HYPERTENSION IN OBESITY AND THE IMPACT OF WEIGHT LOSS (original) (raw)
. Author manuscript; available in PMC: 2018 Aug 24.
Published in final edited form as: Curr Cardiol Rep. 2017 Aug 24;19(10):98. doi: 10.1007/s11886-017-0912-4
Abstract
Purpose of Review
Several interrelated mechanisms play a role in the development of hypertension in obesity, often contributing to end organ damage including cardiovascular disease and chronic kidney disease.
Recent Findings
The treatment of hypertension in obesity is complicated by a high prevalence of resistant hypertension, as well as unpredictable hemodynamic effects of many medications. Weight loss stabilizes neurohormonal activity and causes clinically significant reductions in blood pressure. While lifestyle interventions can improve blood pressure, they fail to consistently yield sustained weight loss and have not demonstrated long-term benefits. Weight loss surgery provides more permanent weight reduction, corresponding with dramatic declines in blood pressure and attenuation of long-term cardiovascular risk.
Summary
Hypertension is closely linked to the prevalence, pathophysiology, and morbidity of obesity. There are multiple barriers to managing hypertension in obesity. Surgical weight loss offers the most promise in reducing blood pressure and decreasing end organ damage in this patient population.
Keywords: Obesity, morbid obesity, hypertension, metabolic syndrome, bariatric surgery, weight loss
INTRODUCTION
The obesity epidemic is a global crisis that is undeniably intensifying. In 2015, there were 603.7 million obese adults worldwide [1]. Over the past 25 years, the prevalence of obesity in both children and adults has doubled in 73 countries [1]. In the Unites States (US), based on the most recent data from the National Health and Nutrition Examination Survey evaluating 2638 adult men and 2817 adult women between 2013 and 2014 [2], the age-adjusted prevalence of obesity was 37.7%, up from 22.9% ten to fifteen years prior [3]. While rates of obesity in US men are starting to plateau, there is a rising trend among women, with the greatest increase in rates of class 3 obesity (defined as a body mass index [BMI] ≥40 kg/m2) [2]. Outside of the US, the prevalence of obesity continues to climb, particularly in developing countries [4].
A critical consequence of the increasing prevalence of obesity is the burden of illness associated with excess body weight. Excess body weight is associated with 7.1% of deaths from any cause and 4.9% of disability worldwide [1]. Elevated body mass index (BMI) is an independent risk factor for the development of type 2 diabetes mellitus, dyslipidemia, chronic kidney disease, ischemic heart disease, stroke, dementia, several malignancies, nonalcoholic fatty liver disease, and obstructive sleep apnea [5–12]. These myriad disease processes drive increased disability, morbidity, and mortality among the obese [1, 13–15], and promote poorer quality of life [16].
Many of the comorbidities associated with obesity are facilitated by or contribute to an extremely high prevalence of hypertension in the obese population [17–19]. About half of hypertensive patients in the US are obese [20]. Accordingly, over one-third of the US obese population has a diagnosis of hypertension, compared to less than one-fifth of normal-weight individuals [21]. The relationship between obesity and hypertension is multifaceted and closely intertwined with other comorbidities present in obesity. The diagnosis and monitoring of hypertension in obesity is often complicated by difficulties in accurately measuring blood pressure in these patients [22]. Furthermore, highly complex underlying pathophysiologic factors contribute to several challenges in treating hypertension in obesity [23], perpetuating the greater morbidity and mortality observed in this population.
