Sedation in Pediatric Esophagogastroduodenoscopy (original) (raw)
Abstract
Pediatric esophagogastroduodenoscopy (EGD) has become an established diagnostic and therapeutic modality in pediatric gastroenterology. Effective sedation strategies have been adopted to improve patient tolerance during pediatric EGD. For children, safety is a fundamental consideration during this procedure as they are at a higher risk of severe adverse events from procedural sedation compared to adults. Therefore, a detailed risk evaluation is required prior to the procedure, and practitioners should be aware of the benefits and risks associated with sedation regimens during pediatric EGD. In addition, pediatric advanced life support by endoscopists or immediate intervention by anesthesiologists should be available in the event that severe adverse events occur during pediatric EGD.
Keywords: Sedation, Child, Esophagogastroduodenoscopy
Introduction
Pediatric esophagogastroduodenoscopy (EGD) has evolved during the last 40 years with an increasing number of diagnoses and treatments in pediatric gastroenterology, and the need for more optimal sedation protocols in pediatric EGD cases is also increasing [1,2]. Young children can often be uncooperative during procedures that they do not understand, and they are also more likely to experience psychological trauma caused by the separation from their parents and pain during the procedure if the intended sedation is not sufficient. In addition, reducing patient distress through appropriate sedation and analgesia protocols is critical for enhancing the effectiveness and feasibility of EGD. The European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommends general anesthesia (GA) for pediatric EGD or deep sedation if GA is not available [3].
Parents also experience anxiety with regard to the safety of pediatric EGD because procedural sedation adverse events (PSAEs) still occur with this procedure despite recent technical advances in patient monitoring and the safety of the sedative drugs now used in pediatric patients [4]. PSAEs may still happen due to the unintended induction of deeper sedation in children [5,6], and appropriate pre-procedural risk evaluation and selection of protocols for treating PSAE are essential in the management of sedated children. Hence, GA administered by a multidisciplinary team led by an anesthesiologist is preferred in cases of pediatric EGD [3]. However, GA is available only in a few large centers because of the limited number of anesthesiologists, and the low cost (about US $100) of pediatric EGD in Korea precludes the incorporation of that anesthesia practice [7,8].
To date, no meta-analysis on the effectiveness and safety of sedatives in pediatric EGD has been conducted owing to limited data. In addition, there are currently no comprehensive guidelines as to the best sedation practices in pediatric EGD [3,9,10], whereas well-established guidelines for general procedural sedation from organizations such as the American Academy of Pediatrics (AAP) have been published [11-13]. Propofol is the most promising sedative in terms of effectiveness and safety for pediatric EGD [14-19]. However, the administration of propofol by non-anesthesiologists is off-label in Korea, which means that medico-legal issues may arise if severe PSAEs occur during or after the procedure. Likewise, propofol is not commonly used in pediatric EGD in Korea [7] and is restricted mainly to anesthesiologists. The aims of this present review are to provide an update on the latest evidence and opinions regarding the best sedation practices for pediatric EGD and to make some reasonable suggestions for the future establishment of structural sedation guidelines for pediatric EGD in Korea.
PROTOCOL ESTABLISHMENT FOR YOUR CENTER
The ideal sedation practice for pediatric EGD should fulfill the following five principals of sedation issued by the AAP [11]: (1) guard patient safety and welfare; (2) minimize physical discomfort; (3) lessen anxiety and psychological trauma; (4) control patient behavior for the safe completion of the procedure; and (5) discharge the patient safely. Both GA and intravenous (iv) sedation have been used to accomplish these goals in pediatric EGD. For these purposes, detailed requirements must be met in a properly equipped venue, and it must be noted that many factors can affect the determination of a sedation protocol according to hospital resources (Fig. 1). A North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) survey identified substantial practice variation among sedation regimens [20]. Practitioners should determine the appropriate level of sedation for EGD (mild to GA) and sedative drug to use based on a presedation risk assessment, the age and size of the patient, purpose of the procedure (diagnostic vs. therapeutic), expectations of the parents, volume of procedures, and effective management of any possible PSAE.
Fig. 1.
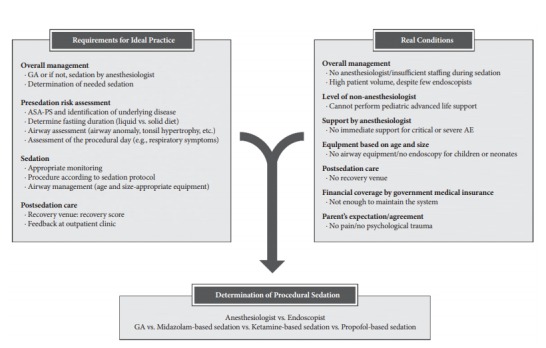
Determination of the sedation protocol for a pediatric esophagogastroduodenoscopy. GA, general anesthesia; ASA-PS, American society of anesthesiologists-physical status; AE, adverse event.
