Effects of a sweetpotato protein digest on lipid metabolism in mice administered a high-fat diet (original) (raw)
Abstract
Sweetpotato peptide (SPP) was prepared by enzyme digestion of sweetpotato protein from starch wastewater. Animal experiments assessed the effect of SPP on body weight, abdominal adipose tissue mass, serum lipids and adipocytokines. Body and liver weight and epididymal and mesenteric fat of mice fed a high-fat diet containing 0.5% or 5% SPP for 28 days were significantly lower than control mice. Triglyceride and cholesterol in VLDL and LDL and leptin levels were significantly lower in the serum of SPP-administered mice compared to control mice. Biomarker arrays showed that adiponectin, melanocyte-stimulating-hormone-alpha and neuromedin U were more than 1.5 times higher, while TNF-alpha was about 1.5 times lower in the livers of SPP-administered mice compared to control mice. These results suggest SPP mitigated leptin resistance in mice administered a high-fat diet, and maintained anorexigenic peptide levels. SPP administration may suppress lipogenesis by increasing adiponectin levels and decreasing TNF-alpha levels in adipocytes.
Keyword: Food science
1. Introduction
Sweetpotato (Ipomoea batatas L.) roots are not only used for human consumption, they are used to make starch materials, processed foods, and distilled spirits in Japan. Starch use accounts for about 15% (131,500 tons) of total sweetpotato production. Starch residues are discharged during starch production and are mainly used in animal feed and compost. Large amounts of the wastewater, which can cause serious environmental problems, are discarded after clarification. Investigation into the uses of the by-products of the sweetpotato starch industry would benefit both the environment and industry.
Sweetpotato has a high nutritional value and phytochemical composition (Mohanraj and Sivasankar, 2014). Proteins, sugars, polyphenols and water-soluble polysaccharides derived from sweetpotato are discharged into the wastewater. Water-soluble polysaccharides from sweetpotato are reported to possess immunostimulatory activity (Zhao et al., 2005). A sweetpotato protein was previously shown to have a high amino acid score and anti-diabetic effects (Ishiguro et al., 2008). In addition, we previously prepared a sweetpotato protein digest (sweetpotato peptide, SPP) with angiotensin I-converting enzyme (ACE) inhibitory activity and demonstrated its hypotensive effects in spontaneously hypertensive rats (SHR) (Ishiguro et al., 2012). Peptides isolated from food proteins have shown various health benefits, such as antioxidation (Zhang et al., 2010), amelioration of glucose tolerance (Otani et al., 2009), inhibition of HIV protease (Yust et al., 2004) and hypocholesterolemic effects (Liu et al., 1994), as well as hypotensive effects (Fujita et al., 2001). Investigation of SPP will provide valuable data on any health benefits.
In this study, we investigated the effects of SPP on weight gain, lipid accumulation and hyperlipidemia; the profile of serum cholesterol and triglyceride in sub-fractionated lipoprotein; serum leptin and adiponectin levels; and the expression levels of obesity related biomarkers, such as adipocytokine and anorexigenic peptides in the livers of high-fat diet-administered mice.
2. Materials and methods
2.1. Materials
Three proteases were kindly donated by Daiwa Kasei K.K. (Hyogo, Japan) and Amano Enzyme Inc. (Aichi, Japan).
2.2. Preparation of a sweetpotato peptide (SPP)
SPP was prepared as previously described (Ishiguro et al., 2012) with slight modification: sweetpotato roots (cv. Shiroyutaka for starch production, 240 kg) were squeezed, without the addition of water, by an attritor fitted with a centrifugal dehydrator (Seno Iron Works Co., Ltd., Hokkaido, Japan). Residue was pressed using a screw press separator (PSS4-J, Nippon Engineering Corporation, Fukuoka, Japan) and the extract was added to the squeezed juice from the centrifugal dehydrator. The starch was separated from the squeezed juice by continuous centrifugal separation in a centrifugal separator (HS-324L, IHI Corporation, Tokyo, Japan). Proteins were collected from the supernatant, corresponding to the wastewater, by isoelectric focusing precipitation. The supernatant was adjusted to pH 4.5, and the precipitate was collected by centrifugation (LAPX 404SGP-31, Alfa Laval, Sweden). The precipitate (6.0 kg), including sweetpotato protein, was freeze dried and kept at −30 °C until use. One hundred and fifty grams of freeze dried precipitate was added to 15 L of pure water. The protein solution was digested with three proteases, Thermoase PC10F (Daiwa Kasei, Hyogo, Japan) (1.5 g), Protease S (Amano Enzyme, Aichi, Japan) (15 g), and Proleather FG-F (Amano Enzyme, Aichi, Japan) (15 g); these were selected because of their high protein degradation and ACE inhibitory activity. The protein solution was adjusted to pH 8.5, and the enzyme reaction was conducted at 65 °C for 16 h. The digest was then boiled for 30 min to inactivate the enzymes. The digest was filtered using activated carbon and spray-dried. The dried digest (SPP) was used in the animal experiments.