PATHOPHYSIOLOGIC MECHANISMS OF HYPERTENSION IN OBESITY
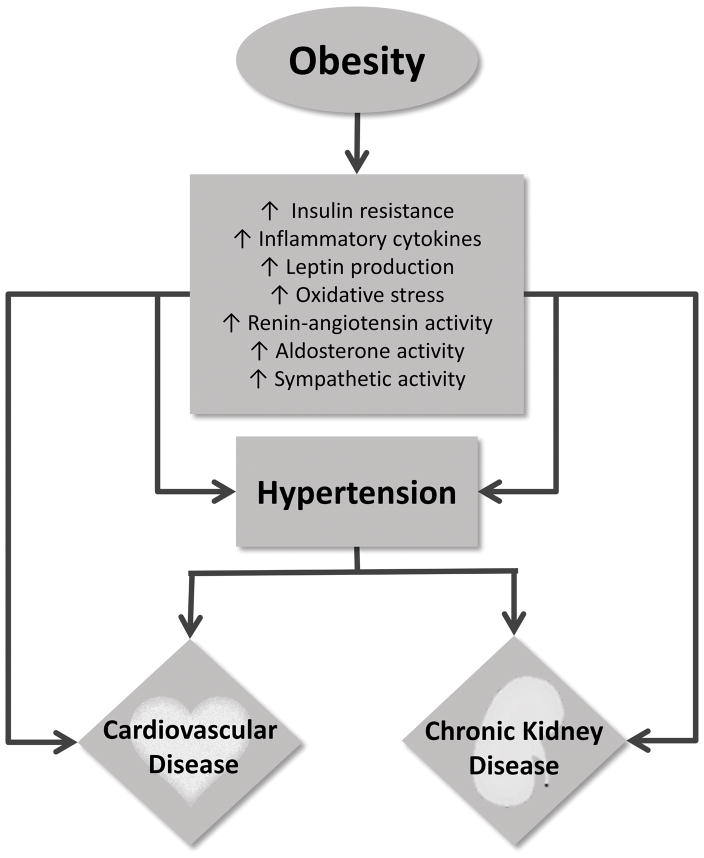
Multiple pathophysiologic mechanisms play a role in the development of hypertension in obesity, which in turn propagate end organ damage including cardiovascular disease and chronic kidney disease (Figure 1). These highly interrelated mechanisms include insulin resistance, inflammation, oxidative stress, adipokines (such as adiponectin and leptin), the sympathetic nervous system, and the renin-angiotensin aldosterone system [24–28]. Many of these factors interact with one another in bidirectional pathways and are exacerbated by greater degrees of adiposity. Broadly, their activity can induce endothelial dysfunction and alter hemodynamics throughout the body, promoting the elevation in blood pressure commonly observed in obesity.
Figure 1.
More specifically, increased adiposity is strongly correlated with endothelial dysfunction, attributed in part to amplified oxidative stress and reduced nitric oxide availability [26, 29]. Obesity is also linked to elevated circulating markers of inflammation including C-reactive protein, erythrocyte sedimentation rate, and plasminogen-activator inhibitor 1, as well as inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 [25]. In addition to oxidative stress, recent literature suggests that altered intestinal microbiota may be an important underlying mechanism in promoting inflammation in obesity by affecting intestinal permeability [25, 30]. The heightened inflammatory activity observed in obesity often results in vascular dysfunction and development of hypertension in this patient population [31, 32].
Insulin resistance and oxidative stress observed in the setting of increased visceral adiposity contribute to heightened sympathetic nervous system activity [33]. Obstructive sleep apnea, which is extremely common in obesity, also causes sympathetic stimulation, further contributing to elevated blood pressure in this patient population [12, 34]. In addition to heightened sympathetic nervous system activity, excess adipose tissue is associated with increased angiotensin type 1 and 2 receptor expression as well as elevated circulating angiotensin II, angiotensin-converting enzyme, and aldosterone levels [28, 35, 36]. This elevated renin-angiotensin aldosterone system activity may in part be due to target organ effects of circulating adipokines [27]. As a result, this elevated renin-angiotensin aldosterone system activity is not systemically regulated, and alters the renal hemodynamics by causing afferent renal arteriolar dilation and efferent renal arteriolar vasoconstriction [36]. The combination of enhanced sympathetic nervous system activity and renin-angiotensin aldosterone system activity in obesity also cause impaired natiuresis, increased renal sodium reabsorption, and extracellular volume expansion, further propagating the development of hypertension in obesity [33, 36].
END ORGAN EFFECTS OF HYPERTENSION IN OBESITY
Much of the end organ damage associated with hypertension in obesity is related to the vascular impact of excess adipose tissue. Even after adjusting for traditional cardiovascular risk factors, obesity is strongly correlated with subclinical measures of atherosclerosis, including coronary artery calcification, increased internal and common carotid artery intimal medial thickness, and enlarged left ventricular mass [37]. Central adiposity is also independently associated with greater risk of arterial stiffness and microvascular disease [38–40], which are likely substantially mediated by the increased prevalence of hypertension in this patient population.