PRESEDATION RISK ASSESSMENT
The rate of GA-related cardiac arrest and resulting mortality in children has been calculated at about 22.2 and 10.7/10,000 procedures, respectively, in a single study [21]. In that study, the described risk factors were urgency, age <1 year, American Society of Anesthesiologists-physical status (ASA-PS) ≥III, and female sex. Of them, ASA-PS ≥III showed the highest odds ratio (6 to 11). The preoperative status of the patient can be classified according to physical conditions such as ASA-PS (Table 1) which has also been reported as a reliable predictor of PSAEs including mortality in both adults and children [22-25]. Despite controversy regarding the inconsistent application of ASA-PS in pediatric patient populations, most practitioners have been regularly using this grading system and one-third of these users have modified the ASA-PS definitions based on their own needs [22].
Table 1.
Presedation Risk Assessment
| Determination of sedation [24] | |
|---|---|
| Mild | Cognitive function and coordination may be impaired, and patients respond normally to verbal commands. |
| Moderate | Patients respond purposefully to verbal commands, but their consciousness is depressed. |
| Deep | Patients have a depressed level of consciousness, but they respond purposefully to repeated or painful stimulation. Ventilatory function may be impaired during deep sedation. |
| General anesthesia | Patients are not arousable, even by painful stimulation. Cardiovascular function may be impaired. |
| Grading of ASA-PS [22] | |
| Class I | Normal healthy patient |
| Class II | Patient with mild systemic disease |
| Class III | Patient with severe systemic disease |
| Class IV | Patient with severe systemic disease that is a constant threat to life |
| Class V | Moribund patient not expected to survive without an emergency procedure |
| Evaluation of airway and potential anomalies [25] | |
| Mallampati score I | Uvula is completely visible |
| Mallampati score II | Partially visible uvula |
| Mallampati score III | Soft palate is visible but not uvula |
| Mallampati score IV | Only hard palate is visible and not soft palate or uvula |
| Airway anomaly | Laryngomalacia, tracheoesophageal fistula, etc. |
| Determination of fasting period [24] | |
| Clear liquids | 2 hr |
| Breast milk | 4 hr |
| Solid meal, nonhuman milk | 6 hr |
| Emergency endoscopy | Not applicable |
In a previous multicenter study of sedation in about 30,000 pediatric cases, desaturation (157/10,000 sedations), vomiting (47/10,000 sedations), excessive secretion (41/10,000 sedations), and unexpected apnea (24/10,000 sedations) were noted [26], suggesting that caution must be exercised in children with potential airway problems or an uncorrected cardiopulmonary anomaly such as laryngomalacia, chromosomal abnormalities, tetralogy of Fallot, and bronchopulmonary dysplasia. In addition, assessment of concurrent respiratory conditions in previously healthy children, such as episodes of upper respiratory infection and wheezing within two weeks, is also important for avoiding laryngospasm, which can be a life-threatening PSAE during an endoscopic procedure.
Before deciding to undertake a pediatric EGD procedure, the author uses the checklist indicated in Table 1 to (1) determine the sedation level; (2) assess the ASA-PS; (3) evaluate the airway; and (4) confirm the fasting period. To prevent any unexpected laryngeal spasms or respiratory PSAEs, the author always postpones the procedure when the patient presents with any upper respiratory symptoms on the day of the operation, particularly in the case of EGD. The risk of laryngeal spasm can be doubled in children during GA with a laryngeal mask airway [27], suggesting that the EGD procedure itself may be an important trigger of this PSAE.
COMMON INTRAVENOUS SEDATIVES
Midazolam has been the conventional drug used for firstline sedation in children, and the typical dosages are listed in Table 2. However, since midazolam alone often provides inadequate sedation, it is commonly used in combination with ketamine or propofol or with narcotic analgesics such as meperidine and fentanyl [28]. For these reasons, the author always uses midazolam as a premedication with ketamine-based sedation. Several PSAEs such as respiratory depression, hypotension, and paradoxical agitation have been described, and paradoxical reactions including agitation, aggressive behavior, and combativeness have also been reported in less than 2% of children who received midazolam sedation during pediatric EGD [29]. If a paradoxical reaction is suspected, cerebral hypoxia must be excluded first, at which point iv administration of flumazenil can be used to reverse the reaction to midazolam [30].
Table 2.
