Do females use their sexual status to gain resource access? Investigating food-for-sex in wolves and dogs (original) (raw)
Abstract
While food sharing among related individuals can be explained by kin selection, food sharing between unrelated individuals has been more of an evolutionary puzzle. The food-for-sex hypothesis provides an explanation for the occurrence of food sharing among nonkin. However, little is known about the socio-ecological factors that can promote such a commodity exchange. A species mating system is a factor potentially influencing food-for-sex patterns of behavior. Here, we compared wolves, which form pair-bonds, with dogs, which are typically promiscuous in free-ranging contexts, to investigate the effect of reproductive stages on the behavior around a food source in 2 different contexts. Furthermore, we considered the roles of both the males and the females in the potential food-for-sex exchange. Results indicate that in both species and for both sexes the breeding period promotes decreased aggression. Additionally, females were more persistent in their attempts to access the food and were able to monopolize the resource more when in heat as compared to outside the breeding period. Finally, in dogs, but not wolves, females spent more time in proximity to the male’s bone and had a shorter latency to start eating it when in heat. Overall, this study demonstrates that the food-for-sex hypothesis plays a part in intersexual food sharing in canids, and highlights the role of females in the interaction. These effects were especially the case in dogs, suggesting a potential effect of mating system on food-for-sex responses.
Keywords: canid, food-for-sex, food sharing, tolerance.
Food sharing constitutes the joint use of a monopolizable food resource by more than 1 individual (Stevens and Gilby 2004) or the transfer of a food item from 1 individual to another (Feistner and McGrew 1989). In many instances, such sharing is a costly act for the donor, which can be explained by kin selection among related individuals (Hamilton 1964). More difficult to explain are the less common, but nevertheless observed, instances of food sharing between unrelated individuals. Investigating the contexts under which nonkin sharing occurs can reveal the evolutionary value of such a costly act.
One reciprocity-based hypothesis that may explain sharing with unrelated individuals is the food-for-sex hypothesis (Kaplan and Hill 1985), which proposes that males may share food with sexually receptive females in exchange for the opportunity to mate with them. This theory emerged as an explanation for the findings across multiple cultures that human males who are more successful hunters have higher reproductive success (Smith 2004). Although this is likely a complex phenomenon involving multiple factors including, but not limited to, prestige and phenotypic correlations with other fitness enhancing qualities (Gurven and von Rueden 2006), a potential basic factor involved is the direct exchange of meat for mating access; whereby good hunters are more successful at attracting mating partners. However, by itself, data on human food sharing, although informative about the course of human evolution, cannot reveal conclusions on the specific evolutionary forces producing such a relationship.
Comparative research across species is of particular use to investigate the potential role of food-for-sex exchange in the enhanced reproductive fitness of good hunters. By studying a variety of species where food sharing and copulations can be quantitatively recorded, we can isolate the socio-ecological factors that may drive the selection of such an exchange and test whether this exchange may provide an explanation for food sharing among nonkin. Therefore, over recent years, researchers have begun to investigate whether nonhuman primates exhibit food-for-sex exchanges.
Initial findings in chimpanzees suggested that the presence of an estrous female did not increase hunting probability or meat sharing with these females over anestrous females (Mitani and Watts 2001; Watts and Mitani 2002; Gilby et al. 2006, 2008). However, these studies focused on the short-term exchange for immediate mating opportunities. When considering a longer time frame, Gomes and Boesch (2009) found that over a period of 22 months, wild chimpanzee females copulated more frequently with males who shared meat with them (after controlling for a number of other alternative explanations), suggesting that at least chimpanzees do in fact exchange meat for sex. Similarly, in a study with orangutans, although food transfers did not result in more mating for males within 3 h, they did allow the association with the female to last longer, potentially resulting in longer-term mating benefits (van Noordwijk and van Schaik 2009). Finally, there is some observational evidence that food-for-sex may also explain intersexual food sharing in bonobos (Hohmann 2015). Furthermore, in a captive setting, Crick et al. (2013) demonstrated that female chimpanzees that copulated more during sessions where males were given high-quality food, gained more food with less perseverance. Even in the highly despotic rhesus macaques, an increase of food sharing has been found between male–female dyads that are engaged in consortship compared to dyads not in consortship (Dubuc et al. 2012).
Taken together the results from promiscuous primates so far suggest that a reproductively relevant context can promote food-for-sex exchange, even in despotic or solitary species that are not usually tolerant in regard to food. An important variable that is, therefore, likely to have an impact is the mating system of a given species. In a comprehensive analysis of food sharing in 68 primate species, Jaeggi and van Schaik (2011) concluded that living in multi-male groups, a proxy for female mate choice in primates, was a significant predictor of sharing from males to females. Chimpanzees, bonobos, and macaques, which show a food-for-sex effect, are promiscuous and females demonstrate mate choice (chimpanzees: Stumpf and Boesch 2006, macaques: Small 1990, bonobos: Hohmann 2015), therefore, males may show food sharing at reproductively crucial periods as a method of competing for mating opportunities (Gwynne 1984). Pair-bonded species, on the other hand, theoretically do not need to use trade or costly signaling during the mating period as they already have a high probability of mating success. However, overall, pair-bonded species show food sharing more often than nonmonogamous species (e.g., Chivers, 1974; Wolovich et al. 2007; St-Pierre et al. 2009), which might suggest that consortship is a precursor to pair-bonding (Dubuc et al. 2012). Until now, the effect of the reproductive period on food sharing in pair-bonded species has received little attention.
Although food sharing is overall more common in pair-bonded species, so far, to our knowledge only 1 pair-bonded species has been studied in regard to the effects of reproductive state on the extent of food sharing. Wolovich et al. (2008) provisioned captive owl monkey pairs with apple pieces during cycling (when conception could occur), pregnancy, and lactation. They found that males preferentially transferred food to their mates when they were lactating rather than when they were cycling. However, the authors did not accurately distinguish between when females could ‘potentially’ conceive and when actual mating was observed. Nevertheless, based on this study, it would seem that in pair-bonded species, where fathers can be more certain of their paternity, the post-parturition period, when care of both mate and offspring is important, is more likely to elicit food sharing behavior/provisioning from the male than the mating period.
