Long-term Outcomes After Proton Beam Irradiation in Patients With Large Choroidal Melanomas (original) (raw)
This single-center cohort study analyzes 10-year outcomes of visual acuity, eye retention, development of neovascular glaucoma, tumor recurrence, and melanoma-related mortality in patients who underwent proton beam irradiation for large choroidal melanomas.
Key Points
Question
What are the 10-year outcomes in patients who underwent proton beam irradiation for large choroidal melanomas?
Finding
In this single-center cohort study of 336 patients who underwent proton beam radiotherapy, the 10-year rate of visual acuity retention of at least 20/200 was 9% and of at least counting fingers was 22%. Ten years after treatment, the eye was retained (70%) and the tumor was controlled (88%) in most cases.
Meaning
Eye conservation may be possible with retention of ambulatory vision in a small proportion of patients with large choroidal melanomas many years after treatment with proton beam irradiation.
Abstract
Importance
Although radiotherapy has been used more frequently in past decades for the management of large melanomas, long-term efficacy of proton beam irradiation (PBI) of large choroidal melanomas has not been reported.
Objective
To evaluate long-term outcomes in patients who underwent PBI for the treatment of large choroidal melanomas.
Design, Setting, and Participants
Data were obtained at a single Boston, Massachusetts, academic tertiary referral practice for this retrospective cohort study. In total, 336 patients with large tumors treated over a 13-year period from January 1, 1985, to December 31, 1997, and followed up until the end points were reached or until December 31, 2008, were included. Data analyses were initially completed in February 2017 and finalized in July 2017. Large tumors were those with a height 10 mm or greater or a longest linear diameter greater than 16 mm or a height greater than 8 mm when the optic nerve was involved.
Intervention
Proton beam irradiation (total 70 Gy) delivered in 5 equal fractions.
Main Outcomes and Measures
The primary outcomes of rates of visual acuity retention, eye retention, tumor recurrence, and melanoma-related mortality were calculated using Kaplan-Meier estimates, and Cox proportional hazards regression analyses were completed to evaluate risk factors for tumor recurrence and melanoma-related mortality.
Results
In this cohort of 336 patients with large tumors, 150 were women and 329 were white; mean (SD) age was 60.0 (14.0) years. Of 178 patients without optic nerve involvement (tumor >1 disc diameter from optic nerve), the mean (SD) largest basal diameter was 18.1 (1.9) mm and mean height was 8.2 (2.7) mm. Optic nerve involvement and tumors greater than 8 mm were observed in 109 patients (32.4% of the cohort). Baseline visual acuity of 20/200 or better was observed in 244 patients (72.6%), and worse than 20/800 in 52 (15.5%). Ten-year rates of visual acuity retention were 8.7% (95% CI, 4.1%-15.6%) for at least 20/200 and 22.4% (95% CI, 15.4%-30.4%) for at least counting fingers. Ten years after PBI therapy, the eye was retained (70.4%; 95% CI, 61.5%-77.6%) and tumor controlled (87.5%; 95% CI, 76.8%-93.5%) in most patients. The 10-year all-cause mortality rate was 60.7% (95% CI, 55.5%-65.9%). Approximately half of the patients died of metastatic uveal melanoma (10-year rate, 48.5%; 95% CI, 43.0%-54.4%).
Conclusions and Relevance
This study demonstrates that eye conservation is possible in most cases, with ambulatory vision retained in a small proportion of patients 10 years after PBI. Tumor recurrence rates were low and mortality rates were comparable to those observed after enucleation.
Introduction
Large choroidal melanomas have been treated historically with enucleation. Over the past decades, radiotherapy has been used more frequently in the management of large melanomas. However, globe-sparing management of large choroidal melanomas is challenging because of secondary complications from the high volume of radiation and an increased rate of local recurrence.
Complications, most often radiation retinopathy, papillopathy, and neovascular glaucoma, are associated with a high risk of vision loss, and many large tumors are located in close proximity to the optic disc, further increasing the risk of such complications. The use of plaque brachytherapy can be technically challenging for large tumors or tumors close to the optic nerve. Placement of large plaques may be necessary, and delivery of an insufficient radiation dose at the tumor apex and uneven tumor coverage in thick tumors adjacent to the optic nerve can occur. The advantage of using a proton beam is that it may spare sensitive structures of the eye while delivering a homogeneous dose of radiation to the tumor. In addition, the radiation dose abruptly decreases outside the treatment area. These properties make proton beam irradiation (PBI) a suitable method to treat large tumors and tumors located near the optic nerve and fovea, delivering the prescribed dose to the entire area of the target tissue and reducing radiation exposure to surrounding healthy tissue. In this article, we present long-term patient outcomes after PBI of large choroidal melanomas.