List of Current Sedatives Used in Pediatric EGD
| Sedatives | Age | Dose | Time to onset | Duration | Repeating dose |
|---|---|---|---|---|---|
| Midazolam | 6 mo–5 yr | 0.05–0.1 mg/kg | 2–3 min | 45–60 min | 0.1 mg/kg/2–5 min (max 0.6 mg/kg) |
| 6–12 yr | 0.025–0.05 mg/kg | 0.1 mg/kg/2–5 min (max 0.4 mg/kg) | |||
| 12 yr | 1–2.5 mg (not per kg) | 1 mg/2–5 min | |||
| Ketamine | 1–1.5 mg/kg | 1–5 min | 15 min | 0.5 mg/kg/10 min | |
| Propofol | 3 mo–3 yr | 2 mg/kg | 0.5–1 min | 3–10 min | 1 mg/kg |
| 3 yr | 1 mg/kg | 0.5 mg/kg | |||
| Meperidine | 0.3–2 mg/kg | 3–6 min | 60–180 min | ||
| Fentanyl | 1–2 μg/kg | 20–40 min | 20–40 min | 1–1.25 μg/kg/3 min |
Ketamine is one of safest known sedative drugs in common use in pediatric patients. Its dissociative effect is induced by N-methyl-D-aspartate receptor binding, and it has been shown to be effective for sedation even in infants [28,31]. The iv administration of ketamine at between 0.5 and 2 mg/kg per dose has been reported, and a 1 mg/kg iv injection has been commonly used as an initial dose. Repeat doses of 0.5 mg/kg every 10 minutes can be used to maintain the desired effect. In a previous randomized controlled trial (RCT), the recommended initial dose of ketamine was suggested to be 1 mg/kg [32]. In that study, combined sedation with midazolam and ketamine required a second dose of ketamine in 57% of the children with a 96% sedation appropriateness, whereas 69% of the group with ketamine only needed a second dose with a 99% appropriateness, suggesting that a single dose of ketamine alone may be appropriate and effective for pediatric EGD. The appropriate dose for the oral route has not yet been validated. However, a randomized double-blind study of pediatric EGD [33] reported that a combination strategy with oral ketamine (5 mg/kg) and iv midazolam (0.1 mg/kg; up to 0.3 mg/kg) provided more effective sedation and faster recovery despite the use of lower doses compared to other combinations of placebo-midazolam or oral fentanyl-iv midazolam.
Laryngospasm is a potentially lethal PSAE resulting from ketamine sedation that occurs in approximately 5%–10% of pediatric cases [32,34]. Fortunately, this PSAE is likely to be transient in most cases and can be resolved by immediate termination of the procedure and supplementation with inhaled oxygen. Ketamine is contraindicated in infants <3 months of age and children with a history of airway instability, tracheal abnormalities, active pulmonary disease, head injury, and central nervous system disorders [28]. In addition, bradycardia and hypotension are potential drawbacks of ketamine use, and extreme caution is required for children with severe heart disease or hypovolemia. The author has experienced several cases of severe respiratory PSAE from a loading combination of iv midazolam at 0.1 mg/kg and iv ketamine at 1 mg/kg. This has led to a reduction in the iv ketamine dose to 0.5 mg/kg, and no severe respiratory PSAEs have been encountered over the last 10 years using this dose (unpublished data). While hallucinogenic emergence reactions to ketamine have been reported in 25% of adult patients, they have been observed in less than 5% of pediatric patients who received midazolam as a premedication before endoscopy [32]. The author has adapted ketamine-based sedation as a primary method for more than 20 years, with 400 pediatric EGDs performed annually. However, data on the use of ketamine for pediatric EGD remain limited.
Propofol, a gamma-aminobutyric acid receptor blocking drug, is characteristic of rapid-onset but short-acting sedatives. The ESPGHAN recommends propofol-based sedation as the safest and most convenient way to achieve deep sedation during pediatric endoscopy [14]. Notably, severe PSAEs from propofol-based sedation are rare (0.3%) in children [1], and no reports have indicated the severity, records of intubation, resuscitation, permanent complications, or death. The duration of propofol use is very short (5–15 minutes) compared to other drugs, and the rate of sedation failure is only 0%–0.4% [17,35], suggesting its potential for use without anesthesiologist support. A retrospective study of the largest pediatric endoscopy population analyzed to date (_n_=36,516) is of interest in this regard [36]. That study included 4,805 EGD and 1,676 colonoscopy cases and describes the side effects of propofol-based sedation (1–2 mg/kg) mainly administered by pediatricians. The most common PSAEs were desaturation (0.4%) and laryngospasm (0.2%), while others such as hypotension and bronchospasm were noted in less than 0.1% of the children. In another report that included the second largest pediatric population (_n_=4,904) to undergo an endoscopic procedure, in which propofol was administered by the endoscopist, only 5% of the children had desaturation [37].
