Radiolabeled cholesteryl ethers: A need to analyze for biological stability before use (original) (raw)
Abstract
Radiolabeled cholesteryl ethers are widely used as non-metabolizable tracers for lipoproteins and lipid emulsions in a variety of in vitro and in vivo experiments. Since cholesteryl ethers do not leave cells after uptake and are not hydrolyzed by mammalian cellular enzymes, these compounds can act as markers for cumulative cell uptakes of labeled particles. We have employed [3H]cholesteryl oleoyl ether to study the uptake and distribution of triglyceride-rich emulsion particles on animal models. However, questionable unexpected results compelled us to analyze the stability of these ethers. We tested the stability of two commercially available radiolabeled cholesteryl ethers - [3H]cholesteryl oleoyl ether and [3H]cholesteryl hexadecyl ether from different suppliers, employing in vitro, in vivo and chemical model systems. Our results show that, among the two cholesteryl ethers tested, one ether was hydrolyzed to free cholesterol in vitro, in vivo and chemically under alkaline hydrolyzing agent. Free cholesterol, unlike cholesteryl ether, can then re-enter the circulation leading to confounding results. The other ether was not hydrolyzed to free cholesterol and remained as a stable ether. Hence, radiolabeled cholesteryl ethers should be analyzed for biological stability before utilizing them for in vitro or in vivo experiments.
Abbreviations: CE, cholesteryl ester; [3H]CEt-ARC, [3H] cholesteryl oleoyl ether-American Radiolabeled Chemicals; [3H]CEt-PE, [3H] cholesteryl hexadecyl ether-PerkinElmer; FC, free cholesterol; hrs, hours; TLC, thin layer chromatography; TG, triglycerides
Keywords: Cholesteryl ether, J774 A2 macrophages, Soy oil emulsion, Thin layer chromatography, triDHA emulsion
Highlights
- •
Tested stability of two commercially available radiolabeled cholesteryl ethers. - •
One ether was hydrolyzed to free cholesterol (FC) in vitro and in vivo. - •
FC, re-entered circulation giving questionable unexpected results in experiments. - •
The other ether was unhydrolyzed in all model systems. - •
Radiolabeled cholesteryl ethers should be analyzed for stability before use.
1. Introduction
Radiolabeled cholesteryl esters (CE) have been used as markers for lipid and lipoprotein metabolic experiments, but CE are degradable following uptake by cells and the radiolabel can be released as free cholesterol (FC) into the cell itself or into blood. FC may readily exchange between lipoproteins and membrane surfaces and re-enter cells leading to confounding results [1], [2].
Availability of CE analogs that are both radiolabeled and not metabolized in mammalian cells have contributed to understanding the metabolism of plasma lipoproteins and lipid emulsions. The structural and thermodynamic similarities of the cholesteryl ethers to the esters allow the use of ethers as non-metabolizable analogs for CE in biological systems [3], [4], [5], [6], [7]. The stable ether bond is not hydrolyzed by mammalian cellular enzymes and ethers do not readily escape from cells. This results in accumulation of cholesteryl ethers in cells and provides a cumulative marker of the labeled lipid particle uptake [8]. Radiolabeled cholesteryl ethers have been proven as useful tracers on a variety of in vitro and in vivo investigations employing lipoproteins [9], [10], [11], lipid emulsions [12], and liposomes [13], [14].
Our research group is involved in experimental projects employing laboratory made triglyceride (TG) emulsions of docosahexaenoic acid (triDHA) [15] and commercially available fish oil and soy oil emulsions in studies in neonatal/ adult mice and in vitro models [16], [17], [18]. A major area of interest within our group is studies on transport and delivery of TG-rich emulsion particles in animal models. We have employed radiolabeled cholesteryl ethers as tracers for these studies [19], [20]. Recently, after obtaining a new supplier for the radiolabeled cholesteryl ether, unexpected results were observed in our well established model systems. These prompted us to investigate the biological stability of commercially purchased radiolabeled cholesteryl ethers. In the present investigation, we have compared the biological stability of two commercial radiolabeled cholesteryl ethers widely used in studies on lipid metabolism, employing previously established in vitro [18], [21] and in vivo [12], [22] model systems from our laboratory. Further, we also tested the chemical stability of radiolabeled cholesteryl ethers under conditions of alkaline hydrolysis.
