Madagascar's ephemeral palaeo-grazer guild: who ate the ancient C4 grasses? (original) (raw)
Madagascar today is 65% grassland. Its grassy biomes were widely interpreted as ‘anthropogenic’ before Bond et al. [1] challenged that view, claiming that grasses colonized Madagascar during the Miocene. Through an analysis of grass clade endemism and specialization, Vorontsova et al. [2] have marshalled compelling support for this argument.
Still, many questions about Madagascar's grasslands remain, and they are critical to debates regarding why so many forest-dwelling animals disappeared after human arrival. We know that forests declined and grasslands expanded, but we do not know precisely when, why or to what extent. Did significant pre-contact expansion occur [3]? Did post-contact aridification trigger this transformation [4]? Did forests disappear as people shifted their subsistence strategy from ephemeral foraging to dedicated agro-pastoralism [5]? Did early human colonizers severely reduce Madagascar's natural grazer community through hunting, radically changing the fire ecology and ultimately destroying forests [6,7]? Normally, grasslands are maintained by natural fire and by grazers, usually large-bodied herbivores. But Madagascar's modern grazers (e.g. cattle and goats) were recently introduced. If pre-contact grasslands were extensive, we would expect to see a significant palaeo-grazer guild. The prime candidates are pygmy hippopotamuses and giant tortoises—animals that, along with giant lemurs and elephant birds, comprised Madagascar's now-extinct megafauna.
According to Vorontsova et al. [2], previously published stable carbon isotope (δ13C) data suggest that (i) Madagascar's hippopotamuses consumed a ‘high proportion of C4 plants’ (i.e. grasses) [8] and (ii) its giant tortoises had diverse diets [9]. We were motivated to write this comment for three reasons. First, Crowley et al. [8] never claimed that Madagascar's hippopotamuses ate a ‘high proportion of C4 plants’. We did note that subfossil hippos, tenrecs, carnivorans and the endemic rodent Hypogeomys australis had higher δ13C values than subfossil primates (except Hadropithecus [10]), but we never defended a high proportion of C4 plants in the diets of any of these animals. Second, Vorontsova et al. [2] omitted important published isotope data on hippos and tortoises from Madagascar [8,11,12]. Third, we believe that the literature would benefit from greater discussion of these data.
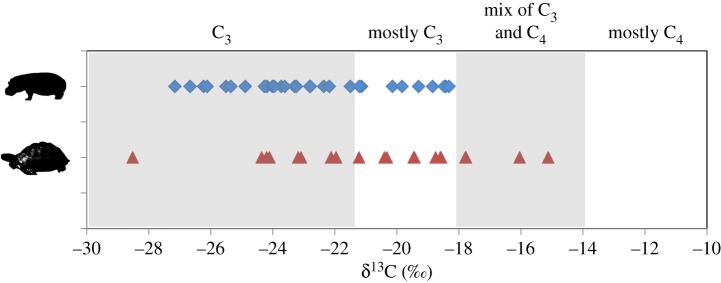
C4 grasses (including those measured in Madagascar [11]) typically have δ13C values between −11 and −14‰, while C3 plants have much lower values, averaging between −26.5 and −31.5‰ for different Madagascan forests [13]. Assuming an offset of +5‰ between herbivore bone collagen and plant diet (reviewed in [14]), pure C4 consumers should have δ13C values more than −9‰, those with pure C3 diets should have δ13C values less than −21.5‰, and those that consumed a relatively even mixture of C3 and C4 plants should have values of −14 to −18‰. Thus, herbivores with values between −18 and −21.5‰ likely consumed more C3 than C4 plants.
Carbon isotope values for herbivores should approximate the relative abundance of C4 grasses in a particular habitat provided that those herbivores are adapted to eating grasses and other C4 plants, and that grazers are not selective in the grasses they consume. Clearly, δ13C values for some vertebrates, such as ratites (which tend to select small fruits) and lemurs (which tend to browse on C3 fruit and leaves), make poor proxies for C4 plant abundance, but δ13C values for grazers like hippos and tortoises should be appropriate. In Africa, the common hippo (Hippopotamus amphibius) feeds predominantly on grasses, and the pygmy hippo (Choeropsis liberiensis) consumes a mixture of terrestrial plants including grasses [15–17]. Tortoises are also important grazers that consume both C3 and C4 plants [18]. In large numbers, they function effectively as lawn mowers, making grasses less vulnerable to natural fires by keeping them short (e.g. [19]).