The results of more advanced studies of propofol-based sedation for pediatric EGD have been reported by anesthesiologists and have indicated that the safety of this drug is adequate [15,38]. In another randomized double-blind study of pediatric EGD, propofol-based sedation (1 mg/kg) in combination with tramadol or fentanyl was found to be efficient and safe [38]. Tosun et al. also conducted a comparative analysis of propofol-based sedation (1.2 mg/kg) with ketamine vs. fentanyl, and propofol was also effective and safe for sedation in pediatric EGD [15].
Infusion pain from propofol can be so strong in young children that they are prone to agitation even before the start of the procedure. A low dose of ketamine, midazolam, or meperidine is therefore often necessary as a premedication to control this pain. In addition, since propofol does not have an analgesic effect, children are given iv ketamine or tramadol prior to propofol-based sedation at the author’s institution. Similarly to anesthesiologist societies in the United States and in European countries, the Korean Society of Anesthesiologists recommends that only an anesthesiologist should administer propofol [39]. Notably, propofol is still an off-label medication in Korea, and propofol-related PSAEs may lead to medico-legal issues, although the evidence from many studies suggests that this drug is safe and effective for use in sedation in both adults and children undergoing an endoscopy.
POSSIBLE COMBINATION REGIMENS FOR PEDIATRIC EGD
The available data are still too limited to draw basic conclusions or establish clear guidelines for sedation during pediatric EGD (Table 3) [1-4,9-13,28,40-42]. Consequently, there is no single sedative or combined regimen of sedative drugs that has been validated as ideal in pediatric EGD, and two previous systematic reviews of pediatric endoscopy studies have not provided clear guidelines [1,2]. The ESPGHAN guidelines for pediatric endoscopy [3] simply recommend GA or propofol-based deep sedation by trained professionals if GA is not available. While Korean endoscopists may in fact agree that GA alone is appropriate for pediatric EGD, under the current medical coverage guidelines of the Korean government, the covered cost of a single endoscopy is about US $100. At this cost level, even 10 procedures per day would not be sufficient to cover the costs of maintaining a multidisciplinary GA team led by anesthesiologists. Therefore, few Korean hospitals will maintain an anesthetic team for pediatric endoscopy procedures owing to the expense [7]. The following practical recommendations for three different combination sedation regimens for pediatric endoscopies are made by the author.
Table 3.
Published Structural Sedation Protocols for Pediatric EGD
| Meta-analysis of sedation regimens for pediatric endoscopy | Comments |
|---|---|
| None | |
| Published organizational guidelines for procedural sedation | |
| European Society of Gastrointestinal Endoscopy and European Society for Pediatric Gastroenterology Hepatology and Nutrition [3] | Recommended GA or deep sedation for pediatric endoscopy |
| American Academy of Pediatrics and American Academy of Pediatric Dentistry [11,12] | Systemic build-up of procedure venue for pediatric sedation |
| North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition [9,10] | Special consideration for pediatric endoscopy |
| National Clinical Guideline Centre (United Kingdom) [13] | Systemic build-up of procedure venue for pediatric sedation |
| Systemic review of pediatric endoscopy | |
| Tringali et al. [4] | Complications resulting from sedation |
| Orel et al. [2] | Sedative drugs |
| van Beek et al. [1] | Sedative drugs |
| Expert opinion on pediatric sedation | |
| Chung et al. [40] | Pediatric endoscopy |
| Green et al. [28] | Ketamine in emergency centers |
| Dar et al. [41] | Pediatric endoscopy |
| Fredette et al. [42] | Pediatric endoscopy |
Midazolam-based combinations
Midazolam-based sedation offers the most established and conventional way to sedate the patient during pediatric endoscopy with significantly decreased risks compared to GA [43,44]. The narcotics meperidine and fentanyl are commonly used together with midazolam. Unintended deep sedation caused by midazolam plus narcotics can be reversed by treatment with flumazenil (0.01 mg/kg) and naloxone (0.1 mg/kg) antagonists, respectively. As there are no reversal agents for ketamine and propofol, the availability of antagonists for midazolam and narcotics is a significant advantage of this combination as a sedation regimen in pediatric EGD. Although four RCTs have investigated the effectiveness and safety of midazolam-based combination sedation [45-48], additional strategies are needed given the procedural issues observed in their studies, such as additional restraints, prolonged time to induce sedation, and frequent sedation failures. Hence, as the effectiveness of midazolam-based sedation is likely to be suboptimal,1 the endoscopist must properly evaluate the pros (safety and available antagonists) and cons (discomfort due to insufficient sedation and occasional need for restraints) of the use of these drugs prior to the procedure.
