seen with illicit drug use, bipolar disorder, and post-traumatic stress disorder. Key strengths of the study were availability of historical data on affected water systems, a relatively high exposure prevalence and wide range of exposure levels, and little confounding. Challenges arose mainly from the historical nature of the exposure assessments.
Conclusions
The Cape Cod Health Study demonstrates how scientists can take advantage of unique “natural experiments” to learn about the health effects of environmental pollution. This body of work has improved our understanding of the long-term health effects of early life exposure to this common environmental contaminant and will help risk assessors and policy makers ensure that U.S. drinking water supplies are safe for vulnerable populations.
Keywords: drinking water, tetrachloroethylene, vulnerable populations, brain
Introduction
Tetrachloroethene (also called tetrachloroethylene, perchloroethylene, perc, or PCE) is a widely used solvent in dry cleaning, textile processing and metal degreasing1. In the United States (U.S.), approximately 1.5 million people are occupationally exposed each year2. Most use is in small, inadequately controlled work settings such as dry cleaning, automobile repair, and machine shops, making PCE a common drinking water contaminant from improper management and disposal1. U.S. surveys of drinking water contaminants have found it in 11% of tested wells3 and 38% of surface water supplies4. Thus, it is not surprising that detectable levels of PCE in biological media were found in 77% of a general U.S. population sample5.
While the typical source for drinking contamination with PCE is improper disposal, an unusual scenario for widespread contamination of drinking water supplies occurred in the Cape Cod region of Massachusetts. The interior surface of water mains produced between 1968 and 1980 contained a plastic liner intended to eliminate taste and odor problems plaguing asbestos-cement pipes carrying the very soft water in the Cape Cod region. The liner was sprayed as a slurry composed of vinyl resin (Piccotex,™ Johns-Manville Corporation, Denver, CO) dissolved in PCE. Because PCE is volatile it was assumed it would evaporate completely during the drying process6. However, water samples taken in 1980 by the Massachusetts Department of Environment Protection (DEP) revealed that a substantial amount of PCE had remained in the liner and was leaching into the public drinking water supplies6.
A survey of local water departments indicated that approximately 660 miles of vinyl-lined asbestos-cement pipes (VL/AC) had been installed in 91 Massachusetts cities and towns7. The largest portion was installed in the Cape Cod region due to substantial residential development. PCE levels found in water samples from affected pipes on Cape Cod ranged from 1.5 to 7,750 µg/L (ppb), depending on the rate of water flow6.
Digging up and replacing the VL/AC pipes was prohibitively expensive, so a program of flushing and bleeding the water distribution system was instituted in the most problematic areas. The objective was to reduce PCE levels below 40 µg/L, the suggested no response level at the time6. However, by the time these risk reduction measures were implemented, tens of thousands of residents had been drinking PCE-contaminated water for up to 15 years. Monitoring now ensures that levels remain below the current maximum contaminant level of 5 µg/L8.
A few years after the PCE contamination was discovered, the Massachusetts Department of Public Health reported elevations in cancer incidence and mortality in the Cape Cod region9. In response to concern about the possible relationship between the elevated cancer rates and pollution in the region, we undertook a case-control study to evaluate the carcinogenic potential of population exposure to air and water pollution, including PCE-contaminated drinking water10, 11.
After completing the cancer case-control studies, we initiated a new round of data collection for a retrospective birth cohort study (“Cape Cod Health Study”) to examine a more comprehensive array of possible health consequences of PCE exposure, particularly exposure early in life. The Cape Cod Health Study initially assessed the short-term impact of prenatal exposure to PCE-contaminated drinking water on reproductive outcomes, including low birth weight12, prematurity12, miscarriage13, stillbirth14, and congenital anomalies15. More recently, we extended the study to examine the long-term impact of both prenatal and childhood exposure on neurological outcomes, including vision16, neuropsychological functioning17, brain structure18, risky behaviors19, and mental illness20. There is a dearth of information on long-term neurotoxic effects of early life exposure despite well-established short-term effects among adults1. The purpose of this review is to describe the methods and findings from the Cape Cod Health Study as well as the strengths and challenges of conducting population-based epidemiological research in this unique setting.
