Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines (original) (raw)
Abstract
Hyponatremia is a common water balance disorder that often poses a diagnostic or therapeutic challenge. Therefore, guidelines were developed by professional organizations, one from within the United States (2013) and one from within Europe (2014). This review discusses the diagnosis and treatment of hyponatremia, comparing the two guidelines and highlighting recent developments. Diagnostically, the initial step is to differentiate hypotonic from nonhypotonic hyponatremia. Hypotonic hyponatremia is further differentiated on the basis of urine osmolality, urine sodium level, and volume status. Recently identified parameters, including fractional uric acid excretion and plasma copeptin concentration, may further improve the diagnostic approach. The treatment for hyponatremia is chosen on the basis of duration and symptoms. For acute or severely symptomatic hyponatremia, both guidelines adopted the approach of giving a bolus of hypertonic saline. Although fluid restriction remains the first-line treatment for most forms of chronic hyponatremia, therapy to increase renal free water excretion is often necessary. Vasopressin receptor antagonists, urea, and loop diuretics serve this purpose, but received different recommendations in the two guidelines. Such discrepancies may relate to different interpretations of the limited evidence or differences in guideline methodology. Nevertheless, the development of guidelines has been important in advancing this evolving field.
Keywords: cerebral edema, copeptin, vasopressin, urea, vasopressin receptor antagonist, osmotic demyelination syndrome
Hyponatremia (serum sodium [SNa] <136 mmol/L) is a common water balance disorder that often poses a diagnostic or therapeutic challenge.1 This may explain why management of hyponatremia is still suboptimal, as also recently illustrated by a hyponatremia registry.2 Hyponatremia is not a disease but rather a pathophysiologic process indicating disturbed water homeostasis.3 Therefore, hyponatremia should be further classified in order to provide directions for diagnosis and treatment (Table 1). These classifications illustrate that hyponatremia is a very heterogeneous disorder. This has complicated clinical studies, because “the” patient with hyponatremia does not exist. Instead, the underlying disease that is complicated by hyponatremia usually characterizes patients with hyponatremia.4,5 The most common causes of hyponatremia are the syndrome of inappropriate antidiuresis (SIAD), diuretic use, polydipsia, adrenal insufficiency, hypovolemia, heart failure, and liver cirrhosis (the latter two are often collectively referred to as “hypervolemic hyponatremia”). Although recent years have seen several developments in the diagnosis and treatment of hyponatremia, the evidence base is still limited. To capture the current approach to hyponatremia, two sets of guidelines have been developed, one by professional organizations from within the United States (“United States guideline”) and one from within Europe (“European guideline,” in which the authors of this review participated).6–9 The professional organizations involved in the United States guideline were Tufts University Office of Continuing Education and In 2 MedEd; the initiative was also supported by an unrestricted educational grant from Otsuka America Pharmaceutical.9 The professional organizations involved in the European guideline were the European Renal Association–European Dialysis and Transplantation Association, the European Society of Endocrinology, and the European Society of Intensive Care Medicine.6–8 The United States guideline refrained from using a quality-of-evidence scoring system due to the limited evidence. Instead, the guideline was on the basis of expert panel recommendations, which relied on a critical evaluation of relevant literature by the panel members. The European guideline did perform systematic reviews of the available evidence using the Grading of Recommendations Assessment Development and Evaluation scoring system. Both guideline committees were interdisciplinary, and the European guideline was endorsed by the European societies of nephrology, endocrinology, and intensive care.6–8 This brief review will compare the two guidelines to discuss the diagnosis and treatment of hyponatremia, while also highlighting recent developments. Because of the breadth of both guidelines, this review will focus on the salient features. To place both guidelines in perspective we will integrate in our discussion the pertinent comments published after their release.10–13
Table 1.
Classifications of hyponatremia
| Classification | Criteria | Limitations of Clinical Utility |
|---|---|---|
| Moderate (125–129 mmol/L) versus severe/profounda (<125 mmol/L) | Absolute SNa concentration | Symptoms do not always correlate with degree of hyponatremia |
| Acute versus chronic | Time of development (cutoff 48 h) | Time of development not always known |
| Symptomatic versus asymptomatic | Presence of symptoms | Many symptoms aspecific; chronic hyponatremia may be symptomatic |
| Hypotonic, isotonic, or hypertonic | Measured serum osmolality | Ineffective osmoles (e.g., urea, ethanol) are also measured |