2.3. Animal experiments
Animal experiments were performed by Skylight Biotech Inc. at Akita University (Akita, Japan). Mice were maintained at a room temperature of 23 ± 2 °C and relative humidity of 55 ± 5% under a 12 h light-dark cycle. Five-week-old male C57BL/6 mice (Japan SLC, Inc., Shizuoka, Japan) were fed High Fat Diet 32 (CLEA Japan, Inc., Tokyo, Japan) (Table 1) and acclimatized for 1 week. Mice were divided into three groups consisting of five animals, according to body weight. The levels of serum cholesterol and triglyceride in mice were not significantly different between the three groups at the beginning of the experiment. The control group was fed High Fat Diet 32, and test groups were fed High Fat Diet 32 containing SPP at a concentration of 5% (w/w) or 0.5% (w/w). Food and sterile water were available ad libitum. All mice were assessed to be in good health throughout the experiments and no forms of toxicity were observed in mice administered SPP. Twenty eight days after initiation of the test diets, body weight was measured and blood samples for the measurement of cholesterol, triglyceride, leptin and adiponectin were obtained from the heart under ketamin anesthesia. The mice were fasted from 22:00 h the day before and sampling was conducted at 10:00 h. After blood sampling, liver, mesenteric fat and epididymal fat were isolated and weighed. Serum samples and livers were stored at −80 °C until use. The animals were treated according to the “Guidelines for Proper Conduct of Animal Experiments” (Science Council of Japan). The study was approved by the Ethics Committee of Akita University.
Table 1.
Composition of the high-fat diet.
| Ingredients | Composition (%) |
|---|---|
| Milk Casein | 24.5 |
| Egg Albumen powder | 5.0 |
| L-cystine | 0.43 |
| Beef fat powder | 15.88 |
| Safflower oil | 20.0 |
| Crystalline cellulose | 5.5 |
| Malto dextrin | 8.25 |
| Lactose | 6.928 |
| Sucrose | 6.75 |
| AIN96 vitamin mixture | 1.4 |
| AIN96 mineral mixture | 5.0 |
| Choline bitartrate | 0.36 |
| _tert_-butylhydroquinone | 0.002 |
2.4. Measurements of serum cholesterol, triglyceride, leptin and adiponectin