Although limited, the above findings suggest that promiscuous species with mate competition are more likely to show food-for-sex exchanges whereas pair-bonded species show no change in food-sharing probability inside and outside sexually receptive periods. This prediction could be tested by using either closely related species with different mating systems or the same methodology across multiple species, but so far no such direct test has been carried out.
In addition to the effect of the mating system, previous studies have alluded to, but neglected to formally analyze, the potential role of the female in this commodity exchange. It is often assumed that the male shares food in order to gain mating opportunities. However, it is also possible that females use sexual receptivity to gain access to food by: 1) using sex to reduce tension and, therefore, create more tolerance in the males, 2) becoming less risk-averse at this time due to hormonal changes and, therefore, challenging males more, and/or 3) being more likely to choose to mate with males which share food with them. For example, Wolovich et al. (2008) found that females begged more when lactating compared to cycling, and Dubuc et al. (2012) reported that female macaques in consortship pairs approached the food containers more and submitted less than control pairs when not in consortship.
Therefore, the current study aimed to: 1) compare the effect of different mating systems in 2 closely related species; wolves and dogs and 2) investigate the role of both the males and females in food sharing during, and outside of, sexually receptive periods.
Monogamy is only present in approximately 5% of mammalian species, but is the most common breeding system among canids (Clutton-Brock 1989). Wolves Canis lupus are one such species, demonstrating pair-bonding over multiple years (Harrington and Mech 1982; Mech 1995; Jenks 2011). They breed only once per year and offspring from previous years remain to help with pup-rearing (Mech and Boitani 2003).
Despite the prevalence of pair-bonding in canids, 1 species, the domestic dog C . lupus familiaris, usually shows a promiscuous mating system more comparable to chimpanzees and macaques (Lord et al. 2013). Free-ranging dogs are those whose movements, activities, and reproduction are not constrained by humans (Cafazzo et al. 2014) and likely represent the largest group of domestic dogs in the world (>80%, Lord et al. 2013). Despite being closely related to wolves (Lindblad-Toh et al. 2005), dogs often live in multi-male, multi-female groups where both males and females have multiple mating partners and females exhibit choice over their mates (Cafazzo et al. 2014).
As well as showing differences in reproductive strategies, it is emerging that wolves and dogs also show different levels of tolerance around food resources. In the wild, wolves are reliant on food sharing as they are cooperative hunters, sharing large prey that the pack brings down together (Mech and Boitani 2003; MacNulty et al. 2012). Furthermore, they show regurgitative food transfer, with the breeding male provisioning the mother and pups while the female as well as other pack members regurgitate for the pups (Mech et al. 1999). However, to date there are no studies quantifying the factors involved in wolf food-sharing behavior.
Free-ranging dogs, on the other hand, are primarily scavengers that do not rely on cooperative hunting (Butler et al. 2004, Vanak and Gompper 2009), but feed on more readily available food sources from human waste. Likewise, although rare instances of alloparental care have been observed (Paul et al. 2014a), dogs do not typically provision puppies (but see Pal 2005), with even the mothers monopolizing food provisions and directly competing with their young offspring (Paul et al. 2014b). In the only direct comparison of food-sharing behavior in captive pack-living wolves and dogs, Range et al. (2015) found that high ranking dogs showed little tolerance to subordinates around food whereas subordinate wolves were more able to challenge their partners for access to the resource. This supports findings by Mech (1999) that regardless of rank, each wolf is able to defend his/her food source, and suggests a less despotic control of resources by dominant individuals in wolves than dogs.
While wolves and dogs show different mating systems and food-sharing tendencies, both species demonstrate linear dominance hierarchies when living in larger groups and males are dominant over females (wolves: Mech 1999, dogs: Cafazzo et al. 2010). This means that outside of the breeding season, the males of both species have priority of access to a food resource. Overall, this suggests they are good candidates for testing the effect of pair-bonding and promiscuity on food-for-sex exchanges.
We tested identically raised and kept pack-living wolves and dogs in male–female dyadic tolerance tests and more naturalistic group feeding observations. Despite testing animals in captivity, where mating preferences maybe more constrained than under free-ranging conditions, we would expect that mating strategies inherent to the specific species should still influence the species’ behaviors. We recorded the levels of food monopolization, aggression, and persistence behaviors of both males and females in these contexts both when females were in heat and when they were out of heat. Based on the limited literature in primates we predicted that the dogs would show a food-for-sex effect by becoming more tolerant when females were in heat, but the pair-bonded wolves would vary less in their tolerance levels across the reproductive stages. Additionally, because it is possible that females become less risk-averse during heat and/or more likely to choose tolerant over aggressive mates, we also predicted that females would have as much of an influence as males on any observed food-for-sex effects. Specifically, we predicted females to show more begging behaviors and less avoidance of the resource during reproductively receptive periods.
Materials and Methods
Subjects
Wolf and dog packs at the Wolf Science Center (www.wolfscience.at) were tested. All subjects were hand-raised in peer groups from the age of 10 days. They were bottle-fed and later hand-fed by humans and had continuous access to humans in the first 5 months of their life. After 5 months they were introduced into the packs of adult animals and currently live in large 2,000–8,000m2 enclosures in these groups (see Range et al. (2015) for more details). As adults they take part in training and various behavioral experiments on a daily basis.
The current experiment tested 9 dogs (5F, 4M) and 8 wolves (3F, 5M, see Tables 1 and 2 for details). Two methods were used to investigate food-for-sex effects, dyadic food tolerance tests and naturalistic bone feedings in the pack environment. All individuals had reached sexual maturity, but because 2 subordinate wolves did not become sexually active (i.e., they were never seen mating or attempting to mate with another individual), although they were present in the naturalistic tests, they did not take part in the dyadic tolerance tests and were not included in any analyses (not included in the number of subjects).