Summary of Methods used in the Cape Cod Health Study
Identification of subjects
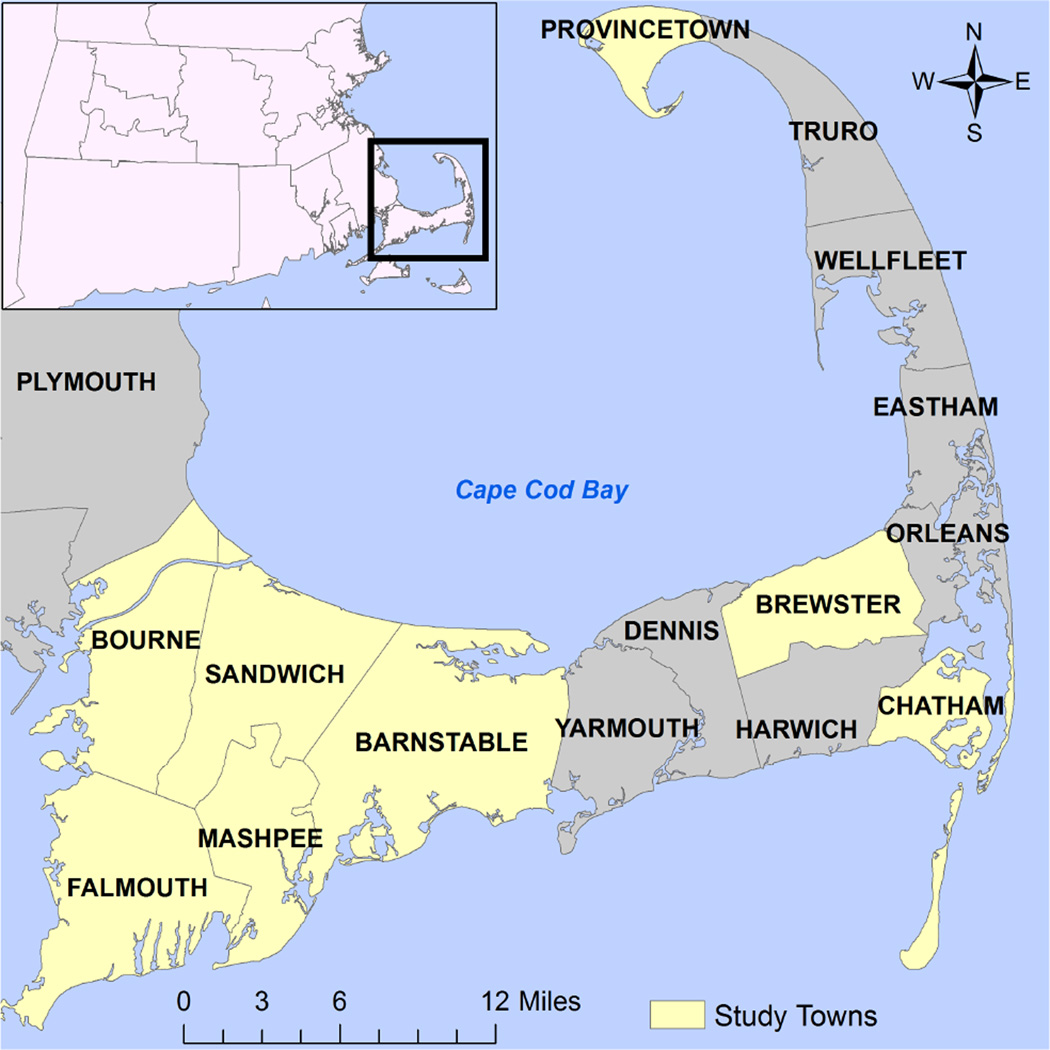
Subjects born between 1969 and 1983 to women who lived in one of eight Cape Cod towns with some VL/AC water pipes (Figure 1) were eligible for enrollment in the birth cohort19. Subjects were identified as likely exposed or likely unexposed by cross matching maternal address on birth certificates with information from local water companies on the location and installation year of the VL/AC pipes. We tentatively designated subjects as “exposed” if their birth residence was either directly adjacent to a VL/AC pipe or adjacent to a pipe connected to a VL/AC pipe and the only possible water flow to the residence was through the VL/AC pipe. Subjects initially designated as “unexposed” were randomly selected from the remaining resident births during this period and frequency matched to “exposed” subjects on month and year of birth.
Figure 1.
Cape Cod Region of Massachusetts (USA) with highlighted study towns.
In order to increase the sample size and assess childhood exposure, we then identified any older siblings of “exposed” and “unexposed” subjects who were born in Massachusetts during 1969–1983 (N=1,202). All older siblings were initially judged to be “unexposed” at birth because they were born while the family resided at an apparently unaffected residence. However, the original designation of all subjects was considered provisional until more extensive exposure assessments, as described below, were completed.
Follow-Up and Data Collection
Starting with the address on the birth certificate, we traced _s_ubjects to obtain their current addresses and telephone numbers using the Internet and other resources19. Letters were sent to all successfully traced subjects in 2007–2008 describing the general purpose of the study and requesting that they complete a survey about risky behaviors such as alcoholic beverage consumption and illicit drug use; health outcomes including mental illnesses; confounding variables; and their residential history, from birth through 1990, including the street address and calendar years of residence for all Cape Cod addresses. The survey also gathered information on a subject’s knowledge of the PCE water pollution and self-assessments of PCE exposure to evaluate the possibility of recall bias.
A total of 5,040 subjects were selected for the study, including 1,910 “exposed” and 1,928 “unexposed” subjects and 1,202 older “unexposed” siblings. About 6.6% could not be located, 45.5% were located but never responded to numerous contact attempts, 3.7% refused to participate and 2.2% were deceased, leaving 1,689 participants who returned the questionnaire. Characteristics of participants and non-participants (including their initial PCE exposure status, age, race, birth weight and gestational duration) were quite similar, suggesting that non-response did not result in selection bias19.
Eligible subjects who completed the survey were also invited to participate in a clinical study comprised of neuropsychological tests to evaluate visuospatial abilities, attention and executive function, short-term memory, motor skills, academic achievement, omnibus intelligence and mood; vision exams to assess color discrimination and contrast sensitivity; and magnetic resonance imaging (MRI) to measure white and gray matter volumes and white matter hypointensities16–18. Prior studies among adults suggested that these outcomes might be affected by early life exposure to solvents21–25. Sixty-three participants completed the vision exams and neuropsychological tests and 42 underwent the MRI.