Total cholesterol, triglyceride, leptin and adiponectin were measured 28 days after the start of the experiment. Total cholesterol and triglyceride was measured using Cholestest CHO and Cholostest TG (Daiichi Pure Chemicals Co. Ltd., Tokyo, Japan), respectively. Serum leptin and adiponectin levels were measured using a Mouse Leptin ELISA Kit (Morinaga Institute of Biological Science, Yokohama, Japan) and a Mouse/Rat Adiponection ELISA Kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), respectively.
2.5. Serum lipoprotein analysis by HPLC
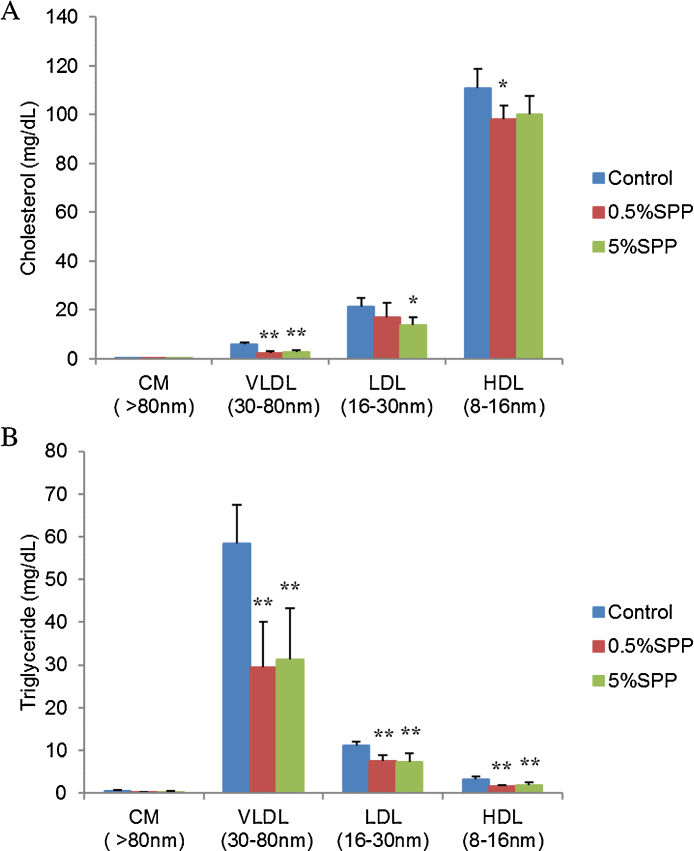
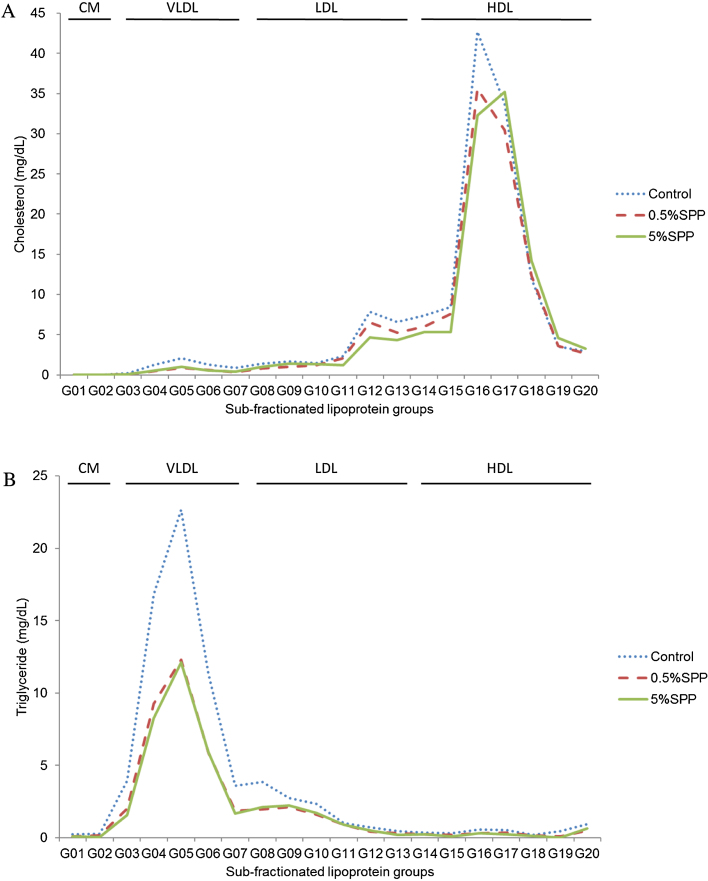
The cholesterol and triglyceride profiles in serum lipoproteins were analyzed by an online dual enzymatic method using HPLC with two tandem connected TSKgel LipopropakXL columns (300 × 7.8 mm; Tosho, Japan) at Skylight Biotech Inc. (Akita, Japan), according to the procedure described by Usui et al., 2002. The concentrations of cholesterol and triglyceride were determined in four fractioned groups according to lipoprotein particle size (diameter) (Fig. 1), chylomicron (CM, >80 nm), very low density lipoprotein (VLDL, 30–80 nm), low density lipoprotein (LDL, 16–30 nm), and high density lipoprotein (HDL, 8–16 nm), using enzymatic reagents (Kyowa Medex, Tokyo Japan). The concentrations of cholesterol and triglyceride were further analyzed in twenty sub-fractioned groups of CM (G01 and 02), VLDL (G03-05: large VLDL, G06: medium VLDL, G07: small VLDL), LDL (G08: large LDL, G09: medium LDL, G10: small LDL, G11-13: very small LDL) and HDL (G14, 15: very large HDL, G16: large HDL, G17: medium HDL, G18: small HDL, G19, 20: very small HDL).
Fig. 1.
Cholesterol (A) and triglyceride (B) concentrations in CM, VLDL, LDL and HDL in the serum of C57BL/6 mice fed a high-fat diet containing SPP for 28 days. Vertical bars represent the mean ± SD. Statistical significance of the difference in cholesterol and triglyceride between the control group and SPP-administered groups was evaluated using the Dunnet test (n = 5; *p < 0.05, **p < 0.01).
