Molecular Imaging of Tumor Hypoxia with Positron Emission Tomography (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 14.
Published in final edited form as: Radiat Res. 2014 Mar 27;181(4):335–349. doi: 10.1667/RR13590.1
Abstract
The problem of tumor hypoxia has been recognized and studied by the oncology community for over 60 years. From radiation and chemotherapy resistance to the increased risk of metastasis, the low oxygen concentrations in tumors have caused patients with many types of tumors to respond poorly to conventional cancer therapies. It is clear that patients with high levels of tumor hypoxia have a poorer overall treatment response and that the magnitude of hypoxia is an important prognostic factor. As a result, the development of methods to measure tumor hypoxia using invasive and non-invasive techniques has become desirable to the clinical oncology community. A variety of imaging modalities have been established to visualize hypoxia in vivo. Positron Emission Tomography (PET) imaging, in particular, has played a key role for imaging tumor hypoxia because of the development of hypoxia-specific radiolabelled agents. Consequently, this technique is increasingly used in the clinic for a wide variety of cancer types. Following a broad overview of the complexity of tumor hypoxia and measurement techniques to date, this review will focus specifically on the accuracy and reproducibility of PET imaging to quantify tumor hypoxia. Despite numerous advances in the field of PET imaging for hypoxia, we continue to search for the ideal hypoxia tracer to both qualitatively and quantitatively define the tumor hypoxic volume in a clinical setting to optimize treatments and predict response in cancer patients.
Introduction
Tumor hypoxia is a characteristic of solid tumors and has been recognized as a crucial factor impacting cancer treatment effectiveness since its discovery (1–3). Hypoxia occurs because of the high rate of oxygen demanded and consumed by the tumor cells, stroma and endothelial cells exceeds that of the supply which is diminished compared to the blood supply of normal tissue (4). The tumor hypoxic microenvironment impairs response to chemotherapy and radiation treatment for cancer patients resulting in poor prognosis and a reduction in overall survival (5). This is caused by the presence of hypoxia in the tumor and the associated cellular oxygen reduction; as well as hypoxia-induced factors that lead to increased tumor proliferation (tumor invasion and metastases) and a more aggressive tumor phenotype (poor differentiation and reduced apoptosis) (5–7).
For these reasons, over the last 60 years, invasive and non-invasive techniques have been developed to measure tumor hypoxia. Although no method is ideal, molecular imaging has become the most commonly used non-invasive modality. Positron Emission Tomography (PET), Single-Photon Emission Computed Tomography (SPECT), Magnetic Resonance Imaging (MRI), and Optical Imaging have become useful tools to characterize the magnitude and variability of hypoxia within a tumor and to guide clinical treatment decisions (8,9). PET, in particular, has become a leader in hypoxia imaging. Several PET radiotracers have been developed and continue to be synthesized to visualize the extent of tumor hypoxia on a patient-specific basis (10). It has been shown that several of these tracers, particularly nitroimidazoles, can directly and reproducibly identify the presence of hypoxia (11,12).
Pre-treatment PET imaging for tumor hypoxia combined with quantitative methods for characterizing the hypoxic status of a tumor could allow the clinician to more precisely stratify patients for randomized clinical trials aimed at overcoming the treatment resistance conferred by tumor hypoxia or alter radiation therapy treatment plans. However, a major problem with PET imaging for tumor hypoxia to date is that currently-implemented methods used to quantify the hypoxic fraction do not represent absolute tumor _p_O2 values. Moreover, existing techniques are inconsistent between clinics, resulting in highly variable and potentially suboptimal methods for defining hypoxic tumor volumes. Improving methods of quantification would enable more accurate identification of inter- and intra-tumor variability in hypoxia, and help the clinician combat increased tumor aggressiveness and improve poor prognosis for cancer patients. The aim of this review is to discuss the current status and promise of PET imaging for tumor hypoxia measurement in a clinical setting and how it can be used to improve the treatment of oncology patients.
Tumor hypoxia affects cancer therapy in a complex way
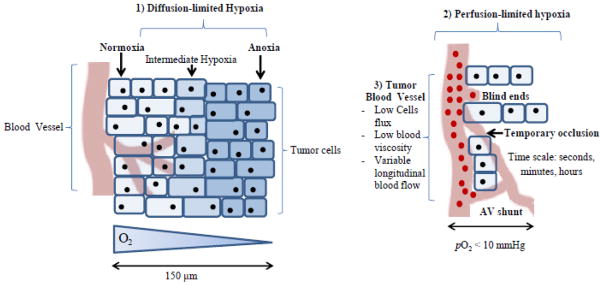
Tumor hypoxia results from several tumor-specific traits as illustrated in Figure 1. These include: [1] chronic hypoxia caused by the limited-diffusion distance of oxygen from blood vessels to the tumor cells (~150–200 μm) (2); [2] acute hypoxia due to the perfusion-limited impairment of the vascular vessels by (a) their structural abnormality and (b) high interstitial pressure caused by the combination of fluid in the tumor matrix and the rapidly proliferating tumor cells leading to constriction of the intra-tumor vasculature (13–18); and [3] tumor blood vessel traits such as a reduced vascular density and arteriolar supply, poor blood vessels networks, varied red blood cell flux and high blood viscosity (19).
Figure 1.
Tumor hypoxia is caused by several tumor-specific traits that result in chronic (diffusion-limited), acute (perfusion-limited) hypoxia. In combination with tumor blood vessel abnormalities, tumor hypoxia is a highly complex biological mechanism, making it difficult to counteract with current treatment techniques.
The adverse impact of tumor hypoxia on cancer treatment response is a result of the reduction in diffusion, perfusion, and delivery of oxygen to cancer cells. Tumor hypoxia has been shown to have a negative impact on the effectiveness of radiation therapy. This resistance is attributed to a reduction in DNA damage under reduced oxygen conditions. There is an intrinsic oxygen dependence of radiation-induced DNA damage which has a higher probability of chemical restoration under hypoxic conditions but may become permanent in the presence of oxygen. (20–22). As a result, the biological effect of megavoltage X-ray radiation is 2–3 times higher in the presence of oxygen (21,23). Reduction in cellular oxygen and nutrients also forms proteomic and genomic changes creating more mutant, adaptive, and aggressive tumor cells (5,16). However, clinical evidence suggests that hypoxia-induced treatment failure is more likely attributed to radiation resistance and not hypoxia-induced metastasis (24). Consequently, the hypoxic fraction in tumors has been correlated with a negative treatment outcome (25,26) and a reduction in overall survival (6,24,27–30).
In the 1990s, the tumor hypoxic environment (oxygen and delivery imbalance) was connected with many tumor-specific traits (31,32). The main effect of the reduced oxygen level was the increased production of hypoxia-inducible factor 1 (HIF-1), a transcription factor that becomes active under hypoxic conditions and regulates oxygen homeostasis under hypoxic conditions (32). The influence of oxygen concentration on the activity of HIF-1 is mostly a result of a delicate balance between prolyl hydrolyase domain-containing proteins and the crucial role of the von Hippel-Lindau tumor suppressor protein (31). HIF-1 activates genes that upregulate glycolysis, angiogenesis and enhances cell survival under oxidative-stress resulting in a causal relationship between hypoxia and tumor metastasis and growth (33,34). Target genes include vascular endothelial growth factor (VEGF), facilitative glucose transporters (GLUTs), hexokinases (HKs), erythropoietin (EPO), carbonic anhydrase IX (CAIX) and are all targets of hypoxic prevention or identification. Such conditions created by hypoxia, and in turn HIF-1, lead to tumors that are more invasive and resistant to cancer treatments such as chemotherapy (32).
Nearly all solid tumors are positive for some form of hypoxia (severe and intermediate levels) or anoxic (no oxygen present) cells and these areas are often heterogeneously distributed within the tumor (5). The presence of hypoxia does not depend on tumor size, stage, pathology or nodal status (13). Moreover, the prevalence of tumor hypoxia and its traits are not cancer-type specific and have been found in a wide range of human malignancies including cancers of the head and neck (H&N), prostate, rectum, breast, uterine cervix as well as brain tumors, soft tissue sarcomas and malignant melanomas (6,25,26,35–37). Hockel et al. (6) showed a drastic decrease in the overall survival in cervical cancer patients with more hypoxic tumors due to hypoxia-induced increased invasiveness and chemoradiation resistance. Brizel et al. (37) also clinically demonstrated an increase in hypoxia-induced invasive through metastatic disease in soft tissue sarcoma patients. It is evident based on the biological complexity, prevalence, and negative prognostic impact of tumor hypoxia in cancer patients that a measurement technique with the capability of detecting and quantifying hypoxia would have substantial clinical implications.