With regard to renal effects of hypertension in obesity, the upregulation of sympathetic nervous system and renin-angiotensin aldosterone system activity appreciated in obesity yields higher cardiac output and extravascular volume expansion [33, 36]. On the glomerular level, these altered hemodynamics expand renal plasma flow and increase glomerular pressure, often leading to glomerular hyperfiltration [33, 36]. These changes may ultimately result in glomerulomegaly, podocytopathy, focal glomerulosclerosis, and proteinuria [36, 41]. As such, obesity is associated with both the development of de novo renal disease as well as greater risk of progression of chronic kidney disease. In particular, class 3 obesity confers a 7-fold increased risk of the development of end stage renal disease compared to normal-weight individuals in the general population [7].
Hypertension in obesity is also highly associated with cardiac remodeling. The endothelial dysfunction caused by heightened sympathetic nervous system activity, renin-angiotensin aldosterone system activity, inflammatory cytokines, and oxidative stress in the setting of excess adipose tissue can contribute to left ventricular hypertrophy, ischemic heart disease, cardiac fibrosis, and cerebrovascular disease [17, 27, 42]. These effects are amplified in severe obesity, with up to a 2-fold increased risk in ischemic heart disease and 6-fold increased risk of congestive heart failure reported in large-scale observational analyses among women with class 3 obesity compared to normal-weight women [43].
CHALLENGES IN THE PHARMACOLOGIC MANAGEMENT OF HYPERTENSION IN OBESITY
The physiologic complexity of hypertension in obesity engenders multiple challenges in its pharmacological management (Table 1), potentially contributing to the greater risk of target organ damage in this patient population. Obesity is strongly correlated with treatment resistant hypertension, often requiring the additive and synergistic effects of several antihypertensive medications to achieve adequate blood pressure control. The etiology of resistant hypertension in these patients is likely multifactorial, including dysfunctional neurohormonal pathways, particularly increased aldosterone secretion, as well as the systemic effects of adipokines such as leptin and adiponectin [27, 28]. Although obesity is not known to alter the oral absorption of medications, the pharmacokinetics and pharmacodynamics of many medications are also impacted by excess adiposity. These changes are mediated through a variety of pathophysiologic mechanisms, including abnormal drug handling, expanded volume of distribution, altered hepatic and renal clearance, and heightened neurohormonal activity [23, 44].
Table 1.
Potential Pharmacologic Challenges in Treating Hypertension in Obesity
| Drug resistant hypertension |
|---|
| Altered neurohormonal pathways |
| Increased renal sodium reabsorption |
| Impaired natiuresis |
| Adipokines |
| Altered volume of distribution |
| Drug lipophilia |
| Expanded plasma volume |
| Altered clearance |
| Dysfunctional hepatic metabolism |
| Altered enzyme activity |
| High prevalence of nonalcoholic fatty liver disease |
| Increased cardiac output |
| Impaired estimation of renal clearance |
| Glomerular hyperfiltration |
| High prevalence of chronic kidney disease |
| Inaccuracies of creatinine clearance equations |
The volume of distribution of a medication describes the overall amount of the medication in a person’s body relative to the concentration of that medication in a particular body compartment, and provides an approximation of the extent to which a medication is delivered into soft tissue [23]. However, obese patients tend to have expanded plasma volume, which can alter the volume of distribution. This contributes to significant differences in plasma concentrations of some medications in obese patients compared to normal-weight patients, despite similar soft tissue concentrations [44]. In particular, the volume of distribution of lipophilic medications is affected by excess adiposity. Lipophilic medications tend to readily disperse into adipose tissue, making it difficult to achieve therapeutic plasma levels [45].
Nonalcoholic fatty liver disease plays in important role in dysfunctional drug clearance in obesity. Hepatic steatosis causes reduced hepatic microvascular blood flow, resulting in altered delivery of medications to the liver [46]. Additionally, nonalcoholic fatty liver disease contributes to abnormal hepatic enzyme function, leading to both increased and decreased rates of hepatic clearance of medications, depending on the enzyme involved in its metabolism [47, 48]. Altered renal clearance is also an important factor that complicates the predictability of medication clearance in obesity. Given that obesity is associated with amplified cardiac output and glomerular hyperfiltration [33, 36], obese patients can experience faster renal clearance of medications compared to normal-weight individuals. Conversely, obesity is also correlated with higher rates of chronic kidney disease, which can lead to reduced renal drug clearance. To further complicate the interpretation of renal drug clearance in obesity, creatinine-based equations for calculating renal clearance are often highly biased in obesity due to inability to appropriately account for large body surface area, inaccuracies at higher levels of glomerular filtration, and difficulty estimating muscle mass (which generates creatinine) in these patients [49, 50].