2.6. Obesity peptide biomarker array analysis
The obesity peptide biomarker array analysis for mouse/rat (Phoenix Pharmaceuticals, Inc., CA, USA) was performed according to the manufacturer’s instruction at Filgen Inc. (Nagoya, Japan). The liver tissues of a mouse selected from each group after 28 days administration of the high-fat diet containing SPP were isolated. The array consists of 44 different biomarkers: ACTH, adiponectin, ADM, AGRP, amylin, apelin-36, autotaxin, bombesin, CART, CCK, CGRP, CRF, desnutrin, dynorphin A, endorphin-beta, ghrelin, GHRF, GLP-1, GLP-2, glucagon, IL-6, IMD/AM-2, INSL-3, INSL-5, KISS-1, leptin, MCH, MSH-alpha, nesfatin, neuromedin U, NPB-29, NPW-23, NPY, orexin A, oxytocin, PACAP38, PrRP-31, PYY, RELM-beta, resistin, TNF-alpha, urocortin, VIP and visfatin. The expression levels of biomarkers in the liver of SPP-administered mice were compared to the control. The data was the average of two replications and the values are the ratio of the expression intensities of biomarkers derived from livers of 0.5% or 5% SPP administered mice to control mice.
2.7. Statistical analysis
Statistical analyses of differences between the control group and the groups that received SPP were conducted using the multiple comparison test of Dunnet.
3. Results
3.1. Animal experiments
Animal experiments were performed to assess the effect of SPP on body weight, abdominal adipose tissue mass, serum lipids and adipocytokines. The body weight of male C57BL/6 mice fed a high-fat diet containing 5% SPP was significantly lower than control mice, although food intake was not statistically different in all groups after 28 days administration (Table 2). Liver weight and epididymal fat were significantly lower in the 0.5% (P < 0.05) and 5% (P < 0.01) SPP-administered groups compared with the control group (Table 2). Mesenteric fat was significantly lower in the 5% (P < 0.01) SPP-administered group compared with the control (Table 2). Fatty liver was identified in control mice (moderately in 3 animals and slightly in 2 animals) and in 0.5% SPP-administered mice (moderately in 2 animals and slightly in 3 animals) according to the autopsy report. No mice in the 5% SPP-administered group were diagnosed with a fatty liver (data not shown).
Table 2.
Body weight, food intake and adipose tissue weights of C57BL/6 mice fed a high-fat diet containing SPP for 28 days.
| Dietary groups | ||||||
|---|---|---|---|---|---|---|
| Control | 0.5% | 5% | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Initial body weight (g) | 21.7 | 0.5 | 21.7 | 0.5 | 21.7 | 0.5 |
| Final body weight (g) | 28.7 | 0.9 | 27.6 | 1.2 | 25.4** | 1.0 |
| Food intake (g) | 69.1 | 4.3 | 74.1 | 19.3 | 72.7 | 8.3 |
| Liver weight (g) | 1.06 | 0.03 | 0.99* | 0.03 | 0.92** | 0.06 |
| Mesenteric fat (g) | 0.31 | 0.05 | 0.24 | 0.05 | 0.16** | 0.05 |
| Epididymal fat (g) | 0.57 | 0.08 | 0.42* | 0.10 | 0.29** | 0.08 |
Serum total cholesterol levels were not statistically different among any groups after 28 days administration of a high-fat diet containing SPP. The serum triglyceride levels were significantly lower in the 0.5% (P < 0.05) and 5% (P < 0.01) SPP-administered group compared with the control (Table 3). There were no significant differences in serum adiponectin levels, while serum leptin levels were significantly lower in the 5% (P < 0.01) SPP-administered group compared with the control (Table 3). These results indicate that SPP prevents body weight gain, lipid accumulation in the fatty tissue and any elevations in serum triglyceride and leptin levels in mice fed a high-fat diet.
Table 3.
Serum total cholesterol, triglyceride, adiponectin and leptin levels of C57BL/6 mice fed a high-fat diet containing SPP for 28 days.