Methods of tumor hypoxia measurement
Tumor hypoxia measurement can be divided into two categories, invasive and non-invasive. Invasive measurements are considered to be the ‘gold standard’ as they provide a direct measure of oxygen concentration in tissue. Numerous studies using the Eppendorf _p_O2 electrode (38,39) have found that real-time _p_O2 measurements are correlated with negative survival in patients with various cancer types (6,25,26,35–37). Although still in use, the Eppendorf electrode is no longer in production or supported by the manufacturer. In 1999, the Oxylite fiber-optic sensor (40) became available to provide a _p_O2 measurements but has not been validated in the clinical setting. Moreover, both methods are user dependent as the invasive technique requires practice and expertise (41). The _p_O2 values are also restricted by sampling error as hypoxia is heterogeneous and it is not possible to extract _p_O2 measurements for the entire tumor. The invasiveness of the procedure limits its use to measure changes in tumor hypoxia and makes it only useful for superficially-located or easily accessible tumors. Also, the electrode cannot differentiate necrotic and anoxic tissue regions of a tumor.
Immunohistochemical (IHC) staining is another invasive technique used to measure tumor hypoxia. This can be done by administering exogenous bioreductive nitroimidazole compounds, e.g., Pimonidazole or EF5 (42,43), before biopsy that bind to hypoxic regions. IHC staining can also be used to detect hypoxia-specific overexpressed endogenous proteins, e.g., carbonic anhydrase IX (44). However, as the tumor biopsy (even if multiple cores are taken) is not representative of the whole tumor _p_O2, under-sampling is also a problem. Moreover, these methods generally provide information on the presence of the hypoxic regions (as opposed to necrotic ones) but not the location and size. As this technique is invasive, repetitive measurement of changes in the hypoxic fraction after cancer treatment is not realistic in a clinical setting (45).
Molecular imaging techniques can be used to perform non-invasive measurements of tumor hypoxia. These include MRI, SPECT, Optical Imaging, and PET. MRI techniques are based on contrast agents (endogenous or exogenous) and include techniques such as electron paramagnetic resonance (EPR) (46,47), dynamic contract MRI (DCE-MRI), magnetic resonance spectroscopy (MRS) (48) and blood oxygen-dependent level (BOLD) imaging (49,50). Optical imaging techniques measure the optical absorption, scattering and fluorescence in a tissue (51). Recent developments in PET have allowed it to surpass SPECT in the availability and number of hypoxia imaging agents. These include halogenated nitroimidazole PET agents, such as 18F and 124I, as well as metallic agents, such as [64Cu]-ATSM. The available PET tracers will be discussed below.
PET imaging to identify tumor hypoxia: current status of PET radiotracers
PET radiotracers consist of a radioisotope and a biologically significant molecule that is specific to the functional measurement, e.g., glucose for glucose metabolism (52). 11C, 15O and 18F are popular radioisotopes due to their short half-lives. In 1981, Chapman et al. (53) were the first to detect tumor hypoxia with nitroimidazole compounds and molecular imaging. The widespread use of PET for imaging hypoxia is made possible through the use of exogenous markers, hypoxia-specific agents that are reduced and covalently bind to intracellular macromolecules in the tumor in the absence of oxygen (54). Figure 2 shows representative clinical images of PET radiotracers for tumor hypoxia. In nitroimidazoles, a common marker for hypoxia, the relationship between the binding of and oxygen tension, is directly correlated with cell retention of the tracer varying significantly over the same range of oxygen concentration that has the largest impact on radiosensitivity (52).
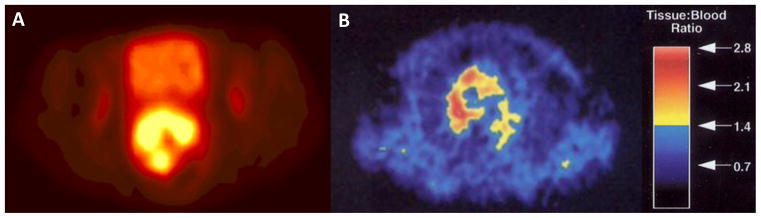
Figure 2.
Representative clinical images of PET radiotracers for tumor hypoxia. (A) 64Cu-ATSM axial image (arbitrary scale) from a static PET scan 30–60 min post-injection in a patient with primary cervical tumor of uterine cervix patients with a tumor-to-muscle activity ratio of 10.3, adapted with permission from Lewis et al. (61). (B) 18F-FMISO axial image from a static 120–160 min PET scan in a non-small cell lung cancer patient with a maximum tumor-to-blood ratio of 2.8, adapted with permission from Koh et al.(131).
Table 1 provides an overview of all of PET radiotracer human studies published to date that have been used in human patients to image tumor hypoxia, their clinical application and conclusions. These include several nitroimidazole radiotracers (10), such as 2-(2-nitro-(1)H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide ([18F]-EF5) (55), [18F]-Fluoroazomycin ([18F]-FAZA),(56), [18F]-Fluoromisonidazole ([18F]-FMISO) (57,58), 3-[18F]fluoro-2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)propan-1-ol ([18F]-HX4) (59), (18)F-fluoroerythronitroimidazole ([18F]-FETNIM) (60), as well as non-nitroimidazole compounds such as [64Cu]diacetyl-bis(_N_4-methylthiosemicarbazone) ([64Cu]-ATSM) (61). [18F]-EF5 is a promising agent stemming from etanidazole radiosensitizer. With higher lipophilicity than [18F]-FMISO, the tracer has shown good tumor diffusion (62). Similarly, [18F]-FAZA is becoming a more popular PET imaging radiotracer for tumor hypoxia as its difference in chemical structure to [18F]-FMISO increases its hydrophilicity allowing for faster clearance and higher tumor-to-background ratio (63). [18F]-FAZA has also been successfully correlated with Pimonidazole immunohistochemical staining in animal models and shown to be a promising clinical marker of hypoxia (64). However, it still requires more clinical testing. [64Cu]-ATSM is a non-nitroimidazole compound that can be trapped intracellularly though the exact hypoxia retention mechanism for [64Cu]-ATSM has yet to be fully elucidated (65). In vitro experiments did not demonstrate hypoxic selectivity with [64Cu]-ATSM (66,67). Moreover, a direct comparison between [64Cu]-ATSM and [18F]-FMISO showed that an increase in oxygenation only resulted in a decrease in uptake of [18F]-FMISO and not [64Cu]-ATSM (68). So despite some clinical use with this agent, more studies are necessary to confirm if it is truly a hypoxia-specific marker (69).
Table 1.