Table 1.
The pack compositions of the dogs and wolves during the period of the study
| Pack | Species | Individual | Sex |
|---|---|---|---|
| Kaspar | Wolf | Kaspar | M |
| Aragorn | M | ||
| Tala | F | ||
| Chitto | M | ||
| Shima | F | ||
| Nanuk 1 | Wolf | Nanuk | M |
| Una | F | ||
| Yukon | F | ||
| Nanuk 2 | Wolf | Nanuk | M |
| Una | F | ||
| Geronimo | Wolf | Geronimo | M |
| Wamblee | M | ||
| Yukon | F | ||
| Meru | Dog | Meru | M |
| Nia | F | ||
| Maisha | Dog | Maisha | M |
| Binti | F | ||
| Nuru | Dog | Nuru | M |
| Layla | F | ||
| Zuri | F | ||
| Asali | Dog | Asali | M |
| Bora | F |
Table 2.
The number of trials each dyad completed in each reproductive stage for both the dyadic and naturalistic tests
| Dyadic test | Naturalistic test | |||||
|---|---|---|---|---|---|---|
| Female | Male | Species | Heat | No heat | Heat | No heat |
| Tala | Kaspar | Wolf | 12 | 12 | 6 | 4 |
| Aragorn | Wolf | 12 | 12 | NA | NA | |
| Yukon | Nanuk | Wolf | 6 | 6 | NA | NA |
| Geronimo | Wolf | 6 | 3 | 6 | 4 | |
| Wamblee | Wolf | 6 | 2 | NA | NA | |
| Una | Nanuk | Wolf | 11 | 12 | 10 | 5 |
| Nia | Meru | Dog | 5 | 10 | 6 | 1 |
| Binti | Maisha | Dog | 12 | 12 | 6 | 5 |
| Layla | Nuru | Dog | 9 | 12 | 3 | 8 |
| Zuri | Dog | 11 | 12 | NA | NA | |
| Bora | Asali | Dog | 9 | 12 | 5 | 6 |
Wolves start breeding in their 2nd year whereas dogs can breed in their 1st year, although this varies between breeds (Lord et al. 2013). In this study all subjects were at least 2 years old. Both wolf and dog females demonstrate the same signs of estrous and were considered to be in heat when they were observed with a swollen vulva and vaginal bleeding (Cafazzo et al. 2014). Within the heat period we also distinguished between peak and nonpeak days. Peak days were when a female allowed males to mount her and attempt copulation and typically lasted 3–7 days. Wolves have 1 breeding season per year, for our subjects this is always between January and March. Female dogs can come into heat at any time and usually do so twice per year. As such, in contrast to male wolves, male dogs are able to breed at any time of the year. Despite this, many of our female pack dogs come into heat around the same time as the wolves, plus another time later in the year.
All subjects were tested in heat; within this period, we ran the sessions as evenly as possible over the peak and nonpeak days. We tested whether there was a difference between peak and nonpeak days, but the models did not improve with this more fine-grained information; therefore, only heat versus nonheat is reported in the results. Additionally, for the nonheat period subjects were tested 1 month before or after heat.
The research was discussed and approved by the institutional ethics committee at the University of Veterinary Medicine, Vienna, in accordance with GSP guidelines and national legislation (03/01/97/2014).
Dyadic tolerance tests
Dyads always comprised 1 male and 1 female from the same pack. The 2 animals were released onto a food source at the same time and tolerance around the food source was measured. Dyads completed up to 12 trials per reproductive stage (some dyads received fewer trials due to pack composition changes, see Table 1 for details), but with no more than 2 trials per day. In order to minimize any order effects that may occur due to habituation to the test over time, subjects were tested in 2 rounds. Round 1 took place from January 2014 to May 2014 and consisted of first testing the dyads when the females were in heat and then after the heat had ended. Round 2 tested the same dyads before the females came into heat and then during their heat and took place from November 2014 to May 2015. While the period of testing was relatively stable for the wolves across the 2 years, it varied somewhat for the dogs due to the fact that dogs can come into heat at any time of the year. Despite this, the same amount of time elapsed between the 2 rounds and between the in-heat and nonheat testing in the 2 species. The tests were always run prior to the routine evening feeding of the animals. All possible male–female dyads were tested, and all dyads were tested both in Round 1 and Round 2.
General set-up
Subjects were placed into separate, but adjacent, side compartments where they could see, but not enter the central enclosure (see Supplementary Movie 1). A sliding door connected each compartment to the central enclosure. The animals were already accustomed to being temporarily separated and to moving through the doors when opened. The experimenter then walked into the central enclosure and visibly placed a shallow plastic bowl baited with 10 meat chunks and a handful of dry dog food (20 cm in diameter for dogs and 40 cm for wolves due to their different head sizes) in front of the animals, centrally between them at a distance of 3 m from each door, and then left again. The meat chunks are a highly desirable food for both the wolves and dogs and the dry food increased the total volume to allow each trial to last longer. The amount of food provided was not enough to satiate the animals. The experimenter filmed all trials from the other side of the fence, at a distance of 5 m from the food location. For all trials, subjects were filmed until the food was finished or for a maximum of 2 min.
Individual trials
At the start of each session each animal was individually released into the central enclosure through a sliding door and allowed to eat a handful of meat and dry food from the bowl before the test began. This was in order to show the animals that food rewards will be placed in the bowl and to ensure food motivation. Each subject received 1 individual trial before testing and the trial was filmed. Although rare, if an animal was not motivated to eat in the individual trial, the test was not continued that day. Although the second individual could watch the individual trial, the order of the individual trials between the 2 subjects was randomized across trials.