Geocoding Residential Addresses
Approximately 95% of reported residential addresses on Cape Cod were geocoded to a latitude and longitude (ArcGIS 8.1, ESRI, Redlands, CA) by a team member who was blind to the exposure and outcome status of the participant19. When possible, addresses were geocoded to a specific parcel (i.e., plot of land). Addresses that could not be geocoded to a specific parcel were geocoded to the closest parcel by street number, the middle of the streets less than a mile long, or the intersection with the nearest cross-street for streets a mile or longer.
PCE Exposure Assessment
An initial exposure status was provisionally assigned to each subject by examining maps of the pipe distribution network surrounding the birth residence. We then used a leaching and transport model to determine the final exposure designation based on an estimated relative mass of PCE delivered to each residence from the prenatal period through the age of five years19. Changes in the water distribution systems in the 1990s prevented assessment of PCE exposure beyond age five.
The leaching model was developed for our cancer studies by Webler and Brown11, 26. It approximates the amount of PCE entering the drinking water using the starting quantity of PCE in the liner, the age of the pipe, and the leaching rate of PCE from the liner into the water. The pipe's initial stock of PCE was estimated using the pipe’s diameter, length and average thickness. Laboratory experiments by Demond suggested that the leaching process followed a simple exponential decay process with rate constant of 2.25 years6.
The PCE delivered to a household depends not only upon how much is leached, but where it is subsequently transported by the water distribution system. The transport algorithm requires an evaluation of water flow rate and direction, which are functions of the pipe geometry and the number of water users. We incorporated the Webler and Brown leaching algorithm into the open source code of EPANET water distribution modeling software to estimate PCE transport throughout a town’s entire distribution system. EPANET, which was developed by the U.S. EPA to help utilities devise water quality monitoring programs27, has been applied in several epidemiological studies assessing health effects of drinking water contaminants28,29.
We used maps of subject residences and the distribution systems to create a diagram representing the water sources, pipe characteristics, and nodes, denoting points of water use along the pipe. Information on the locations, installation dates and diameters of all VL/AC water pipes was obtained from local water departments and the Massachusetts DEP. Each subject residence was assigned to the closest node on the distribution system. We assumed that all land parcels represented water users, all water users in the network drew the same amount of water, and water sources did not change over the study period. These assumptions were supported by observations that the study area was primarily comprised of residences, and the distribution system changed little between the 1960s and 1980s, except for adding water sources to accommodate population growth.
The combined leaching and transport model simulated the flow of water through each town’s distribution system to estimate the annual mass of PCE distributed to each node and all subject residences connected to the node. We assumed that residences served by a private well or located in towns with no VL/AC pipes had no PCE exposure at that address. We considered this reasonable because available records indicated little or no PCE contamination of these water sources.
Exposure during the prenatal period was estimated by multiplying the annual mass of PCE that entered the subject’s residence during their birth year by 9/12. We estimated exposure during early childhood by summing the estimated mass of PCE that entered their residences from the month and year following birth through the month and year of the fifth birthday. Simple proportions were used to estimate exposure during partial years. Poor recall of bottle water use and bathing practices during pregnancy and childhood precluded incorporating these behavioral data into the exposure calculations19. These factors were also shown to have little effect on the exposure distribution in our prior cancer study30.
Data Analysis
The analysis compared the occurrence of each outcome among subjects with prenatal and early childhood PCE exposure to unexposed subjects. We examined the impact of any PCE exposure and, whenever possible, we assessed dose-response relationships according to exposure level. We were unable to study the separate impact of prenatal exposure alone because nearly all subjects with prenatal exposure also had childhood exposure. We were also unable to examine the impact of exposure only during childhood because there were too few subjects in this category to provide stable results.
Risk ratios (RR) were used to estimate the strength of the association between PCE exposure and the occurrence of dichotomous outcomes (e.g., any illicit drug use). Mean differences were used for assessing relationships with continuous outcomes (e.g., color confusion index). Ninety-five percent confidence intervals (95% CI) and _p_-values were used to measure the precision of the associations. Confounding variables considered for adjusted analyses included demographic characteristics, key risk factors for the behavioral and health outcomes under study, and non-drinking water sources of solvent exposure. Depending on the outcome, variables that changed the crude estimate by more than 10–30% were included in final multivariate models.
Results
Participant Characteristics
As shown in Table 1, the characteristics of modeled exposed and unexposed participants were comparable19. Cape Cod Health Study participants were predominantly female, white, college-educated, married or cohabitating, employed and were, on average, 29 years old when they completed the study questionnaires. Few participants had possible occupational exposure to solvents but many had potential exposure from hobbies.
Table 1.