| Dietary groups | ||||||
|---|---|---|---|---|---|---|
| Control | 0.5% | 5% | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Total cholesterol (mg/dL) | 137.5 | 11.5 | 128.1 | 10.6 | 127.6 | 9.3 |
| Triglyceride (mg/dL) | 78.2 | 12.0 | 51.5* | 17.4 | 45.5** | 11.9 |
| Adiponectin (μg/mL) | 15.4 | 1.9 | 14.3 | 2.6 | 13.6 | 1.0 |
| Leptin (ng/mL) | 9.4 | 2.9 | 7.0 | 3.8 | 2.8** | 2.0 |
3.2. Cholesterol and triglyceride profiles in serum lipoproteins
Cholesterol and triglyceride levels in serum lipoproteins, CM, VLDL, LDL and HDL, after 28 days administration of a high-fat diet containing SPP were analyzed by HPLC. Cholesterol levels were significantly lower in the SPP-administered group compared with the control in serum VLDL and LDL (Fig. 1A). Triglyceride levels were also lower in the SPP-administered group compared with the control in serum VLDL, LDL and HDL (Fig. 1B). Cholesterol and triglyceride profiles were further analyzed in 20 sub-fractioned groups of CM, VLDL, LDL and HDL. Cholesterol levels were lower in the serum of SPP-administered mice compared with the control mice in G04-G06 (large VLDL and medium VLDL), G11-15 (very small LDL and very large HDL) and G16-G18 (large, medium and small HDL) (Fig. 2A). SPP dose-dependent responses in cholesterol levels were observed, in particular, in very small LDL (G11-13). Triglyceride levels were considerably lower in the serum of SPP-administered mice in fractions G03-G08 (large, medium and small VLDL and large LDL) (Fig. 2B). These results indicate that the reductions in triglyceride and cholesterol levels by SPP was dependent on the particle size in serum lipoproteins.
Fig. 2.
Cholesterol (A) and triglyceride (B) concentrations in 20 sub-fractionated groups of CM, VLDL, LDL and HDL in the serum of C57BL/6 mice fed a high-fat diet containing SPP for 28 days. Each sub-fractionated group was assigned to CM (G01 and 02), VLDL (G03-05: large VLDL, G06: medium VLDL, G07: small VLDL), LDL (G08: large LDL, G09: medium LDL, G10: small LDL, group G11-13: very small LDL) and HDL (G14, 15: very large HDL, G16: large HDL, G17: medium HDL, G18: small HDL, G19, 20: very small HDL), according to their particle size.
3.3. Metabolic disease related biomarker array analysis
In order to evaluate the effect of SPP on factors related to metabolic diseases, biomarker array analysis was performed. Adrenocorticotrophic hormone (ACTH), adiponectin, KISS-1, melanocyte-stimulating-hormone-alpha (MSH-alpha), nesfatin, neuromedin U, neuropeptide W-23 (NPW-23), orexin A and resistin were more than 1.5 times higher in the liver of 0.5% and/or 5% SPP-administered mice compared to the control mice. TNF-alpha and desnutrin were about 1.5 times lower in the 0.5% or 5% SPP-administered mice compared to the control mice (Table 4). These results suggest that SPP administration ameliorates the abnormality of secretion of some adipocytokines and neuropeptides related to appetite, and contributes to the control of lipid metabolism in mice fed a high-fat diet.
Table 4.
Changes in expression of obesity biomarkers in the liver of C57BL/6 mice fed a high-fat diet containing SPP.
| Biomarkers | 0.5% SPP | 5% SPP | Biomarkers | 0.5% SPP | 5% SPP |
|---|---|---|---|---|---|
| ACTH | 1.568846 | 1.207352 | INSL-3 | 1.247944 | 1.004722 |
| Adiponectin | 1.869254 | 2.018212 | INSL-5 | 1.128766 | 1.006280 |
| ADM | 0.942962 | 0.996387 | KISS-1 | 2.374750 | 1.311740 |
| AGRP | 1.217677 | 1.201734 | Leptin | 1.213525 | 1.004622 |
| Amylin | 1.222357 | 1.156575 | MCH | 1.284525 | 1.285361 |
| Apelin-36 | 1.198470 | 1.085409 | MSH-alpha | 2.260421 | 1.556036 |
| Autotaxin | 1.073183 | 0.982489 | Nesfatin | 2.199554 | 1.381554 |
| Bombesin | 1.221198 | 1.060198 | Neuromedin U | 2.305536 | 1.638912 |
| CART | 1.070417 | 1.063943 | NPB-29 | 1.198133 | 1.029302 |
| CCK | 1.142432 | 1.033288 | NPW-23 | 1.581845 | 1.153735 |
| CGRP | 1.325779 | 1.167294 | NPY | 1.246320 | 1.073343 |
| CRF | 1.077451 | 0.960503 | Orexin A | 2.120926 | 1.415045 |
| Desnutrin | 0.460312 | 1.086319 | Oxytocin | 1.236405 | 0.917337 |
| Dynorphin A | 1.110873 | 0.922335 | PACAP38 | 1.290654 | 1.026850 |
| Endorphin-beta | 1.311076 | 1.084468 | PrRP-31 | 1.130610 | 1.020291 |
| Ghrelin | 1.241355 | 1.112199 | PYY | 1.144237 | 0.981967 |
| GHRF | 1.264400 | 1.145316 | RELM-beta | 1.152111 | 0.962001 |
| GLP-1 | 1.151995 | 1.069993 | Resistin | 2.731225 | 1.439922 |
| GLP-2 | 1.274524 | 1.363427 | TNF-alpha | 0.701973 | 0.589492 |
| Glucagon | 1.190494 | 1.129924 | Urocortin | 1.188631 | 1.001872 |
| IL-6 | 1.129821 | 0.858137 | VIP | 0.828300 | 1.023039 |
| IMD/AM-2 | 1.343179 | 1.044383 | Visfatin | 1.197846 | 1.093286 |
4. Discussion