Test trials
Immediately after the individual trials, the subjects received 1 test trial where, after the experimenter placed the baited bowl in the central enclosure, the animals were simultaneously released into the enclosure through the sliding doors (see Supplementary Movie 1). The test ended when all the food was consumed or after 2 min. If more than 1 trial was run in a day (max 2), the subjects were always given a short break of 2–5 min in between each and another individual trial was run prior to the second trial to ensure the animals were still food motivated.
Naturalistic bone feedings
In the bone feeding trials, large bones were provided to each of the male members of the pack by a trainer who handed it to them through the fence (see Table 1 for pack compositions). The bones were 10–40 cm in length, but when multiple males were present, all received a bone of the same size within a trial. Although the bones varied in size, bones of different sizes were randomly allocated to “in-heat” and “out of heat” trials. Trials took place in the pack’s home enclosure and all pack members were present during every trial. The behavior of both the males and females was filmed from outside the enclosure. A session was only started if each male accepted the bone into their mouth right away, thus demonstrating food motivation, and lasted until 1) the bones had been consumed, 2) 20 min after the female gained the bone, or 3) a maximum of 90 min. Again, trials were run both when the females were in heat and when they were not in heat (Table 2).
Coding and inter-observer analyses
For both types of test, the videos of each session were coded with Solomon Coder Beta 15.01.13 (Copyright András Péter, http://solomoncoder.com). The ethograms used for the coding can be found in the Supplementary Materials 1. The coding of the food tolerance tests was carried out by RD with 20% coded by Laura Stott (blind to the hypotheses) for reliability. Cohen’s kappa coefficient revealed a high level of agreement on all binomial variables, considering the guidelines by Landis and Koch (1977; >0.75 as excellent and 0.4–0.75 as good; aggression, 1.0; affiliation, 1.0; feeding alone, 0.68). The bone feedings were coded by Teresa Schmidjell, who was blind to the hypotheses, with 20% coded by RD for reliability. Again, Cohen’s kappa showed a high level of agreement for all variables (aggression, 0.92; begging, 0.71; waiting, 0.86; scrounging, 0.71; submission, 1; close proximity, 0.98; latency to eat, 0.83).
All analyses were carried out in R version 3.2.2.
Analyses
For both tests we were interested in whether specific behaviors (Dyadic tests: aggression, affiliation, co-feeding, and food monopolization. Naturalistic tests: aggression, begging, waiting, scrounging, and submission. See Supplementary Materials for definitions) were more likely to occur depending on the reproductive stage (no heat or heat). In line with our aim, we carried out generalized linear mixed models (GLMM) (glmer function in the lme4 package) with a binomial distribution and logit link function: our dependent variable was the occurrence of specific behaviors/behavioral categories (0/1). As explanatory variable, we tested fixed categorical effects of reproductive stage (heat/no-heat), species, and sex and the interactions between reproductive stage and species and reproductive stage and sex. As some subjects were tested in multiple dyads and each dyad was tested repeatedly we created a variable whereby each individual–dyad combination was given a unique identifier. This variable was then used as the random effect in the models to control for dyad behavior and repeated testing. For all GLMMs, no overdispersion was found. The following construct depicts the basic model used for all GLMMs and linear mixed models (LMMs):
Responseijklm∼heati+sexj+heat*sexij+speciesk+species*heatjk+animalijkl+eijklm
In this model, heat_i_ is the fixed categorical effect of heat, sex_j_ is the fixed categorical effect of sex, heat*sex_ij_ is the interaction between heat and sex, species_k_ is the fixed categorical effect of species, species*heat_ik_ is the interaction effect between heat and species. Animal_ijkl_ is the random animal within dyad effect with mean zero and e_ijklm_ is the random residual with mean zero.
Because the trials of the dyadic tests only lasted between 5 and 120 s, durational data was not considered, rather we analyzed whether the likelihood of a behavior occurring in that short-time span was affected by our independent variables (binomial analyses). However, due to the large range in dyadic trial lengths we controlled for trial length using the offset function in the GLMM.
To allow for comparison between tests, likelihood of specific behavior occurring was also analyzed in the naturalistic tests. However, since these lasted longer, additionally, the time spent in proximity of the bone, latency to access the bone and duration of bone monopolization were analyzed using a LMM (lmer function in lme4 package). Again, a variable whereby each individual–dyad combination was given a different name was included as the random effect. The fixed categorical effects of reproductive stage (heat/no-heat), species, and sex and the interactions between reproductive stage and species and reproductive stage and sex were included as factors in the model. Finally, 1 of our predictions related to the females being more persistent during heat than out of heat. Begging, waiting, scrounging, submissive behaviors, and time spent in proximity to the bone (see ethogram for full definitions) can be considered measures of “persistence,” or how willing females are to actively attempt to access the bone. Thus, we ran the models on each of these behaviors in the females only. Finally, in order to assess whether any additional access to the bones by females when in heat was down to the behavior of males or females, we analyzed whether male proximity to the bone was affected by female reproductive stage. All LMMs respected the assumptions of the model.
All analyses were carried out in R version 3.2.2 (R Core Team, 2015). Although we included sex and species as fixed effects in the model, our questions relates specifically to reproductive state, and its potential interactions with species and sex; therefore, any main effect of species or sex alone are not reported below.
Results
Dyadic tests
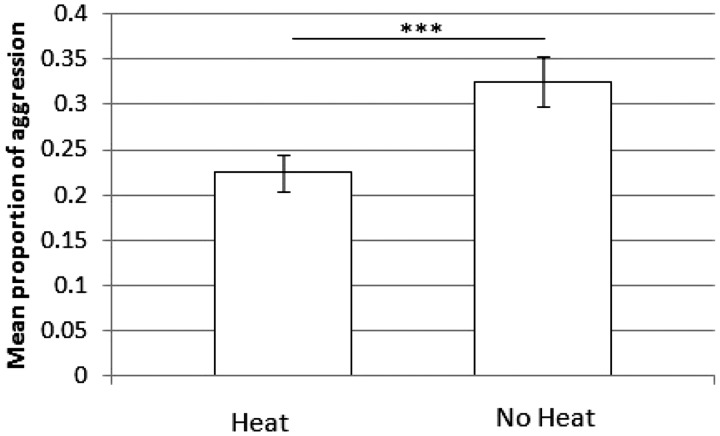
First, there was no interaction between heat and species (_χ_2 = 0.14, df = 1, P = 0.7) or heat and sex (_χ_2 = 0.007, df = 1, P = 0.9) on the likelihood of aggression. However, overall there was significantly less likelihood of aggression when females were in heat than out of heat (_χ_2 = 23.79, df = 1, P < 0.0001; Figure 1).