| Hypovolemic, euvolemic, hypervolemic | Clinical assessment of volume status | Clinical assessment of volume status has low sensitivity and specificity |
Differential Diagnosis of Hyponatremia
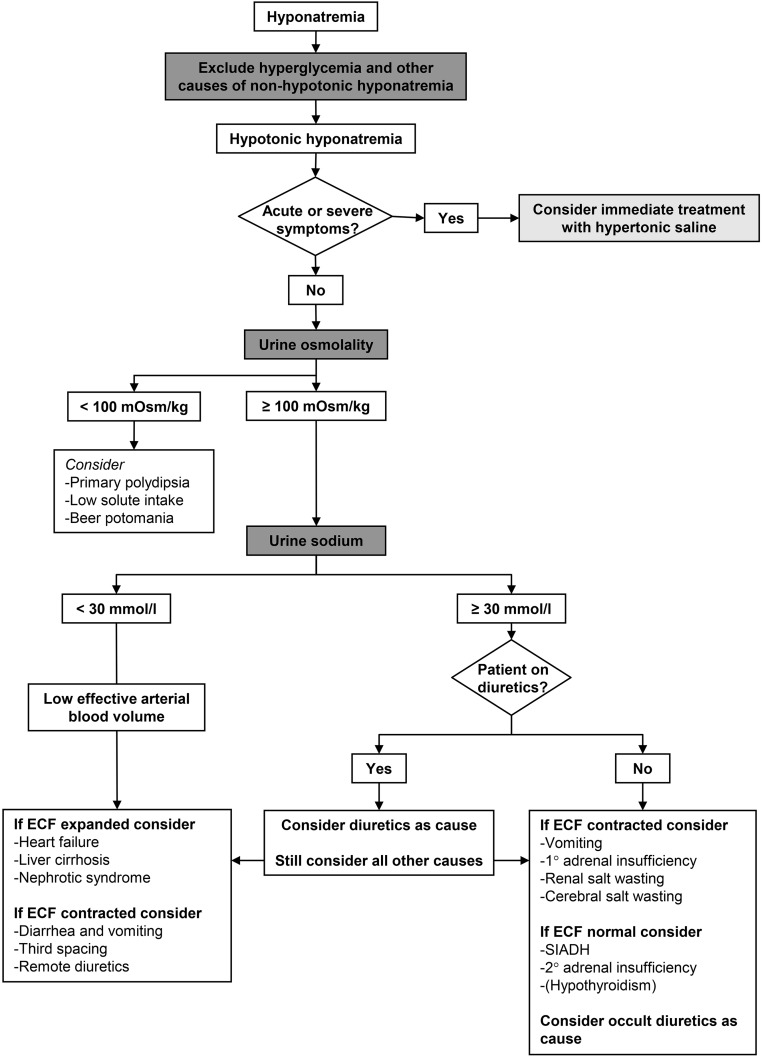
Although the United States guideline did not present a diagnostic algorithm, the classifications of hyponatremia on the basis of tonicity and volume status were discussed.9 The initial differentiation in hypotonic and nonhypotonic hyponatremia is important, because management is different.14 Nonhypotonic hyponatremia is usually caused by hyperglycemia, but may also be caused by the administration of mannitol or hypertonic radiocontrast.7 In these settings, management is usually conservative, although a decrease in extracellular tonicity may occur during treatment.15 Nonhypotonic hyponatremia can also be caused by pseudohyponatremia, a laboratory artifact that may occur with high concentrations of triglycerides, cholesterol, or protein.16 The United States guideline subsequently divided hypotonic hyponatremia into hypovolemic, euvolemic, and hypervolemic hyponatremia.9 Although this represents the most traditional and commonly used approach to hypotonic hyponatremia, it deserves scrutiny. Hypovolemic and euvolemic hyponatremia are notoriously difficult to differentiate on the basis of physical examination,17 whereas hypervolemic hyponatremia is usually clinically obvious (presence of edema or ascites). Two studies that analyzed the diagnostic performance of the clinical assessment of volume status in patients with hyponatremia reported low sensitivity (50%–80%) and specificity (30%–50%).18,19 Previously, we showed that clinicians often misclassify hyponatremia when using algorithms that start with clinical assessment of volume status.20 Similarly, physicians in training had a better diagnostic performance than senior physicians when using an algorithm in which urine osmolality (UOsm) and urine sodium (UNa) concentration are prioritized over assessment of volume status.21 Because the kidneys will respond to hypovolemia or a low effective arterial blood volume with sodium retention, UNa<30 mmol/L can be used to identify both hypovolemic and hypervolemic hyponatremia. Three caveats, however, should be emphasized: (_1_) UNa will also be low in patients consuming a low sodium diet (rare in the western populations), (_2_) the (recent) use of diuretics will increase UNa, and (_3_) patients with CKD may be less able to reabsorb sodium.7,22 In addition, advanced CKD usually impairs water excretion, complicating the evaluation of the role of vasopressin in water balance.23 These considerations prompted the European guideline committee to propose an algorithm that prioritizes UOsm and UNa over volume status (Figure 1). It also incorporates the limitations of UNa. In addition, it recommends early identification of acute or symptomatic hyponatremia to identify patients in whom immediate treatment is indicated. Two additional diagnostic tests for hyponatremia merit discussion, including a trial of volume expansion and the fractional uric acid excretion (FEUA). A trial of volume expansion with isotonic saline can be used to diagnose hypovolemic hyponatremia.18 Although a rise in SNa in response to isotonic saline would be consistent with hypovolemic hyponatremia, another possibility would be that the stimulus for vasopressin release in a patient with SIAD abated. Such stimuli are often nonspecific and transient, including pain or nausea.14,24 In addition, SNa has been shown to improve upon saline infusion in patients with SIAD with UOsm<500 mOsm/kg.25 Conversely, isotonic saline may sometimes worsen hyponatremia, a phenomenon called “desalination.”26 In response to the United States guideline, Gross raised the issue of how to deal with mixed forms of hyponatremia, for example SIAD and hypovolemia.10 Indeed, we previously showed that patients often have two to three possible causes for hyponatremia (although it was unclear if and to which extent each cause contributed).27 In addition to a trial of volume repletion, an alternative approach to mixed pathogenesis would be to combine hypertonic saline with desmopressin.28,29 Although the literature on this approach is limited, it offers a rational approach to prevent a rapid rise in SNa that may occur once hypovolemia has been corrected. Fenske _et al._ found that FEUA>12% had the highest sensitivity and specificity to diagnose SIAD with or without diuretic use.30 This study is of interest because it formally tested the diagnostic performance of several parameters using receiver operating curves. More recently, a larger study confirmed that FEUA>12% had the best sensitivity and specificity for SIAD.31 In absolute terms, however, the performance of FEUA was still moderate, and UNa>30 mmol/L and FEUrea>55% had better sensitivity and specificity for SIAD, respectively. We frequently analyze FEUA in patients with hyponatremia, but mainly use it as supporting information. FEUA is high in both SIAD and cerebral salt wasting, but normalizes in SIAD only during treatment.32 Of note, however, is that even in neurosurgical patients with hyponatremia, cerebral salt wasting is rare and has remained an enigmatic and not widely accepted clinical entity.33,34
Figure 1.
Diagnostic algorithm for hyponatremia. Based on the European guideline.7 ECF, extracellular fluid.