We expect that Madagascan hippos and tortoises living in dry forests or wooded savannahs would have consumed bulk foliage in the understorey, including herbaceous terrestrial and aquatic vegetation as well as C3 (i.e. shade-loving) grasses. If C4 grasslands were widespread in pre-contact Madagascar, there is good reason to expect that they would have been attractive to hippos and tortoises, and C4 grass consumption should be reflected in the δ13C values for these animals.
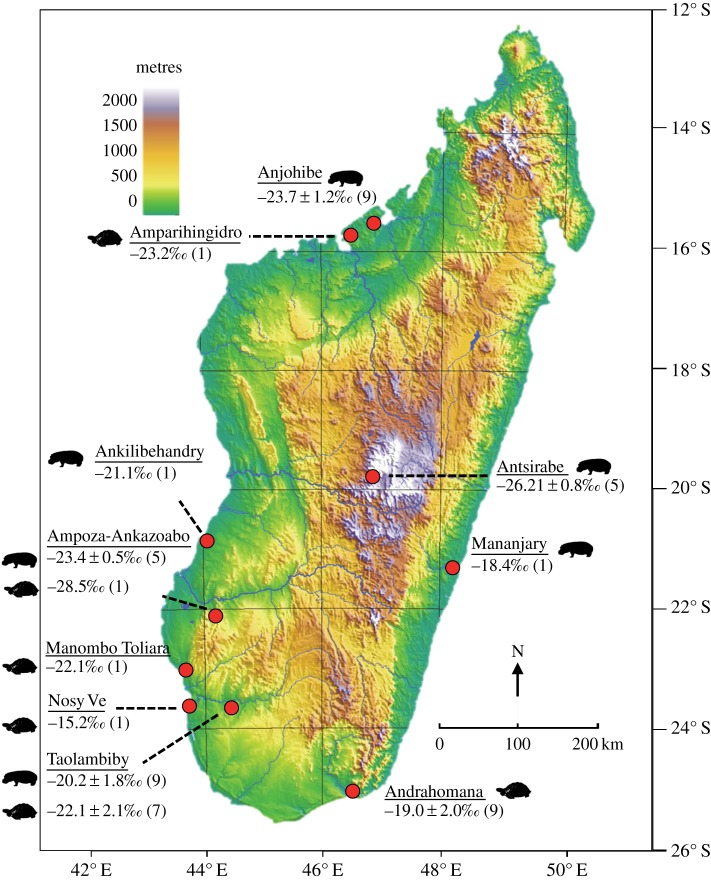
Figures 1 and 2 summarize all published δ13C data (to our knowledge) for the three extinct Hippopotamus and two extinct tortoise (Aldabrachelys) species from Madagascar [7,9–12] (doi:10.5061/dryad.68s67). We subtracted 1.2‰ from each value to correct for differences in atmospheric CO2 before and after the industrial revolution [20], allowing direct comparison of subfossil animal and modern plant values. All subfossil data presented below have been corrected. We present results for hippos and tortoises by site rather than by species because we are interested in reconstructing palaeohabitats, and because not all species attributions are secure.
Figure 1.
Tortoise and hippopotamus subfossil localities with associated corrected δ13C data. All data available from the Dryad Digital Repository (http://dx.doi.org/doi:10.5061/dryad.68s67). (Online version in colour.)
Figure 2.
Corrected δ13C values for hippopotamuses and tortoises. Expected isotopic ranges for different diets are indicated. (Online version in colour.)
The δ13C data for hippos suggest dedicated C3 plant consumption at Anjohibe, Antsirabe and Ampoza, and a C3-dominated diet at Ankilibehandry, Taolambiby and Mananjary (figure 1). Ironically, the sites with the greatest C3 plant consumption by hippos (Anjohibe in the northwest and Antsirabe in the Central Highlands) are in exactly those regions now blanketed by C4 savannah. The most recent radiocarbon dates for hippos at Anjohibe are 2635 and 2940 calendar years before present (Cal BP), and their δ13C values are −22.2 and −22.4‰, respectively, indicating a C3 diet. Uranium–thorium dating of the speleothems from Anjohibe has demonstrated a transformation from a C3- to a C4-dominated landscape between 890 and approximately 1000 CE [5], which postdates the decline of hippos in the northwest [12]. The timing for grassland expansion in the Central Highlands is less constrained [21], but the youngest hippos at Antsirabe (1075 and 1150 Cal BP) have δ13C values that indicate pure C3 plant consumption (−26.7 and −26.2‰, respectively) even at these late dates. There is no temporal trend in δ13C values during the time span (7150–1075 Cal BP) represented by our entire hippo sample.