2. Materials and methods
2.1. Ethics statement
All research studies were carried out according to protocols approved by the Columbia University Institutional Animal Care and Use Committee and in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
2.2. Materials
TriDHA was purchased from Nu-Chek Prep, Inc. (Elysian, MN). Egg yolk phosphatidylcholine was obtained from Avanti Polar-Lipids, Inc. (Alabaster, AL). The soy oil emulsion (IntralipidRx) was manufactured by Fresenius Kabi (Sweden) and was purchased from Baxter Healthcare Corporation (Deerfield, IL). The fish oil emulsion (OmegavenRx) was obtained from Fresenius Kabi (Austria). We used radiolabeled cholesteryl ethers from two companies; cholesteryl oleoyl ether (ART 1319) from American Radiolabeled Chemicals (St. Louis, MO) herein named as ([3H]CEt-ARC) and cholesteryl hexadecyl ether (NET 859001MC) from PerkinElmer (Boston, MA) herein named as ([3H]CEt-PE).
2.3. Lipid emulsions
TriDHA emulsions (10% by TG weight/100 mL emulsion) were laboratory made using sonication and centrifugation with TG oil and egg yolk phospholipid as previously detailed [15]. The emulsions were analyzed for the amount of TG and phospholipids (PL) by enzymatic procedure using a commercial kit according to the accompanying instructions (Wako Chemicals USA, Inc., Richmond, VA). The TG: phospholipid mass ratio was 5.0 ± 1.0, similar to very low density lipoprotein (VLDL)-sized particles.
We labeled commercially available fish oil/ soy oil emulsions and laboratory-made triDHA emulsions with radiolabeled cholesteryl ethers as we previously described [12]. Briefly, in a small glass vial, desired amounts of [3H]CEt-ARC or [3H]CEt-PE were mixed with 20–50 μL of 100% of ethanol. The vial was rotated to coat the vial wall evenly under N2 gas until the solvents evaporated completely. Immediately upon the vial becoming dry, 150 μL of the emulsion was added to the vial. The vial was mixed with gentle non-vortex shaking and allowed to sit for 30 min. After the same procedure, another two portions of emulsion were added to a total volume of 500 μL. The emulsion was sonicated three times on ice for 20 s, each at a power setting of 40 W using a Branson Sonifier Cell Disruptor (model W185) (Branson Scientific, Inc., Plainview, NY) to incorporate the [3H]cholesteryl ethers into the core of the emulsion particles.
2.4. Cells
Monolayer cultures of J774 A2 macrophage were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) fetal bovine serum, streptomycin (100 μg/mL), penicillin (100 U/mL), and glutamine as described previously (21). For each experiment, the cells were plated onto 6 well plates at 37 °C in an atmosphere containing 5% CO2, 95% air. Experiments were performed at 24 h after plating and 80% confluent cells were used for these studies.
2.5. Incubations
Prior to incubation, cells were washed with PBS at 37 °C. Then PBS was exchanged with experimental media. Cells were incubated for 4 & 24 h with commercially available soy oil emulsions labeled with [3H]CEt-ARC or [3H]CEt-PE. The incubation was performed at 37 °C on a rocker. At the end of incubation, medium was removed. The cells were chilled on ice and washed twice with ice cold PBS containing 0.2% BSA (1 and 5 min washes) and twice with ice cold PBS alone. Cell lipids were extracted by hexane/isopropanol (3:2) as described previously [23].
2.6. Animals
Wild type C57BL/6 J adult and neonatal mice were purchased from Jackson Laboratories (Bar Harbor). Adult mice were anesthetized by intraperitoneal injection of ketamine/xylazine. The mice were intraperitoneally administered fish oil emulsion labeled with [3H]CEt-ARC. After emulsion injection, retro-orbital blood was drawn at fixed time intervals (0, 1.5, 6, 8 and 24 h) by heparinized capillary tubes. Mice were sacrificed immediately after collection of the final blood sample at 24 h. Liver was dissected out after perfusion with 0.9% NaCl-containing heparin (2 units/mL). Total plasma TG was enzymatically measured by Wako TG determination kit. The amount of radioactivity in blood was measured by liquid scintillation spectrometry and was expressed as the percent (%) of the injected dose remaining in the whole blood [12].
Neonatal mice (p10) were intraperitoneally injected with laboratory-made triDHA emulsions labeled with [3H]CEt-ARC or [3H]CEt-PE. The animals were sacrificed at 1, 2, 4 and 24 h after the injection of radiolabeled triDHA emulsion. Blood was collected by intracardiac puncture. Liver was dissected out following perfusion with 0.9% NaCl-containing heparin (2 units/mL).
Liver samples were homogenized using a Polytron Tissue Disruptor (Omni TH, Kenneswa, GA). Chloroform/methanol (2:1) was used to extract lipids from liver homogenates, plasma and radiolabeled emulsions as described previously [16].