Visceral adipose tissue accumulation is an important risk factor of metabolic and cardiovascular disorders (Bastard et al., 2006). Increased low-density lipoprotein cholesterol (LDL-C) or decreased high-density lipoprotein cholesterol (HDL-C) and increased triglyceride are widely known risk factors of coronary heart disease (CHD) (Okazaki et al., 2005). A previous study showed that the visceral fat area positively correlated with very low-density lipoprotein cholesterol (VLDL-C) and LDL-C in 62 male subjects (Okazaki et al., 2006). In this study, we demonstrated that body weight gain, accumulation of visceral fat and fatty liver symptoms were suppressed in mice administered a SPP diet (Table 2). Serum VLDL-C and LDL-C levels were significantly lower in SPP-administered mice compared to control mice (Fig. 1A), although serum total cholesterol was not significantly different among the groups (Table 3). Triglyceride, which is abundant in VLDL, was also clearly lower in SPP-administered groups compared with the control (Fig. 1B). These results suggest an intriguing possibility that SPP has the potential to mitigate hyperlipidemia and obesity.
The profiles of 20 lipoprotein sub-fractions from the serum of mice fed a high-fat diet suggested SPP had a dose-dependent effect on cholesterol levels, especially in very small LDL (G11-13) (Fig. 2A). The predominance of small, dense LDL has been accepted as an important cardiovascular risk factor (Lamarche et al., 1997; Superko and Gadesam, 2008) and characteristic of the dyslipidemic state seen in type 2 diabetes (Qiu et al., 2007). A relationship between predominance of small, dense LDL particles, high triglyceride levels and low HDL-C concentrations and cardiovascular diseases have been reported (Austin et al., 1990; Campos et al., 1992; Okazaki et al., 2006). Our results suggest SPP may be advantageous in subjects with lipid abnormalities and heart disease by reducing the levels of VLDL-C, very small LDL-C and VLDL-triglyceride.
Lipid and sugar metabolism is maintained by various adipocytokines and neuropeptides related to food intake. Leptin is an anorexigenic adipocytokine, primarily secreted by adipocyte cells. Plasma leptin levels are elevated in subjects who are overweight, suggesting obesity may be associated with leptin resistance (Schwartz et al., 1996). Leptin also influences the activity of various orexigenic and anorexigenic peptides in the hypothalamus (Klok et al., 2006). Leptin resistance disrupts hypothalamic control of energy homeostasis, which results in obesity and increased lipid production (Yang and Barouch, 2007). MSH-alpha decreases body fat in humans, regulates energy balance, appetite control and glucose transport in rat adipocytes (Miller et al., 2003). MSH-alpha, together with ACTH and endorphin-beta, are synthesized by proteolytic processing of the proopiomelanocortin (POMC) precursor in the pituitary and brain: POMC levels are increased by leptin (Yang and Barouch, 2007). Neuromedin U is also a hypothalamic neuropeptide, that regulates body weight through the effects of anorexigenic activity and energy expenditure, independent of the leptin signaling pathway (Hanada et al., 2004). Adiponectin plays an important role in metabolic regulation processes in various tissues: it suppresses gluconeogenesis, lipogenesis and triglyceride levels in the liver (Ruan and Dong, 2016). TNF-alpha, a proinflammatory cytokine, is increased in the adipose tissue of obese mice and subjects (Puglisi and Fernandez, 2008). TNF-alpha reduces adiponectin expression and induces the overproduction of VLDL particles, which might explain its direct relationship with serum triglycerides (Qin et al., 2008). The secretion of TNF-alpha is stimulated by leptin (Yang and Barouch, 2007). In our study, serum leptin levels were dose-dependently suppressed in SPP-administered mice after 28 days administration of a high-fat diet containing SPP (Table 3). In addition, biomarkers associated with obesity and lipid abnormality were increased in the livers of SPP-administered mice, in particular, the levels of adiponectin, MSH-alpha and neuromedin U were more than 1.5 times higher in both the 0.5% and 5% SPP-administered groups compared with the control (Table 4). TNF-alpha was about 1.5 times lower in the 5% SPP-administered group compared with the control group (Table 4). These results suggest that SPP mitigated leptin resistance in mice administered a high-fat diet, and maintained anorexigenic peptide levels, such as MSH-alpha and neuromedin U. SPP administration may suppress lipogenesis and triglyceride levels by increasing adiponectin levels and decreasing TNF-alpha levels in adipocyte tissues.