PET radiotracer imaging studies to visualize tumor hypoxia in humans subjects
| PET Tracer | Study | Cancer type | No. of patients (n) | Type and duration of scan + tracer uptake metric | Study conclusion |
|---|---|---|---|---|---|
| [18F]-FMISO | Koh _et al._1992 (57) | Non-small cell lung cancer | 8 | Static scan at 120 minsTBR ≥ 1.4 | 18[F]-FMISO imaging may help select patients for trials targeting radioresistant cancers |
| [18F]-FMISO | Valk _et al._1992 (58) | Glioma | 3 | Static scan at 120–180 minsTumor-to-plasmaNo threshold | 18[F]-FMISO imaging is feasible to detect hypoxia in human gliomas |
| [18F]-FMISO | Koh _et al._1995 (131) | Non-small cell lung cancer | 7 | Static scan at 120–160 minsTBR ≥ 1.4 | 18[F]-FMISO imaging elucidated the changes in tumor oxygenation during radiotherapy |
| [18F]-FMISO | Rajendran et al. 2003 (83) | Soft Tissue Sarcomas | 19 | Static scan at 120 minsTBR ≥ 1.2 | Tumor hypoxia as defined by [18F]-FMISO PET imaging does not correlate with metabolism via [18F]-FDG imaging |
| [18F]-FMISO | Gagel et al. 2004 (77) | Head & Neck | 16 | Static scan at 120 minsTMR.No threshold | [18F]-FMISO PET provides non-invasive measurement of tumor hypoxia in H&N cancer patients |
| [18F]-FMISO | Bruehlmeier et al. 2004 (75) | Glioblastoma | 11 | Dynamic scan for 90 mins adStatic scan at 150–170 minsDistribution Volume >1 | [18F]-FMISO shows hypoxia in brain tumors |
| [18F]-FMISO | Eschmann et al. 2005 (92) | Head & Neck and NSCLC | 40 | Static scan at 240 minsSUVTMR > 2 | Prediction of radiotherapy outcome is feasible based on [18F]-FMISO uptake in tumor |
| [18F]-FMISO | Loi et al. 2005 (99) | Rectal cancer | 16 | Static scan at 120 minsSUVNo threshold | Difficult to use [18F]-FMISO PET imaging to stratify patients for this site but hypoxia was detectable |
| [18F]-FMISO | Thorwarth et al. 2005 (94) | Head & Neck | 16 | Dynamic scan from 15–60 mins and static scan at 120 mins and 180 minsSUVmax HRPNo threshold | Local control information for H&N cancer patients may be derived from the perfusion and diffusion of [18F]-FMISO PET imaging |
| [18F]-FMISO | Hicks et al. 2005 (93) | Head & Neck | 15 | Static scan at 120minsSUVmaxIndependent scoring systemNo threshold | High prevalence of [18F]-FMISO uptake in patient cohort may support prediction of adverse prognosis through PET imaging |
| [18F]-FMISO | Cher et al. 2006 (85) | Glioma | 17 | Static scan at 120 minsSPM99 value | [18F]-FMISO PET imaging provides prognostic information for glioma based on hypoxia status |
| [18F]-FMISO | Cherk et al. 2006 (84) | Non-small cell lung cancer | 21 | Static scan at 120 minsSUVmaxNo threshold | [18F]-FMISO and [18F]-FDG imaging did not show a significant correlation between hypoxia and glucose metabolism |
| [18F]-FMISO | Gagel et al. 2006 (91) | Non-small cell lung cancer | 8 | Static scan at 180 minsSUVmaxSUVmeanTMRNo threshold | [18F]-FMISO PET allowed the definition of hypoxic sub-regions that may correspond to areas of local recurrence |
| [18F]-FMISO | Rischin et al. 2006 (98) | Head & Neck | 45 | Static scan at 120 minsSUVmaxIndependent hypoxic score (93) | Showed the prognostic value of [18F]-FMISO detection of hypoxia in patients stratified to chemoradiation with or without tirapazamine |
| [18F]-FMISO | Thorwarth et al. 2006 (95) | Head & Neck | 12 | Static scans at 120, 240 minsSUV > 1.4 | [18F]FMISO and [18F]FDG PET provide independent tumor information |
| [18F]-FMISO | Eschamnn et al. 2007 (107) | Head & Neck | 14 | Static scan at 240 minsSUV TMRNo threshold | Changes in [18F]-FMISO PET uptake during radiation therapy are indicative of reoxygenation |
| [18F]-FMISO | Lee et al. 2008 (106) | Head & Neck | 10 | Static scan at 120–150 minsTBR ≥ 1.3 | In silico dose escalation is possible in H&N cancer patients based on [18F]-FMISO PET imaging |
| [18F]-FMISO | Lin et al. 2008 (114) | Head & Neck | 7 | Static scan at 120–150 minsTBR ≥ 1.3 | Changes in tumor hypoxia spatial distribution as shown by 18[F]-FMISO PET indicate that coverage of volumes by dose-painting may not be achievable |
| [18F]-FMISO | Nehmeh _et al._2008 (132) | Head & Neck | 20 | Static scan at 117–195 minsSUVTBR > 1.2 | Changes in [18F]-FMISO PET tumor uptake between scans in the same patient can occur |
| [18F]-FMISO | Dirix et al. 2009 (133) | Head & Neck | 15 | Static scan at 120–160 minsTBR > 1.2 | Imaging with [18F]-FMISO PET provides added value to radiotherapy planning in H&N patients |
| [18F]-FMISO | Yamane et al. 201l (90) | Head & Neck | 13 | Static scan at 150minsSUVmax TMRNo threshold | A reduction in [18F]-FMISO uptake after neoadjuvant chemotherapy was seen in H&N patients |
| [18F]-FMISO | Kawai et al. 2011 (134) | Glioblastoma | 10 | Static scan at 15mins, 130–160minsTBR > 1.2 | [18F]-FMISO PET detected hypoxia can be related to the neovascularization shown on by gadolinium-enhanced MRI |
| [18F]-FMISO | Kikuchi et al. 2011 (89) | Head & Neck | 17 | Static scan at 150 minsSUVmaxTBR > 1.3 | [18F]-FMISO PET may predict radiotherapy and survival outcomes in H&N patients |
| [18F]-FMISO | Hugonnet et al. 2011 (135) | Metastatic renal cell carcinoma | 53 | Static scan at 120–240 minsSUVmaxTBR > 1.2 | Sunitinib reduced hypoxia in metastases and did not induce significant hypoxia in non-hypoxic metastases |
| [18F]-FMISO | Hirata et al. 2012 (96) | Glioblastoma | 23 | Static scan at 240 minsSUVmax > 1.3x mean SUV | [18F]-FMISO PET may distinguish glioblastoma from lower grade gliomas based on tumor hypoxia status |
| [18F]-FMISO | Zips et al. 2012 (87) | Head & Neck | 25 | Static scan at 120 minsSUVmax TBRNo threshold | 18[F]-FMISO PET imaging provides strong prognostic information for treatment |
| [18F]-FMISO | Toma-Dasu et al. 2012 (117) | Head & Neck | 7 | Static scan at 120–160 minsUptake values converted to TCP | Incorporation of 18[F]-FMISO PET imaging with this approach to treatment planning may provide improved treatment results for H&N cancer patients |
| [18F]-FMISO | Mammar et al. 2012 (136) | Chordoma | 7 | Static scan at 120 minsSUVmaxTumor-to-cerebellum >1 | [18F]-FMISO PET/CT enables imaging of the tumor hypoxia in residual chordomas |
| [18F]-FMISO | Askoxylakis et al. 2012 (88) | Non-small cell lung cancer | 15 | Dynamic scan for 60 minsTracer kinetic parameter (K1, k2, k3 and k4)No threshold | Correlated functional MRI and [18F]-FMISO PET tumor hypoxic regions and evaluated tumor local response using RECIST criteria |
| [18F]-FMISO | McKeage et al. 2012 (97) | Advanced solid tumors | 42 | Static scan at 90–120 minsTBR > 1.2 | The presence or absence of [18F]-FMISO defined tumor hypoxia did not correlate with response to PR-104-based combination chemotherapy |
| [18F]-FMISO | Okamoto et al. 2013 (82) | Head & Neck | 11 | Static scan at 240 minsSUVmaxTBR ≥ 1.5TMR > 1.25 | [18F]-FMISO PET defined tumor hypoxic regions were reproducible in subsequent scans |
| [18F]-FMISO | Segard et al. 2013 (137) | Pancreatic | 10 | Static scan at 120 minsSUVmax TBRNo threshold | Hypoxia was minimally detected using [18F]-FMISO PET imaging in pancreatic cancer |
| [18F]-FMISO | Chang et al. 2013 (112) | Head & Neck | 8 | Static scan at 120 minsTMR > 1.5Threshold = 1.5 | Hypoxia dose-painting in H&N patients using [18F]-FMISO PET imaging is feasible |
| [18F]-FMISO | Cheng et al. 2013 (86) | Breast cancer | 11 | Static scan at 120, 240 minsSUV ≥ 2.1TBR ≥ 1.2 | In breast cancer patients [18F]-FMISO PET imaging can predict endocrine resistance |
| [18F]-FAZA | Grosu et al. 2007 (110) | Head & Neck | 18 | Static scan at 120 minsSUV ≥ 1.5TMRNo threshold | [18F]-FAZA PET imaging has the potential to be used for hypoxia-driven radiotherapy treatment planning |
| [18F]-FAZA | Postema et al. 2009 (138) | Head & Neck, Lung, glioma and lymphoma | 50 | Static scan at 120–180minsSUVmax TBRNo threshold | Imaging with [18F]-FAZA PET can visualize hypoxic lesions in patients with glioma |
| [18F]-FAZA | Schuetz et al. 2010 (139) | Uterine cervix | 15 | Dynamic scan 60, 120 minsSUVmax TMRNo threshold | [18F]-FAZA PET imaging is feasible in uterine cervix cancer patients |