Figure 1.
Subjects were more likely to show aggression out of heat than in heat in dyadic tests.
There were also no interactions between reproductive stage and species (_χ_2 = 0.22, df = 1, P = 0.6) or sex (_χ_2 = 0.04, df = 1, P = 0.8) on the likelihood of affiliative behaviors occurring during testing. Furthermore, across both species and sexes, subjects were not more likely to show affiliative behaviors when females were in heat than when not in heat (_χ_2 = 1.98, df = 1, P = 0.1).
There was no interaction between species and reproductive stage (_χ_2 = 1.77, df = 1, P = 0.2) on the likelihood of subjects to monopolize the food, indicating reproductive period had a similar effect in wolves and dogs. There was, however, an interaction between sex and reproductive stage (_χ_2 = 9.93, df = 1, P = 0.002). Accordingly, we ran separate models for males and females. There was a slight tendency for males to monopolize the food less when females were in heat (_χ_2 = 3.13, df = 1, P = 0.08). Females, on the other hand, were significantly more likely to feed alone when in heat than when not in heat (_χ_2 = 7.4, df = 1, P = 0.007). See Supplementary Table S1 for an overview of responses at the dyadic level. Conversely, in both species and sexes, co-feeding was less likely to occur in heat than out of heat (_χ_2 = 12.06, df = 1, P = 0.0005), with no interactions between sex and heat (_χ_2 = 0.03, df = 1, P = 0.8) or species and heat (_χ_2 = 1.42, df = 1, P = 0.2).
Naturalistic tests
In neither species nor sex was there an effect of reproductive stage on the likelihood of aggression being shown in the naturalistic tests (_χ_2 = 0.81, df = 1, P = 0.3). Nor was there an effect of reproductive stage on the duration of food monopolization in either species or sex (F = 0.09, df = 1, P = 0.8).
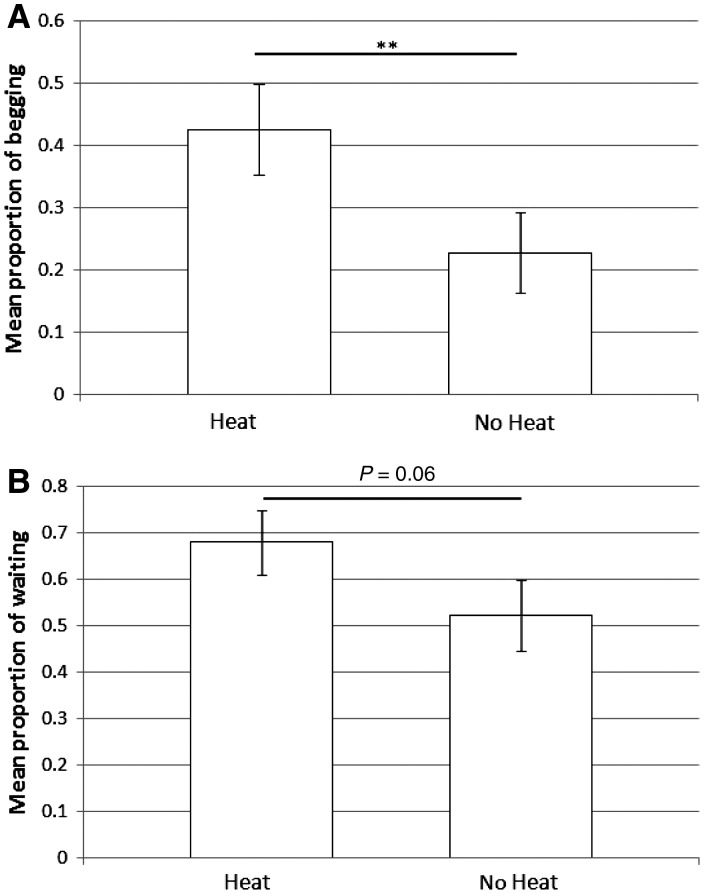
Both wolf and dog females were more likely to beg (_χ_2 = 6.9, df = 1, P = 0.009) and there was a tendency for them to be more likely to wait (_χ_2 = 3.48, df = 1, P = 0.06) when in heat than out of heat (Figure 2). There was no effect of heat, however, on the likelihood of scrounging (_χ_2 = 1.44, df = 1, P = 0.2) or submissive behaviors (_χ_2 = 0.14, df = 1, P = 0.7) in either species.
Figure 2.
Females were more likely to beg (A) and show a tendency to wait more (B) when in heat than out of heat.
There was an interaction between species and reproductive stage on the proportion of time spent in close proximity to the bone (F = 4.26, df = 1, P = 0.04). Dog females spent more time in close proximity (within 1 body length) to bones when in heat than when out of heat (F = 4.2, df = 1, P = 0.05), further suggesting increased persistence of dog females when they are in heat. There was no effect of heat on the proportion of time wolf females spent in proximity to the bone (F = 0.91, df = 1, P = 0.3). Interestingly, there was no interaction between heat and species (F = 0.31, df = 1, P = 0.5), nor was there a main effect of heat (F = 1.04, df = 1, P = 0.3) on the proportion of time males spent in proximity to the bone.