Methods
Patients and Treatment
Patients with large tumors of the eye treated during a 13-year period (January 1, 1985, to December 31, 1997) were included in this study. Patients were followed up until the end points were reached or until December 31, 2008. The Collaborative Ocular Melanoma Study (COMS) criteria were used as a guideline to classify large tumors, that is, tumors with a height greater than 10 mm, a height greater than or equal to 2 mm and a greatest linear diameter greater than 16 mm, or a height greater than 8 mm with optic nerve involvement (defined as a tumor margin within 1 disc diameter [DD] of the optic disc). All patients were identified through the Ocular Melanoma Registry at Massachusetts Eye and Ear (MEE). The study was approved by the institutional review board of MEE, which also waived the need for informed patient consent.
The recommended follow-up protocol for patients was evaluation every 6 months for the first 5 years after treatment and then annually in the ocular oncology clinic at MEE or by their local ophthalmologists. The ophthalmologic examination at each visit completed at MEE included Snellen visual acuity testing, slitlamp biomicroscopy, indirect ophthalmoscopy, fundus photography, and ultrasonography. Adherence to the schedule of follow-up visits varied; 50 of 336 patients (14.9%) completed at least 15 follow-up visits, the minimum expected if the protocol were followed.
Mortality surveillance was completed annually through direct contact with participants, their next of kin, and local ophthalmologists, internists, oncologists, or other specialists monitoring the patient’s overall health. Whenever possible, cause of death was determined by obtaining hospital medical records. The National Death Index was used to ascertain cause of death for patients who were lost to follow-up. Cause of death provided by next of kin only was accepted when other confirmation methods were unavailable.
The treatment planning protocol for proton therapy of uveal melanomas has been described previously. A 3-dimensional treatment planning computer program facilitated the selection of the appropriate fixation angle, which was chosen to minimize irradiation of the lens, fovea, and optic disc. Patient immobilization was achieved via a bite block and individually contoured plastic masks mounted into a frame on a head holder. Proper patient positioning was confirmed radiographically, and patients were monitored during treatment with a video camera to ensure that they maintained fixation on the predetermined point. In this series, all patients received a total dose to the tumor of 70 Gy (gray, relative biological effectiveness) delivered in 5 equal fractions. The margins of the surrounding normal tissue that were included in the radiation field were 1.5 mm to 90% of the dose and 3 mm to 70% of the dose.
Study End Points
Two visual acuity end points were evaluated: visual acuity of 20/200 or better and visual acuity of counting figures (CF) or better. For the 20/200 end point, 244 participants with a baseline visual acuity of at least 20/200 (72.6% of the cohort) were included. Additional end points were eye retention, tumor recurrence, melanoma-related mortality, and neovascular glaucoma.
Statistical Analysis
Data analyses were initially completed February 2017 and finalized July 2017. All cumulative rates were calculated with the Kaplan-Meier estimator using Stata, version 12 (StataCorp). P < .05 was considered statistically significant.
Results
Baseline Characteristics
In total, 336 patients were included in the study; of these, 150 were women, and the mean (SD) age was 60.0 (14.0) years. Median follow-up time for survival was 7 years (range, 2.8 months to 23.9 years), and 131 patients (39.0%) in the cohort had at least 10 years of follow-up. For ocular outcomes, the median follow-up was 3 years (range, 1.4 months to 15.4 years), and 27 patients (8.0%) had at least 10 years of follow-up. The median age at treatment was 61 years (range, 24-90 years), with more men than women diagnosed with large tumors. Most participants (97.9%) self-reported their race as white. Hispanic and African American participants each represented less than 1% of the cohort. Median largest tumor dimensions were 18 mm (range, 9.0-24.0 mm) for basal diameter and 8.7 mm (range, 2.0-17.1 mm) for height. Of 178 patients without optic nerve involvement (tumor >1 disc diameter from optic nerve), the mean (SD) largest basal diameter was 18.1 (1.9) mm and mean height was 8.2 (2.7) mm. Optic nerve involvement and tumors greater than 8 mm were observed in 109 patients (32.4% of the cohort). With use of the TNM staging system of the American Joint Committee on Cancer and the International Union Against Cancer for uveal melanomas, most tumors (318 of 336 [94.6%]) were classified as T3 tumors. Retinal detachment was present at baseline in most patients (256 [76.2%]), and 205 (61.0%) had tumors within 2 DD of the optic nerve. Visual acuity at initial evaluation was equal to or better than 20/40 in 131 patients (39.0%), at least 20/200 in 244 (72.6%), and worse than 20/800 in 52 (15.5%) (Table 1).