Ketamine-based combinations
Despite the high effectiveness of ketamine in adults, there has only been one pediatric RCT comparing the effectiveness of midazolam as a premedication for ketamine-based sedation [32]. Ketamine is suggested to be safe and effective for pediatric endoscopy, although the data remain limited [1,2]. A ketamine-based sedation regimen (comprising premedication with midazolam at 1 mg/kg and ketamine at 0.5 mg/kg) is principally used at the author’s hospital and has caused no lethal complications in over 3,000 cases of pediatric EGD since 2007 (unpublished data). It must be noted that these data may have a selection bias as a pediatric endoscopy is never performed if the patient has any upper respiratory symptoms within two weeks of the planned procedure. The author has experienced cases where additional restraints were required during a pediatric EGD under this ketamine-based sedation regimen and, in rare instances, has received complaints from older children due to insufficient sedation.
Propofol-based combinations
Five RCTs of propofol-based combinations have been conducted to date [15-18] in which propofol was identified as the most effective and safest sedative for pediatric EGD. These trials have also shown that both the effectiveness and patient tolerance of propofol can be enhanced by combining it with ketamine, fentanyl, meperidine, or midazolam. Most notably, ketamine plus narcotics offer analgesic benefits in cases of infusion pain in children under propofol sedation. Propofol is still contraindicated for any procedural sedation in children under current Korean government medical coverage guidelines.
MONITORING AND RESCUE STRATEGIES
The AAP guidelines for procedural sedation recommend (1) continuous pulse oximetry and (2) heart rate monitoring as essential for patient monitoring [11]. Different levels of sedation may require different scales of monitoring. Additional monitoring modalities such as electrocardiography, capnography, and blood pressure measurements at specific intervals are recommended under moderate or deep sedation.
In a previous Food and Drug Administration report of sedation-related mortality among 95 pediatric cases in the United States in which PSAEs were caused by an unintended deep sedation, about 70% of the children experienced respiratory difficulties, such as respiratory depression and arrest as a first episode [49]. However, a lack of hospital-based facilities, which possibly means a lack of access to immediate and appropriate resuscitation, was reported to be related to the cardiac arrest that occurred as the second episode in these cases. In addition, mortality was significantly higher among the cases in which a hospital-based facility was lacking (93% vs. 37%, respectively). This study strongly suggests that a prompt response to PSAEs and appropriate resuscitation by trained practitioners are critical for the prevention of cascades that could lead to cardiac arrest in sedated children. According to the NASPGHAN guidelines, all endoscopists performing pediatric procedures should be certified for pediatric advanced life support and be familiar with resuscitation protocols [10].
POSTPROCEDURAL MONITORING AND DISCHARGE
The recovery venues for pediatric EGD cases should be equipped with appropriate monitoring devices and must be managed by specialized staff. A full recovery to consciousness must be confirmed prior to discharge, and the recovery and discharge criteria presented in Table 4 must be fulfilled [50-52]. In particular, additional attention must be paid to children of a young age, patients with an ASA class >III, and any cases with anatomic airway abnormalities including enlarged tonsils. Of particular note, infants and toddlers may undergo re-sedation after discharge due to residual sedative effects and may then be susceptible to airway obstruction when placed in a car safety seat. Flumazenil is occasionally not sufficient to prevent re-sedation because its half-life is shorter than that of midazolam. There are several discharge criteria that can be applied for children including a variety of sedation scoring systems. For young children, the author uses the discharge criteria specified by the AAP [50], whereas the ASA discharge criteria and scoring system have been used for older children [51,52].
Table 4.
Recommended Discharge Criteria Following Pediatric EGD
| AAP discharge criteria [50] | ||
|---|---|---|
| 1. Cardiovascular function and airway patency are satisfactory and stable. | ||
| 2. The patient is easily arousable, and protective reflexes are intact. | ||
| 3. The patient can talk (if age appropriate). | ||
| 4. The patient can sit up unaided (if age appropriate). | ||
| 5. For a very young or handicapped child incapable of the typically expected responses, the presedation level of responsiveness or a level as close as possible to the normal level for that child should be achieved. | ||
| 6. The state of hydration is adequate. | ||