| [18F]-FAZA | Le et al. 2012 (140) | Head & Neck | 39 | Static scan at 120 minsSUVmax TBRNo threshold | Circulating hepatocyte growth factor correlated with [18F]-FAZA tumor uptake values |
| [18F]-FAZA | Mortensen et al. 2012 (64) | Head & Neck | 40 | Static scan at 120 minsTMR ≥ 1.4 | [18F]-FAZA provides suitable prognostic detection in H&N cancer patients with hypoxic tumors |
| [64Cu]-ATSM | Dietz et al. 2008 (141) | Rectal carcinoma | 19 | Dynamic scan for 60minsTMR > 2.6 | [60Cu]-ATSM-PET may predict survival and tumor response to neoadjuvant chemoradiotherapy for rectal cancer patients |
| [64Cu]-ATSM | Lewis et al. 2008 (61) | Uterine cervix | 10 | Static 30–60 mins scanTMRNo threshold | [64Cu]-ATSM can be used to image tumor hypoxia in uterine cervix patients |
| [62Cu]-ATSM | Lohith et al. 2009 (142) | Lung (adenocarcinoma and SCC) | 8 | Dynamic scan and static at 30mins and 66 minsSUVmeanNo threshold | Pathohistologic lung cancer type may result in different [62Cu]-ATSM tumor uptake |
| [18F]-HX4 | van Loon et al. 2010 (59) | Solid cancer | 12 | Static scan at 30, 60 and 120minsSUVmax TMRNo threshold | Detecting tumor hypoxia with [18F]-HX4 is not associated with patient toxicity |
| [18F]-HX4 | Zegers et al. 2013 (143) | Non-small cell lung cancer | 15 | Static scan at 120 and 240 minsSUVmaxTBR > 1.5 | Most of the NSCLC lesions exhibited [18F]-HX4 uptake and the 4 hour imaging timepoint provided better contrast |
| [18F]-EF3 | Mahy et al. 2008 (144) | Head & Neck | 10 | Static scans at 30, 65, 115, 180, 245 and 310 and 375mins% of injected dose per gram of tissueNo threshold | [18F]EF3 PET imaging in head & neck cancer patients is feasible and hypoxic tumor regions could be visualized |
| [18F]-EF5 | Komar et al. 2008 (62) | Head & Neck | 15 | Dynamic scan for 60mins and static scans at 120, 180 and 240 minsTMR > 1.5 | [18F]-EF5 PET imaging can be used to identify hypoxia in H&N patients |
| [18F]-EF5 | Koch et al. 2010 (145) | Glioblastoma | 10 | 3 dynamic PET scans 210minsSUV | [18F]-EF5 is safe to use in patients to identify tumor hypoxia |
| [18F]-FETNIM | Lehtio et al. 2003 (146) | Head & Neck | 10 | Dynamic scan for 20 and 80–120 minsDV TMRNo threshold | [18F]-FETNIM PET imaging is a hypoxia marker in oncological patients and uptake is better understood using dynamic image analysis |
| [18F]-FRP-170 | Shibahara et al. 2010 (147) | Brain cancers | 8 | Static scan at 120 minsSUVmaxNo threshold | FRP-170 PET imaging can identify tumor hypoxia lesions in patients with various brain cancers as verified by histology |
Since its development (70), numerous studies, both pre-clinical and clinical, consider [18F]-FMISO and PET imaging to be the most promising method for hypoxia quantification since the tracer binds in hypoxic cells selectively (57,58,71,72). As result, it is a lead contender in the in vivo and clinical assessment of hypoxia and is the most extensively studied PET hypoxia tracer (73,74). Koh et al. (57) and Valk et al. (58) first demonstrated that [18F]-FMISO could detect hypoxia in human tumors. Rasey et al. (71) further validated the tracer sensitivity as a hypoxic marker in 37 patients and confirmed the prevalence, presence, and variability of hypoxia in human tumors. Bruehlmeier et al. (75) also supported the use of [18F]-FMISO for hypoxia quantification and showed the lack of influence of perfusion and the blood-brain-barrier on tracer hypoxia detection. This implies tracer differentiation and specificity. Only Bentzen et al. (76) has shown an inability for [18F]-FMISO to detect hypoxia in human tumors. However, this discrepancy could be attributed to the trial protocol design, as [18F]-FMISO was used to characterize tumors, not to explicitly detect hypoxia. Most significantly, Gagel et al. (77) showed the tracer was representative of intracellular _p_O2 as [18F]-FMISO uptake correlated with Eppendorf _p_O2 probe measurements, while this was not the case for [18F]-FDG (78). Statistically significant correlations have also been found between [18F]-FMISO uptake and immunohistochemistry staining (79–81) and the HIF1-alpha hypoxic-inducible factor (76). Okamoto et al. (82) showed the [18F]-FMISO PET could provide reproducible hypoxic volumes in H&N cancer patients. In the clinic, [18F]-FMISO has been shown to detect hypoxia in a variety of tumor types from soft-tissue sarcoma, H&N cancer, non-small cell lung cancer, breast cancer, and brain tumors (71,77,83–86).
PET imaging of tumor hypoxia: Predicting treatment response and developing more optimal radiotherapy treatments
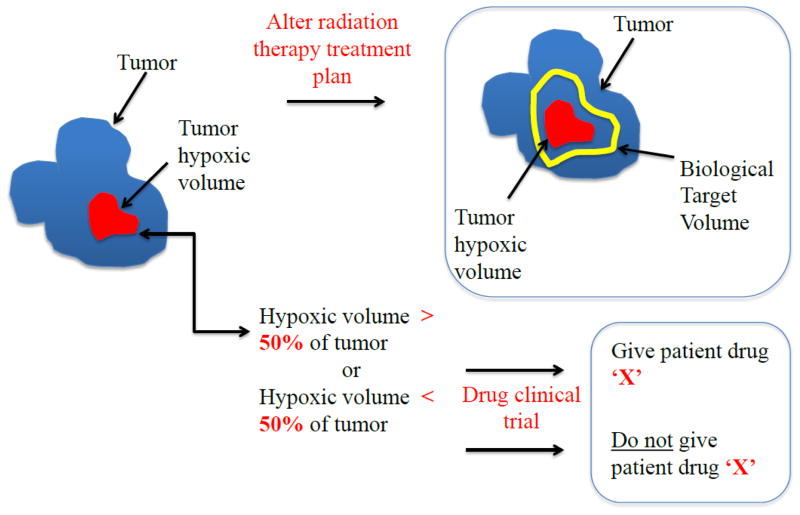
The schematic in figure 3 shows two potential therapeutic uses of hypoxia imaging in the clinical setting. It is widely accepted that PET imaging can identify tumor hypoxia in cancer patients to predict prognosis to treatment (85,87–95) to stratify them into responding and non-responding groups or provide more targeted treatments for the poor responders (86,96–100). Such a process could also prevent some patients from receiving unnecessary treatments and consequential side-effects or indicate the use of hypoxia-selective drugs such as tirapazamine (98) or the hypoxic radiosensitizer nimorazole (100). However, a potential limitation of this application is that a single pre-therapy scan (used to define hypoxic patient group) may be an inadequate measure of tumor hypoxia as oxygenation is a dynamic process and one single pre-treatment imaging time point may not be adequate.
Figure 3.
A schematic illustrating two potential uses of hypoxia PET imaging in a clinical setting in an effort to (a) develop more optimal radiotherapy treatments and to (b) use hypoxia status to stratify patients for a drug trial.
Lee et al. (11) reviewed the clinical data of over 300 patients and concluded [18F]-FMISO is a predictor of treatment response and prognosis for cancer patients. Direct comparisons between [18F]-FDG and [18F]-FMISO show the latter to be a similar or stronger predictor of outcome (77,101). This predictive information is vital as hypoxia is correlated with poor local control and a high incidence of metastasis (102). Eschmann et al. (92) and other groups (87–91,93,95) reported that [18F]-FMISO could predict local recurrence in H&N cancer and non-small cell lung cancer (NSCLC) patients. Zips et al. (87) also confirmed that [18F]-FMISO PET could predict local recurrence in 25 patients. Additional studies in various cancer types have confirmed that [18F]-FMISO is a prognostic factor in glioma (85) and breast (86) cancer patients.
PET imaging of tumor hypoxia is also used to modify radiation therapy treatment to improve therapy outcomes, such as accelerated radiotherapy with carbogen and nicotinamide (ARCON) (103) and dose escalation (104,105). Lee et al. (106) showed in an 11 patient treatment planning feasibility study that boosting the hypoxic volume (GTVH) was feasible based on [18F]-FMISO scans and provided a theoretical improved local tumour control without exceeding the normal tissue tolerance. Both Eschmann et al. (107) and Popple et al. (104) dose escalated [18F]-FMISO-defined hypoxic volumes and predicted a theoretical increase in tumour control. This is true for [64Cu]-ATSM treatment planning studies that showed an IMRT dose boost was feasible with respect to normal tissue constraints (108). However, most of the studies (105,106,109–113) on dose escalation are in silico and are not actually delivered to patients. Temporal changes in the spatial distribution of hypoxia on [18F]-FMISO may also be problematic and lead to insufficient dose coverage of the tumor hypoxic region if hypoxic volumes change significantly during treatment (114). Practical dose escalation strategies may therefore require serial imaging and adaptive planning which would be more feasible in a hypofractionation paradigm (115). In addition, tumor hypoxia is diffusely distributed and thus heterogeneity can restrict the feasibility of defining a hypoxic subvolume (110).