Several studies have demonstrated differing responses to treatment with certain antihypertensive medications in obese compared to non-obese patients, highlighting that this patient population in susceptible to atypical responses to medications. Understanding that sympathetic nervous system activity and renin-angiotensin aldosterone system activity are upregulated in obesity, inhibition of these neurohormonal pathways has generated amplified hemodynamic responses in obese patients who do not have resistant hypertension. In an in vivo study directly measuring renal hemodynamics, obese patients had a greater renal vasodilatory response to short term angiotensin converting enzyme inhibition with captopril than normal-weight individuals [51]. Although there are no measurable changes in pharmacokinetic handling of beta blockers in obesity [52], another study demonstrated that obese patients had significantly greater blood pressure response compared to lean patients after treatment with combined alpha- and beta-adrenergic blockade [53]. We recently performed an observational study evaluating the renal effects of time-updated exposure to renin-angiotensin system blockade compared to any other antihypertensive therapy in over 200,000 obese, non-diabetic, hypertensive patients in the United Kingdom. The study demonstrated that incident renin-angiotensin system inhibition increased the risk of clinically significant, often-transient renal dysfunction in this patient population [54]. These findings may be due to exaggerated intrarenal hemodynamic effects of renin-angiotensin system inhibition, potentially as a result of the chronically heightened activity of this neurohormonal pathway in obese patients.
Several small studies have investigated the relative effects of different antihypertensive classes in obese patients, with mixed results. Combination angiotensin receptor blocker and thiazide therapy demonstrated a significantly greater decline in systolic blood pressure compared to combination calcium channel blocker and thiazide therapy (20.6 vs. 14.5 mmHg, p=0.011) in obese patients [55]. Obese patients also demonstrated a stronger reduction of systolic blood pressure when randomized to aliskiren monotherapy versus thiazide monotherapy (16.7 vs. 12.2 mmHg, p <0.001) [56]. A post hoc analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial demonstrated higher frequency of blood pressure control in obese patients randomized to chlorthalidone compared to Lisinopril [57]. Collectively, these data suggest that renin-angiotensin system blockers seems to have a stronger effect on blood pressure control than calcium channel blockers in obese patients; the data are otherwise inconsistent or inconclusive. Additional studies are needed to elucidate optimal pharmacologic management of hypertension in this patient population.
Understanding the important role of aldosterone in driving sodium retention, endothelial dysfunction, cardiac fibrosis, and glomerulosclerosis in obesity [27, 28], mineralocorticoid receptor antagonists remain a promising option in the targeted treatment of hypertension in obese patients. Several studies have demonstrated renoprotective and cardioprotective effects of treatment with mineralocorticoid receptor antagonists in high-risk populations [58–61]. Data from a small, uncontrolled study suggest that mineralocorticoid receptor antagonists may have a greater beneficial effect on endothelial function in patients with higher BMI and abdominal adiposity [62]; however, a small randomized controlled trial found no difference in endothelial function in obese patients treated with a short-term course of spironolactone vs. placebo [63]. Nonetheless, animal studies have demonstrated a reduction in the development of obesity-associated podocyte injury, systolic heart failure, and diastolic heart failure in rats treated with mineralocorticoid antagonists, independent of blood pressure lowering effects [64–66]. There have been no studies evaluating target organ effects of mineralocorticoid antagonists specifically in obese humans. Furthermore, the potential role of spironolactone as monotherapy in this patient population, rather than its usual role as adjunctive therapy, remains unclear.
EFFECTS OF LIFESTYLE INTERVENTIONS ON HYPERTENSION IN OBESITY
Several studies have demonstrated that weight loss corresponds to clinically significant declines in renin-angiotensin aldosterone system and sympathetic nervous system activity, which can have substantial effects on systemic blood pressure [67–70]. Observational evidence of non-surgical weight loss education demonstrates successful declines in body mass index, waist circumference, and blood pressure [71]. Non-surgical weight loss is also associated with improvement in renal function, both in conjunction with and independently of remission of hypertension [68, 72]. However, lifestyle interventions such as diet and exercise are limited in their magnitude of effect and sustainability. Although patients who undergo lifestyle interventions often see initial or modest improvements in blood pressure, behavioral counseling along with diet, exercise, or both does not result in persistent weight loss, and fails to attenuate long-term adverse cardiovascular outcomes resulting from obesity [73–75].