| ASA discharge guidelines [51] | ||
| 1. Patients should be alert and oriented; infants and patients whose mental status was initially abnormal should have returned to their baseline status. Practitioners and parents must be aware that pediatric patients are at risk of airway obstruction should the head fall forward while the child is secured in a car seat. | ||
| 2. Vital signs should be stable and within acceptable limits. | ||
| 3. Use of scoring systems may assist in the documentation of fitness for discharge. | ||
| 4. Sufficient time (up to 2 hr) should have elapsed after the last administration of reversal agents (naloxone, flumazenil) to ensure that patients do not become resedated after reversal effects have worn off. | ||
| 5. Outpatients should be discharged in the presence of a responsible adult who will accompany them home and be able to report any postprocedural complications. | ||
| 6. Outpatients and their escorts should be provided with written instructions as to postprocedure diet, medications, activities, and a phone number to be called in case of emergency. | ||
| Aldrete post-anesthesia recovery score [52] | ||
| Activity | Able to move 4 extremities voluntarily or on command | 2 |
| Able to move 2 extremities voluntarily or on command | 1 | |
| Unable to move extremities voluntarily or on command | 0 | |
| Respiration | Able to breathe deeply and cough freely | 2 |
| Dyspnea or limited breathing | 1 | |
| Apneic | 0 | |
| Circulation | Blood pressure ±20% of pre-anesthetic level | 2 |
| Blood pressure ±20% to 49% of pre-anesthetic level | 1 | |
| Blood pressure ±50% of pre-anesthetic level | 0 | |
| Consciousness | Fully awake | 2 |
| Arousable on calling | 1 | |
| Not responding | 0 | |
| O2 saturation | Able to maintain O2 saturation >92% on room air | 2 |
| Needs O2 inhalation to maintain O2 saturation >90% | 1 | |
| O2 saturation <90% even with O2 supplementation | 0 | |
| The total score must be >8 before discharging the patient. |
CONCLUSIONS
Both the safety and effectiveness of the sedation approach are fundamental when undertaking an EGD procedure in children who are at a higher risk of severe PSAEs compared to adults. Therefore, a detailed presedation risk assessment is a prerequisite for pediatric EGD procedures. Based on the available clinical resources, costs, and parent expectations, the endoscopist should carefully select the type of sedation for pediatric EGD if GA is not available. All practitioners should clearly understand the benefits and risks associated with sedation regimens for pediatric EGD. Pediatric advanced life support or immediate intervention by anesthesiologists should be readily available when PSAEs occur, especially in the context of propofol- or ketamine-based sedation. If these resources are unavailable, the author recommends a midazolam plus narcotics sedation regimen, since effective antidotes can be used if needed. During and after pediatric EGD, the quality of monitoring and strictness of the discharge criteria are non-negotiable requirements for successful outcomes given that PSAEs can occur in children irrespective of the sedation type, combination regimen, or practitioner.
Footnotes
**Conflicts of Interest:**The author has no financial conflicts of interest.
REFERENCES
- 1.van Beek EJ, Leroy PL. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr. 2012;54:171–185. doi: 10.1097/MPG.0b013e31823a2985. [DOI] [PubMed] [Google Scholar]
- 2.Orel R, Brecelj J, Dias JA, et al. Review on sedation for gastrointestinal tract endoscopy in children by non-anesthesiologists. World J Gastrointest Endosc. 2015;7:895–911. doi: 10.4253/wjge.v7.i9.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tringali A, Thomson M, Dumonceau JM, et al. Pediatric gastrointestinal endoscopy: European society of gastrointestinal endoscopy (ESGE) and European society for paediatric gastroenterology hepatology and nutrition (ESPGHAN) guideline executive summary. Endoscopy. 2017;49:83–91. doi: 10.1055/s-0042-111002. [DOI] [PubMed] [Google Scholar]
- 4.Tringali A, Balassone V, De Angelis P, Landi R. Complications in pediatric endoscopy. Best Pract Res Clin Gastroenterol. 2016;30:825–839. doi: 10.1016/j.bpg.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Paterson N, Waterhouse P. Risk in pediatric anesthesia. Paediatr Anaesth. 2011;21:848–857. doi: 10.1111/j.1460-9592.2010.03366.x. [DOI] [PubMed] [Google Scholar]
- 6.von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773–783. doi: 10.1016/S0140-6736(10)61193-2. [DOI] [PubMed] [Google Scholar]
- 7.Ryoo E, Kim KM, Scientific Committee of the Korean Society of Pediatric Gastroenterology, Hepatology, and Nutrition Pediatric endoscopic sedation in Korea: a survey of the Korean society of pediatric gastroenterology, hepatology and nutrition. Korean J Pediatr Gastroenterol Nutr. 2008;11:21–27. [Google Scholar]