Selected Characteristics of Participants in the Cape Cod Health Study
| Characteristic | Both Prenatal and EarlyChildhood Exposure(N=831) | Unexposed(N=547) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age* (n, mean, sd) | 831 | 29.2 (3.6) | 547 | 29.6 (3.8) |
| Gender | ||||
| Male | 331 | 39.8 | 216 | 39.5 |
| Female | 500 | 60.2 | 331 | 60.5 |
| % White race | 818 | 98.4 | 539 | 98.5 |
| Educational Level* | ||||
| High school graduate or less | 128 | 15.4 | 67 | 12.2 |
| Some college | 192 | 23.1 | 144 | 26.3 |
| Four year college grad or higher | 510 | 61.4 | 335 | 61.2 |
| Missing | 1 | 0.1 | 1 | 0.2 |
| Work Status* | ||||
| Employed | 719 | 86.5 | 487 | 89.0 |
| Not Employed | 92 | 11.1 | 54 | 9.9 |
| Missing | 20 | 2.4 | 6 | 1.1 |
| Marital status* | ||||
| Single | 272 | 32.7 | 157 | 28.7 |
| Married or cohabitating | 536 | 64.5 | 371 | 67.8 |
| Other | 19 | 2.3 | 12 | 2.2 |
| Missing | 4 | 0.5 | 7 | 1.3 |
| History of work-related solvent exposure* | ||||
| Yes | 123 | 14.8 | 71 | 13.0 |
| No | 687 | 82.7 | 461 | 84.3 |
| Missing | 21 | 2.5 | 15 | 2.7 |
| History hobby with solvent exposure* | ||||
| Yes | 700 | 84.2 | 462 | 84.5 |
| No | 124 | 14.9 | 79 | 14.4 |
| Missing | 7 | 0.8 | 6 | 1.1 |
| Mother’s age at subject’s birth (n, mean (sd)) | 831 | 27.2 (4.7) | 547 | 27.5 (4.4) |
| Mother’s educational level at subject’s birth | ||||
| High school graduate or less | 327 | 39.4 | 178 | 32.5 |
| Some college | 243 | 29.2 | 188 | 34.4 |
| Four year college grad or higher | 260 | 31.3 | 180 | 32.9 |
| Missing | 1 | 0.1 | 1 | 0.2 |
| Mother received prenatal care during subject’s gestation | ||||
| Yes | 794 | 95.5 | 520 | 95.1 |
| No | 4 | 0.5 | 0 | 0.0 |
| Missing | 33 | 4.0 | 27 | 4.9 |
PCE Exposure Levels
Cumulative PCE exposure levels from the prenatal period through age five years spanned several orders of magnitude, ranging from 11 milligrams to 4,668 grams19. Mean and median cumulative exposure levels were 142 and 34 grams, respectively, reflecting a long upper tail to the distribution. When we converted our modeled PCE exposure estimates to annual point concentrations, we estimated that PCE concentrations in water entering the study homes ranged from less than 1 ug/L to 5,197 ug/L, levels consistent with public water analyses during the study period6.
Exposure Self-Assessments
Comparison of each participant’s self-assessed exposure status to that derived from the modeled assessment revealed very few participants who knew their exposure status: 7% of participants considered exposed by the modeled assessment thought their drinking water was polluted by PCE, while 29% of exposed subjects thought their water was not polluted; 64% were uncertain20. Similarly, 31% of subjects considered unexposed by the modeled assessment thought that their drinking water was not polluted while 5% thought that their drinking water was polluted; 63% were uncertain.
Risky Behaviors
We defined risky behavior to be smoking, alcoholic beverage consumption and illicit drug use. The occurrence of risky behaviors was more common among participants who were highly exposed during gestation and early childhood19. In particular, increases in the risk of initiating smoking at a younger age (RR: 1.4, 95% CI: 0.8–2.5) and heavy smoking (RR: 1.3, 95% CI: 0.9–2.0), drinking frequently as a teenager (RR: 1.6, 95% CI: 1.1–2.3) and drinking heavily in the past 30 days (RR: 1.3, 95% CI: 1.0–1.7) were reported by participants in the highest exposure tertile.
Highly exposed participants also had increases in the risk of using more than one major drug of abuse as a teenager and as an adult (RR for teen use=1.6, 95 % CI: 1.2–2.2; and RR for adult use=1.5, 95 % CI: 1.2–1.9). Specific drugs for which elevated risks were observed included crack/cocaine, psychedelics/hallucinogens, club/designer drugs, Ritalin without a prescription, and heroin. Limiting the analyses to participants without prenatal exposure to maternal cigarette smoking, marijuana use and alcohol consumption strengthened these associations.
Mental Health
We examined the occurrence of depression, bipolar disorder, post-traumatic stress disorder and schizophrenia in relation in the study population20. Participants with any exposure during gestation and early childhood had elevations in the risk of bipolar disorder (RR: 1.8, 95% CI: 0.9–1.4) and post-traumatic stress disorder (RR: 1.5, 95% CI: 0.9–2.5) that were further increased among participants in the highest exposure tertile (RR: 2.7, 95% CI: 1.3–5.6 for bipolar disorder and RR: 1.7, 95% CI: 0.9–3.2 for post-traumatic stress disorder). By contrast, the risk of depression was not associated with prenatal and childhood PCE exposure (RR: 1.1, 95% CI: 0.9–1.4). The occurrence of schizophrenia was too rare to produce reliable conclusions.
Vision
The 63 participants with early life exposure whose vision was examined also had evidence of long-term subclinical visual dysfunction16. Participants exposed to high PCE levels exhibited lower contrast sensitivity at intermediate and high spatial frequencies compared to unexposed participants. Exposed participants also exhibited poorer color discrimination than unexposed participants [mean difference in Farnsworth Color Confusion Index= 0.05 (p=0.04)]. No differences in visual acuity were observed.