Many weight loss medications are effective in contributing to clinically significant weight reduction [76]; however, their influence on hypertension is mixed. In randomized controlled trials of hypertensive adults comparing weight loss medications to placebo, orlistat and phentermine/topiramate reduced blood pressure, while sibutramine increased blood pressure [77]. Many commercially available weight loss medications have not been studied in hypertensive populations, and data on their impact on long-term morbidity and mortality is greatly limited. Sibutramine has been removed from the market due to considerable risks of adverse outcomes, and phentermine/topiramate is not marketed in Europe due to similar concerns [77].
Given the close interplay between obstructive sleep apnea, obesity, and hypertension, there is a great deal of interest in understanding how management of obstructive sleep apnea can impact hypertension in obesity. A recent randomized controlled trial by Chirinos et al. evaluated the effects of weight loss, continuous positive airway pressure (CPAP) therapy, or both on markers of inflammation, insulin resistance, and intermediate measures of cardiovascular outcomes including blood pressure. Among patients who adhered to their assigned protocol, patients had a mean weight of 112–114 kg at the start of the study; patients in the weight loss intervention groups achieved 6.8–7 kg of weight reduction. The combined intervention group achieved the greatest reduction in systolic blood pressure (14.1 mmHg), although all three intervention groups experienced clinically significant declines in blood pressure [78]. The combined intervention was also associated with greater reduction in insulin resistance and serum triglycerides compared to either intervention alone. Thus, combined CPAP therapy and non-surgical weight loss can have important effects on blood pressure and other cardiovascular risk factors in obese patients who have both hypertension and obstructive sleep apnea. No data exist regarding the effects of these interventions on long-term morbidity and mortality.
EFFECTS OF BARIATRIC SURGERY ON HYPERTENSION IN OBESITY
Surgical interventions have a more profound and lasting impact on weight loss and intermediate risk factors for cardiovascular disease than non-surgical interventions. Large magnitude weight loss following bariatric surgery is associated with high rates of hypertension remission. In a meta-analysis of randomized controlled trials, 50% of obese patients who underwent bariatric surgery had a diagnosis of hypertension prior to undergoing surgery; these patients experienced a 75% remission of hypertension [79]. These results were corroborated by more recent randomized controlled trials [80, 81]. A recent small prospective study demonstrated substantial, sustained declines in plasma leptin and muscle sympathetic nerve traffic following bariatric surgery, which likely facilitated the decline in blood pressure observed [82].
With regard to end organ effects of hypertension in obesity, echocardiography demonstrated regression of left ventricular hypertrophy and improvement in diastolic function following bariatric surgery [83]. The improvement in intermediate risk factors following surgical weight loss seems to be correlated to improved long-term renal and cardiovascular outcomes and mortality. Although there is no existing randomized controlled data, in observational studies, bariatric surgery is associated with a significant reduction in proteinuria [84], longitudinal renal function decline, and development of end stage renal disease [85]. Several observational studies have also demonstrated substantial reduction in long-term cardiovascular events and mortality in patients who underwent bariatric surgery compared to usual care [72, 86–88].
Surgical weight loss does confer perioperative risks due to the procedures themselves, including surgical complications, reoperation, acute kidney injury, and mortality [79, 89]. Nonetheless these risks have relatively low rates of occurrence [79], and seem to be outweighed by the improved morbidity and mortality observed following weight loss surgery.
CONCLUSION
Given the rising prevalence of obesity, it is critical to address modifiable risk factors to reduce the burden of illness in these patients. However, the closely overlapping and complex pathophysiology contributing to hypertension in obesity greatly complicates its management, further exacerbated by challenges in accurately measuring blood pressure in these patients. Moreover, obese patients have higher rates of resistant hypertension, altered drug handling, and greater burden of comorbidities, engendering additional barriers to appropriate treatment. More investigations are needed to better understanding the optimal pharmacologic management of hypertension in this challenging patient population.