Vasopressin
Arginine vasopressin (the antidiuretic hormone) plays a central role in the pathogenesis of hyponatremia. In one study, nonosmotic secretion of vasopressin was detected in 97% of patients with hyponatremia.35 Because hypotonicity normally suppresses vasopressin, the reasons for nonosmotic vasopressin release should be considered.36 “Appropriate” vasopressin release is due to hypovolemia or low effective arterial blood volume, both of which activate baroreceptors to cause vasopressin release. Although one might expect thiazide-induced hyponatremia to be due to hypovolemia secondary to saliuresis, this is not the case.37 Instead, the pathogenesis appears to be a combination of polydipsia and impaired urea-mediated water excretion.37,38 “Inappropriate” vasopressin release is usually caused by the effect of an underlying disease or drugs on central osmoreceptors; alternatively, vasopressin can be produced ectopically (e.g., in small cell lung cancer or olfactory neuroblastoma).3,39,40 In addition, hypocortisolism increases vasopressin release, because corticotropin-releasing hormone normally suppresses vasopressin.41 Although rare, secondary and even primary adrenal insufficiency may mimic SIAD and can be missed without appropriate testing.42–44 Although the kidney usually limits the degree of hyponatremia in SIAD (“vasopressin escape”45), it can also cause antidiuresis independent of vasopressin.46,47 A specific example is gain-of-function mutations of the vasopressin type 2 receptor causing hereditary hyponatremia (“nephrogenic SIAD”).48 Despite the pathogenetic role of vasopressin in hyponatremia, plasma vasopressin is rarely measured in clinical practice. This has two reasons. First, UOsm accurately reflects vasopressin activity, and, therefore, this more readily available parameter can be used instead. Second, vasopressin is difficult to measure reliably in nonexpert laboratories, because it binds to platelets, it is unstable in isolated plasma, and commercial assays are not very sensitive for low concentrations.49 These limitations, however, have largely been resolved by the development of an assay for copeptin.50
Copeptin
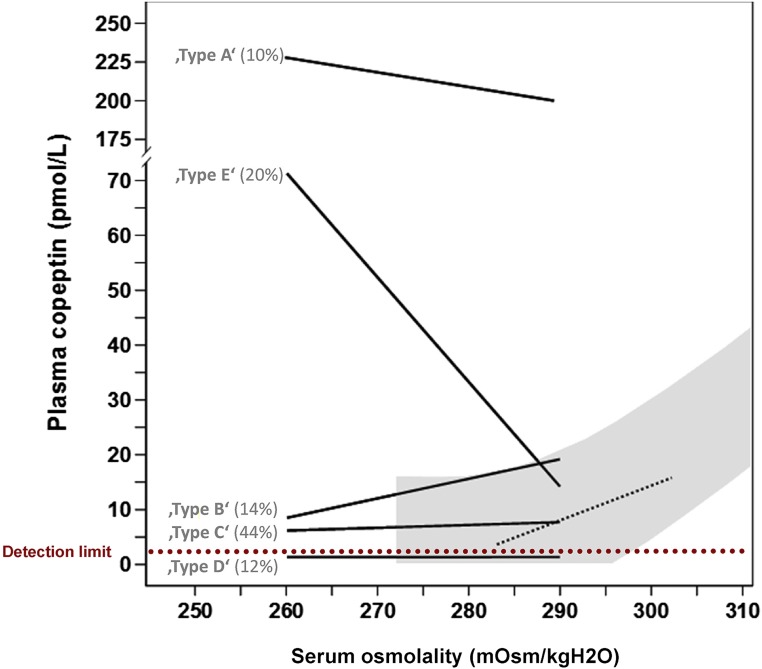
Enzymatic cleavage of the vasopressin prohormone produces not only vasopressin, but also neurophysin and copeptin (also called C-terminal proarginine vasopressin).51 Because copeptin is more stable, it can be measured more easily. Copeptin can therefore be used as a surrogate marker for vasopressin. Although both guidelines only briefly discuss copeptin, emerging data justify a brief discussion on the diagnostic utility of this novel marker. Fenske et al. found that plasma copeptin levels were higher in patients with hypo- or hypervolemic hyponatremia than in patients with SIAD.52 This was demonstrated previously35 and likely reflects an “osmoreceptor gain,” the phenomenon in which angiotensin II amplifies vasopressin release in the context of a low effective arterial blood volume.53,54 Because hypovolemic hyponatremia is characterized by high plasma copeptin and low UNa, the plasma copeptin to UNa ratio may be especially useful to differentiate it from SIAD. Although the study by Fenske et al. did indeed demonstrate this,52 the specificity of copeptin/UNa for SIAD in a more recent and larger study was less high.31 An interesting approach was the use of plasma copeptin to differentiate SIAD subtypes.55 Using hypertonic saline, SIAD subtypes were defined on the basis of their relationship between serum osmolality and plasma copeptin (Figure 2). As expected, low plasma copeptin levels are diagnostic for hyponatremia due to polydipsia.31,52 Arguably, the need for a novel diagnostic marker for this cause of hyponatremia is limited, as it is usually obvious from the clinical setting and the low UOsm. In addition to plasma copeptin, two additional circulating markers were recently evaluated in patients with hyponatremia, including apelin and midregional proatrial natriuretic peptide (MR-proANP).56,57 Physiologically, apelin and vasopressin are regulated in opposite directions by volemic and osmotic stimuli.56 Apelin not only inhibits vasopressin release centrally, but also counteracts the antidiuretic effect in the kidney.58 However, in patients with hyponatremia due to SIAD or heart failure, plasma apelin was insufficiently suppressed, possibly contributing to antidiuresis in these settings.56 Similar to plasma copeptin, MR-proANP levels were higher in patients with hypovolemic or hypervolemic hyponatremia than in patients with SIAD (although these levels were still higher than in healthy subjects).57 High MR-proANP in hypovolemic hyponatremia is counterintuitive, but may be explained by lower GFR secondary to volume depletion.59 Although plasma copeptin, apelin, and MR-proANP increase insight into the pathophysiology of hyponatremia, the true diagnostic potential of these parameters remains to be determined. In addition, one single parameter is unlikely to achieve optimal discriminatory power. A relevant question is whether a combination of diagnostic parameters might improve management.
Figure 2.
Copeptin-based classification of five subtypes of the syndrome of inappropriate antidiuresis (SIAD). The shaded gray area and the black dashed line show the normal physiologic relationship between serum osmolality and plasma copeptin (as surrogate marker for vasopressin). In SIAD type B this relationship is intact, but the osmotic threshold for vasopressin release has decreased. In SIAD types A and C vasopressin release is no longer regulated by serum osmolality. In SIAD type D plasma copeptin levels are undetectable. In SIAD type E the normal relationship between serum osmolality and copeptin has reversed. This phenomenon has been coined “barostat reset,” as it may indicate increased sensitivity of baroreceptors to increased vasopressin release. Percentages indicate how often each subtype was present in one study of 50 patients. Data on the basis of Fenske et al.55 and figure modified from Fenske et al.116 with permission.