- 8.Lee MC. Sedation for pediatric endoscopy. Pediatr Gastroenterol Hepatol Nutr. 2014;17:6–12. doi: 10.5223/pghn.2014.17.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leichtner AM, Gillis LA, Gupta S, et al. NASPGHAN guidelines for training in pediatric gastroenterology. J Pediatr Gastroenterol Nutr. 2013;56(Suppl 1):S1–S8. doi: 10.1097/MPG.0b013e31827a78d6. [DOI] [PubMed] [Google Scholar]
- 10.ASGE Standards of Practice Committee. Lightdale JR, Acosta R, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2014;79:699–710. doi: 10.1016/j.gie.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Coté CJ, Wilson S, American Academy of Pediatrics. American Academy of Pediatric Dentistry Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures: update 2016. Pediatrics. 2016;138 [PubMed] [Google Scholar]
- 12.Guideline for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures: update 2016. Pediatr Dent. 2016;38:77–106. [PubMed] [Google Scholar]
- 13.National Clinical Guideline Centre (UK) National Institute for Health and Care Excellence (UK) National institute for health and care excellence: clinical guidelines. London: Royal College of Physicians (UK); 2010. Sedation in children and young people: sedation for diagnostic and therapeutic procedures in children and young people. CG112. [PubMed] [Google Scholar]
- 14.Thomson M, Tringali A, Dumonceau JM, et al. Paediatric gastrointestinal endoscopy: European society for paediatric gastroenterology hepatology and nutrition and European society of gastrointestinal endoscopy guidelines. J Pediatr Gastroenterol Nutr. 2017;64:133–153. doi: 10.1097/MPG.0000000000001408. [DOI] [PubMed] [Google Scholar]
- 15.Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983–988. doi: 10.1111/j.1460-9592.2007.02206.x. [DOI] [PubMed] [Google Scholar]
- 16.Paspatis GA, Charoniti I, Manolaraki M, et al. Synergistic sedation with oral midazolam as a premedication and intravenous propofol versus intravenous propofol alone in upper gastrointestinal endoscopies in children: a prospective, randomized study. J Pediatr Gastroenterol Nutr. 2006;43:195–199. doi: 10.1097/01.mpg.0000228099.04702.39. [DOI] [PubMed] [Google Scholar]
- 17.Barbi E, Petaros P, Badina L, et al. Deep sedation with propofol for upper gastrointestinal endoscopy in children, administered by specially trained pediatricians: a prospective case series with emphasis on side effects. Endoscopy. 2006;38:368–375. doi: 10.1055/s-2005-921194. [DOI] [PubMed] [Google Scholar]
- 18.Disma N, Astuto M, Rizzo G, et al. Propofol sedation with fentanyl or midazolam during oesophagogastroduodenoscopy in children. Eur J Anaesthesiol. 2005;22:848–852. doi: 10.1017/S0265021505001432. [DOI] [PubMed] [Google Scholar]
- 19.Kaddu R, Bhattacharya D, Metriyakool K, Thomas R, Tolia V. Propofol compared with general anesthesia for pediatric GI endoscopy: is propofol better? Gastrointest Endosc. 2002;55:27–32. doi: 10.1067/mge.2002.120386. [DOI] [PubMed] [Google Scholar]
- 20.Lightdale JR, Mahoney LB, Schwarz SM, Liacouras CA. Methods of sedation in pediatric endoscopy: a survey of NASPGHAN members. J Pediatr Gastroenterol Nutr. 2007;45:500–502. doi: 10.1097/MPG.0b013e3180691168. [DOI] [PubMed] [Google Scholar]
- 21.Bharti N, Batra YK, Kaur H. Paediatric perioperative cardiac arrest and its mortality: database of a 60-month period from a tertiary care paediatric centre. Eur J Anaesthesiol. 2009;26:490–495. doi: 10.1097/EJA.0b013e328323dac0. [DOI] [PubMed] [Google Scholar]
- 22.Aplin S, Baines D, DE Lima J. Use of the ASA physical status grading system in pediatric practice. Paediatr Anaesth. 2007;17:216–222. doi: 10.1111/j.1460-9592.2006.02094.x. [DOI] [PubMed] [Google Scholar]
- 23.Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg. 2015;18:184–190. doi: 10.1016/j.ijsu.2015.04.079. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. American Academy of Pediatric Dentistry. Coté CJ, Wilson S, Work Group on Sedation Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587–2602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 25.Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–490. doi: 10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 26.Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the pediatric sedation research consortium. Pediatrics. 2006;118:1087–1096. doi: 10.1542/peds.2006-0313. [DOI] [PubMed] [Google Scholar]
- 27.von Ungern-Sternberg BS, Boda K, Schwab C, Sims C, Johnson C, Habre W. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology. 2007;107:714–719. doi: 10.1097/01.anes.0000286925.25272.b5. [DOI] [PubMed] [Google Scholar]
- 28.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461. doi: 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Massanari M, Novitsky J, Reinstein LJ. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr (Phila) 1997;36:681–684. doi: 10.1177/000992289703601202. [DOI] [PubMed] [Google Scholar]