Weight loss can result in dramatic improvements in physiologic parameters contributing to hypertension. However, while excess food intake and inactivity are major contributors to the development of obesity, lifestyle interventions such as diet and exercise are often insufficient to generate clinically significant weight loss. Although associated with greater short-term risk, surgical interventions provide more sustained declines in body mass, with greater likelihood of reversal of hypertension. While data are limited regarding long-term outcomes, surgical weight loss seems to confer a significant renal, cardiovascular, and mortality benefit in obese patients.
Acknowledgments
This publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL133843. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest
Jordana B. Cohen declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article contains information on a retrospective study performed by the author. For this type of study formal consent is not required. The article does not contain any other studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017 doi: 10.1056/NEJMoa1614362. This systematic review of epidemiologic evidence evaluated trends in obesity across 195 countries, evaluating the global impact of obesity on mortality and disability. Unlike previous studies, these analyses addressed important issues including the relationship between BMI and economic development and the influence of of epidemiologic and demographic transition on disease burden in obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 8.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring) 2013;21(1):E51–5. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A, et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239(2):629–33. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. British Medical Journal. 2016:353. doi: 10.1136/bmj.i2156. ARTN i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 19.Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126(25):2983–9. doi: 10.1161/CIRCULATIONAHA.112.117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014;130(19):1692–9. doi: 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saydah S, Bullard KM, Cheng Y, Ali MK, Gregg EW, Geiss L, et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring) 2014;22(8):1888–95. doi: 10.1002/oby.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca-Reyes S, de Alba-Garcia JG, Parra-Carrillo JZ, Paczka-Zapata JA. Effect of standard cuff on blood pressure readings in patients with obese arms. How frequent are arms of a ‘large circumference’? Blood Press Monit. 2003;8(3):101–6. doi: 10.1097/01.mbp.0000085763.28312.03. [DOI] [PubMed] [Google Scholar]
- 23.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004;44(2):127–33. doi: 10.1161/01.HYP.0000137982.10191.0a. [DOI] [PubMed] [Google Scholar]
- 25.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–15. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. The Adipocyte-Derived Hormone Leptin is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.018226. This in vitro study evaluating human adrenal tissue demonstrated that leptin acts directly on adrenal glomerulus cells to stimulate aldosterone production and pro-fibrotic cardiac markers. Anti-mineralocorticoid administration was effective in attenuating aldosterone secretion as well as the pro-fibrotic markers. [DOI] [PubMed] [Google Scholar]
- 28.Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, et al. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65(1):45–53. doi: 10.1161/HYPERTENSIONAHA.114.03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamez-Mendez AM, Vargas-Robles H, Rios A, Escalante B. Oxidative Stress-Dependent Coronary Endothelial Dysfunction in Obese Mice. PLoS One. 2015;10(9):e0138609. doi: 10.1371/journal.pone.0138609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7(5):e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121(17):1896–903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 32.Caillon A, Schiffrin EL. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr Hypertens Rep. 2016;18(3):21. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 33.Lohmeier TE, Iliescu R. The sympathetic nervous system in obesity hypertension. Curr Hypertens Rep. 2013;15(4):409–16. doi: 10.1007/s11906-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32(7):1551–65. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10(2):93–8. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168(9):928–35. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wohlfahrt P, Somers VK, Cifkova R, Filipovsky J, Seidlerova J, Krajcoviechova A, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235(2):625–31. doi: 10.1016/j.atherosclerosis.2014.05.958. [DOI] [PubMed] [Google Scholar]