General Approach to Treatment
A cutoff of 48 hours is usually used to differentiate acute from chronic hyponatremia (Table 1).7 This classification is useful because acute and chronic hyponatremia may be complicated by different neurologic conditions. Acute hyponatremia can cause cerebral edema when cells have insufficient time to adapt to the hypotonic extracellular environment. In chronic hyponatremia brain cell adaptation has occurred and, in this setting, an acute increase in extracellular tonicity induced by treatment can cause osmotic demyelination syndrome (ODS).60,61 Therefore, for each patient with profound hyponatremia (SNa<125 mmol/L), it is useful to consider whether cerebral edema or ODS should be suspected.62,63 This automatically leads to the often heated debate on optimal correction rates in hyponatremia.64 Both guidelines reached consensus that the limit (not the goal) should be around 10 mmol/L per day for both acute and chronic hyponatremia (Table 2).7,9 Of note, the United States guideline recommends a lower limit of 8 mmol/L per day if there is a high risk of ODS (e.g., in patients with hypokalemia, alcoholism, malnutrition, or liver disease).9 In response to these recommendations, Adrogué and Madias proposed even more conservative limits of 6–8 mmol/L per day regardless of duration or symptoms.11 Although we agree that this is likely to be both sufficient and safe, the data to support this are still limited. It is of interest to see how over the years the recommended correction rates have gradually become more conservative (with recommended correction rates as high as 20 mmol/L per day around 1990).65 A subject directly related to correction rates is overcorrection. Both guidelines recommend frequent monitoring of SNa during the active correction phase (i.e., all treatments except fluid restriction). An aspect that was overlooked by both guidelines is that the measurement of SNa may not offer the precision required for this monitoring. Tormey et al. calculated the so-called “reference change value” for SNa using a common analyzer and demonstrated that only changes in SNa ≥4 mmol/L were certain to be real.12 If overcorrection is detected, both guidelines used different criteria for when to relower SNa: when initial SNa was <120 mmol/L (United States guideline) or when limits are exceeded (European guideline, Table 2). Both guidelines recommend hypotonic fluids or desmopressin for relowering SNa. A combination of hypotonic fluids and desmopressin may be required for treating overcorrection in hypovolemic hyponatremia, because a persistent water diuresis may ensue after correction of hypovolemia.66 Experimental data indicate improved outcomes with reinduction of hyponatremia after rapid overcorrection.67 Another point that merits discussion is the consistent association of hyponatremia with worse outcomes.63,68 This may indicate that hyponatremia has adverse effects beyond the classic neurologic symptoms.69,70 However, in the absence of randomized intervention studies indicating that correction of hyponatremia improves outcomes, it remains unclear whether these associations are causal.5
Table 2.
Comparison of the United States and European guidelines
| Subject | United States Guideline | European Guideline |
|---|---|---|
| Acute or symptomatic hyponatremia | Severe symptoms: Bolus 3% NaCl (100 ml over 10 min × 3 as needed) | Severe symptoms: Bolus 3% NaCl (150 ml over 20 min 2–3 times as needed) |
| Moderate symptoms: Continuous infusion 3% NaCl (0.5–2 ml/kg per h) | Moderate symptoms: Bolus 3% NaCl (150 ml 3% over 20 min once) | |
| Chronic hyponatremia | ||
| SIAD | Fluid restriction (first line) | Fluid restriction (first line) |
| Demeclocycline, urea, or vaptan (second line) | Urea or loop diuretics + oral NaCl (second line) | |
| Do not recommend or recommend against vaptana | ||
| Recommend against lithium or demeclocycline | ||
| Hypovolemic hyponatremia | Isotonic saline | Isotonic saline or balanced crystalloid solution |
| Hypervolemic hyponatremia | Fluid restriction | Fluid restriction |
| Vaptansb | Recommend against vaptan | |
| Correction rates | Minimum: 4–8 mmol/L per d, 4–6 mmol/L per d (high risk of ODS) | No minimum |
| Limits: 10–12 mmol/L per d, 8 mmol/L per d (high risk of ODS) | Limit: 10 mmol/L per d | |
| Management of overcorrection | Baseline SNa≥120 mmol/L: probably unnecessary | Start once limit is exceeded |
| Baseline SNa<120 mmol/L: start relowering with electrolyte-free water or desmopressin after correction exceeds 6–8 mmol/L per d | Consult an expert to discuss infusion containing electrolyte-free water (10 ml/kg) with or without 2 _μ_g desmopressin iv |
Treatment of Acute Hyponatremia
Several settings predispose to acute hyponatremia, especially if combined with increased free water intake.71 Among others, these include the postoperative period, exercise, and the use of 3,4-methylenedioxymethamphetamine (“Ecstasy”), haloperidol, thiazide diuretics, desmopressin, oxytocin, or intravenous cyclophosphamide.71–74 A specific situation is the use of irrigants (glycine, sorbitol, mannitol) during transurethral or hysteroscopic procedures. Although absorption of the irrigants glycine and sorbitol may cause hypotonic hyponatremia, the degree of hypotonicity and therefore the risk of cerebral edema depends on the type of irrigant and the time course in osmolar shifts.75,76 In contrast, mannitol causes hypertonic hyponatremia without a risk for cerebral edema. In daily practice, the distinction between acute and chronic hyponatremia is difficult, because the time in which hyponatremia developed is usually unknown. The United States and European guidelines approached this challenge differently. The United States guideline adhered to acute versus chronic hyponatremia, but did subdivide acute hyponatremia on the basis of the presence of severe or mild-to-moderate symptoms (Table 2).9 The European guideline based its recommendations primarily on the presence and severity of symptoms rather than on duration.7 Both guidelines recommend hypertonic saline (typically 3% NaCl) for acute or symptomatic hyponatremia.7,9 Hypertonic saline is an effective and potentially life-saving treatment for cerebral edema due to hyponatremia, as the high extracellular sodium concentration immediately removes water from the intracellular space. In patients with hypervolemic hyponatremia, hypertonic saline may be combined with loop diuretics.9 The required volume of hypertonic saline to reach a predefined increase in SNa can be estimated using the Adrogué–Madias or Barsoum–Levine formulae.77,78 Although predictions with these formulae are fairly accurate,66 a switch toward giving hypertonic saline as fixed bolus has occurred in recent years.79 No studies have systematically tested this approach, but there are a number of appealing aspects. First, especially in patients with cerebral edema, it is desirable to achieve a rapid partial correction in SNa. Second, a fixed bolus omits the need for calculations in a patient with an acute problem, limiting potential calculation errors. Third, bolus therapy limits the risk of overcorrection, which does occur commonly with a continuous infusion of hypertonic saline.80 On the basis of these considerations, both guidelines recommend bolus therapy, albeit with slightly different specifications (Table 2).7,9 Recently, Ayus and colleagues reported their experience using a different protocol (500 ml 3% NaCl over 6 hours) in 64 patients with hyponatremic encephalopathy (SNa<130 mmol/L and neurologic symptoms).81 On average, this protocol increased SNa with 12 and 14 mmol/L in the first 24–48 hours, and improved symptoms without evidence of ODS.81 However, the severity of hyponatremia (SNa frequently <110 mmol/L) and the duration of symptoms suggest that some of these patients had chronic hyponatremia. If so, these correction rates would exceed currently recommended limits (Table 2).