Table 1. Baseline Characteristics of 336 Patients and Tumors.
| Characteristic | Initial Screening |
|---|---|
| Follow-up time, median (range), y | |
| Survival | 7 (2.8 mo to 23.9 y) |
| Ocular outcomes | 3 (1.4 mo to 15.4 y) |
| Age at treatment, median (range), y | 61 (24-90) |
| Male sex, No. (%) | 186 (55.4) |
| White, No. (%) | 329 (97.9) |
| LTD, median (range), mm | 18.0 (9.0-24.0) |
| Tumor height, median (range), mm | 8.7 (2.0-17.1) |
| Retinal detachment, No. (%) | 256 (76.2) |
| Tumor within 2 DD of optic disc, No. (%) | 205 (61.0) |
| Distance of tumor from optic disc, median (range), DD | 1.5 (0-7) |
| Baseline visual acuity, No. (%) | |
| 20/40 or better | 131 (39.0) |
| 20/50-20/100 | 74 (22.0) |
| 20/125-20/800 | 79 (23.5) |
| CF-NLP | 52 (15.5) |
Visual Acuity Outcomes
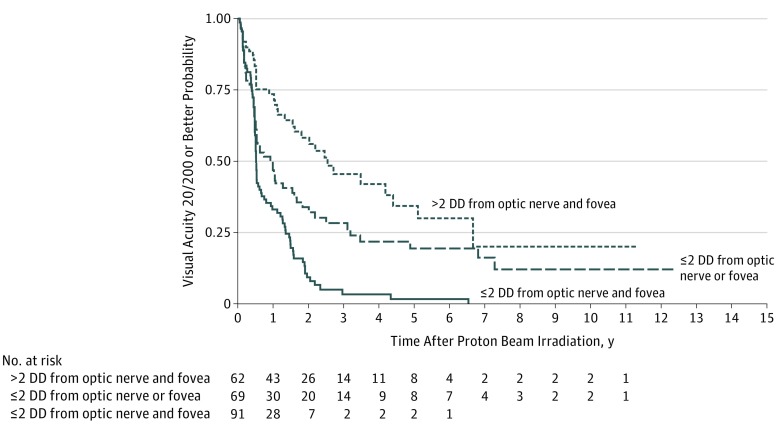
Visual acuity in 48.6% of patients (95% CI, 41.8%-55.0%) was 20/200 or better 1 year after treatment, but rates diminished considerably thereafter, with visual acuity retention 20/200 or better in only 22.6% (95% CI, 16.9%-28.9%) 3 years after PBI, 15.9% (95% CI, 10.6%-22.1%) at 5 years, and 8.7% (95% CI, 4.1%-15.6%) at 10 years (Table 2). Decreased visual acuity was dependent on the proximity of the tumor to the optic nerve and fovea: 73.5% (95% CI, 60.3%-82.8%) of patients with tumors located greater than 2 DD from the optic nerve and the fovea had visual acuity of 20/200 or better 1 year after treatment, and this proportion decreased to 45.4% (95% CI, 30.9%-58.9%) at 3 years, 34.3% (95% CI, 19.7%-49.5%) at 5 years, and 20.1% at 10 years. By contrast, 33% (95% CI, 23.5%-42.9%) of patients with tumors located within 2 DD of both structures retained visual acuity of 20/200 or better 1 year after treatment, and retention at 3 years and beyond approached 0% (3-year rate, 3.3% [95% CI, 0.7%-9.7%]; 5-year rate, 1.7% [95% CI, 0.2%-7.6%]); P < .001, log-rank test) (Figure 1). Similar results were observed for visual acuity retention of CF or better in patients with a baseline vision of at least CF, with 10-year retention rates of 22.4% (95% CI, 15.4%-30.4%) overall, 47.7% (95% CI, 27.1%-65.7%) for patients with tumors located greater than 2 DD from both structures, and 4.8% (95% CI, 1.2%-12.5%) for patients with tumors located within 2 DD of both structures (P < .001, log-rank test).