2.7. Chemical hydrolysis of radiolabeled cholesteryl ethers
To check the stability of radiolabeled cholesteryl ethers under conditions of alkaline hydrolysis, [3H]CEt-ARC or [3H]CEt-PE were mixed with 40 µg cholesteryl oleate and incubated with 0.1 mL of 1 M ethanolic KOH at 80 °C for 1 h [24]. Lipids were extracted using chloroform/methanol (2:1) as described previously [16].
2.8. Analyses
2.8.1. Thin layer chromatography
Cells/ liver extracts, radiolabeled emulsions and chemically hydrolyzed radiolabels were assayed for hydrolysis of cholesteryl ethers by determining radioactivity in the FC and CE bands on thin layer chromatography (TLC) with the solvent system hexane: diethyl ether: acetic acid (70:30:1) [23]. Samples were run in parallel lanes with standards and individual spots identified by iodine vapour, which were then scraped to determine the radioactivity in each band region.
2.8.2. Liquid scintillation counting
Tritium in blood and TLC samples were determined with liquid scintillation counting procedure. In brief, the samples were suspended in the scintillation fluid (Ultima Gold scintillation fluid, PerkinElmer, Boston, MA), mixed well and 3H dpm were assayed in a Perkin Elmer Tri-Carb liquid scintillation spectrometer 5110 TR.
2.9. Statistical analysis
Data are presented as mean ± SE. We compared plasma TG and radioactivity levels between different time points after intraperitoneal (i.p.) injection of radiolabeled fish oil emulsion. Paired _t_-tests were used to determine significant differences between time points. To compare the differences between radiolabel recovered in different TLC band regions from emulsions and cells, one-way ANOVA followed by post hoc Tukey test was used. Unpaired _t_-tests were used to compare the significant differences between different groups/time points on chemical and in vivo models.
3. Results
3.1. TG levels and blood clearance in mice injected with [3H]CEt-ARC labeled fish oil emulsion
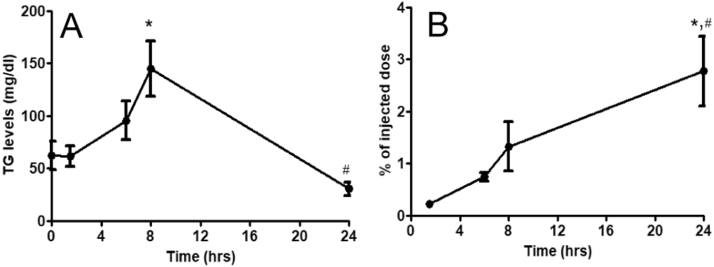
After injection of radiolabeled fish oil emulsion in mice, blood TG levels increased up to 2.3 fold at 8 h compared to baseline. This was followed by a decrease of TG levels to below baseline at 24 h (Fig. 1A). In contrast, the [3H] radioactivity levels in blood continuously increased over 24 h. Compared to the blood levels of radioactivity observed at 1.5 h, the levels continued to increase up to 5.7 fold at 8 h and 12 fold at 24 h after the injection (Fig. 1B).
Fig. 1.
Plasma TG concentrations and radiolabel measurements. (A) Plasma TG concentrations in mice acutely injected (i.p.) with [3H]CEt-ARC labeled lipid emulsion (B) Blood clearance in mice acutely injected (i.p.) with [3H]CEt-ARC labeled lipid emulsion. Emulsion particles remaining in blood were calculated as % of the injected dose. Values are mean ± SE, n = 5 at each data point. *p < 0.05, compared to 0 and 1.5 h time points; #p < 0.05, compared to 8 h time point.
When lipid extracts from mice liver samples collected at 24 h after the injection of radiolabeled fish oil emulsions were run on TLC, the percent of radiolabel recovered at different band regions were found to be: CE-7%; TG-20%; FC- 62%. These unexpected findings led us to carry out the following assays and experiments.