Treatment of Chronic Hyponatremia
Except for hypovolemic hyponatremia, the treatment of chronic hyponatremia relies on reducing free water intake and/or increasing renal free water excretion (Table 2). Fluid restriction (<1 L/d) is often the cornerstone of the therapy for chronic hyponatremia.24 The urine to serum electrolyte ratio ([UNa + urine potassium concentration]/SNa) indicates if the patient is in an antidiuretic or aquaretic phase, and can also help estimate the degree of fluid restriction required to increase SNa.3,11,24,82 For patients with a ratio>1 (indicating concentrated urine), <500 ml fluid/d is recommended, which is difficult to adhere to. Winzeler et al. recently showed that in patients with SIAD fluid restriction is effective in 59% of patients.83 Predictors of nonresponse were a UNa≥130 mmol/L and UOsm≥500 mOsm/kg.83 This implies that in patients with chronic hyponatremia pharmacologic therapy is often required to increase renal free water excretion. This can be achieved by treatment with loop diuretics, urea, vasopressin receptor antagonists (“vaptans”), or demeclocycline. The two guidelines diverge in their recommendations regarding pharmacologic therapy for SIAD and hypervolemic hyponatremia (Table 2). This was the case especially for vaptans, which will therefore be discussed in more detail below.
Vaptans
Vaptans block vasopressin type 2 receptors in collecting duct principal cells and therefore induce aquaresis (for comprehensive review, see Berl,84 Hoorn and Zietse,85 Lehrich et al.,86 Rozen-Zvi et al.,87 and Greenberg and Verbalis88). Several vaptans were developed, including tolvaptan, satavaptan, lixivaptan, and conivaptan (which also targets vasopressin type 1a receptors). On the basis of their mechanism of action, vaptans are a logical and targeted therapy for hyponatremic patients with excess vasopressin. Indeed, several large clinical trials have shown that vaptans are clearly effective in increasing SNa in patients with hyponatremia due to SIAD, heart failure, or liver cirrhosis.89,90 Both guidelines agree that there is no place for vaptans in patients with acute or severely symptomatic hyponatremia, for which hypertonic saline is the treatment of choice.7,9 Still, it has been difficult to position vaptans in the therapeutic arsenal of chronic hyponatremia.11,84,91,92 The United States guideline lists vaptans as one of the pharmacologic options, if fluid restriction has failed (Table 2).9 The European guideline did not recommend vaptans in moderate hyponatremia.7 The reason to do so was the absence of evidence for improved hard outcomes with correction of SNa. Meanwhile, one meta-analysis has suggested improved survival with correction of hyponatremia,93 although bias is difficult to exclude because no randomized controlled trials are available. Furthermore, there is evidence for potential harm of vaptans, including overcorrection, and liver toxicity.7,87,94–96 Because ODS has mainly been reported after overcorrection of profound hyponatremia, the European guideline recommended against vaptans in this setting.7 Recently, Tzoulis et al. reported “real-life experience” with tolvaptan in 61 patients with resistant hyponatremia due to SIAD.97 The average rise in SNa after 24 hours was 9.0±3.9 mmol/L. Excessive correction of hyponatremia (>12 mmol/L per day) was observed in 23% of patients (all with profound hyponatremia), although none of them developed signs of ODS.97 ODS was reported in one patient with heart failure in whom 15 mg tolvaptan caused SNa to increase from 126 to 142 mmol/L in the first day and to further increase to 187 mmol/L in subsequent days.94 On the other hand, improvement of symptoms has been shown with the use of vaptans. This includes improvements in some neurocognitive symptoms,98 performance status in cancer patients,99 dyspnea in patients with heart failure,100 and ascites in patients with liver cirrhosis.101 Therefore, in our view, an unresolved question with regard to the use of vaptans remains, of whether symptomatic improvement outweighs the risk of overcorrection, even if ODS is rare.
Urea
Both guidelines suggest an interesting alternative to vaptans for chronic hyponatremia due to SIAD, namely urea.102 Urea induces an osmotic diuresis, thereby increasing renal free water excretion. Decaux and colleagues pioneered the use of urea in the 1980s for SIAD, but also for other forms of hyponatremia.103–109 More recently, in 12 patients with SIAD, Soupart et al. compared the treatment with satavaptan to urea (both treatment periods 1 year).110 Interestingly, both therapies had a similar efficacy and side-effect profile. Although urea does not prevent overcorrection, it may reduce the risk of the associated brain damage. In a rat model of experimental SIAD, Gankam Kenge et al. compared the neurologic outcomes after overcorrection (approximately 30 mmol/L per day) with hypertonic saline, lixivaptan, or urea.111 Quite strikingly, neurologic scores and survival were better in the animals treated with urea. Histologic analysis showed that, in comparison to the two other treatments, urea reduced demyelination, microglial activation, and changes in the blood-brain barrier, and increased astrocyte viability.111 Although one should be careful to extrapolate these findings to humans, this may explain why patients with ESRD and hyponatremia do not develop ODS after treatment with hemodialysis.112 One specific disadvantage of urea used to be its palatability. This problem has been solved by developing a formulation in which urea is combined with sodium bicarbonate, citric acid, and sucrose (see European guideline for prescription7) and by the development of a commercially available urea powder drink mix (Ure-Na by Nephcentric).
Summary and Conclusions
Our impression is that the development of the United States and European guidelines has helped to standardize and improve the management of hyponatremia. The two guidelines are more often in agreement than in disagreement. The discrepancies are likely related to the interpretation of the limited evidence and the methodology used to draft the guidelines.113 Nagler et al. evaluated all available international guidelines on hyponatremia and analyzed how well they met the Appraisal of Guidelines for Research and Evaluation criteria.114 They identified considerable variation in methodologic rigor in the development of guidelines, potentially explaining inconsistencies in recommendations.114 Because hyponatremia is a heterogeneous disorder rather than a clear-cut disease, not all patients can be covered by guidelines. That said, which evidence does the field need for the coming years? First, it would be useful to evaluate if a combination of the traditional and newer diagnostic tests would improve not only diagnosis but also outcomes. Second, the approach of giving a bolus of hypertonic saline should be studied to address the optimal volume, whether this should be on the basis of (ideal) body weight, and how often it should be repeated to reach the desired increase in SNa.115 Third, the role of vaptans in the treatment of chronic hyponatremia remains a logical focus. For example, it would be important to analyze whether the copeptin-based subtypes of SIAD respond differently to vaptans (Figure 2). Finally, studies analyzing the effect of a vaptan in comparison with another active treatment (rather than placebo) on patient-relevant outcomes (rather than SNa) are warranted.
Disclosures
None.