Table 2. Unadjusted Rates of Primary Ocular Outcomes.
| Outcome | Time After Proton Beam Irradiation, y | |||
|---|---|---|---|---|
| 1 | 3 | 5 | 10 | |
| Visual acuity 20/200 or better | ||||
| No. at risk | 222 | 53 | 22 | 4 |
| % (95% CI) | 48.6 (41.8-55.0) | 22.6 (16.9-28.9) | 15.9 (10.6-22.1) | 8.7 (4.1-15.6) |
| Eye retention | ||||
| No. at risk | 334 | 221 | 132 | 37 |
| % (95% CI) | 95.1 (92.1-97.0) | 85.8 (80.9-89.5) | 77.4 (71.1-82.5) | 70.4 (61.5-77.6) |
Figure 1. Kaplan-Meier Estimates of Visual Acuity Retention (>20/200) Over a 10-Year Follow-up Period, Adjusted for Tumor Location (Proximity of Tumor to Optic Nerve and/or Fovea).
DD indicates disc diameters.
Eye Retention
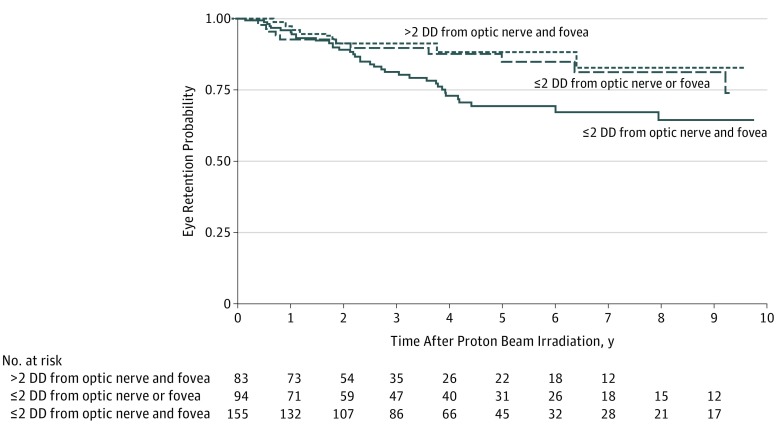
Eye conservation rates were high for all patients regardless of tumor location. Unadjusted rates of eye retention were 95.1% (95% CI, 92.1%-97.0%) 1 year after irradiation, 77.4% (95% CI, 71.1%-82.5%) at 5 years, and 70.4% (61.5%-77.6%) at 10 years (Table 2). With longer follow-up, patients with tumors located farther away (>2 DD) from critical structures experienced better outcomes than those with tumors in proximity to the optic nerve and fovea, with 88.2% vs 69.4% at 5 years and 82.7% vs 64.5% at 10 years (P = .048, log-rank test; Figure 2).
Figure 2. Kaplan-Meier Estimates of Eye Retention Over a 10-Year Follow-up Period, Adjusted for Tumor Location (Proximity of Tumor to Optic Nerve and/or Fovea).
DD indicates disc diameters.
Neovascular Glaucoma
Among the 336 patients, 85 (25.3%) developed neovascular glaucoma. The median time from treatment to diagnosis of neovascular glaucoma was 19 months (interquartile range, 12.2-25.5 months). By 5 years after irradiation, more than one-third of patients (34.9%) had developed neovascular glaucoma. Patients with tumors located within 2 DD of the optic nerve and/or fovea had higher rates of neovascular glaucoma (rates at 10 years, 44.8%; 95% CI, 34.8-54.6 for tumors ≤2 DD from optic nerve and fovea and 36.2%; 95% CI, 25.1%-50.2% for tumors ≤2 DD from optic nerve or fovea) than patients with tumors farther away from both structures (rate at 10 years, 20.6%; 95% CI, 11.3%-35.6%) (P = .01, log-rank test; Table 3).