Some studies implemented dose painting by numbers (116,117), however, the results yielded clinically implausible boost volumes to achieve the desired tumor-control probability. Clinical implementation of dose painting is dependent on accurate quantification of the hypoxic region and the capability of delivering large dose gradients on small spatial scales which may be challenging with current techniques (118). This challenge in radiation delivery is of great concern during hypofractionated treatments due to increased radioresistance of the hypoxic fraction in these cases (119). Moreover, simulations found that less dose was required for cell kill in a given PET voxel when based on oxygen concentration and not treating the voxel as a homogenous level of hypoxia (120). This suggests dose painting by numbers may be useful (121), but can we get a reliable image of hypoxia? In a recent review, Geets et al. (122) agree that, although dose painting is an attractive concept, it restricted by low contrast, high noise, and poor spatial resolution of the PET image as well as degradation by errors in treatment delivery (set-up error and patient motion). Animal studies may make further investigation possible to perhaps confirm the benefit of dose painting or provide evidence for or against uniform target boosting or redistribution of dose to increase local control.
PET imaging for tumor hypoxia – where to go from here?
PET imaging for tumor hypoxia is an essential part of radiation therapy treatment. However, there are still a number of important challenges that need to be overcome.
The ideal PET tracer for tumor hypoxia
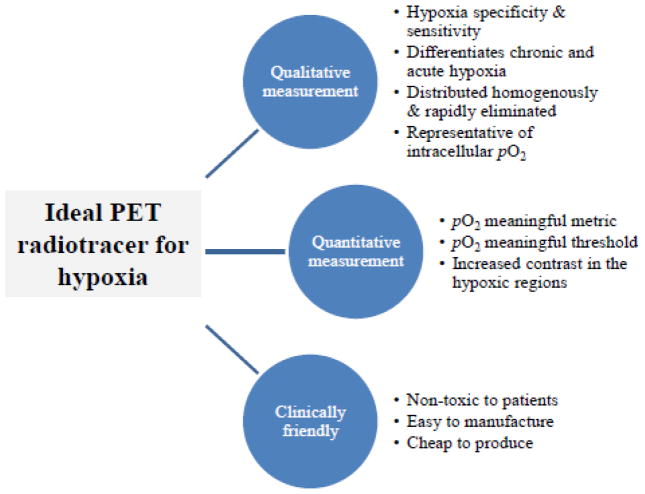
The criterion for a clinical PET radiotracer is outlined in figure 4. The first hurdle is the choice of PET tracer. It is evident that each PET tracer for hypoxia has positive and negative attributes. Even [18F]-FMISO has its limitations despite wide clinical implementation. A PET tracer should be capable of providing both qualitative and quantitative measurements. The PET image should show hypoxia only, not regions of necrosis or normoxia and be representative of tumor cell _p_O2 levels within the clinically-relevant hypoxic range (e.g., 0–10 mmHg) irrespective of the tumor grade or cell type (10). Often tracers are only able to detect very low _p_O2 levels, but the cells with ‘intermediate’ levels of hypoxia can be more important than the maximally resistant cells in determining tumor response to fractionated radiotherapy (123). As radiotracers require a biologically-significant molecule, e.g., a nitroimidazole combined with a radioactive component such as 18F, it is crucial that the radioisotope does not interfere with the biological properties of the exogenous marker. For example, the lipophilic and hydrophilic balance of the marker is responsible for tracer distribution and clearance of the unbound tracer from the tumor to provide a better tumor to background ratio. Much of radiotracer selection stems from the availability of the tracer, ease of synthesis and tumor type/model.
Figure 4.
The characteristics of the ‘ideal’ PET radiotracer for imaging hypoxia to allow for both qualitative and quantitative measurement of the tumor hypoxic fraction and be available in a clinical setting.
The second challenge is developing a reliable method for tracer validation. PET tracers are often validated against an accepted standard of hypoxia measurement. However, no ‘true’ gold standard exists. While the Eppendorf _p_O2 electrode is often considered the gold standard, it is limited by user-dependence and sampling error (41). Comparisons have been made against immunohistochemical staining; however, the spatial registration can be challenging. On imaging, necrotic and normoxic regions appear to be the same (no uptake) while some immunohistochemical stains can differentiate between these regions. (124). Moreover, this highlights one of the fundamental limitations of PET imaging – the discrepancy between the microscopic scale of hypoxia and the macroscopic resolution of the PET voxel. Thus, through imaging, we are missing much of heterogeneity of hypoxia via the partial volume effect and large voxel sizes. If we compare the PET voxel to Eppendorf _p_O2 values or microscopic immunohistochemistry, it is essential to average the measurement values. We also do not know the accuracy of these comparisons at low or intermediate _p_O2 values that show the most resistance to treatment (125).
The third obstacle is assessing tracer uptake and the tumor hypoxic fraction in a quantitative and reproducible way. The most commonly used metric is the tumor-to-blood ratio (TBR) which is dependent on an arbitrary threshold value. So what threshold should be used? Monnich et al. (126) correlated the tumor voxel _p_O2 as determined by the tissue oxygenation and tracer diffusion dynamic simulations to attempt to identify an [18F]-FMISO parameter that was representative of or correlated with a _p_O2 value for the same voxel (127). The main limitation is that the _p_O2 value is cycling while a static [18F]-FMISO scan only represents a ‘snapshot’ of hypoxia. Moreover, when the tracer is compared to a _p_O2 value such as in Chang et al. (128), the correlative [18F]-FMISO values may be low for _p_O2 due to the sampling error of the electrode. Furthermore, in a situation where hemoglobin levels are low (low _p_O2 gradient), but perfusion is high, [18F]-FMISO retention will be high.
What is realistic in a clinical setting?
Considering all of these challenges, is it realistic to quantify tumor hypoxia with PET in a clinical setting? [18F]-FMISO uptake, for example, appears to vary across tumor types and sites within the same patient (71,77,83). This inconsistency reduces the hypoxia differentiation of the tracer but may be attributed to the hypoxia definition used, e.g., the maximum Standard uptake value (SUVmax) or TBR. Differentiating between hypoxic levels based on TBR can be ambiguous as ratios can vary across patients and tumor types. However, dynamic as opposed to static scanning further elucidates the movement of the tracer from the blood into the tumor and the hypoxic regions. This method provides both temporal and spatial information for the tracer and hypoxia and is more reliable and accurate than the SUV measurement. As a result, static scans are insufficient as a dynamic or early scan is needed to provide information about the input function and the perfusion of the tumor vasculature within the tumor voxels (94,126). The Thorwarth model (129), which has been clinically validated (94), accounts for perfusion, diffusion, and binding of the tracer and can distinguish between necrosis and severely hypoxic regions. Monnich et al. (126) proposed a scan at an early time-point, 15 minutes post-injection, and a late scan at 4 hours. Ideally, the tracer kinetic model would be sensitive to patient specific tracer pharmacokinetics, e.g., clearance and metabolite formation. In addition, models must be robust enough to account for large voxel sizes (3–4 mm in each dimension), noise, organ motion, and technical challenges associated with image registration. Despite the introduction of kinetic modeling techniques to analyze dynamic PET data, several challenges remain before this analysis technique can become a clinical reality. Perhaps a model that could accommodate a clinically realistic imaging schedule would be better, i.e., to image at an early and late time point and use the model to calculate the hypoxic fraction. Such a model could provide more information on the best imaging time points during radiotherapy (87,107). Alternatively, the [18F]-FMISO imaging data could be predictive of response to RT instead of being used to provide adaptive information (94).
Conclusions and Outlook
It is evident that PET imaging is a powerful tool for visualizing tumor hypoxia in patients in a clinical setting. The feasibility of imaging hypoxia with PET has been clinically demonstrated in a number of cancer types using several existing radiotracers. PET has allowed the prediction of treatment response and demonstrated the potential for optimizing radiotherapy plans. However, due to the complexity of tumor hypoxia, technological advances to combat low image contrast, high noise, and poor spatial resolution are needed to improve our ability to routinely quantify the hypoxic fraction in human tumors. Improvements in spatial resolution with PET, MRI, or bioluminescent imaging may bring us closer to clinically incorporating the hypoxic fraction of the patient tumor in routine cancer therapy. Moreover, the development of improved tracers and image analysis techniques are essential to provide better hypoxia specificity and quantification metrics. A systematic review of randomized clinical trials demonstrates a clinical benefit of adding hypoxic modification to radiotherapy for H&N squamous cell carcinomas (130). This, combined with the advancement of PET imaging techniques and tracer development coupled with more _in viv_o studies, PET imaging could make hypoxic modification a standard of care in the clinical setting for many cancer types.