Burney et al. [7] published two δ13C values (−8.0 and −8.7‰) for a single ‘Mananjary’ hippopotamus that suggest pure C4 grass consumption, but the specimen is a skull of an H. amphibius from continental Africa [22] that was apparently sent to Madagascar for comparative research. Two dates for this specimen are very recent (130 and 155 Cal BP [7]), lending credence to this inference. The only isotope datum for an actual Mananjary hippo derives from an older specimen (2250 Cal BP) whose δ13C value (−18.4‰ [7]) is more consistent with values for hippos from elsewhere on the island (figure 1).
Overall, tortoises have slightly higher δ13C values than hippos (figure 2), and their values suggest dedicated C3 plant consumption at Amparihingidro, Ampoza, Manombo Toliara and Taolambiby (figure 1). Data from Andrahomana and ‘near Nosy Ve’ reveal a mixed diet, although the δ13C value from ‘near Nosy Ve’ is considerably elevated, suggesting greater grass consumption. The age of the latter individual (1120 Cal BP) may post-date regional landscape transformation. Dates for other tortoises average 2776.5 Cal BP and range from 635 Cal BP (Manombo Toliara) to 7260 Cal BP (Amparihingidro). Almost all tortoise dates fall well before the landscape transformation documented in speleothem records [5] from the northwest.
In conclusion, there exists strong evidence that Madagascar's pygmy hippopotamuses and giant tortoises consumed primarily C3 plants. We must consider the possibility that the grassy biomes that characterize so much of Madagascar today are young, and that ‘old-growth’, pre-contact grasslands were small in extent and maintained largely by natural fire with limited megafaunal assistance [23,24]. While Madagascar's grasses may be ancient, its vast grassy biomes could be products of recent anthropogenic disturbance. Vorontsova et al. [2] showed that some of Madagascar's endemic grasses cannot tolerate grazing and trampling by introduced ungulates, and they concluded that the grasses evolved under less intensive disturbance than exists today. This is entirely consistent with our observation that not a single Madagascan megafaunal species can be called a committed C4 grazer on the basis of stable isotope data collected to date.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.68s67.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Bond WJ, Silander JA, Ranaivonasy J, Ratsirarson J. 2008. The antiquity of Madagascar's grasslands and the rise of C4 grassy biomes. J. Biogeogr. 35, 1743–1758. ( 10.1111/j.1365-2699.2008.01923.x) [DOI] [Google Scholar]
- 2.Vorontsova MS, et al. 2016. Madagascar's grasses and grasslands: anthropogenic or natural? Proc. R. Soc. B 283, 20152262 ( 10.1098/rspb.2015.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quéméré E, Amelot X, Pierson J, Crouay-Roy B, Chikhi L. 2012. Genetic data suggest a natural prehuman origin of open habitats in northern Madagascar and question the deforestation narrative in this region. Proc. Natl Acad. Sci. _USA_109, 13 028–13 033. ( 10.1073/pnas.1200153109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virah-Sawmy M, Willis KJ, Gillson L. 2010. Evidence for drought and forest declines during the recent megafaunal extinctions in Madagascar. J. Biogeogr. 37, 506–519. ( 10.1111/j.1365-2699.2009.02203.x) [DOI] [Google Scholar]
- 5.Burns SJ, Godfrey LR, Faina P, McGee D, Hardt B, Ranivoharimanana L, Randrianasy J. 2016. Rapid human-induced landscape transformation in Madagascar at the end of the first millennium of the Common Era. Q. Sci. Rev. 134, 92–99. ( 10.1016/j.quascirev.2016.01.007) [DOI] [Google Scholar]
- 6.Burney DA, Robinson GS, Burney LP. 2003. Sporormiella and the late Holocene extinctions in Madagascar. Proc. Natl Acad. Sci. USA 100, 10 800–10 805. ( 10.1073/pnas.1534700100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burney DA, Burney LP, Godfrey LR, Jungers WL, Goodman SM, Wright HT, Jull AJT. 2004. A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63. ( 10.1016/j.jhevol.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 8.Crowley BE, Godfrey LR, Irwin MT. 2011. A glance to the past: subfossils, stable isotopes, seed dispersal and lemur species loss in southern Madagascar. Am. J. Primatol. 73, 25–37. ( 10.1002/ajp.20817) [DOI] [PubMed] [Google Scholar]