Table 3. Kaplan-Meier Estimates of Neovascular Glaucoma.
| Tumor Location | Time After Proton Beam Irradiation, ya | P Value | |||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 10 | ||
| Unadjusted (n = 336)b | 6.5 (4.2-9.9) | 28.4 (23.2-34.5) | 34.9 (28.9-41.7) | 36.1 (29.8-43.2) | |
| ≤2 DD from optic nerve and fovea (n = 156) | 8.9 (5.3-14.9) | 35.8 (27.9-45.0) | 41.8 (33.2-51.6) | 44.8 (34.8-54.6) | .01 |
| ≤2 DD from optic nerve or fovea (n = 95) | 6.1 (2.6-14.0) | 31.0 (21.2-43.8) | 36.2 (25.1-50.2) | 36.2 (25.1-50.2) | |
| >2 DD from optic nerve and fovea (n = 83) | 2.6 (0.7-9.9) | 11.5 (5.9-21.6) | 20.6 (11.3-35.6) | 20.6 (11.3-35.6) |
Tumor Recurrence
Tumors recurred locally in 2.3% (95% CI, 1.1%-4.8%) of patients 1 year after PBI treatment, 5.0% (95% CI, 2.9%-8.5%) at 3 years, 7.8% (95% CI, 4.8%-12.6%) at 5 years, and 12.5% (95% CI, 6.5%-23.2%) at 10 years. Tumors at least 18 mm in largest basal diameter were more likely to recur than smaller tumors (relative risk [RR], 3.5; 95% CI, 1.27-9.75; P = .02), but risk of local recurrence was not significantly associated with thicker tumors, which were defined as at least 8.7 mm (the median size in our cohort).
Melanoma-Related Mortality
One year after treatment, 2.4% (95% CI, 1.22%-4.80%) of patients died of uveal melanoma. This percentage increased to 48.5% (95% CI, 43.0%-54.4%) by 10 years after PBI. Patients with larger tumors were at increased risk of dying of melanoma; a 1-mm increase in basal tumor diameter was associated with a 20% increase in risk (RR, 1.20; 95% CI, 1.11-1.29; P < .001).
Discussion
We evaluated outcomes after PBI therapy in patients with large tumors using COMS criteria to classify tumor size. Although enucleation is sometimes recommended for these tumors, proton irradiation is an attractive alternative treatment because of the delivery of a homogeneous radiation dose across the entire tumor and the sharp decrease in radiation dose distal to the tumor. Favorable outcomes were demonstrated in our cohort with regard to eye retention and local control. By contrast, visual acuity retention (20/200 or better) was observed in less than 20% of patients (eFigure in the Supplement). Poor visual prognosis was not unexpected given that tumor dimensions, both basal diameter and thickness, are established risk factors for visual acuity loss in patients treated with radiotherapy. Nevertheless, one-third of patients with tumors located more than 2 DD from the optic nerve and fovea retained at least 20/200 visiual acuity 10 years after PBI. Although tumor size is also associated with an increased risk of tumor recurrence and eye loss, local tumor recurrence was relatively infrequent, and eye conservation was possible for most patients in our cohort.
Our findings are similar to several studies in patients who received proton therapy for large choroidal melanomas, although direct comparisons are difficult because of variable definitions of size. Some investigators used the COMS criteria, while others used tumor height as a sole criterion for large tumors or the TNM staging classification.
Bensoussan et al reported findings similar to ours with respect to visual acuity retention (better than 20/200), eye retention, and melanoma-related mortality. Poorer results were observed in a shorter-term study of patients classified as having extra-large tumors treated with PBI, particularly with regard to local tumor recurrence and eye conservation. The melanomas included in that study were likely skewed toward the largest of tumors (median tumor diameter of patients included was 19.2 mm).
Large choroidal melanomas can also be successfully treated with plaque radiotherapy. The disadvantages of using this modality for treating large tumors include scleral melting from the high dose of radiation to the base of the tumor and insufficient dose at the tumor apex. Higher rates of tumor recurrence have been reported in patients treated with plaque radiotherapy than in those receiving radiotherapy using charged particles. However, Shields et al reported the same rate of recurrence 10 years after treatment with iodine-125 plaque radiotherapy as that reported herein. However, their series included smaller tumors than those reported here (median largest tumor diameter [LTD], 14 mm vs 18 mm; minimum LTD, 5 mm vs 9 mm; and maximum LTD, 21 mm vs 24 mm). Visual acuity outcomes appear to be fairly similar between the 2 modalities.