There are many reports of enzyme digests derived from food protein with activities which potentially prevent lifestyle diseases: angiotensin I-converting enzyme inhibition (Suetsuna, 1998; Fujita et al., 2001; Yonekura and Tanaka, 2003), vasorelaxing activity (Yoshikawa et al., 2000), hypocholesterolemic activity (Yoshikawa et al., 2000; Liyanage et al., 2008; Liu et al., 1994), immunostimulating activity (Yoshikawa et al., 2000), antioxidation (Zhang et al., 2010), amelioration of glucose tolerance (Otani et al., 2009) and inhibition of HIV protease (Yust et al., 2004). We report that SPP, derived from sweetpotato, might contribute to the amelioration of lipid abnormality and have identified four peptides, I-T-P, G-Q-Y, I-I-P and S-T-Y-Q-T, which may be responsible for the activity. Further research into the roles of these peptides are required.
5. Conclusion
SPP was prepared from sweetpotato starch wastewater using a combination of three proteases. SPP inhibited body weight gain, visceral fat gain and alleviation of fatty liver symptoms. In addition, SPP lowered the levels of triglyceride, very small LDL-C and VLDL-C in serum. The involvement of SPP in the regulation of expression of anorexigenic neuropeptides and adipocytokines was experimentally inferred. It is expected that SPP could be used as a functional food material for people with metabolic disorders. However, the administration level of SPP would be required for human health is uncertain at this study. Human intervention studies are needed for confirmation of the effect of SPP administration on the disorder of lipid metabolism.
Declarations
Author contribution statement
Koji Ishiguro: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rie Kurata, Takashi Kume: Performed the experiments.
Yoshikazu Shimada, Yoto Sameshima: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Studies on Biorecycling of Waste from the Agriculture, Forestry, and Fisheries Sector − Development of Technology for Sustainable Utilization and Recycling Systems from Food Industry, Agriculture, and Fisheries).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Austin M.A., King M.C., Vranizan K.M., Krauss R.M. Atherogenic lipoprotein phenotype: A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- Bastard J.P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H., Capeau J., Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Campos H., Genest J.J., Jr., Blijlevens E., McNamara J.R., Jenner J.L., Ordovas J.M., Wilson P.W.F., Schaefer E.J. Low density lipoprotein particle size and coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamagami T., Ohshima K. Effects of an ace-inhibitory agent, Katsuobushi oligopeptide: in the spontaneously hypertensive rat and in borderline and mildly hypertensive subjects. Nutr. Res. 2001;21:1149–1158. [Google Scholar]
- Hanada R., Teranishi H., Pearson J.T., Kurokawa M., Hosoda H., Fukushima N., Fukue Y., Serino R., Fujihara H., Ueta Y., Ikawa M., Okabe M., Murakami N., Shirai M., Yoshimatsu H., Kangawa K., Kojima M. Nuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat. Med. 2004;10:1067–1073. doi: 10.1038/nm1106. [DOI] [PubMed] [Google Scholar]
- Ishiguro K., Yoshimoto M., Tsubata M., Takagaki K., Sameshima Y., Kume T., Ikeda K. Anti-diabetic effect of sweetpotato protein with trypsin inhibitor activity derived from by-product of starch industry. J. Clin. Biochem. Nutr. 2008;43(Suppl.1):410–412. [Google Scholar]
- Ishiguro K., Sameshima Y., Kume T., Ikeda K., Matsumoto J., Yoshimoto M. Hypotensive effect of a sweetpotato protein digest in spontaneously hypertensive rats and purification of angiotensin I-converting enzyme inhibitory peptides. Food Chem. 2012;13:774–779. [Google Scholar]
- Klok M.D., Jakobsdottir S., Drent M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. 2006;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Lamarche B., Tchernof A., Moorjani S., Cantin B., Dagenais G.R., Lupien P.J., Despre’s J.P. Small: dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- Liu A., Takeichi K., Sugiyama M., Shimabukuro T., Yamamoto S. Effect of dietary peptides on plasma lipids and its mechanism studied in rats and mice. Nutr. Res. 1994;4:1661–1669. [Google Scholar]