Acknowledgments
E.J.H. is supported by the Dutch Kidney Foundation (KSP-14OK19).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Adrogué HJ, Madias NE: The challenge of hyponatremia. J Am Soc Nephrol 23: 1140–1148, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg A, Verbalis JG, Amin AN, Burst VR, Chiodo JA 3rd , Chiong JR, Dasta JF, Friend KE, Hauptman PJ, Peri A, Sigal SH: Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int 88: 167–177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterns RH: Disorders of plasma sodium--causes, consequences, and correction. N Engl J Med 372: 55–65, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Winzeler B, Jeanloz N, Nigro N, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum C, Bock A, Huber A, Mueller B, Christ-Crain M: Long-term outcome of profound hyponatremia: A prospective 12 months follow-up study. Eur J Endocrinol 175: 499–507, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD: Mortality and serum sodium: Do patients die from or with hyponatremia? Clin J Am Soc Nephrol 6: 960–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group : Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 170: G1–G47, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group : Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant 29[Suppl 2]: i1–i39, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E: Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 40: 320–331, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ: Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med 126[Suppl 1]: S1–S42, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Gross P: Panel recommendations on hyponatremia. Am J Med 127: e29, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Adrogué HJ, Madias NE: Diagnosis and treatment of hyponatremia. Am J Kidney Dis 64: 681–684, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Tormey WP, Carney M, Cuesta M, Sreenan S: Reference change values for sodium are ignored by the American and European treatment guidelines for hyponatremia. Clin Chem 61: 1430–1432, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Avila M: The clinical practice guideline on diagnosis and treatment of hyponatraemia: A response from Otsuka Pharmaceutical Europe Ltd. Eur J Endocrinol 171: L1–L3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen DM, Ellison DH: Evaluating hyponatremia. JAMA 313: 1260–1261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoorn EJ, Carlotti AP, Costa LA, MacMahon B, Bohn G, Zietse R, Halperin ML, Bohn D: Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr 150: 467–473, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Turchin A, Seifter JL, Seely EW: Clinical problem-solving. Mind the gap. N Engl J Med 349: 1465–1469, 2003 [DOI] [PubMed] [Google Scholar]
- 17.McGee S, Abernethy WB 3rd , Simel DL: The rational clinical examination. Is this patient hypovolemic? JAMA 281: 1022–1029, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Musch W, Thimpont J, Vandervelde D, Verhaeverbeke I, Berghmans T, Decaux G: Combined fractional excretion of sodium and urea better predicts response to saline in hyponatremia than do usual clinical and biochemical parameters. Am J Med 99: 348–355, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Chung HM, Kluge R, Schrier RW, Anderson RJ: Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med 83: 905–908, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Hoorn EJ, Halperin ML, Zietse R: Diagnostic approach to a patient with hyponatraemia: Traditional versus physiology-based options. QJM 98: 529–540, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S: Utility and limitations of the traditional diagnostic approach to hyponatremia: A diagnostic study. Am J Med 123: 652–657, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Hoorn EJ, Hotho D, Hassing RJ, Zietse R: Unexplained hyponatremia: Seek and you will find. Nephron, Physiol 118: 66–71, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Roussel R, Fezeu L, Marre M, Velho G, Fumeron F, Jungers P, Lantieri O, Balkau B, Bouby N, Bankir L, Bichet DG: Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab 99: 4656–4663, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Ellison DH, Berl T: Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 356: 2064–2072, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Musch W, Decaux G: Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM 91: 749–753, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Steele A, Gowrishankar M, Abrahamson S, Mazer CD, Feldman RD, Halperin ML: Postoperative hyponatremia despite near-isotonic saline infusion: A phenomenon of desalination. Ann Intern Med 126: 20–25, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Hoorn EJ, Lindemans J, Zietse R: Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant 21: 70–76, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Sood L, Sterns RH, Hix JK, Silver SM, Chen L: Hypertonic saline and desmopressin: A simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis 61: 571–578, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Perianayagam A, Sterns RH, Silver SM, Grieff M, Mayo R, Hix J, Kouides R: DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol 3: 331–336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenske W, Störk S, Koschker AC, Blechschmidt A, Lorenz D, Wortmann S, Allolio B: Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab 93: 2991–2997, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum CA, Nickel CH, Bingisser R, Bock A, Huber A, Müller B, Christ-Crain M: Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: ‘The Co-MED Study’ [published online ahead of print September 22, 2016]. Clin Endocrinol (Oxf) 10.1111/cen.13243 [DOI] [PubMed] [Google Scholar]
- 32.Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E: Is it cerebral or renal salt wasting? Kidney Int 76: 934–938, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Sherlock M, O’Sullivan E, Agha A, Behan LA, Owens D, Finucane F, Rawluk D, Tormey W, Thompson CJ: Incidence and pathophysiology of severe hyponatraemia in neurosurgical patients. Postgrad Med J 85: 171–175, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML: Cerebral salt wasting: Truths, fallacies, theories, and challenges. Crit Care Med 30: 2575–2579, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Anderson RJ, Chung HM, Kluge R, Schrier RW: Hyponatremia: A prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med 102: 164–168, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Hoorn EJ, Zietse R: Hyponatremia revisited: Translating physiology to practice. Nephron, Physiol 108: 46–59, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Friedman E, Shadel M, Halkin H, Farfel Z: Thiazide-induced hyponatremia. Reproducibility by single dose rechallenge and an analysis of pathogenesis. Ann Intern Med 110: 24–30, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Frenkel NJ, Vogt L, De Rooij SE, Trimpert C, Levi MM, Deen PM, van den Born BJ: Thiazide-induced hyponatraemia is associated with increased water intake and impaired urea-mediated water excretion at low plasma antidiuretic hormone and urine aquaporin-2. J Hypertens 33: 627–633, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Robertson GL: Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med 119[Suppl 1]: S36–S42, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Hoorn EJ, Monserez