3.2. Cholesteryl ether radiolabel position on TLC
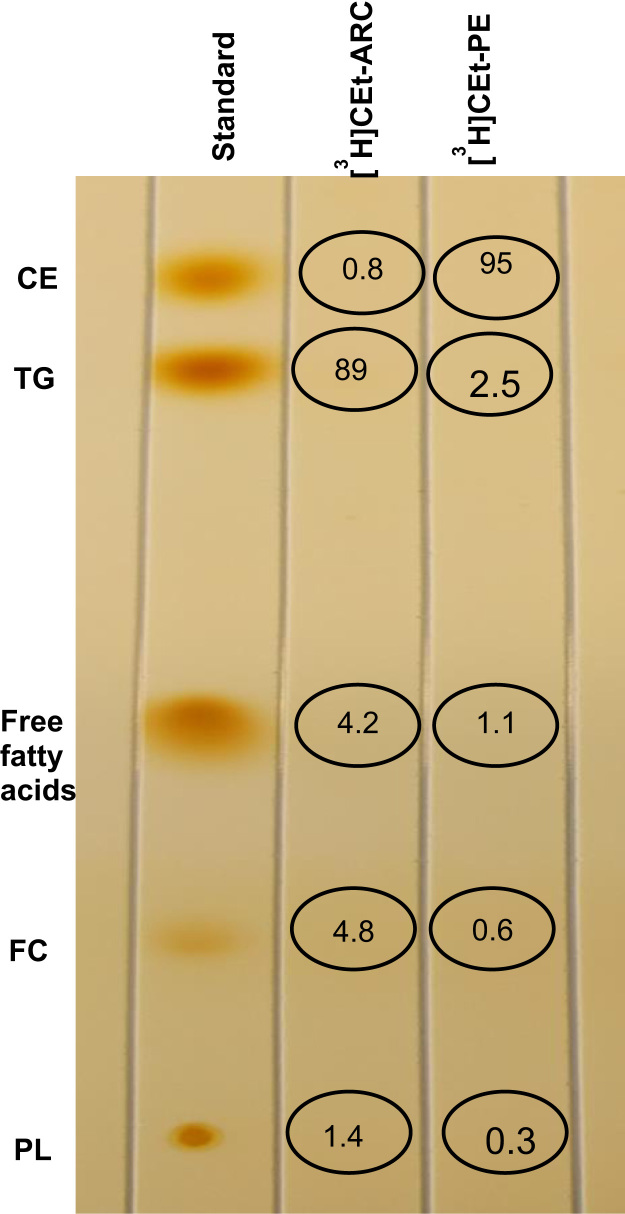
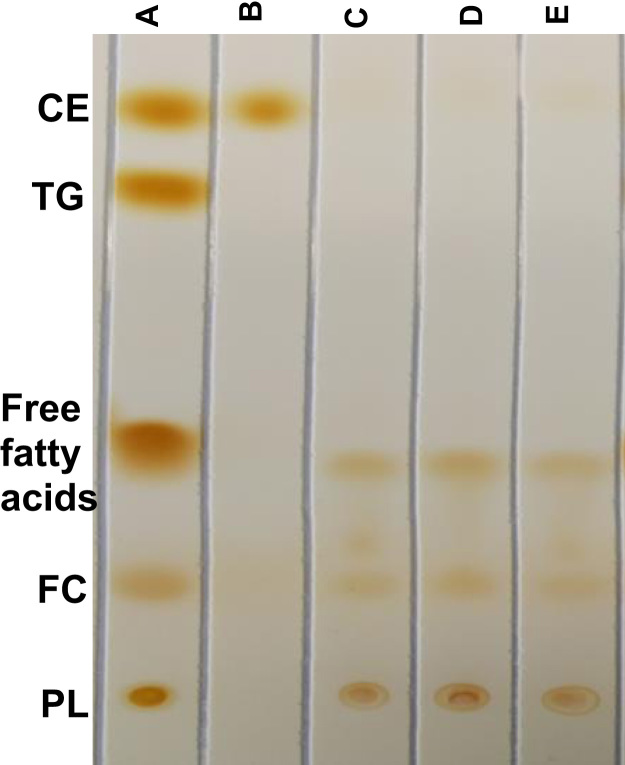
When radiolabeled cholesteryl ethers were run on TLC, 89% of the label from [3H]CEt-ARC was found to be at the TG band region. In contrast, 95% of [3H]CEt-PE label was found at the CE band region (Fig. 2).
Fig. 2.
Thin layer chromatographs of radiolabels. Thin layer chromatographs of [3H]CEt-ARC and [3H]CEt-PE. Percent values of radioactivity in each band region are means of duplicate determinations for each ether.
3.3. Cholesteryl oleoyl ether-ARC catabolized to FC in vitro
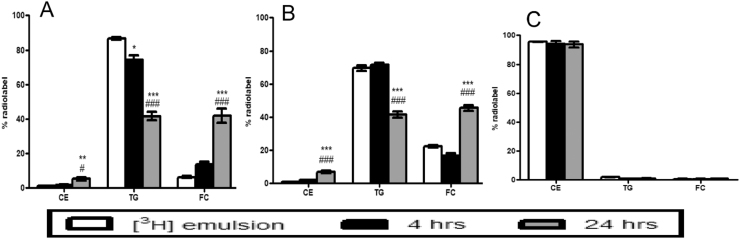
We analyzed the in vitro stability of [3H]CEt-ARC employing radiolabeled soy oil emulsions incubated with J774 A2 macrophages. In an initial experiment, we found that the radiolabel extracted from the soy oil emulsion contained 6% of the label as FC. Further, among cells incubated with soy oil emulsions labeled with [3H]CEt-ARC, 14% of the recovered intracellular label was found as FC at 4 h and 42% was found as FC at 24 h (Fig. 3A). In a following experiment, we found that the radiolabel extracted from the soy oil emulsion contained 22% of the label as FC, indicating that [3H]CEt-ARC was not stable during storage and was hydrolyzed to FC over time. Among cells incubated with soy oil emulsions labeled with [3H]CEt-ARC, 16% of the recovered intracellular label was found as FC at 4 h and 46% was found as FC at 24 h (Fig. 3B).