- Liyanage R., Han K.H., Watanabe S., Shimada K., Sekikawa M., Ohba K., Tokuji Y., Ohnishi M., Shibayama S., Nakamori T., Fukushima M. Potato and soy peptide diets modulate lipid metabolism in rats. Biosci. Biotechnol. Biochem. 2008;72:943–950. doi: 10.1271/bbb.70593. [DOI] [PubMed] [Google Scholar]
- Miller R., Aaron W., Toneff T., Vishnuvardhan D., Beinfeld M.C., Hook V.Y.H. Obliteration of α-melanocyte-stimulationg hormone derived from POMC in pituitary and brains of PC2-deficient mice. J. Neurochem. 2003;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Mohanraj R., Sivasankar S. Sweet Potato (Ipomoea batatas [L.] Lam)-A valuable medicinal food: A review. J. Med. Food. 2014;17:733–741. doi: 10.1089/jmf.2013.2818. [DOI] [PubMed] [Google Scholar]
- Okazaki M., Usui S., Ishigami M., Sakai N., Nakamura T., Matsuzawa Y., Yamashita S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler. Thromb. Vasc. Biol. 2005;25:578–584. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- Okazaki M., Usui S., Fukui A., Kubota I., Tomoike H. Component analysis of HPLC profiles of unique lipoprotein subclass cholesterols for detection of coronary artery disease. Clin. Chem. 2006;52:2049–2053. doi: 10.1373/clinchem.2006.070094. [DOI] [PubMed] [Google Scholar]
- Otani L., Ninomiya T., Murakami M., Osajima K., Kato H., Murakami T. Sardine peptide with angiotensin I-converting enzyme inhibitory activity improves glucose tolerance in stroke-prone spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009;73:2203–2209. doi: 10.1271/bbb.90311. [DOI] [PubMed] [Google Scholar]
- Puglisi M.J., Fernandez M.L. Modulation of C-reactive protein, tumor necrosis factor-α, and adiponectin by diet, exercise and weight loss. J. Nutr. 2008;138:2293–2296. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- Qin B., Anderson R.A., Adeli K. Tumor necrosis factor-α directly stimulates the overproduction of hepatic apolipoprotein B100-containing VLDL via impairment of hepatic insulin signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G1120–1129. doi: 10.1152/ajpgi.00407.2007. [DOI] [PubMed] [Google Scholar]
- Qiu C., Rudra C., Austin M.A., Williams M.A. Association of gestational diabetes mellitus and low-density lipoprotein (LDL) particle size. Physiol. Res. 2007;56:571–578. doi: 10.33549/physiolres.931073. [DOI] [PubMed] [Google Scholar]
- Ruan H., Dong L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016;8:101–109. doi: 10.1093/jmcb/mjw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.W., Peskind E., Raskind M., Boyko E.J., Porte D., Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat. Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- Superko H.R., Gadesam R.R. Is it LDL particle size or number that correlates with risk for cardiovascular disease? Curr. Atheroscler. Rep. 2008;10:377–385. doi: 10.1007/s11883-008-0059-2. [DOI] [PubMed] [Google Scholar]
- Suetsuna K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998;6:163–167. [PubMed] [Google Scholar]
- Usui S., Hara Y., Hosaki S., Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 2002;43:805–814. [PubMed] [Google Scholar]
- Yang R., Barouch L.A. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- Yonekura M., Tanaka A. Isolation and application of physiologically active peptides from soybean whey and okura proteins. Prot. Res. 2003;6:88–93. (in Japanese) [Google Scholar]
- Yoshikawa M., Fujita H., Matoba N., Takenaka Y., Yamamoto T., Yamauchi R., Tsuruki H., Takahata K. Bioactive peptides derived from food proteins preventing lifestyle-related diseases. BioFactors. 2000;12:143–146. doi: 10.1002/biof.5520120122. [DOI] [PubMed] [Google Scholar]
- Yust M.M., Pedroche J., Megías C., Girón-Calle J., Alaiz M., Millán F. Rapeseed protein hydrolysates: a source of HIV protease peptide inhibitors. Food Chem. 2004;87:387–392. [Google Scholar]
- Zhang J., Zhang H., Wang L., Guo X., Wang X., Yao H. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010;119:226–234. [Google Scholar]
- Zhao G., Kan J., Li Z., Chen Z. Characterization and immunostimulatory activity of an (1-> 6)-a-D-glucan from the root of Ipomoea batatas. Int. Immunopharmacol. 2005;5:1436–1445. doi: 10.1016/j.intimp.2005.03.012. [DOI] [PubMed] [Google Scholar]