Neuropsychological Testing
Among participants who completed neuropsychological testing, there was no evidence of major decrements in neuropsychological performance in relation to early life exposure to PCE17. There were modest associations between exposure and diminished performance on tasks that assessed visuospatial function (mean difference =−0.3, 95% CI: −0.6 to +0.1), motor functioning (e.g., mean difference Neurobehavioral Evaluation System finger tapping= −1.8, 95% CI: −5.7 to +2.2), learning and memory (mean difference= −0.2, 95% CI: −0.6 to +0.1), and attention and executive function (mean difference= −0.2, 95% CI: −0.5 to +0.1)[17]. No associations were seen for decrements in performance on tests of omnibus intelligence, academic achievement or language.
Structural Magnetic Resonance Imaging
MRI studies were performed on 42 participants. Early life exposure to PCE was not associated with alterations in the brain structures as seen in structural neuroimaging studies18. In particular, no statistically significant differences were observed between exposed and unexposed subjects on measures of white and gray matter volumes and white matter hypointensities.
Strengths and Limitations of Cape Cod Health Study
Like John Snow's cholera investigation in 1854 London31, the Cape Cod Health Study demonstrates how scientists can take advantage of unique “natural experiments” to learn about the health effects of environmental pollution. The unusual circumstances that led to the contamination of the public water supplies on Cape Cod presented both strengths and challenges for carrying out our research.
While there is now considerable evidence to support PCE’s designation as a probable or likely human carcinogen1, 32, 33, its cancer-causing potential was suspected in 1980 when the water contamination was first publicized34. Thus, the discovery that PCE was leaching into public water supplies prompted state and local authorities to conduct a thorough investigation of the extent of the contamination and to swiftly develop and implement a remediation plan. The comprehensive investigation resulted in an extensive repository of water system records without which our research would have been impossible to conduct.
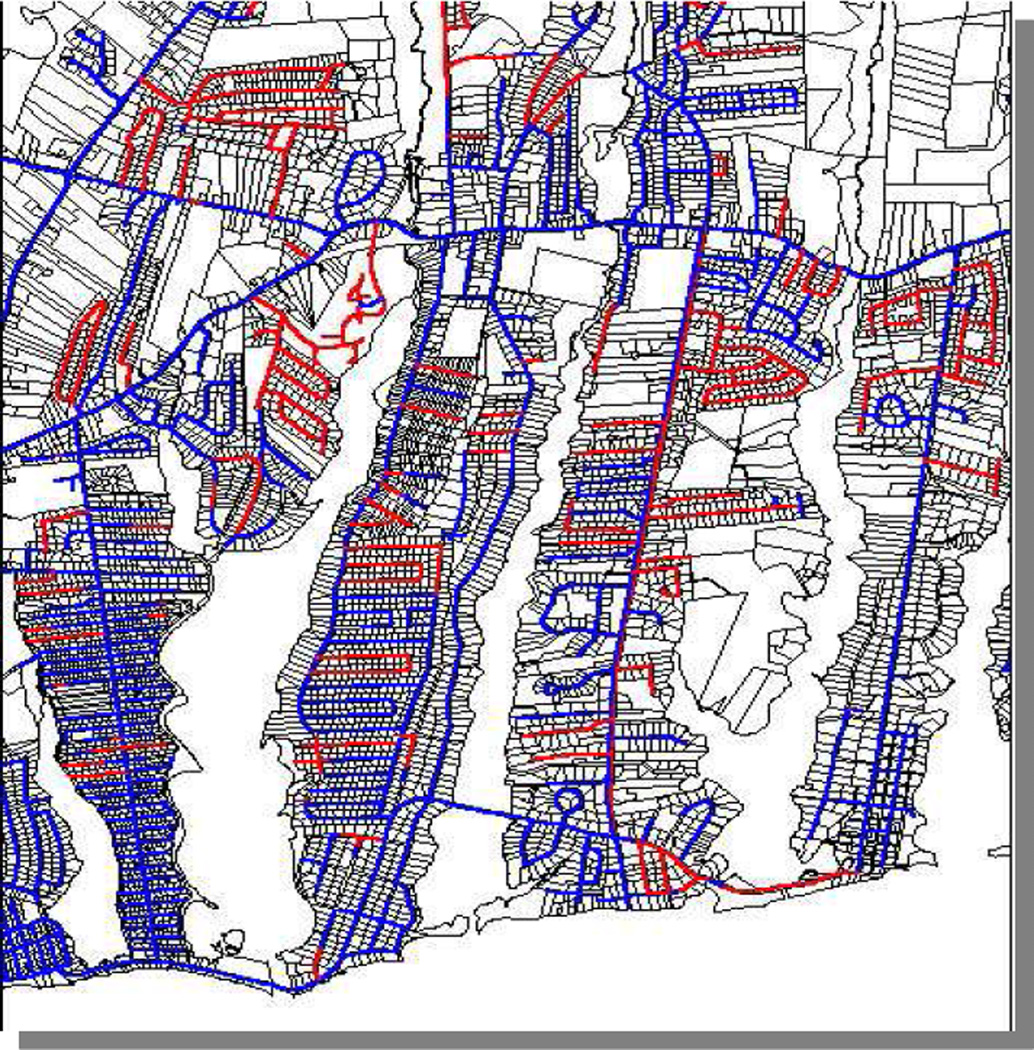
The widespread nature and irregular pattern of contamination were also fortuitous circumstances for our study. First, the high exposure prevalence made it feasible to identify a sufficient number of exposed participants. Second, because VL/AC pipes were installed in response to expansion and replacement needs in a town’s water system, adjacent streets and even adjacent houses had different pipes and thus different exposures (Figure 2), resulting in minimal confounding by participant characteristics (Table 1). This also meant that PCE exposures were not correlated with other environmental contaminants such as trihalomethames. The diverse settings where VL/AC pipes were installed, for example high-flow locations along main thoroughfares and low-flow areas such as dead end streets, also led to a wide range of exposure levels, another serendipitous circumstance of our study. Thus, key strengths were availability of historical data on affected water systems, a relatively high exposure prevalence and wide range of exposure levels, and little confounding.