Fig. 3.
In vitrostability of radiolabeled cholesteryl ethers. Percent of radiolabel recovered in CE, TG and FC band regions on TLC from [3H]CEt-ARC and [3H]CEt-PE labeled emulsions and J774 A2 cells incubated with these emulsions for 4 and 24 h (A- study 1 [3H]CEt-ARC; B- study 2 [3H]CEt-ARC; C- [3H]CEt-PE). Values are mean ± SE, n = 2–6. *p < 0.05, **p < 0.01, ***p < 0.001 compared to radiolabeled emulsion; ###p < 0.001 compared to 4 h time point.
Among cells exposed to soy oil emulsions labeled with [3H]CEt-PE, 94% of the label was unhydrolyzed at both 4 & 24 h. Only trace amount of FC was detected in cells (Fig. 3C).
3.4. Cholesteryl oleoyl ether-ARC catabolized to FC in vivo
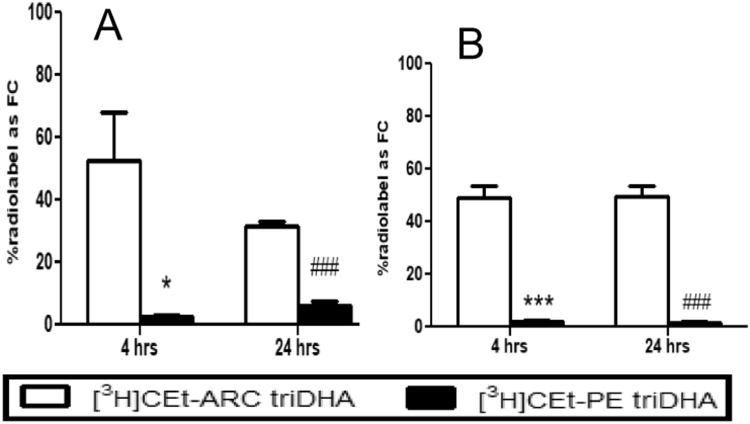
When lipid extracts from plasma samples collected at different time points after the injection of [3H]CEt-ARC labeled emulsions were run on TLC, 53% of the [3H]CEt-ARC label was found as FC at 4 h and 32% of the label was found as FC at 24 h. However, among mice injected with [3H]CEt-PE labeled tri-DHA emulsion, the label was not hydrolyzed (Fig. 4A). Similarly in liver, 49% of the [3H]CEt-ARC label was found as FC at 4 h and 50% of the label was found as FC at 24 h. However, among mice injected with [3H]CEt-PE labeled triDHA emulsion, the label was unhydrolyzed at either 4 or 24 h (Fig. 4B).
Fig. 4.
In vivostability of radiolabeled cholesteryl ethers. Percent of radiolabel recovered as FC on TLC from plasma (A) and liver (B) of mice injected with triDHA emulsions labeled with [3H]CEt-ARC or [3H]CEt-PE at 4 and 24 h after the injection. Values are mean ± SE, n = 3–4. *p < 0.05, ***p < 0.001 compared to [3H]CEt-ARC labeled emulsions at 4 h time point; ###p < 0.001 compared to [3H]CEt-ARC labeled emulsions at 24 h time point.
3.5. Cholesteryl oleoyl ether-ARC chemically hydrolyzed to FC
With the above results, we tested the effects of a strong saponification method to check the chemical stability of radiolabeled cholesteryl ethers. On incubation with 1 M ethanolic KOH at 80 °C for 1 h, cholesteryl oleate was completely hydrolyzed. The brownish spot visualized on phospholipid (PL) region of the TLC plate was due to KOH, since KOH alone stained the PL region of the plate. Under alkaline conditions which completely hydrolyzed cholesteryl oleate, [3H]CEt-ARC was hydrolyzed to FC, as 88% of the recovered radiolabel was found as FC in TLC band regions. However, [3H]CEt-PE label was unhydrolyzed (Fig. 5, Table 1).