- 30.Weinbroum AA, Szold O, Ogorek D, Flaishon R. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol. 2001;18:789–797. doi: 10.1046/j.1365-2346.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 31.Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med. 2004;44:460–471. doi: 10.1016/S0196064404006365. [DOI] [PubMed] [Google Scholar]
- 32.Brecelj J, Trop TK, Orel R. Ketamine with and without midazolam for gastrointestinal endoscopies in children. J Pediatr Gastroenterol Nutr. 2012;54:748–752. doi: 10.1097/MPG.0b013e31824504af. [DOI] [PubMed] [Google Scholar]
- 33.Motamed F, Aminpour Y, Hashemian H, Soltani AE, Najafi M, Farahmand F. Midazolam-ketamine combination for moderate sedation in upper GI endoscopy. J Pediatr Gastroenterol Nutr. 2012;54:422–426. doi: 10.1097/MPG.0b013e3182323c75. [DOI] [PubMed] [Google Scholar]
- 34.Green SM, Klooster M, Harris T, Lynch EL, Rothrock SG. Ketamine sedation for pediatric gastroenterology procedures. J Pediatr Gastroenterol Nutr. 2001;32:26–33. doi: 10.1097/00005176-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Larsen R, Galloway D, Wadera S, et al. Safety of propofol sedation for pediatric outpatient procedures. Clin Pediatr (Phila) 2009;48:819–823. doi: 10.1177/0009922809337529. [DOI] [PubMed] [Google Scholar]
- 36.Chiaretti A, Benini F, Pierri F, et al. Safety and efficacy of propofol administered by paediatricians during procedural sedation in children. Acta Paediatr. 2014;103:182–187. doi: 10.1111/apa.12472. [DOI] [PubMed] [Google Scholar]
- 37.Long Y, Liu HH, Yu C, et al. Pre-existing diseases of patients increase susceptibility to hypoxemia during gastrointestinal endoscopy. PLoS One. 2012;7:e37614. doi: 10.1371/journal.pone.0037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedirli N, Egritas O, Cosarcan K, Bozkirli F. A comparison of fentanyl with tramadol during propofol-based deep sedation for pediatric upper endoscopy. Paediatr Anaesth. 2012;22:150–155. doi: 10.1111/j.1460-9592.2011.03707.x. [DOI] [PubMed] [Google Scholar]
- 39.Roh WS, Kim DK, Jeon YH, et al. Analysis of anesthesia-related medical disputes in the 2009-2014 period using the Korean society of anesthesiologists database. J Korean Med Sci. 2015;30:207–213. doi: 10.3346/jkms.2015.30.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HK, Lightdale JR. Sedation and monitoring in the pediatric patient during gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 2016;26:507–525. doi: 10.1016/j.giec.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Dar AQ, Shah ZA. Anesthesia and sedation in pediatric gastrointestinal endoscopic procedures: a review. World J Gastrointest Endosc. 2010;2:257–262. doi: 10.4253/wjge.v2.i7.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredette ME, Lightdale JR. Endoscopic sedation in pediatric practice. Gastrointest Endosc Clin N Am. 2008;18:739–751, ix. doi: 10.1016/j.giec.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Squires RH, Jr, Morriss F, Schluterman S, Drews B, Galyen L, Brown KO. Efficacy, safety, and cost of intravenous sedation versus general anesthesia in children undergoing endoscopic procedures. Gastrointest Endosc. 1995;41:99–104. doi: 10.1016/s0016-5107(05)80589-9. [DOI] [PubMed] [Google Scholar]
- 44.Liacouras CA, Mascarenhas M, Poon C, Wenner WJ. Placebo-controlled trial assessing the use of oral midazolam as a premedication to conscious sedation for pediatric endoscopy. Gastrointest Endosc. 1998;47:455–460. doi: 10.1016/s0016-5107(98)70244-5. [DOI] [PubMed] [Google Scholar]
- 45.Ali S, Davidson DL, Gremse DA. Comparison of fentanyl versus meperidine for analgesia in pediatric gastrointestinal endoscopy. Dig Dis Sci. 2004;49:888–891. doi: 10.1023/b:ddas.0000030106.01928.b4. [DOI] [PubMed] [Google Scholar]
- 46.Khoshoo V, Thoppil D, Landry L, Brown S, Ross G. Propofol versus midazolam plus meperidine for sedation during ambulatory esophagogastroduodenoscopy. J Pediatr Gastroenterol Nutr. 2003;37:146–149. doi: 10.1097/00005176-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Fishbein M, Lugo RA, Woodland J, Lininger B, Linscheid T. Evaluation of intranasal midazolam in children undergoing esophagogastroduodenoscopy. J Pediatr Gastroenterol Nutr. 1997;25:261–266. doi: 10.1097/00005176-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Hofley MA, Hofley PM, Keon TP, Gallagher PR, Poon C, Liacouras CA. A placebo-controlled trial using intravenous atropine as an adjunct to conscious sedation in pediatric esophagogastroduodenoscopy. Gastrointest Endosc. 1995;42:457–460. doi: 10.1016/s0016-5107(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 49.Coté CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105(4 Pt 1):805–814. doi: 10.1542/peds.105.4.805. [DOI] [PubMed] [Google Scholar]
- 50.American Academy of Pediatrics. American Academy of Pediatric Dentistry. Coté CJ, Wilson S, Work Group on Sedation Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Paediatr Anaesth. 2008;18:9–10. doi: 10.1111/j.1460-9592.2007.02404.x. [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 52.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]