Figure 2.
Irregular Pattern of VL/AC Pipes in Cape Cod, Massachusetts, USA
Key: Vinyl-lined pipe 
Unlined pipe 
Nevertheless, conducting the Cape Cod Health Study also presented considerable challenges. These arose mainly from the historical nature of the exposure assessments. These assessments did not account for behavioral characteristics (which were poorly recalled), and necessitated many assumptions, both of which led to non-differential exposure misclassification and likely attenuated the associations.
We were fortunate to locate a small number of drinking water sample test results from 1980 for comparison with our modeled exposure assessments. While these historical samples were not completely satisfactory as a “gold standard” because they were only used to give a rough indication of where a problem existed and how severe it was, we found good correlation between our modeled estimates and PCE concentrations in the historical water samples (Spearman correlation coefficient (ρ)=0.65, p<0.00010)35, bolstering our confidence in the validity of our assessments and suggesting that the extent of exposure misclassification may be relatively modest.
Conclusions
PCE is a widespread environmental and occupational contaminant1, 2, a probable carcinogen32, 33 and an acknowledged neurotoxicant in the workplace setting32, 33. Until now, few studies have shown non-cancer effects at typical environmental exposure levels. An unusual scenario that resulted in PCE contamination of the drinking water in the Cape Cod region of Massachusetts afforded a unique opportunity to examine long-term neurotoxic effects of prenatal and early childhood exposures to PCE.
Results from the Cape Cod Health Study to date provide evidence that early life exposure to PCE-contaminated drinking water has long-term neurotoxic effects (Table 2). Moderate associations were seen with illicit drug use, bipolar disorder, and post-traumatic stress disorder. In addition, modest associations were observed between early life exposure and subsequent risky behaviors such as cigarette smoking and alcoholic beverage consumption; diminished performance on tests of visuospatial function, learning and memory, attention and executive functioning, motor speed and mood; and subclinical decrements in color vision and contrast sensitivity. In contrast, no evidence of an association was seen for depression; diminished performance on tests of omnibus intelligence, academic achievement and language; visual acuity; and alterations in brain structure as seen on neuroimaging. Because the study population is highly educated and predominantly white, these results may not be generalizable to more ethnically diverse and disadvantaged populations.
Table 2.
Summary Long-Term Neurotoxic Effects of Early Life Exposure to Tetrachloroethylene-contaminated Drinking Water in the Cape Cod Health Study Population
| | Health/Test Outcome | Strength of Association* | | | ----------------------------------- | ---------------------------------- | - | | Risky Behaviors | Cigarette Smoking | + | | | | | | Alcoholic Beverage Consumption | + | | | | | | | Illicit Drug Use | ++ | | | | | | | Mental Illness | Depression | 0 | | | | | | Bipolar Disorder | ++ | | | | | | | PTSD | ++ | | | | | | | Vision Tests | Visual Acuity | 0 | | | | | | Contrast Sensitivity | + | | | | | | | Color Vision | + | | | | | | | Neuropsychological Tests | Omnibus Intelligence | 0 | | | | | | Academic Achievement | 0 | | | | | | | Language | 0 | | | | | | | Visuospatial Function | + | | | | | | | Learning and Memory | + | | | | | | | Attention and Executive Functioning | + | | | | | | | Motor Speed | + | | | Mood | + | | | | | | | Brain Structure according to MRI | White Matter Volume | 0 | | | | | | Gray Matter Volume | 0 | | | | | | | White Matter Hypointensities | 0 | |
While the Cape Cod Health Study is the first to report these long-term neurotoxic effects, the results are concordant with many other investigations of shorter-term neurological effects among adults and children following solvent exposure1. The mode of action by which PCE may cause neurotoxicity is unknown. However, this small fat-soluble molecule easily crosses the blood brain barrier and has high affinity for the lipophilic tissues of the brain. In addition, experimental evidence suggests several possible mechanisms, including peroxidation of cell membrane lipids36, loss of myelin37, fatty acid changes in the brain38, and interference with neurotransmitters and GABAA receptors39. Because PCE is a common environmental contaminant, it is important to understand its long-term impact on the health of vulnerable populations such as pregnant women and their developing fetuses. This body of work has taken an important step towards improving our understanding of the long-term neurotoxic effects of early life exposure to PCE and will help risk assessors and policy makers ensure that U.S. drinking water supplies are safe for all to consume.