Fig. 5.
Chemical hydrolysis of cholesteryl ethers. Thin layer chromatographs of lipid extracts from chemically hydrolyzed samples of [3H]CEt-ARC or [3H]CEt-PE with cholesteryl oleate. A- Standard; B- cholesteryl oleate; C- KOH cholesteryl oleate; D- KOH cholesteryl oleate & [3H]CEt-ARC; E- KOH cholesteryl oleate & [3H]CEt-PE.
Table 1.
Percent radioactivity in cholesteryl ester, triglycerides, free fatty acids, free cholesterol and phospholipids band regions determined by thin layer chromatography of chemically hydrolyzed [3H]CEt-ARC and [3H]CEt-PE. Values are mean ± SE. n = 3–4. ***p < 0.001 compared to [3H]CEt-ARC.
| % Radioactivity | ||
|---|---|---|
| [3H]CEt-ARC | [3H]CEt-PE | |
| Cholesteryl ester | 1.1 ± 0.1 | 95.5 ± 0.2*** |
| Triglycerides | 1 ± 0.1 | 0.9 ± 0.1 |
| Free fatty acids | 3.4 ± 0.1 | 2.1 ± 0.1*** |
| Free cholesterol | 88.2 ± 0.2 | 0.6 ± 0.01*** |
| Phospholipids | 6.3 ± 0.2 | 0.9 ± 0.1*** |
4. Discussion
To study blood clearance, tissue uptake and distribution of lipid emulsions and lipoproteins, it is often informative to include a marker, which will incorporate into cells and will remain metabolically inert under the experimental conditions. As well, the marker should not freely exchange between membranes and extracellular fractions. The cumulative deposition of the labeled particles provides data on the accumulation of this marker in cells and organs [1], [8]. Results observed during our initial studies employing [3H]CEt-ARC as tracers for lipid emulsion clearance in our rodent models show increasing levels of radioactivity rather than decreasing levels at 24 h. To clarify this observation, the radiolabel extracted from the mice liver samples were run on TLC, where we found more than half of the label to be in the form of FC at 24 h after the injection, providing the explanation for our results. The radiolabeled FC, unlike cholesteryl ether, re-entered the circulation resulting in increased levels of radioactivity in blood after TG levels were decreased. This finding led us to conduct the experiments herein comparing the biological stability of two commercially available radiolabeled cholesteryl ethers employing in vitro and in vivo model systems.
When radiolabeled cholesteryl ethers were run on TLC, most of the radiolabel with [3H]CEt-ARC was found to appear at the TG bands instead of CE. The reason behind this observation is not clear, as cholesteryl ether and CE having very similar physical and thermodynamic properties, and should migrate to the same region on TLC [1], [7]. This abnormal TLC cholesteryl ether migration pattern suggests additional polar groupings for [3H]CEt-ARC, which may make it susceptible to cellular hydrolysis. The position of the radiolabel at the TG region on TLC also rule out the possibility that the company is selling radiolabeled cholesteryl ester which is incorrectly marked as cholesteryl ether.
Our further studies on in vitro, in vivo and chemical model systems clearly confirmed that [3H]CEt-ARC was hydrolyzed to FC. 1 h after the injection, almost half of the [3H]CEt-ARC label was found to be in the form of FC in the liver (data not shown). FC can be excreted from liver cells into bile or transformed into bile acids, or it can be secreted into plasma through liver release of VLDL in the form of free and esterified cholesterol [25], [26]. Radiolabeled FC can re-enter the circulation, and be taken up by lipoproteins and other tissues leading to the confounding estimates of the uptake and distribution of emulsion particles. During our studies on distribution of triDHA emulsion particles in the neonatal mice, we found that among animals injected with triDHA emulsion labeled with [3H]CEt-ARC, the percent recovered dose of emulsions decreased in liver and increased in muscle between 4 and 24 h. Interestingly, we did not observe this effect when neonatal mice were injected with [3H]CEt-PE labeled triDHA emulsion (data not shown). Although the underlying reason behind this observation is not clear, we assume that [3H]CEt-ARC catabolized to FC and re-entered circulation leading to this questionable observation. For the studies reported here, we have used one batch of [3H]CEt-ARC. However, we have also observed the questionable TLC patterns previously from two other batches of [3H]CEt-ARC, where 75–98% of the radiolabel appeared at the TG band region (data not shown).