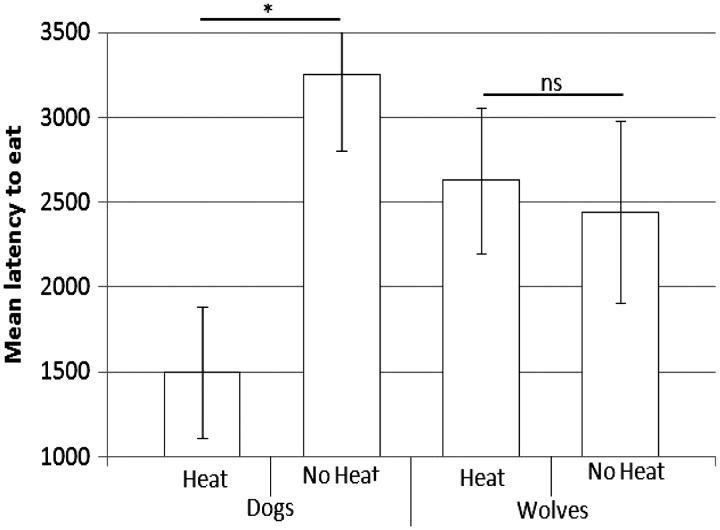
There was a similar interaction between species and reproductive stage on when the males gave up the bone (F = 4.7, df = 1, P = 0.03). Female dogs showed a shorter latency to begin eating the bone when in heat than out of heat (F = 8.7, df = 1, P = 0.006), but no such effect was seen in female wolves (F = 0.1, df = 1, P = 0.7. Figure 3). Supplementary Table S2 shows the responses of each dyad both in and out of heat for each behavioral variable. Overall, dog, but not wolf, females spent more time in proximity to the bone and had a shorter latency to begin eating the bone when in heat than out of heat.
Figure 3.
Female dogs showed a shorter latency to eat the bone when in heat than out of heat. This effect was not seen in wolf females.
Discussion
Overall, the results from the current study show that the behavior of the animals changed according to whether or not the females were in heat. Specifically, in the dyadic tests we saw a decrease in aggression in both sexes and species. Furthermore, females were more likely to monopolize the resource when in heat. In the naturalistic tests there was no effect of heat on the likelihood of aggression. However, females were more likely to show persistent behaviors (specifically, begging and waiting) when in heat than out of heat. Lastly, female dogs, but not wolves, spent more time in proximity to the bone, and had a shorter latency to gain access to the bone, when in heat than out of heat.
These results support our hypothesis that both females and males are playing a role in the food-for-sex exchange. In the dyadic tests both sexes showed less likelihood of aggression during heat. Moreover, in the naturalistic tests, female showed many more behaviors in an attempt to access the bone, such as begging and waiting, and for the dogs, spending more time in proximity to the bone, when in heat than out of heat. There are 2 possible explanations for these changes in behavior: 1) males are more tolerant when the females are in heat and/or 2) the females are more “assertive.”
In their study with chimpanzees, Crick et al. (2013) found a short-term association between copulations and food transfers, but this was not related to the chances of conception and furthermore they did not find any long-term influence of food sharing on mate choice. Similarly, Dubuc et al. (2012) found that female rhesus macaques tested with a male they were currently in consortship with approached the food source more and submitted less than females not in a consortship. Although they actually provide little evidence to support their interpretation, the authors argue against the possibility that these findings are due to increased risk-taking by females during reproductively receptive periods and instead suggest that consortship promotes male tolerance. Therefore, in the reproductive context, both Dubuc et al. (2012) and Crick et al. (2013) concluded that sex reduces tension in macaques and chimpanzees, respectively, both normally intolerant species. Further support for the tolerance-promotion hypothesis has been found in female bonobos, which use nonreproductive sex to reduce tension and facilitate food tolerance (Parish 1994; Hohmann 2015).
Therefore, it appears that within primates, species with varying social systems use sex to facilitate food tolerance. In line with this argument, in our tests, males reduced aggression in the dyadic tests and male dogs left the bones earlier in the naturalistic tests, suggesting that sex might have been used to reduce tension and, therefore, promote tolerance. Based on this explanation, it is possible that, since wolves and dogs only use sex for reproduction, in both species, regardless of mating system, the breeding period promotes food sharing between the sexes as overall tension is reduced. However, our results suggest in fact that, in dogs and wolves, an increase in female assertiveness and risk-taking may also come into play. Indeed, despite levels of aggression remaining constant across reproductive stages in the naturalistic tests, females still showed more persistence to access the food when in heat, suggesting that female assertion may also be an important factor.
The fact that the females monopolized the food more often in the dyadic tests when in heat (as seen by increased food monopolization by the females, and less likelihood of co-feeding and monopolization by the males) suggests a certain level of inhibition on the part of the males to not only allow a female in heat to eat, but to leave a food resource early and allow females to monopolize it. These results may suggest that males are allowing females to feed more in reproductive periods in order to enhance their likelihood of mating success. This is further demonstrated in the dogs as male dogs, but not wolves, left the bone earlier in the naturalistic test when females were in heat (seen by the shorter latency of female’s access to the food). Indeed, Cafazzo et al. (2014) found that female free-ranging dogs were more likely to mate with affiliative males and avoided males which showed intimidation. Therefore, the reduced aggression and food monopolization may be a strategy by the males to make females more likely to allow copulation. On the other hand, since females show more persistence during heat, the shorter latency on the part of the males, in the dogs at least, to give up the food resource may reflect harassment avoidance of a more assertive female, on the part of the males. Jaeggi and van Schaik (2011) suggest harassment-induced sharing is inextricably linked to the social relationships of the individuals involved. Rejecting the harassment of potential mates could result in social costs such as a loss in mating opportunities, further leading to reproductive costs (van Noordwijk and van Schaik 2009).