References
- 1.Mottram JC. A Factor of Importance in the Radio Sensitivity of Tumours. British Journal of Radiology. 1936;9:606–14. [Google Scholar]
- 2.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–48. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–45. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 6.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 7.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 8.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 9.Ballinger JR. Imaging hypoxia in tumors. Semin Nucl Med. 2001;31:321–9. doi: 10.1053/snuc.2001.26191. [DOI] [PubMed] [Google Scholar]
- 10.Mees G, Dierckx R, Vangestel C, Van de Wiele C. Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging. 2009;36:1674–86. doi: 10.1007/s00259-009-1195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18f-fluoromisonidazole. Semin Nucl Med. 2007;37:451–61. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Rasey JS, Martin GV, Krohn KA, editors. Quantifying hypoxia with radiolabeled fluoromisonidazole: pre-clinical and clinical studies. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. [Google Scholar]
- 13.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 15.Brown JM. Tumor hypoxia, drug resistance, and metastases. J Natl Cancer Inst. 1990;82:338–9. doi: 10.1093/jnci/82.5.338. [DOI] [PubMed] [Google Scholar]
- 16.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. The oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 17.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–6. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 19.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Progress in nucleic acid research and molecular biology. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart RD, Yu VK, Georgakilas AG, Koumenis C, Park JH, Carlson DJ. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat Res. 2011;176:587–602. doi: 10.1667/rr2663.1. [DOI] [PubMed] [Google Scholar]
- 22.Alper T, Howard-Flanders P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature. 1956;178:978–9. doi: 10.1038/178978a0. [DOI] [PubMed] [Google Scholar]
- 23.Carlson DJ, Stewart RD, Semenenko VA. Effects of oxygen on intrinsic radiation sensitivity: A test of the relationship between aerobic and hypoxic linear-quadratic (LQ) model parameters. Med Phys. 2006;33:3105–15. doi: 10.1118/1.2229427. [DOI] [PubMed] [Google Scholar]
- 24.Rofstad EK, Sundfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354–9. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–9. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 26.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 27.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–35. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 28.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 29.Hickman JA, Potten CS, Merritt AJ, Fisher TC. Apoptosis and cancer chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1994;345:319–25. doi: 10.1098/rstb.1994.0112. [DOI] [PubMed] [Google Scholar]
- 30.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 33.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 34.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends in biochemical sciences. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 35.Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–56. doi: 10.1016/s0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 36.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 38.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–22. [PubMed] [Google Scholar]
- 39.Donnelly ET, Liu Y, Fatunmbi YO, Lee I, Magda D, Rockwell S. Effects of texaphyrins on the oxygenation of EMT6 mouse mammary tumors. Int J Radiat Oncol Biol Phys. 2004;58:1570–6. doi: 10.1016/j.ijrobp.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Br J Radiol. 1999;72:627–30. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 41.Adam MF, Dorie MJ, Brown JM. Oxygen tension measurements of tumors growing in mice. Int J Radiat Oncol Biol Phys. 1999;45:171–80. doi: 10.1016/s0360-3016(99)00157-1. [DOI] [PubMed] [Google Scholar]
- 42.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)a cet amide]: analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer. 1995;72:869–74. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler WC, Jr, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–8. [PubMed] [Google Scholar]
- 44.Olive PL, Aquino-Parsons C, MacPhail SH, Liao SY, Raleigh JA, Lerman MI, et al. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61:8924–9. [PubMed] [Google Scholar]
- 45.Chitneni SK, Palmer GM, Zalutsky MR, Dewhirst MW. Molecular imaging of hypoxia. J Nucl Med. 2011;52:165–8. doi: 10.2967/jnumed.110.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed. 2004;17:240–62. doi: 10.1002/nbm.900. [DOI] [PubMed] [Google Scholar]
- 47.Pan X, Xia D, Halpern H. Targeted-ROI imaging in electron paramagnetic resonance imaging. J Magn Reson. 2007;187:66–77. doi: 10.1016/j.jmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Kwock L, Gill M, McMurry HL, Beckman W, Raleigh JA, Joseph AP. Evaluation of a fluorinated 2-nitroimidazole binding to hypoxic cells in tumor-bearing rats by 19F magnetic resonance spectroscopy and immunohistochemistry. Radiat Res. 1992;129:71–8. [PubMed] [Google Scholar]
- 49.Landuyt W, Hermans R, Bosmans H, Sunaert S, Beatse E, Farina D, et al. BOLD contrast fMRI of whole rodent tumour during air or carbogen breathing using echo-planar imaging at 1. 5 T. Eur Radiol. 2001;11:2332–40. doi: 10.1007/s003300100996. [DOI] [PubMed] [Google Scholar]
- 50.Dunn JF, O’Hara JA, Zaim-Wadghiri Y, Lei H, Meyerand ME, Grinberg OY, et al. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. J Magn Reson Imaging. 2002;16:511–21. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- 51.Skala MC, Fontanella A, Lan L, Izatt JA, Dewhirst MW. Longitudinal optical imaging of tumor metabolism and hemodynamics. J Biomed Opt. 2010;15:011112. doi: 10.1117/1.3285584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh WJ, Griffin TW, Rasey JS, Laramore GE. Positron emission tomography. A new tool for characterization of malignant disease and selection of therapy. Acta Oncol. 1994;33:323–7. doi: 10.3109/02841869409098424. [DOI] [PubMed] [Google Scholar]
- 53.Chapman JD, Franko AJ, Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981;43:546–50. doi: 10.1038/bjc.1981.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laubenbacher C, Schwaiger M, editors. Blood Perfusion and Microenvironment of Human Tumors. Berlin: Springer; 2000. The potential role of positron emission tomography in investigation of microenvironment. [Google Scholar]
- 55.Ziemer LS, Evans SM, Kachur AV, Shuman AL, Cardi CA, Jenkins WT, et al. Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-EF5. Eur J Nucl Med Mol Imaging. 2003;30:259–66. doi: 10.1007/s00259-002-1037-5. [DOI] [PubMed] [Google Scholar]
- 56.Sorger D, Patt M, Kumar P, Wiebe LI, Barthel H, Seese A, et al. [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]Fluoromisonidazole (18FMISO): a comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol. 2003;30:317–26. doi: 10.1016/s0969-8051(02)00442-0. [DOI] [PubMed] [Google Scholar]
- 57.Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys. 1992;22:199–212. doi: 10.1016/0360-3016(92)91001-4. [DOI] [PubMed] [Google Scholar]
- 58.Valk PE, Mathis CA, Prados MD, Gilbert JC, Budinger TF. Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med. 1992;33:2133–7. [PubMed] [Google Scholar]
- 59.van Loon J, Janssen MH, Ollers M, Aerts HJ, Dubois L, Hochstenbag M, et al. PET imaging of hypoxia using [18F]HX4: a phase I trial. Eur J Nucl Med Mol Imaging. 2010;37:1663–8. doi: 10.1007/s00259-010-1437-x. [DOI] [PubMed] [Google Scholar]
- 60.Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, et al. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology. 1995;194:795–800. doi: 10.1148/radiology.194.3.7862981. [DOI] [PubMed] [Google Scholar]
- 61.Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, Siegel BA. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J Nucl Med. 2008;49:1177–82. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komar G, Seppanen M, Eskola O, Lindholm P, Gronroos TJ, Forsback S, et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med. 2008;49:1944–51. doi: 10.2967/jnumed.108.053785. [DOI] [PubMed] [Google Scholar]
- 63.Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–13. [PubMed] [Google Scholar]
- 64.Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–60. [PubMed] [Google Scholar]
- 66.Burgman P, O’Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nucl Med Biol. 2005;32:623–30. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 67.O’Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys. 2005;61:1493–502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto K, Szajek L, Krishna MC, Cook JA, Seidel J, Grimes K, et al. The influence of tumor oxygenation on hypoxia imaging in murine squamous cell carcinoma using [64Cu]Cu-ATSM or [18F]Fluoromisonidazole positron emission tomography. Int J Oncol. 2007;30:873–81. [PubMed] [Google Scholar]