Recent investigations have employed [3H]CEt-ARC radiolabel for studies on lipoprotein uptake and metabolism [27], [28], [29]. Since commercially available radiolabeled cholesteryl ethers are generally accepted as biologically stable and metabolically inert tracers, researchers use this compound assuming that the source is of non-degradable quality. Our findings of [3H]CEt-ARC being catabolized to FC is of significance, since this can lead to confounding observations. We, thus suggest that commercially available radiolabeled cholesteryl ethers should be analyzed for biological stability prior to use in experiments.
Acknowledgements
This study was supported by National Institutes of Health Grant R01NS088197. The authors gratefully acknowledge Dr. Rajasekhar Ramakrishnan for assistance with statistical analysis.
Footnotes
Appendix A. Transparency document
Supplementary material
References
- 1.Pool G.L., French M.E., Edwards R.A., Huang L., Lumb R.H. Use of radiolabeled hexadecyl cholesteryl ether as a liposome marker. Lipids. 1982;17:448–452. doi: 10.1007/BF02535225. [DOI] [PubMed] [Google Scholar]
- 2.Terpstra A.H. Radiolabeled cholesteryl ethers trace LDL cholesteryl esters but not HDL cholesteryl esters in the rat. Atherosclerosis. 1995;112:1–6. doi: 10.1016/0021-9150(94)05390-5. [DOI] [PubMed] [Google Scholar]
- 3.Stein Y., Halperin G., Stein O. Biological stability of [3H]cholesteryl oleyl ether in cultured fibroblasts and intact rat. FEBS Lett. 1980;111:104–106. doi: 10.1016/0014-5793(80)80771-x. [DOI] [PubMed] [Google Scholar]
- 4.Halperin G., Stein O., Stein Y. Synthesis of ether analogs of lipoprotein lipids and their biological applications. Methods Enzymol. 1986;129:816–848. doi: 10.1016/0076-6879(86)29107-7. [DOI] [PubMed] [Google Scholar]
- 5.Phillips J.E., Preston Mason R. Inhibition of oxidized LDL aggregation with the calcium channel blocker amlodipine: role of electrostatic interactions. Atherosclerosis. 2003;168:239–244. doi: 10.1016/s0021-9150(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 6.Varban M.L., Rinninger F., Wang N., Fairchild-Huntress V., Dunmore J.H., Fang Q., Gosselin M.L., Dixon K.L., Deeds J.D., Acton S.L., Tall A.R., Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deckelbaum R.J., Halperin G., Atkinson D. Thermal transitions and structural properties of synthetic cholesterol alkyl and alkenyl ethers: analogues of biological cholesterol esters. J. Lipid Res. 1983;24:657–661. [PubMed] [Google Scholar]
- 8.Green S.R., Beltz W.F., Goldberg D.I., Pittman R.C. Cholesteryl oleyl and linoleyl ethers do not trace their ester counterparts in animals with plasma cholesteryl ester transfer activity. J. Lipid Res. 1989;30:1405–1410. [PubMed] [Google Scholar]
- 9.Seo T., Qi K., Chang C., Liu Y., Worgall T.S., Ramakrishnan R., Deckelbaum R.J. Saturated fat-rich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J. Clin. Investig. 2005;115:2214–2222. doi: 10.1172/JCI24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson J.L.C., Gautier T., Nijstad N., Tölle M., Schuchardt M., van der Giet M., Tietge U.J.F. High density lipoprotein (HDL) particles from end-stage renal disease patients are defective in promoting reverse cholesterol transport. Sci. Rep. 2017;7:41481. doi: 10.1038/srep41481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holme R.L., Miller J.J., Nicholson K., Sahoo D. Tryptophan 415 is critical for the cholesterol transport functions of scavenger receptor BI. Biochemistry. 2016;55:103–113. doi: 10.1021/acs.biochem.5b00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi K., Seo T., Al-Haideri M., Worgall T.S., Vogel T., Carpentier Y.A., Deckelbaum R.J. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry. 2002;41:3119–3127. doi: 10.1021/bi015770h. [DOI] [PubMed] [Google Scholar]
- 13.Kaess K., Fahr A. Liposomes as solubilizers for lipophilic parenteral drugs: transfer of drug and lipid marker to plasma proteins. Eur. J. Lipid Sci. Technol. 2014;116:1137–1144. [Google Scholar]