- 9.Burleigh R, Arnold EN. 1986. Age and dietary differences of recently extinct Indian-Ocean tortoises (Geochelone s. lat.) revealed by carbon isotope analysis. Proc. R. Soc. Lond. B 227, 137–144. ( 10.1098/rspb.1986.0014) [DOI] [Google Scholar]
- 10.Godfrey LR, Crowley BE, Muldoon KM, Kelley EA, King SJ, Best AW, Berthaume MA. In press What did Hadropithecus eat, and why should paleoanthropologists care? Am. J. Primatol. ( 10.1002/ajp.22506) [DOI] [PubMed] [Google Scholar]
- 11.Crowley BE, Godfrey LR. 2013. Why all those spines? Anachronistic defenses in the Didiereoideae against now extinct lemurs. S. Afr. J. Sci. 109, 70–76. ( 10.1590/sajs.2013/1346) [DOI] [Google Scholar]
- 12.Crowley BE, Samonds KE. 2013. Stable carbon isotope values confirm a recent increase in grasslands in northwestern Madagascar. Holocene 23, 1066–1073. ( 10.1177/0959683613484675) [DOI] [Google Scholar]
- 13.Crowley BE, et al. 2011. Explaining geographical variation in the isotope composition of mouse lemurs (Microcebus). J. Biogeogr. 38, 2106–2121. ( 10.1111/j.1365-2699.2011.02551.x) [DOI] [Google Scholar]
- 14.Tykot RH. 2004. Stable isotopes and diet: you are what you eat. In Proc. of the Int. School of Physics ‘Enrico Fermi’ Course CLIV (eds Martini M, Milazzo M, Piecentini M), pp. 433–444. Amsterdam, The Netherlands: IOS Press. [Google Scholar]
- 15.Eltringham SK. 1999. The hippos. London, UK: Academic Press. [Google Scholar]
- 16.Boisserie JR, Zazzo A, Merceron G, Blondel C, Vignaud P, Likius A, Mackaye HT, Brunet M. 2005. Diets of modern and late Miocene hippopotamids: evidence from carbon isotope composition and micro-wear of tooth enamel. Palaeogeogr. Palaeoclimatol. _Palaeoecol._221, 153–174. ( 10.1016/j.palaeo.2005.02.010) [DOI] [Google Scholar]
- 17.Cerling TE, Harris JM, Hart JA, Kaleme P, Klingel H, Leakey MG, Levin NE, Lewison RL, Passey BH. 2008. Stable isotope ecology of the common hippopotamus. J. Zool. 276, 204–212. ( 10.1111/j.1469-7998.2008.00450.x) [DOI] [Google Scholar]
- 18.van der Sluis LG, Hollund HI, Buckley M, De Louw PGB, Rijsdijk KF, Kars H. 2014. Combining histology, stable isotope analysis and ZooMS collagen fingerprinting to investigate the taphonomic history and dietary behaviour of extinct giant tortoises from the Mare aux Songes deposit on Mauritius. Palaeogeogr. Palaeoclimatol. _Palaeoecol._416, 80–91. ( 10.1016/j.palaeo.2014.06.003) [DOI] [Google Scholar]
- 19.Burney DA, Hume JP, Middleton GJ, Steel L, Burney LP, Porch N. 2015. Stratigraphy and chronology of karst features on Rodrigues Island, Southwestern Indian Ocean. J. Cave Karst Stud. 77, 37–51. ( 10.4311/2013PA0132) [DOI] [Google Scholar]
- 20.Keeling RF, Piper SC, Bollenbacher AF, Walker SJ. 2010. Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. In Trends: a compendium of data on global change. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; See http://cdiac.esd.ornl.gov/trends/co2/iso-sio/iso-sio.html. [Google Scholar]
- 21.Gasse F, Van Campo E. 1998. A 40 000 yr pollen and diatom record from Lake Tritrivakely, Madagascar, in the southern tropics. Quat. Res. 49, 299–311. ( 10.1006/qres.1998.1967) [DOI] [Google Scholar]
- 22.Faure M, Guérin C, Genty D, Gommery D, Ramanivosoa B. 2010. Le plus ancien hippopotame fossile (Hippopotamus laloumena) de Madagascar (Belobaka, Province de Mahajanga). C. R. Palévol. 9, 155–162. ( 10.1016/j.crpv.2010.04.002) [DOI] [Google Scholar]
- 23.Bond WJ, Keeley JE. 2005. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394. ( 10.1016/j.tree.2005.04.025) [DOI] [PubMed] [Google Scholar]
- 24.Veldman JW, et al. 2015. Toward an old-growth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 13, 154–162. ( 10.1890/140270) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.68s67.