An alternative explanation for the reduced food monopolization in males is that they are so interested in mating that they are less interested in food during this time. Two lines of evidence suggest this is not the case. First, successful mating was never observed during the dyadic test sessions, so males were not gaining direct mating access during these sessions. Second, the males were always food motivated. The tests were only run when the males fed in the individual trials before testing and food monopolization was seen by the males in all but 8 of the 204 total test trials (all of which were from the same male and were across heat and nonheat sessions). Therefore, although reduced food motivation may partially explain the reduced food monopolization by males during heat periods, this does not fully explain the findings. These explanations are certainly not mutually exclusive and it is likely that multiple factors are affecting the behavior of the males.
Overall, our results demonstrate that there is a change in food tolerance between reproductive periods. Furthermore, we did find some interactions between species and reproductive state. In the naturalistic tests we found that dog females spent more time in close proximity to the bone when in heat than out of heat, but no such effect was seen in wolf females. This result may be tapping into the crucial beginnings of a divergence between wolves and dogs in nonkin sharing tendencies. We know from a previous study (Range et al. 2015) that subordinate wolves are more likely to attempt to get access to food than subordinate dogs, where the dominant individual easily monopolizes the resource. Male dogs are dominant over females (Cafazzo et al. 2010); therefore, perhaps the female dogs are respecting the lack of tolerance by males, except, interestingly, when they are in heat.
Furthermore, female dogs showed a shorter latency to start eating the bone when in heat than out of heat, whereas no such effect of reproductive stage was found in wolf females. At the moment we cannot establish exactly how persistence, proximity to the bone and a shorter latency to access the bone are related. However, we also found that male proximity to the bone was not affected by female reproductive stage, suggesting that regardless of the male’s behavior, the female dogs’ behavior was affected by her sexual status. Therefore, the females appeared to start to eat the bone earlier when in heat than when not in heat, despite the fact that the male’s proximity to the bone remained the same across reproductive periods. This could be due to increased confidence when in heat as has been suggested by Dubuc et al. (2012) based on their findings in macaques.
Overall, in dogs, but not wolves, females spent more time in proximity to the bone and had a shorter latency to access the bone when in heat than out of heat. These findings are in line with our hypothesis that dogs would show more of an effect of reproductive stage than wolves. However, we also observed a number of similarities between the species; in that, both dogs and wolves showed less aggression as well as more female food monopolization and persistence when females were in heat. There are a number of potential explanations as to why this may be the case. First, since species differences were only found in the naturalistic tests, it may be that the dyadic tests are somehow too artificial or short to capture the species differences. Second, our sample size is limited and, therefore, further studies will be required to truly tease apart the potential effect of the mating system in food-for-sex exchanges. Furthermore, the dogs in our sample did not have a mating structure representative of most free-ranging dogs, as during the period of this study they mostly lived in male–female pairs rather than multi-male, multi-female groups. This different social environment may have led to a more wolf-like mating system in our animals. Furthermore, our sample is a captive population with ready access to food. Although we make the assumption that the ecology has shaped the species’ behaviors, and these will be expressed regardless of keeping/living conditions, results may vary in free-ranging populations with higher competition over resources. An expansion of the current study to wild populations would be a necessary next step.
In sum, interactions around a food source were affected by reproductive status in both wolves and dogs. This study highlights that it is not just an increase in male tolerance, but there is a bidirectional influence of both sexes on food sharing across reproductive stages in wolves and dogs, resulting in overall more food monopolization by females when they are in heat. Our study suggests that food-for-sex can explain nonkin, intersexual sharing in canids and highlights the role of females in this process. Finally, some results from the naturalistic tests were in line with the hypothesis that promiscuity may promote a greater sensitivity to food-for-sex effects. However, future studies with free-ranging populations of wolves and dogs would be necessary to confirm this relationship.
Supplementary Material
Supplementary Data
Acknowledgments
The Wolf Science Center was established by Zsófia Virányi, Kurt Kotrschal, and Friederike Range and we thank all the helpers who made this possible, hence indirectly supporting this research. We further thank Laura Stott for assistance with data collection and video coding and Teresa Schmidjell for video coding. Additionally, we thank Marlies Dolezal of the Bioinformatics and Biostatistics platform at the University of Veterinary Medicine for support with the statistical analyses and all the staff and students of the Wolf Science Center (WSC) for their help with the tests and care of the animals. We further thank many private sponsors, including Royal Canin, for financial support and the Game Park Ernstbrunn for hosting the WSC.
Funding
The research was supported by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC [Grant Agreement no. 311870].