- 14.Al-Jamal W.T., Al-Ahmady Z.S., Kostarelos K. Pharmacokinetics & tissue distribution of temperature-sensitive liposomal doxorubicin in tumor-bearing mice triggered with mild hyperthermia. Biomaterials. 2012;33:4608–4617. doi: 10.1016/j.biomaterials.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Williams J.J., Mayurasakorn K., Vannucci S.J., Mastropietro C., Bazan N.G., Ten V.S., Deckelbaum R.J. N-3 fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice. PLoS One. 2013;8:e56233. doi: 10.1371/journal.pone.0056233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Bennekum A.M., Kako Y., Weinstock P.H., Harrison E.H., Deckelbaum R.J., Goldberg I.J., Blaner W.S. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J. Lipid Res. 1999;40:565–574. [PubMed] [Google Scholar]
- 17.Zirpoli H., Abdillahi M., Quadri N., Ananthakrishnan R., Wang L., Rosario R., Zhu Z., Deckelbaum R.J., Ramasamy R. Acute administration of n-3 rich triglyceride emulsions provides cardioprotection in murine models after ischemia-reperfusion. PLoS One. 2015;10:e0116274. doi: 10.1371/journal.pone.0116274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aviram M., Williams K.J., McIntosh R.A., Carpentier Y.A., Tall A.R., Deckelbaum R.J. Intralipid infusion abolishes ability of human serum to cholesterol-load cultured macrophages. Arterioscler. 1989;9:67–75. doi: 10.1161/01.atv.9.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Qi K., Seo T., Jiang Z., Carpentier Y.A., Deckelbaum R.J. Triglycerides in fish oil affect the blood clearance of lipid emulsions containing long- and medium-chain triglycerides in mice. J. Nutr. 2006;136:2766–2772. doi: 10.1093/jn/136.11.2766. [DOI] [PubMed] [Google Scholar]
- 20.Chang C.L., Seo T., Matsuzaki M., Worgall T.S., Deckelbaum R.J. n-3 fatty acids reduce arterial LDL-cholesterol delivery and arterial lipoprotein lipase levels and lipase distribution. Arterioscler. Thromb. Vasc. Biol. 2009;29:555–561. doi: 10.1161/ATVBAHA.108.182287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwiegelshohn B., Presley J.F., Gorecki M., Vogel T., Carpentier Y.A., Maxfield F.R., Deckelbaum R.J. Effects of apoprotein E on intracellular metabolism of model triglyceride-rich particles are distinct from effects on cell particle uptake. J. Biol. Chem. 1995;270:1761–1769. doi: 10.1074/jbc.270.4.1761. [DOI] [PubMed] [Google Scholar]
- 22.Qi K., Al-Haideri M., Seo T., Carpentier Y.A., Deckelbaum R.J. Effects of particle size on blood clearance and tissue uptake of lipid emulsions with different triglyceride compositions. J. Parenter. Enteral Nutr. 2003;27:58–64. doi: 10.1177/014860710302700158. [DOI] [PubMed] [Google Scholar]
- 23.Ho Y.Y., Al-Haideri M., Mazzone T., Vogel T., Presley J.F., Sturley S.L., Deckelbaum R.J. Endogenously expressed apolipoprotein E has different effects on cell lipid metabolism as compared to exogenous apolipoprotein E carried on triglyceride-rich particles. Biochemistry. 2000;39:4746–4754. doi: 10.1021/bi992294a. [DOI] [PubMed] [Google Scholar]
- 24.Owen J.S., Mederios J.S., Ramalho V., Cechinel Y. Determination of the proportion of cholesteryl ester in plasma. Ann. Clin. Biochem. 1978;15:226–227. doi: 10.1177/000456327801500148. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons G.F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem. J. 1990;268:1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemes K., Åberg F., Gylling H., Isoniemi H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: from molecular mechanisms to clinical implications. World J. Hepatol. 2016;8:924–932. doi: 10.4254/wjh.v8.i22.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chadwick A.C., Holme R.L., Chen Y., Thomas M.J., Sorci-Thomas M.G., Silverstein R.L., Pritchard K.A., Sahoo D. Acrolein impairs the cholesterol transport functions of high density lipoproteins. PLoS One. 2015;10:e0123138. doi: 10.1371/journal.pone.0123138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kartz G.A., Holme R.L., Nicholson K., Sahoo D. SR-BI/CD36 chimeric receptors define extracellular subdomains of SR-BI critical for cholesterol transport. Biochemistry. 2014;53:6173–6182. doi: 10.1021/bi500706x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard R.D., Blesso C.N., Zabalawi M., Fulp B., Gerelus M., Zhu X., Lyons E.W., Nuradin N., Francone O.L., Li X.-A., Sahoo D., Thomas M.J., Sorci-Thomas M.G. Procollagen C-endopeptidase enhancer protein 2 (PCPE2) reduces atherosclerosis in mice by enhancing scavenger receptor class B1 (SR-BI)-mediated high-density lipoprotein (HDL)-cholesteryl ester uptake. J. Biol. Chem. 2015;290:15496–15511. doi: 10.1074/jbc.M115.646240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material