Acknowledgments
The authors would like to acknowledge the study participants who took the time to share their experiences, and the assistance of the local water companies and the Massachusetts Department of Environmental Protection. This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program 5P42ES000738.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All of the authors had access to the data and a role in writing this manuscript.
References
- 1.U.S. Environmental Protection Agency. Toxicological Review of Tetrachloroethylene (Perchloroethylene). In Support of Summary Information on the Integrated Risk Information System (IRIS) Washington DC: US EPA; 2012. Feb, [Google Scholar]
- 2.Ruder AM. Potential health effects of occupational solvents exposure. Ann NY Acad Sci. 2006;1076:207–227. doi: 10.1196/annals.1371.050. [DOI] [PubMed] [Google Scholar]
- 3.Moran MJ, Zogorski JS, Squillace PJ. Chlorinated solvents in groundwater of the United States. Environ Sci Technol. 2007;41:74–81. doi: 10.1021/es061553y. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry. Toxicological profile for tetrachloroethylene. Department of Health and Human Services, Public Health Service; 1997. [PubMed] [Google Scholar]
- 5.Lin YS, Egeghy PP, Rappaport SM. Relationships between levels of volatile organic compounds in air and blood from the general population. J Exp Sci Environ Epidemiol. 2008;18:421–429. doi: 10.1038/sj.jes.7500635. [DOI] [PubMed] [Google Scholar]
- 6.Demond AH. A source of tetrachloroethylene in the drinking water of New England: an evaluation of toxicity of tetrachloroethylene and the prediction of its leaching rates from vinyl-lined asbestoscement pipe. Massachusetts Institute of Technology; 1982. [Google Scholar]
- 7.Massachusetts Department of Environmental Quality Engineering. Status report on tetrachloroethylene contamination of public drinking water supplies by vinyl-lined asbestos cement pipe. 1982 [Google Scholar]
- 8.Massachusetts Department of Environmental Protection. Vinyl-Lined Asbestos-Cement Pipe (VLAC) Monitoring Program SOP for Tetrachloroethylene, a/k/a Perchloroethylene (PCE) [(Accessed July 10, 2015)]; http://www.mass.gov/eea/docs/dep/water/drinking/alpha/i-thru-z/vlacsop.pdf.
- 9.Massachusetts Department of Public Health. Cancer incidence in Massachusetts, 1982–1986. Boston, MA: Massachusetts Department of Public Health; 1990. [Google Scholar]
- 10.Paulu C, Aschengrau A, Ozonoff D. Tetrachloroethyene-contaminated drinking water in Massachusetts and the risk of colon-rectum, lung and other cancers. Environ Health Perspect. 1999;107:265–271. doi: 10.1289/ehp.99107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aschengrau A, Rogers S, Ozonoff D. Tetrachloroethylene-contaminated drinking water and the risk of breast cancer: additional results from Cape Cod, Massachusetts. Environ Health Perspect. 2003;111:167–174. doi: 10.1289/ehp.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aschengrau A, Weinberg J, Rogers S, Gallagher L, Winter M, Vieira V, Webster T, Ozonoff D. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of adverse birth outcomes. Environ Health Perspect. 2008;116:814–820. doi: 10.1289/ehp.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aschengrau A, Weinberg J, Gallagher L, Winter M, Vieira V, Webster T, Ozonoff D. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of pregnancy loss. Water Qual Expo Health. 2009;1:23–34. doi: 10.1007/s12403-009-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carwile JL, Winter M, Mahalingaiah S, Aschengrau A. Prenatal drinking-water exposure to tetrachloroethylene and placental dysfunction disorders. Environ Health. 2014;13:72. doi: 10.1186/1476-069X-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aschengrau A, Janulewicz P, Weinberg J, Gallagher L, Winter M, Vieira V, Webster T, Ozonoff D. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of congenital anomalies: a retrospective cohort study. Environ Health. 2009;8:44. doi: 10.1186/1476-069X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getz K, Janulewicz P, Rowe S, Weinberg J, Winter M, Martin B, Webster TF, Vieira V, Aschengrau A. Prenatal and early childhood exposure to tetrachloroethylene and adult vision. Environ Health Perspect. 2012;120:1327–1332. doi: 10.1289/ehp.1103996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janulewicz P, White RF, Martin B, Winter MR, Weinberg JM, Vieira V, Aschengrau A. Adult Neuropsychological performance following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicol Teratol. 2012;34:350–359. doi: 10.1016/j.ntt.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janulewicz P, Killiany RJ, White RF, Martin BM, Winter MR, Weinberg JM, Aschengrau A. Structural magnetic resonance imaging in an adult cohort following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicol Teratol. 2013;38:13–20. doi: 10.1016/j.ntt.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aschengrau A, Weinberg JM, Janulewicz PA, Romano ME, Gallagher LG, Winter MR, Martin BR, Vieira VM, Webster TF, White RF, Ozonoff DM. Affinity for risky behaviors following prenatal and childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water. Environ Health. 