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
References
- Butler JRA, Du Toit JT, Bingham J, 2004. Free-ranging domestic dogs Canis familiaris as predators and prey in rural Zimbabwe: threats of competition and disease to large wild carnivores. Biol Cons 115:369–378. [Google Scholar]
- Cafazzo S, Bonanni R, Valsecchi P, Natoli E, 2014. Social variables affecting mate preferences, copulation and reproductive outcome in a pack of free-ranging dogs. PLoS ONE 9:e98594.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafazzo S, Valsecchi P, Bonanni R, Natoli E, 2010. Dominance in relation to age, sex, and competitive contexts in a group of free-ranging domestic dogs. Behav Ecol 21:443–455. [Google Scholar]
- Chivers DJ, 1974. The siamang in Malaya: a field study of a primate in tropical rain forest. Contrib Primatol 4:1–335. [PubMed] [Google Scholar]
- Clutton-Brock TH, 1989. Mammalian mating systems. Proc R Soc B 236:239–372. [DOI] [PubMed] [Google Scholar]
- Crick J, Suchak M, Eppley TM, Campbell MW, de Waal FBM, 2013. The roles of food quality and sex in chimpanzee sharing behavior Pan troglodytes. Behaviour 150:1203–1224. [Google Scholar]
- Dubuc C, Hughes KD, Cascio J, Santos LR, 2012. Social tolerance in a despotic primate: co-feeding between consortship partners in rhesus macaques. Am J Phys Anthropol 148:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feistner A, McGrew W, 1989. Food sharing primates: a critical review. Perspect Prim Biol 3:21–36. [Google Scholar]
- Gilby IC, Eberly LE, Pintea L, Pusey AE, 2006. Ecological and social influences on the hunting behaviour of wild chimpanzees Pan troglodytes schweinfurthii. Anim Behav 72:169–180. [Google Scholar]
- Gilby IC, Eberly LE, Wrangham RW, 2008. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Anim Behav 75:351–360. [Google Scholar]
- Gomes CM, Boesch C, 2009. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE 4:e5116.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, von Rueden C, 2006. Hunting, social status and biological fitness. Soc Biol 53:81–99. [DOI] [PubMed] [Google Scholar]
- Gwynne DT, 1984. Courtship feeding increases female reproductive success in bushcrickets. Nature 307:361–363. [Google Scholar]
- Hamilton WD, 1964. The genetical evolution of social behaviour. II. J Theor Biol 7:17–52. [DOI] [PubMed] [Google Scholar]
- Harrington FH, Mech LD, 1982. Patterns of homesites attendance in two Minnesota wolf packs In: Harrington FH, Paquet PC, editors.. Wolves of the World: Perspectives of Behaviour, Ecology and Conservation. Park Ridge (NJ): Noyes Publications, 81–105. [Google Scholar]
- Hohmann G, 2015. Bonobos In: Whelehan P, Bolin A, editors. The International Encyclopedia of Human Sexuality. Malden (MA): John Wiley & Sons, Inc. [Google Scholar]
- Jaeggi AV, van Schaik CP, 2011. The evolution of food sharing in primates. Behav Ecol Sociobiol 65:2125–2140. [Google Scholar]
- Jenks SM, 2011. A longitudinal study of the sociosexual dynamics in a captive family group of wolves: the University of Connecticut wolf project. Behav Genet 41:810–829. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, 1985. Food sharing among ache foragers: tests of explanatory hypotheses. Curr Anthropol 26:223–246. [Google Scholar]
- Landis J, Koch G, 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB. et al. , 2005. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819. [DOI] [PubMed] [Google Scholar]
- Lord K, Feinstein M, Smith B, Coppinger R, 2013. Variation in reproductive traits of members of the genus Canis with special attention to the domestic dog Canis familiaris. Behav Process 92:131–142. [DOI] [PubMed] [Google Scholar]
- MacNulty DR, Smith DW, Mech LD, Vucetich JA, Packer C, 2012. Nonlinear effects of group size on the success of wolves hunting elk. Behav Ecol 23:75–82. [Google Scholar]
- Mech DL, Wolf PC, Packard JM, 1999. Regurgitative food transfer among wild wolves. Can J Zool 77:1192–1195. [Google Scholar]
- Mech LD, 1995. A ten-year history of the demography and productivity of an Arctic wolf pack. Arctic 48:329–332. [Google Scholar]
- Mech LD, 1999. Alpha status, dominance, and division of labor in wolf packs. Can J Zool 77:1196–1203. [Google Scholar]
- Mech LD, Boitani L, 2003. Wolves: Behavior, Ecology, and Conservation. Chicago: University of Chicago Press. [Google Scholar]
- Mitani JC, Watts DP, 2001. Why do chimpanzees hunt and share meat? Anim Behav 61:915–924. [Google Scholar]
- Pal SK, 2005. Parental care in free-ranging dogs. _Appl_Anim Behav Sci 90:31–47. [Google Scholar]
- Parish AR, 1994. Sex and food control in the “uncommon chimpanzee”: how bonobo females overcome a phylogenetic legacy of male dominance. Ethol Sociobiol 15:157–179. [Google Scholar]
- Paul M, Majumder SS, Bhadra A, 2014a. Grandmotherly care: a case study in Indian free-ranging dogs. J Ethol 32:75–82. [Google Scholar]
- Paul M, Majumder SS, Bhadra A, 2014b. Selfish mothers? An empirical test of parent-offspring conflict over extended parental care. Behav Process 103:17–22. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2015. R: a language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Range F, Ritter C, Viranyi Z, 2015. Testing the myth: tolerant dogs and aggressive wolves. Proc R Soc B 282:20150220.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small MF, 1990. Consortships and conceptions in captive rhesus macaques Macaca mulatta. Primates 31:339–350. [Google Scholar]
- Smith EEA, 2004. Why do good hunters have higher reproductive success? Hum Nat 15:343–364. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Gilby IC, 2004. A conceptual framework for nonkin food sharing: timing and currency of benefits. Anim Behav 67:603–614. [Google Scholar]
- St-Pierre A, Larose K, Dubois F, 2009. Long-term social bonds promote cooperation in the iterated Prisoner’s Dilemma. Proc R Soc B 276:4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf RM, Boesch C, 2006. The efficacy of female choice in chimpanzees of the Tai forest, Cote d’Ivoire. Behav Ecol Sociobiol 60:749–765. [Google Scholar]
- Vanak AT, Gompper ME, 2009. Dietary niche separation between sympatric free-ranging domestic dogs and Indian foxes in central India. J Mammal 90:1058–1065. [Google Scholar]
- van Noordwijk MA, van Schaik CP, 2009. Inter sexual food transfer among orangutans: do females test males for coercive tendency? Behav Ecol Sociobiol 63:883–890. [Google Scholar]
- Watts DP, Mitani JC, 2002. Hunting Behavior of Chimpanzees at Ngogo, Kibale National Park, Uganda. Int J Primatol 23:1–28. [Google Scholar]
- Wolovich CK, Evans S, French JA, 2008. Dads do not pay for sex but do buy the milk: food sharing and reproduction in owl monkeys (Aotus spp.). Anim Behav 75:1155–1163. [Google Scholar]
- Wolovich CK, Perea-Rodriguez JP, Fernandez-Duque E, 2007. Food transfers to young and mates in wild owl monkeys Aotus azarai. Am J Primatol 69:1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data