DA, Fenton RA, Overdevest I, Apperloo AJ, Zietse R, Hardillo JA: Olfactory neuroblastoma with hyponatremia. J Clin Oncol 33: e88–e92, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Tamura Y, Yoshida S: Effect of administration of corticotropin-releasing hormone and glucocorticoid on arginine vasopressin response to osmotic stimulus in normal subjects and patients with hypocorticotropinism without overt diabetes insipidus. J Clin Endocrinol Metab 69: 396–401, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Cuesta M, Garrahy A, Slattery D, Gupta S, Hannon AM, Forde H, McGurren K, Sherlock M, Tormey W, Thompson CJ: The contribution of undiagnosed adrenal insufficiency to euvolaemic hyponatraemia: Results of a large prospective single-centre study. Clin Endocrinol (Oxf) 85: 836–844, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Smith JC, Siddique H, Corrall RJ: Misinterpretation of serum cortisol in a patient with hyponatraemia. BMJ 328: 215–216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Hoek J, Hoorn EJ, de Jong GM, Janssens EN, de Herder WW: Severe hyponatremia with high urine sodium and osmolality. Clin Chem 55: 1905–1908, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG: Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bragança AC, Moyses ZP, Magaldi AJ: Carbamazepine can induce kidney water absorption by increasing aquaporin 2 expression. Nephrol Dial Transplant 25: 3840–3845, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Moyses ZP, Nakandakari FK, Magaldi AJ: Fluoxetine effect on kidney water reabsorption. Nephrol Dial Transplant 23: 1173–1178, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE: Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352: 1884–1890, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses AM, Clayton B: Impairment of osmotically stimulated AVP release in patients with primary polydipsia. Am J Physiol 265: R1247–R1252, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Morgenthaler NG, Struck J, Alonso C, Bergmann A: Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Christ-Crain M, Fenske W: Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol 12: 168–176, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Fenske W, Störk S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B: Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 94: 123–129, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Robertson GL, Athar S: The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 42: 613–620, 1976 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Bourque CW: Amplification of transducer gain by angiotensin II-mediated enhancement of cortical actin density in osmosensory neurons. J Neurosci 28: 9536–9544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fenske WK, Christ-Crain M, Hörning A, Simet J, Szinnai G, Fassnacht M, Rutishauser J, Bichet DG, Störk S, Allolio B: A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. J Am Soc Nephrol 25: 2376–2383, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanchard A, Steichen O, De Mota N, Curis E, Gauci C, Frank M, Wuerzner G, Kamenicky P, Passeron A, Azizi M, Llorens-Cortes C: An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J Clin Endocrinol Metab 98: 2084–2089, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum CA, Nickel CH, Bingisser R, Bock A, Rentsch Savoca K, Huber A, Müller B, Christ-Crain M: Mid-regional pro-atrial natriuretic peptide and the assessment of volaemic status and differential diagnosis of profound hyponatraemia. J Intern Med 278: 29–37, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Hus-Citharel A, Bodineau L, Frugière A, Joubert F, Bouby N, Llorens-Cortes C: Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology 155: 4483–4493, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Tzikas S, Keller T, Wild PS, Schulz A, Zwiener I, Zeller T, Schnabel RB, Sinning C, Lubos E, Kunde J, Münzel T, Lackner KJ, Blankenberg S: Midregional pro-atrial natriuretic peptide in the general population/Insights from the Gutenberg Health Study. Clin Chem Lab Med 51: 1125–1133, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Sterns RH, Riggs JE, Schochet SS Jr: Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med 314: 1535–1542, 1986 [DOI] [PubMed] [Google Scholar]
- 61.Sterns RH, Silver SM: Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 119[Suppl 1]: S12–S16, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Berl T: Treating hyponatremia: Damned if we do and damned if we don’t. Kidney Int 37: 1006–1018, 1990 [DOI] [PubMed] [Google Scholar]
- 63.Hoorn EJ, Zietse R: Hyponatremia and mortality: Moving beyond associations. Am J Kidney Dis 62: 139–149, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Berl T: Treating hyponatremia: What is all the controversy about? Ann Intern Med 113: 417–419, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Martin RJ: Central pontine and extrapontine myelinolysis: The osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 75[Suppl 3]: iii22–iii28, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liamis G, Kalogirou M, Saugos V, Elisaf M: Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant 21: 1564–1569, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Gankam Kengne F, Soupart A, Pochet R, Brion JP, Decaux G: Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int 76: 614–621, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Electrolyte disorders in community subjects: Prevalence and risk factors. Am J Med 126: 256–263, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Hoorn EJ, Zietse R: Hyponatremia and mortality: How innocent is the bystander? Clin J Am Soc Nephrol 6: 951–953, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Schrier RW, Sharma S, Shchekochikhin D: Hyponatraemia: More than just a marker of disease severity? Nat Rev Nephrol 9: 37–50, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Hsu YJ, Chiu JS, Lu KC, Chau T, Lin SH: Biochemical and etiological characteristics of acute hyponatremia in the emergency department. J Emerg Med 29: 369–374, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Achinger SG, Arieff AI, Kalantar-Zadeh K, Ayus JC: Desmopressin acetate (DDAVP)-associated hyponatremia and brain damage: A case series. Nephrol Dial Transplant 29: 2310–2315, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Moses AM, Miller M: Drug-induced dilutional hyponatremia. N Engl J Med 291: 1234–1239, 1974 [DOI] [PubMed] [Google Scholar]
- 74.Liamis G, Milionis H, Elisaf M: A review of drug-induced hyponatremia. Am J Kidney Dis 52: 144–153, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Agarwal R, Emmett M: The post-transurethral resection of prostate syndrome: Therapeutic proposals. Am J Kidney Dis 24: 108–111, 1994 [DOI] [PubMed] [Google Scholar]
- 76.Ayus JC, Arieff AI: Glycine-induced hypo-osmolar hyponatremia. Arch Intern Med 157: 223–226, 1997 [PubMed] [Google Scholar]
- 77.Adrogué HJ, Madias NE: Hyponatremia. N Engl J Med 342: 1581–1589, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Barsoum NR, Levine BS: Current prescriptions for the correction of hyponatraemia and hypernatraemia: Are they too simple? Nephrol Dial Transplant 17: 1176–1180, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Moritz ML, Ayus JC: 100 cc 3% sodium chloride bolus: A novel treatment for hyponatremic encephalopathy. Metab Brain Dis 25: 91–96, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH: Hypertonic saline for hyponatremia: Risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2: 1110–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Ayus JC, Caputo D, Bazerque F, Heguilen R, Gonzalez CD, Moritz ML: Treatment of hyponatremic encephalopathy with a 3% sodium chloride protocol: A case series. Am J Kidney Dis 65: 435–442, 2015 [DOI] [PubMed] [Google Scholar]