- 69.Holland JP, Lewis JS, Dehdashti F. Assessing tumor hypoxia by positron emission tomography with Cu-ATSM. Q J Nucl Med Mol Imaging. 2009;53:193–200. [PMC free article] [PubMed] [Google Scholar]
- 70.Rasey JS, Koh WJ, Grierson JR, Grunbaum Z, Krohn KA. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int J Radiat Oncol Biol Phys. 1989;17:985–91. doi: 10.1016/0360-3016(89)90146-6. [DOI] [PubMed] [Google Scholar]
- 71.Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–28. doi: 10.1016/s0360-3016(96)00325-2. [DOI] [PubMed] [Google Scholar]
- 72.Rasey JS, Casciari JJ, Hofstrand PD, Muzi M, Graham MM, Chin LK. Determining hypoxic fraction in a rat glioma by uptake of radiolabeled fluoromisonidazole. Radiat Res. 2000;153:84–92. doi: 10.1667/0033-7587(2000)153[0084:dhfiar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 73.Imam SK. Review of positron emission tomography tracers for imaging of tumor hypoxia. Cancer Biother Radiopharm. 2010;25:365–74. doi: 10.1089/cbr.2009.0740. [DOI] [PubMed] [Google Scholar]
- 74.Padhani AR, Krohn KA, Lewis JS, Alber M. Imaging oxygenation of human tumours. Eur Radiol. 2007;17:861–72. doi: 10.1007/s00330-006-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J Nucl Med. 2004;45:1851–9. [PubMed] [Google Scholar]
- 76.Bentzen L, Keiding S, Nordsmark M, Falborg L, Hansen SB, Keller J, et al. Tumour oxygenation assessed by 18F-fluoromisonidazole PET and polarographic needle electrodes in human soft tissue tumours. Radiother Oncol. 2003;67:339–44. doi: 10.1016/s0167-8140(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 77.Gagel B, Reinartz P, Dimartino E, Zimny M, Pinkawa M, Maneschi P, et al. pO(2) Polarography versus positron emission tomography ([(18)F] fluoromisonidazole, [(18)F]-2-fluoro-2′-deoxyglucose). An appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol. 2004;180:616–22. doi: 10.1007/s00066-004-1229-y. [DOI] [PubMed] [Google Scholar]
- 78.Zimny M, Gagel B, DiMartino E, Hamacher K, Coenen HH, Westhofen M, et al. FDG--a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur J Nucl Med Mol Imaging. 2006;33:1426–31. doi: 10.1007/s00259-006-0175-6. [DOI] [PubMed] [Google Scholar]
- 79.Dubois L, Landuyt W, Haustermans K, Dupont P, Bormans G, Vermaelen P, et al. Evaluation of hypoxia in an experimental rat tumour model by [(18)F]fluoromisonidazole PET and immunohistochemistry. Br J Cancer. 2004;91:1947–54. doi: 10.1038/sj.bjc.6602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Troost EG, Laverman P, Kaanders JH, Philippens M, Lok J, Oyen WJ, et al. Imaging hypoxia after oxygenation-modification: comparing [18F]FMISO autoradiography with pimonidazole immunohistochemistry in human xenograft tumors. Radiother Oncol. 2006;80:157–64. doi: 10.1016/j.radonc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 81.Troost EG, Laverman P, Philippens ME, Lok J, van der Kogel AJ, Oyen WJ, et al. Correlation of [18F]FMISO autoradiography and pimonidazole [corrected] immunohistochemistry in human head and neck carcinoma xenografts. Eur J Nucl Med Mol Imaging. 2008;35:1803–11. doi: 10.1007/s00259-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 82.Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K, et al. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J Nucl Med. 2013;54:201–7. doi: 10.2967/jnumed.112.109330. [DOI] [PubMed] [Google Scholar]
- 83.Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 84.Cherk MH, Foo SS, Poon AM, Knight SR, Murone C, Papenfuss AT, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-Fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–6. [PubMed] [Google Scholar]
- 85.Cher LM, Murone C, Lawrentschuk N, Ramdave S, Papenfuss A, Hannah A, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–8. [PubMed] [Google Scholar]
- 86.Cheng J, Lei L, Xu J, Sun Y, Zhang Y, Wang X, et al. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med. 2013;54:333–40. doi: 10.2967/jnumed.112.111963. [DOI] [PubMed] [Google Scholar]
- 87.Zips D, Zophel K, Abolmaali N, Perrin R, Abramyuk A, Haase R, et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105:21–8. doi: 10.1016/j.radonc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 88.Askoxylakis V, Dinkel J, Eichinger M, Stieltjes B, Sommer G, Strauss LG, et al. Multimodal hypoxia imaging and intensity modulated radiation therapy for unresectable non-small-cell lung cancer: the HIL trial. Radiat Oncol. 2012;7:157. doi: 10.1186/1748-717X-7-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kikuchi M, Yamane T, Shinohara S, Fujiwara K, Hori SY, Tona Y, et al. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann Nucl Med. 2011 doi: 10.1007/s12149-011-0508-9. [DOI] [PubMed] [Google Scholar]
- 90.Yamane T, Kikuchi M, Shinohara S, Senda M. Reduction of [(18)F]fluoromisonidazole uptake after neoadjuvant chemotherapy for head and neck squamous cell carcinoma. Mol Imaging Biol. 2011;13:227–31. doi: 10.1007/s11307-010-0365-2. [DOI] [PubMed] [Google Scholar]
- 91.Gagel B, Reinartz P, Demirel C, Kaiser HJ, Zimny M, Piroth M, et al. [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography in response evaluation after chemo-/radiotherapy of non-small-cell lung cancer: a feasibility study. BMC Cancer. 2006;6:51. doi: 10.1186/1471-2407-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, et al. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–60. [PubMed] [Google Scholar]
- 93.Hicks RJ, Rischin D, Fisher R, Binns D, Scott AM, Peters LJ. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur J Nucl Med Mol Imaging. 2005;32:1384–91. doi: 10.1007/s00259-005-1880-2. [DOI] [PubMed] [Google Scholar]
- 94.Thorwarth D, Eschmann SM, Scheiderbauer J, Paulsen F, Alber M. Kinetic analysis of dynamic 18F-fluoromisonidazole PET correlates with radiation treatment outcome in head-and-neck cancer. BMC Cancer. 2005;5:152. doi: 10.1186/1471-2407-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thorwarth D, Eschmann SM, Holzner F, Paulsen F, Alber M. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol. 2006;80:151–6. doi: 10.1016/j.radonc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 96.Hirata K, Terasaka S, Shiga T, Hattori N, Magota K, Kobayashi H, et al. (1)(8)F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging. 2012;39:760–70. doi: 10.1007/s00259-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 97.McKeage MJ, Jameson MB, Ramanathan RK, Rajendran J, Gu Y, Wilson WR, et al. PR-104 a bioreductive pre-prodrug combined with gemcitabine or docetaxel in a phase Ib study of patients with advanced solid tumours. BMC Cancer. 2012;12:496. doi: 10.1186/1471-2407-12-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98. 02. J Clin Oncol. 2006;24:2098–104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 99.Loi S, Ngan SY, Hicks RJ, Mukesh B, Mitchell P, Michael M, et al. Oxaliplatin combined with infusional 5-fluorouracil and concomitant radiotherapy in inoperable and metastatic rectal cancer: a phase I trial. Br J Cancer. 2005;92:655–61. doi: 10.1038/sj.bjc.6602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol. 1998;46:135–46. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 101.Rajendran JG, Schwartz DL, O’Sullivan J, Peterson LM, Ng P, Scharnhorst J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–41. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):129S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 103.Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30:1777–83. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- 104.Popple RA, Ove R, Shen S. Tumor control probability for selective boosting of hypoxic subvolumes, including the effect of reoxygenation. Int J Radiat Oncol Biol Phys. 2002;54:921–7. doi: 10.1016/s0360-3016(02)03007-9. [DOI] [PubMed] [Google Scholar]
- 105.Alber M, Paulsen F, Eschmann SM, Machulla HJ. On biologically conformal boost dose optimization. Phys Med Biol. 2003;48:N31–5. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- 106.Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eschmann SM, Paulsen F, Bedeshem C, Machulla HJ, Hehr T, Bamberg M, et al. Hypoxia-imaging with (18)F-Misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. Radiother Oncol. 2007;83:406–10. doi: 10.1016/j.radonc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–82. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 109.Rajendran JG, Hendrickson KR, Spence AM, Muzi M, Krohn KA, Mankoff DA. Hypoxia imaging-directed radiation treatment planning. Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):44–53. doi: 10.1007/s00259-006-0135-1. [DOI] [PubMed] [Google Scholar]