- 39.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, et al. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension. 2015;66(2):294–300. doi: 10.1161/HYPERTENSIONAHA.115.05494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boillot A, Zoungas S, Mitchell P, Klein R, Klein B, Ikram MK, et al. Obesity and the microvasculature: a systematic review and meta-analysis. PLoS One. 2013;8(2):e52708. doi: 10.1371/journal.pone.0052708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33(1):23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32(1):16–25. doi: 10.1097/HJH.0b013e328364fb58. [DOI] [PubMed] [Google Scholar]
- 43.McTigue KM, Chang YF, Eaton C, Garcia L, Johnson KC, Lewis CE, et al. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity (Silver Spring) 2014;22(3):801–10. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 44.Hollenstein UM, Brunner M, Schmid R, Muller M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int J Obes Relat Metab Disord. 2001;25(3):354–8. doi: 10.1038/sj.ijo.0801555. [DOI] [PubMed] [Google Scholar]
- 45.Zuckerman M, Greller HA, Babu KM. A Review of the Toxicologic Implications of Obesity. J Med Toxicol. 2015;11(3):342–54. doi: 10.1007/s13181-015-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291(6):684–92. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 47.Brill MJ, van Rongen A, Houwink AP, Burggraaf J, van Ramshorst B, Wiezer RJ, et al. Midazolam pharmacokinetics in morbidly obese patients following semi-simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53(10):931–41. doi: 10.1007/s40262-014-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Bouquegneau A, Vidal-Petiot E, Vrtovsnik F, Cavalier E, Rorive M, Krzesinski JM, et al. Modification of Diet in Renal Disease versus Chronic Kidney Disease Epidemiology Collaboration equation to estimate glomerular filtration rate in obese patients. Nephrol Dial Transplant. 2013;28(Suppl 4):iv122–iv30. doi: 10.1093/ndt/gft329. [DOI] [PubMed] [Google Scholar]
- 50.Sinkeler SJ, Visser FW, Krikken JA, Stegeman CA, Homan van der Heide JJ, Navis G. Higher body mass index is associated with higher fractional creatinine excretion in healthy subjects. Nephrol Dial Transplant. 2011;26(10):3181–8. doi: 10.1093/ndt/gfq850. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed SB, Fisher ND, Stevanovic R, Hollenberg NK. Body mass index and angiotensin-dependent control of the renal circulation in healthy humans. Hypertension. 2005;46(6):1316–20. doi: 10.1161/01.HYP.0000190819.07663.da. [DOI] [PubMed] [Google Scholar]
- 52.Wojcicki J, Jaroszynska M, Drozdzik M, Pawlik A, Gawronska-Szklarz B, Sterna R. Comparative pharmacokinetics and pharmacodynamics of propranolol and atenolol in normolipaemic and hyperlipidaemic obese subjects. Biopharm Drug Dispos. 2003;24(5):211–8. doi: 10.1002/bdd.357. [DOI] [PubMed] [Google Scholar]
- 53.Wofford MR, Anderson DC, Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14(7 Pt 1):694–8. doi: 10.1016/s0895-7061(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 54.Cohen JB, Stephens-Shields AJ, Denburg MR, Anderson AH, Townsend RR, Reese PP. Obesity, Renin-Angiotensin System Blockade and Risk of Adverse Renal Outcomes: A Population-Based Cohort Study. Am J Nephrol. 2016;43(6):431–40. doi: 10.1159/000446862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raij L, Egan BM, Zappe DH, Purkayastha D, Samuel R, Sowers JR. Office and ambulatory blood pressure-lowering effects of combination valsartan/hydrochlorothiazide vs. hydrochlorothiazide-based therapy in obese, hypertensive patients. J Clin Hypertens (Greenwich) 2011;13(10):731–8. doi: 10.1111/j.1751-7176.2011.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Bush C, Keefe DL. Aliskiren-based therapy lowers blood pressure more effectively than hydrochlorothiazide-based therapy in obese patients with hypertension: sub-analysis of a 52-week, randomized, double-blind trial. J Hypertens. 2009;27(7):1493–501. doi: 10.1097/HJH.0b013e32832be593. [DOI] [PubMed] [Google Scholar]
- 57.Reisin E, Graves JW, Yamal JM, Barzilay JI, Pressel SL, Einhorn PT, et al. Blood pressure control and cardiovascular outcomes in normal-weight, overweight, and obese hypertensive patients treated with three different antihypertensives in ALLHAT. J Hypertens. 2014;32(7):1503–13. doi: 10.1097/HJH.0000000000000204. discussion 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70(12):2116–23. doi: 10.1038/sj.ki.5001854. [DOI] [PubMed] [Google Scholar]
- 59.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 2015;314(9):884–94. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 60.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 61.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 62.Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, et al. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 2013;125(11):513–20. doi: 10.1042/CS20130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg R, Kneen L, Williams GH, Adler GK. Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab. 2014;16(3):268–72. doi: 10.1111/dom.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65(5):1082–8. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youcef G, Olivier A, Nicot N, Muller A, Deng C, Labat C, et al. Preventive and chronic mineralocorticoid receptor antagonism is highly beneficial in obese SHHF rats. Br J Pharmacol. 2016;173(11):1805–19. doi: 10.1111/bph.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17(12):3438–46. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 67.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356–62. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 68.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 69.Straznicky NE, Grima MT, Lambert EA, Eikelis N, Dawood T, Lambert GW, et al. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29(3):553–64. doi: 10.1097/HJH.0b013e3283418875. [DOI] [PubMed] [Google Scholar]