- 82.Rose BD: New approach to disturbances in the plasma sodium concentration. Am J Med 81: 1033–1040, 1986 [DOI] [PubMed] [Google Scholar]
- 83.Winzeler B, Lengsfeld S, Nigro N, Suter-Widmer I, Schütz P, Arici B, Bally M, Blum C, Bock A, Huber A, Müller B, Christ-Crain M: Predictors of nonresponse to fluid restriction in hyponatraemia due to the syndrome of inappropriate antidiuresis. J Intern Med 280: 609–617, 2016 [DOI] [PubMed] [Google Scholar]
- 84.Berl T: Vasopressin antagonists. N Engl J Med 372: 2207–2216, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Hoorn EJ, Zietse R: Vasopressin-receptor antagonists. Future Cardiol 6: 523–534, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Lehrich RW, Ortiz-Melo DI, Patel MB, Greenberg A: Role of vaptans in the management of hyponatremia. Am J Kidney Dis 62: 364–376, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U: Vasopressin receptor antagonists for the treatment of hyponatremia: Systematic review and meta-analysis. Am J Kidney Dis 56: 325–337, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Greenberg A, Verbalis JG: Vasopressin receptor antagonists. Kidney Int 69: 2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, Czerwiec FS; SALTWATER Investigators : Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 21: 705–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators : Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Gross PA, Wagner A, Decaux G: Vaptans are not the mainstay of treatment in hyponatremia: Perhaps not yet. Kidney Int 80: 594–600, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Borne RT, Krantz MJ: Lixivaptan for hyponatremia--the numbers game. JAMA 308: 2345–2346, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Corona G, Giuliani C, Verbalis JG, Forti G, Maggi M, Peri A: Hyponatremia improvement is associated with a reduced risk of mortality: Evidence from a meta-analysis. PLoS One 10: e0124105, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malhotra I, Gopinath S, Janga KC, Greenberg S, Sharma SK, Tarkovsky R: Unpredictable nature of tolvaptan in treatment of hypervolemic hyponatremia: Case review on role of vaptans. Case Rep Endocrinol 2014: 807054, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarges P, Steinberg JM, Lewis JH: Drug-induced liver injury: Highlights from a review of the 2015 literature. Drug Saf 39: 801–821, 2016 [DOI] [PubMed] [Google Scholar]
- 96.Woodhead JL, Brock WJ, Roth SE, Shoaf SE, Brouwer KL, Church R, Grammatopoulos TN, Stiles L, Siler SQ, Howell BA, Mosedale M, Watkins PB, Shoda LK: Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug-induced liver injury and identify patient susceptibility factors [published online ahead of print September 21, 2016]. Toxicol Sci 10.1093/toxsci/kfw193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tzoulis P, Waung JA, Bagkeris E, Carr H, Khoo B, Cohen M, Bouloux PM: Real-life experience of tolvaptan use in the treatment of severe hyponatraemia due to syndrome of inappropriate antidiuretic hormone secretion. Clin Endocrinol (Oxf) 84: 620–626, 2016 [DOI] [PubMed] [Google Scholar]
- 98.Verbalis JG, Ellison H, Hobart M, Krasa H, Ouyang J, Czerwiec FS; Investigation of the Neurocognitive Impact of Sodium Improvement in Geriatric Hyponatremia: Efficacy and Safety of Tolvaptan (INSIGHT) Investigators : Tolvaptan and neurocognitive function in mild to moderate chronic hyponatremia: A randomized trial (INSIGHT). Am J Kidney Dis 67: 893–901, 2016 [DOI] [PubMed] [Google Scholar]
- 99.Petereit C, Zaba O, Teber I, Lüders H, Grohé C: A rapid and efficient way to manage hyponatremia in patients with SIADH and small cell lung cancer: Treatment with tolvaptan. BMC Pulm Med 13: 55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators : Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA 297: 1319–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Yan L, Xie F, Lu J, Ni Q, Shi C, Tang C, Yang J: The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: A meta-analysis of randomized controlled trials. BMC Gastroenterol 15: 65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sterns RH, Silver SM, Hix JK: Urea for hyponatremia? Kidney Int 87: 268–270, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Decaux G, Brimioulle S, Genette F, Mockel J: Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med 69: 99–106, 1980 [DOI] [PubMed] [Google Scholar]
- 104.Decaux G, Soupart A: Urea treatment for exercise-associated hyponatremia. Clin J Sport Med 16: 276, author reply 276, 2006 [DOI] [PubMed] [Google Scholar]
- 105.Decaux G, Andres C, Gankam Kengne F, Soupart A: Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care 14: R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cauchie P, Vincken W, Decaux G: Urea treatment for water retention in hyponatremic congestive heart failure. Int J Cardiol 17: 102–104, 1987 [DOI] [PubMed] [Google Scholar]
- 107.Verhoeven A, Musch W, Decaux G: Treatment of the polydipsia-hyponatremia syndrome with urea. J Clin Psychiatry 66: 1372–1375, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Decaux G, Unger J, Brimioulle S, Mockel J: Hyponatremia in the syndrome of inappropriate secretion of antidiuretic hormone. Rapid correction with urea, sodium chloride, and water restriction therapy. JAMA 247: 471–474, 1982 [PubMed] [Google Scholar]
- 109.Decaux G, Mols P, Cauchie P, Flamion B, Delwiche F: Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea. Nephron 44: 337–343, 1986 [DOI] [PubMed] [Google Scholar]
- 110.Soupart A, Coffernils M, Couturier B, Gankam-Kengne F, Decaux G: Efficacy and tolerance of urea compared with vaptans for long-term treatment of patients with SIADH. Clin J Am Soc Nephrol 7: 742–747, 2012 [DOI] [PubMed] [Google Scholar]
- 111.Gankam Kengne F, Couturier BS, Soupart A, Decaux G: Urea minimizes brain complications following rapid correction of chronic hyponatremia compared with vasopressin antagonist or hypertonic saline. Kidney Int 87: 323–331, 2015 [DOI] [PubMed] [Google Scholar]
- 112.Oo TN, Smith CL, Swan SK: Does uremia protect against the demyelination associated with correction of hyponatremia during hemodialysis? A case report and literature review. Semin Dial 16: 68–71, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Van Biesen W, Vanholder R: Clinical practice guidelines on diagnosis and treatment of hyponatraemia: Response to letter from Otsuka Ltd. Eur J Endocrinol 171: L5–L6, 2014 [DOI] [PubMed] [Google Scholar]
- 114.Nagler EV, Vanmassenhove J, van der Veer SN, Nistor I, Van Biesen W, Webster AC, Vanholder R: Diagnosis and treatment of hyponatremia: A systematic review of clinical practice guidelines and consensus statements. BMC Med 12: 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spital A: Treatment of hyponatremic encephalopathy. Am J Kidney Dis 66: 540, 2015 [DOI] [PubMed] [Google Scholar]
- 116.Fenske W, Sandner B, Christ-Crain M: A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. Best Pract Res Clin Endocrinol Metab 30: 219–233, 2016 [DOI] [PubMed] [Google Scholar]