- 110.Grosu AL, Souvatzoglou M, Roper B, Dobritz M, Wiedenmann N, Jacob V, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:541–51. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 111.Choi W, Lee SW, Park SH, Ryu JS, Oh SJ, Im KC, et al. Planning study for available dose of hypoxic tumor volume using fluorine-18-labeled fluoromisonidazole positron emission tomography for treatment of the head and neck cancer. Radiother Oncol. 2010;97:176–82. doi: 10.1016/j.radonc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Chang JH, Wada M, Anderson NJ, Lim Joon D, Lee ST, Gong SJ, et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using (18)F-FMISO PET: A biological modeling study. Acta Oncol. 2013 doi: 10.3109/0284186X.2012.759273. [DOI] [PubMed] [Google Scholar]
- 113.Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol. 2011;101:369–75. doi: 10.1016/j.radonc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin Z, Mechalakos J, Nehmeh S, Schoder H, Lee N, Humm J, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008;70:1219–28. doi: 10.1016/j.ijrobp.2007.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carlson DJ, Yenice KM, Orton CG. Tumor hypoxia is an important mechanism of radioresistance in hypofractionated radiotherapy and must be considered in the treatment planning process. Med Phys. 2011;38:6347–50. doi: 10.1118/1.3639137. [DOI] [PubMed] [Google Scholar]
- 116.Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 117.Toma-Dasu I, Uhrdin J, Antonovic L, Dasu A, Nuyts S, Dirix P, et al. Dose prescription and treatment planning based on FMISO-PET hypoxia. Acta Oncol. 2012;51:222–30. doi: 10.3109/0284186X.2011.599815. [DOI] [PubMed] [Google Scholar]
- 118.Bowen SR, Flynn RT, Bentzen SM, Jeraj R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol. 2009;54:1483–501. doi: 10.1088/0031-9155/54/6/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carlson DJ, Keall PJ, Loo BW, Jr, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol Biol Phys. 2011;79:1188–95. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petit SF, Dekker AL, Seigneuric R, Murrer L, van Riel NA, Nordsmark M, et al. Intra-voxel heterogeneity influences the dose prescription for dose-painting with radiotherapy: a modelling study. Phys Med Biol. 2009;54:2179–96. doi: 10.1088/0031-9155/54/7/022. [DOI] [PubMed] [Google Scholar]
- 121.Thorwarth D, Alber M. Implementation of hypoxia imaging into treatment planning and delivery. Radiother Oncol. 2010;97:172–5. doi: 10.1016/j.radonc.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 122.Geets X, Gregoire V, Lee JA. Implementation of hypoxia PET imaging in radiation therapy planning. Q J Nucl Med Mol Imaging. 2013;57:271–82. [PubMed] [Google Scholar]
- 123.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–50. [PubMed] [Google Scholar]
- 124.Busk M, Horsman MR, Overgaard J. Resolution in PET hypoxia imaging: voxel size matters. Acta Oncol. 2008;47:1201–10. doi: 10.1080/02841860802307716. [DOI] [PubMed] [Google Scholar]
- 125.Bartlett RM, Beattie BJ, Naryanan M, Georgi JC, Chen Q, Carlin SD, et al. Image-guided PO2 probe measurements correlated with parametric images derived from 18F-fluoromisonidazole small-animal PET data in rats. J Nucl Med. 2012;53:1608–15. doi: 10.2967/jnumed.112.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Monnich D, Troost EG, Kaanders JH, Oyen WJ, Alber M, Zips D, et al. Correlation between tumor oxygenation and (18)F-fluoromisonidazole PET data simulated based on microvessel images. Acta Oncol. 2013;52:1308–13. doi: 10.3109/0284186X.2013.812796. [DOI] [PubMed] [Google Scholar]
- 127.Monnich D, Troost EG, Kaanders JH, Oyen WJ, Alber M, Thorwarth D. Modelling and simulation of [(18)F]fluoromisonidazole dynamics based on histology-derived microvessel maps. Phys Med Biol. 2011;56:2045–57. doi: 10.1088/0031-9155/56/7/009. [DOI] [PubMed] [Google Scholar]
- 128.Chang J, Wen B, Kazanzides P, Zanzonico P, Finn RD, Fichtinger G, et al. A robotic system for 18F-FMISO PET-guided intratumoral pO2 measurements. Med Phys. 2009;36:5301–9. doi: 10.1118/1.3239491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thorwarth D, Eschmann SM, Paulsen F, Alber M. A kinetic model for dynamic [18F]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol. 2005;50:2209–24. doi: 10.1088/0031-9155/50/10/002. [DOI] [PubMed] [Google Scholar]
- 130.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Koh WJ, Bergman KS, Rasey JS, Peterson LM, Evans ML, Graham MM, et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [F-18]fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys. 1995;33:391–8. doi: 10.1016/0360-3016(95)00170-4. [DOI] [PubMed] [Google Scholar]
- 132.Nehmeh SA, Lee NY, Schroder H, Squire O, Zanzonico PB, Erdi YE, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–42. doi: 10.1016/j.ijrobp.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dirix P, Vandecaveye V, De Keyzer F, Stroobants S, Hermans R, Nuyts S. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50:1020–7. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 134.Kawai N, Maeda Y, Kudomi N, Miyake K, Okada M, Yamamoto Y, et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2011;38:441–50. doi: 10.1007/s00259-010-1645-4. [DOI] [PubMed] [Google Scholar]
- 135.Hugonnet F, Fournier L, Medioni J, Smadja C, Hindie E, Huchet V, et al. Metastatic renal cell carcinoma: relationship between initial metastasis hypoxia, change after 1 month’s sunitinib, and therapeutic response: an 18F-fluoromisonidazole PET/CT study. J Nucl Med. 2011;52:1048–55. doi: 10.2967/jnumed.110.084517. [DOI] [PubMed] [Google Scholar]
- 136.Mammar H, Kerrou K, Nataf V, Pontvert D, Clemenceau S, Lot G, et al. Positron emission tomography/computed tomography imaging of residual skull base chordoma before radiotherapy using fluoromisonidazole and fluorodeoxyglucose: potential consequences for dose painting. Int J Radiat Oncol Biol Phys. 2012;84:681–7. doi: 10.1016/j.ijrobp.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 137.Segard T, Robins PD, Yusoff IF, Ee H, Morandeau L, Campbell EM, et al. Detection of hypoxia with 18F-fluoromisonidazole (18F-FMISO) PET/CT in suspected or proven pancreatic cancer. Clinical nuclear medicine. 2013;38:1–6. doi: 10.1097/RLU.0b013e3182708777. [DOI] [PubMed] [Google Scholar]
- 138.Postema EJ, McEwan AJ, Riauka TA, Kumar P, Richmond DA, Abrams DN, et al. Initial results of hypoxia imaging using 1-alpha-D: -(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA) Eur J Nucl Med Mol Imaging. 2009;36:1565–73. doi: 10.1007/s00259-009-1154-5. [DOI] [PubMed] [Google Scholar]
- 139.Schuetz M, Schmid MP, Potter R, Kommata S, Georg D, Lukic D, et al. Evaluating repetitive 18F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol. 2010;49:941–7. doi: 10.3109/0284186X.2010.510145. [DOI] [PubMed] [Google Scholar]
- 140.Le QT, Fisher R, Oliner KS, Young RJ, Cao H, Kong C, et al. Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clin Cancer Res. 2012;18:1798–807. doi: 10.1158/1078-0432.CCR-11-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dietz DW, Dehdashti F, Grigsby PW, Malyapa RS, Myerson RJ, Picus J, et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51:1641–8. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lohith TG, Kudo T, Demura Y, Umeda Y, Kiyono Y, Fujibayashi Y, et al. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. J Nucl Med. 2009;50:1948–53. doi: 10.2967/jnumed.109.069021. [DOI] [PubMed] [Google Scholar]
- 143.Zegers CM, van Elmpt W, Wierts R, Reymen B, Sharifi H, Ollers MC, et al. Hypoxia imaging with [F]HX4 PET in NSCLC patients: Defining optimal imaging parameters. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 144.Mahy P, Geets X, Lonneux M, Leveque P, Christian N, De Bast M, et al. Determination of tumour hypoxia with [18F]EF3 in patients with head and neck tumours: a phase I study to assess the tracer pharmacokinetics, biodistribution and metabolism. Eur J Nucl Med Mol Imaging. 2008;35:1282–9. doi: 10.1007/s00259-008-0742-0. [DOI] [PubMed] [Google Scholar]
- 145.Koch CJ, Scheuermann JS, Divgi C, Judy KD, Kachur AV, Freifelder R, et al. Biodistribution and dosimetry of (18)F-EF5 in cancer patients with preliminary comparison of (18)F-EF5 uptake versus EF5 binding in human glioblastoma. Eur J Nucl Med Mol Imaging. 2010;37:2048–59. doi: 10.1007/s00259-010-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lehtio K, Oikonen V, Nyman S, Gronroos T, Roivainen A, Eskola O, et al. Quantifying tumour hypoxia with fluorine-18 fluoroerythronitroimidazole ([18F]FETNIM) and PET using the tumour to plasma ratio. Eur J Nucl Med Mol Imaging. 2003;30:101–8. doi: 10.1007/s00259-002-1016-x. [DOI] [PubMed] [Google Scholar]
- 147.Shibahara I, Kumabe T, Kanamori M, Saito R, Sonoda Y, Watanabe M, et al. Imaging of hypoxic lesions in patients with gliomas by using positron emission tomography with 1-(2-[18F] fluoro-1-[hydroxymethyl]ethoxy)methyl-2-nitroimidazole, a new 18F-labeled 2-nitroimidazole analog. Journal of neurosurgery. 2010;113:358–68. doi: 10.3171/2009.10.JNS09510. [DOI] [PubMed] [Google Scholar]