2011;10(1):102. doi: 10.1186/1476-069X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschengrau A, Weinberg JM, Janulewicz PA, Romano ME, Gallagher LG, Winter MR, Martin BR, Vieira VM, Webster TF, White RF, Ozonoff DM. Occurrence of mental illness following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: a retrospective cohort study. Environ Health. 2012;11:2. doi: 10.1186/1476-069X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockelmann I, Darius S, McGauran N, Robra BP, Peter B, Pfister EA. The psychological effects of exposure to mixed organic solvents on car painters. Disabil Rehabil. 2002;24:455–461. doi: 10.1080/09638280110102126. [DOI] [PubMed] [Google Scholar]
- 22.Bowler RM, Lezak M, Booty A, Hartney C, Mergler D, Levin J, Zisman F. Neuropsychological dysfunction, mood disturbance, and emotional status of munitions workers. Appl Neuropsychol. 2001;8:74–90. doi: 10.1207/S15324826AN0802_2. [DOI] [PubMed] [Google Scholar]
- 23.Condray R, Morrow LA, Steinhauer SR, Hodgson M, Kelley M. Mood and behavioral symptoms in individuals with chronic solvent exposure. Psychiatry Res. 2000;97:191–206. doi: 10.1016/s0165-1781(00)00217-1. [DOI] [PubMed] [Google Scholar]
- 24.Echeverria D, White RF, Sampaio C. A behavioral evaluation of PCE exposure in patients and dry cleaners: a possible relationship between clinical and preclinical effects. J Occup Environ Med. 1995;37:667–680. doi: 10.1097/00043764-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Grosch JW, Neale AV, Demers RY. Neurobehavioral and health-related deficits in solventexposed painters. Am J Ind Med. 1996;30:623–632. doi: 10.1002/(SICI)1097-0274(199611)30:5<623::AID-AJIM11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Webler T, Brown HS. Exposure to tetrachloroethylene via contaminated drinking water pipes in Massachusetts: a predictive model. Arch Environ Health. 1993;48:293–297. doi: 10.1080/00039896.1993.9936716. [DOI] [PubMed] [Google Scholar]
- 27.Rossman LA. EPANET users manual. Cincinnati, OH: U.S. Environmental Protection Agency, Risk Reduction Engineering Laboratory; 1994. [Google Scholar]
- 28.Aral MM, Maslia ML, Ulirsch GV, Reyes JJ. Estimating exposure to volatile organic compounds from municipal water-supply systems: use of a better computational model. Arch Environ Health. 1996;51:300–309. doi: 10.1080/00039896.1996.9936029. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher MD, Nuckols JR, Stallones L, Savitz DA. Exposure to trihalomethanes and adverse pregnancy outcomes. Epidemiol. 1998;9:484–489. [PubMed] [Google Scholar]
- 30.Vieira V, Aschengrau A, Ozonoff D. Assessing the impact of tetrachloroethylene-contaminated drinking water on the risk of breast cancer using a dose model. Environ Health. 2005;4:3. doi: 10.1186/1476-069X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow J. A reprint of two papers of John Snow, MD together with a biographical memoir by B.W. Richardson, MD and an Introduction by Wade Hampton Frost, MD. Facsimile of 1936 Edition. New York: Hafner Publishing Co; 1965. Snow on cholera. [Google Scholar]
- 32.Guyton KZ, Hogan KA, Scott CS, Cooper GS, Bale AS, Kopylev L, Barone S, Makris SL, Glenn B, Subramaniam RP, Gwinn MR, Dzubow RC, Chiu WA. Human health effects of tetrachloroethylene: key findings and scientific issues. Environ Health Perspect. 2014;122:325–334. doi: 10.1289/ehp.1307359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Agency for Cancer Research (IARC) Dry cleaning, some chlorinated solvents, and other industrial chemicals. IARC Monogr Eval Carcinog Risks Hum. 1995;63 [PMC free article] [PubMed] [Google Scholar]
- 34.Ackerman J. Pipes pollute some N.E. water. Boston Globe: 1980. Apr 11, [Google Scholar]
- 35.Gallagher LG, Vieira VM, Ozonoff D, Webster TF, Aschengrau A. Risk of breast cancer following exposure to tetrachloroethylene-contaminated drinking water in Cape Cod, Massachusetts: reanalysis using a modified exposure assessment. Environ Health. 2011;10:47. doi: 10.1186/1476-069X-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cojocel C, Beuter W, Muller W, Mayer D. Lipid peroxidation: a possible mechanism of trichloroethylene-induced nephrotoxicity. Toxicol. 1989;55:131–141. doi: 10.1016/0300-483x(89)90180-7. [DOI] [PubMed] [Google Scholar]
- 37.Isaacson LG, Taylor DH. Maternal exposure to 1,1,2-trichloroethylene affects myelin in the hippocampal formation of the developing rat. Brain Res. 1989;488:403–407. doi: 10.1016/0006-8993(89)90739-7. [DOI] [PubMed] [Google Scholar]
- 38.Kyrklund T, Haglid K. Brain lipid composition in guinea pigs after intrauterine exposure to perchloroethylene. Pharmacol Toxicol. 1991;68:146–148. doi: 10.1111/j.1600-0773.1991.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 39.Olney JW, Farber NB, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect. 2000;108:383–388. doi: 10.1289/ehp.00108s3383. [DOI] [PMC free article] [PubMed] [Google Scholar]