- 70.Hirsch J, Leibel RL, Mackintosh R, Aguirre A. Heart rate variability as a measure of autonomic function during weight change in humans. Am J Physiol. 1991;261(6 Pt 2):R1418–23. doi: 10.1152/ajpregu.1991.261.6.R1418. [DOI] [PubMed] [Google Scholar]
- 71.Rothberg AE, McEwen LN, Kraftson AT, Ajluni N, Fowler CE, Nay CK, et al. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res Care. 2017;5(1):e000341. doi: 10.1136/bmjdrc-2016-000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson BL, Blackhurst DW, Latham BB, Cull DL, Bour ES, Oliver TL, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545–56. doi: 10.1016/j.jamcollsurg.2012.12.019. discussion 56–8. [DOI] [PubMed] [Google Scholar]
- 73.Mastellos N, Gunn LH, Felix LM, Car J, Majeed A. Transtheoretical model stages of change for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2014;2:CD008066. doi: 10.1002/14651858.CD008066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral Counseling to Promote a Healthy Lifestyle for Cardiovascular Disease Prevention in Persons With Cardiovascular Risk Factors: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. US Preventive Services Task Force Evidence Synthesis. 2014 [PubMed] [Google Scholar]
- 75.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. This large, multi-center randomized controlled study evaluated the longitudinal effects of lifestyle interventions (including calorie-restricted diet and increased physical activity) versus diabetes support and education in patients with type 2 diabetes. The study was discontinued early because of a failure to detect a difference in cardiovascular events between the treatment and control groups after a median follow up of 9.6 years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016;315(22):2424–34. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siebenhofer A, Jeitler K, Horvath K, Berghold A, Posch N, Meschik J, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD007654. doi: 10.1002/14651858.CD007654.pub4. [DOI] [PubMed] [Google Scholar]
- 78.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–75. doi: 10.1056/NEJMoa1306187. This randomized controlled trail of patients with obesity and moderate to severe sleep apnea evaluated the effects of weight loss, CPAP, or both on several intermediate markers of cardiovascular risk. Combination therapy resulted in greater reduction of insulin resistance, triglyceride levels, and blood pressure than either treatment alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. This randomized controlled trial of obese type 2 diabetics compared intensive medical therapy to bariatric surgery. Patients who underwent bariatric surgery had much more dramatic weight loss and improved quality of life compared to medical therapy alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 82.Seravalle G, Colombo M, Perego P, Giardini V, Volpe M, Dell’Oro R, et al. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension. 2014;64(2):431–7. doi: 10.1161/HYPERTENSIONAHA.113.02988. [DOI] [PubMed] [Google Scholar]
- 83.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24):1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 84.Li K, Zou J, Ye Z, Di J, Han X, Zhang H, et al. Effects of Bariatric Surgery on Renal Function in Obese Patients: A Systematic Review and Meta Analysis. PLoS One. 2016;11(10):e0163907. doi: 10.1371/journal.pone.0163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney International. 2016;90(1):164–71. doi: 10.1016/j.kint.2016.02.039. This propensity score matched cohort study was the first study to evaluate the effect of bariatric surgery on longitudinal renal outcomes. In multivariable adjusted analyses, the study demonstrated a 58% lower risk of 30% decline in renal function and a 57% lower risk of doubling of serum creatinine or end stage renal disease in obese patients who underwent bariatric surgery compared to matched controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–8. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 87.Scott JD, Johnson BL, Blackhurst DW, Bour ES. Does bariatric surgery reduce the risk of major cardiovascular events? A retrospective cohort study of morbidly obese surgical patients. Surg Obes Relat Dis. 2013;9(1):32–9. doi: 10.1016/j.soard.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 89.Weingarten TN, Gurrieri C, McCaffrey JM, Ricter SJ, Hilgeman ML, Schroeder DR, et al. Acute kidney injury following bariatric surgery. Obes Surg. 2013;23(1):64–70. doi: 10.1007/s11695-012-0766-1. [DOI] [PubMed] [Google Scholar]