A concern of using globe-sparing therapies to treat large tumors is that survival may be compromised. Our results suggest that this concern is unfounded; the 5-year rate of melanoma-related mortality in our cohort was 32%, which is somewhat lower than that reported in a group of patients with large tumors who underwent enucleation. In the COMS clinical trial evaluating treatments for large tumors, unadjusted 10-year rates of histologically confirmed melanoma-related deaths were 45% and 41% for the radiotherapy combined with enucleation arm and the enucleation alone arm, respectively. The 10-year melanoma-related mortality in that study was similar to ours at 48.5% (95% CI, 43.9%-54.4%). Shields et al reported a 10-year metastasis rate of 55% in patients with large tumors treated with iodine-125 plaques.
Limitations
Limitations of this study include its retrospective nature with loss to follow-up. In addition, the use of Kaplan-Meier estimates does not account for those who may recover vision after initial loss.
Conclusions
This study provided long-term follow-up in patients with large choroidal melanomas treated with PBI, confirming the potential benefits of this therapy. Our findings suggested that 61.5% to 77.6% of patients retained the eye through 10 years, although only a small proportion of patients retained ambulatory vision many years after treatment. Tumor recurrence rates were low, and mortality rates were comparable to those previously reported following enucleation.
Supplement.
eFigure. Clinical Case
References
- 1.Diener-West M, Reynolds SM, Agugliaro DJ, et al. ; Collaborative Ocular Melanoma Study Group Report 23 . Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group report 23. J Clin Oncol. 2004;22(12):2438-2444. [DOI] [PubMed] [Google Scholar]
- 2.Augsburger JJ, Corrêa ZM, Trichopoulos N. An alternative hypothesis for observed mortality rates due to metastasis after treatment of choroidal melanomas of different sizes. Trans Am Ophthalmol Soc. 2007;105:54-59. [PMC free article] [PubMed] [Google Scholar]
- 3.Mooy CM, De Jong PT. Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol. 1996;41(3):215-228. [DOI] [PubMed] [Google Scholar]
- 4.Seddon JM, Gragoudas ES, Polivogianis L, et al. Visual outcome after proton beam irradiation of uveal melanoma. Ophthalmology. 1986;93(5):666-674. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES. The Bragg peak of proton beams for treatment of uveal melanoma. Int Ophthalmol Clin. 1980;20(2):123-133. [PubMed] [Google Scholar]
- 6.Collaborative Ocular Melanoma Study Group The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, II: initial mortality findings: COMS report No. 10. Am J Ophthalmol. 1998;125(6):779-796. [DOI] [PubMed] [Google Scholar]
- 7.Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2006;47(11):4666-4673. [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120(12):1665-1671. [DOI] [PubMed] [Google Scholar]
- 9.Bechrakis NE, Bornfeld N, Zöller I, Foerster MH. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology. 2002;109(10):1855-1861. [DOI] [PubMed] [Google Scholar]
- 10.Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62(5):1405-1411. [DOI] [PubMed] [Google Scholar]
- 11.Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut-Orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65(3):780-787. [DOI] [PubMed] [Google Scholar]
- 12.Bensoussan E, Thariat J, Maschi C, et al. Outcomes after proton beam therapy for large choroidal melanomas in 492 patients. Am J Ophthalmol. 2016;165:78-87. [DOI] [PubMed] [Google Scholar]
- 13.Conway RM, Poothullil AM, Daftari IK, Weinberg V, Chung JE, O’Brien JM. Estimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch Ophthalmol. 2006;124(6):838-843. [DOI] [PubMed] [Google Scholar]
- 14.Mosci C, Lanza FB, Barla A, et al. Comparison of clinical outcomes for patients with large choroidal melanoma after primary treatment with enucleation or proton beam radiotherapy. Ophthalmologica. 2012;227(4):190-196. [DOI] [PubMed] [Google Scholar]
- 15.Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (≥8-mm thick) in 354 consecutive patients. Ophthalmology. 2002;109(10):1838-1849. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Nabhan M, Schild SE, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2013;86(1):18-26. [DOI] [PubMed] [Google Scholar]
- 17.Puusaari I, Heikkonen J, Summanen P, Tarkkanen A, Kivelä T. Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology. 2003;110(11):2223-2234. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins BS; Collaborative Ocular Melanoma Study Group . The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, IV: ten-year mortality findings and prognostic factors: COMS report number 24. Am J Ophthalmol. 2004;138(6):936-951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement.
eFigure. Clinical Case