Prevalence of pulmonary hypertension in patients with systemic sclerosis and mixed connective tissue disease (original) (raw)
Abstract
Systemic sclerosis (SSc) and mixed connective tissue disease (MCTD) are 2 conditions in which pulmonary hypertension (PH) can develop.
We retrospectively analyzed the probability of PH in case of 83 patients (69 SSc and 14 MCTD). The European Society of Cardiology/European Respiratory Society (ESC/ERS) echocardiographic guidelines of 2015 were used for the evaluation.
On the basis of an echocardiography, the patients were divided into 2 subgroups: patients with elevated probability of PH (EP) (n = 16) versus the group with a low probability of PH (LP) (n = 67). Of the 16 patients in the EP group, 15 were SSc patients and 1 was an MCTD patient, respectively, that is, 21.7% and 7.1% of all patients. Of the 16 patients with EP, 10 with SSc had right-heart catheterization, which excluded PH in 7 patients; hence, PH was estimated to be 11.6% in the SSc group. The distribution of the individual causes of PH was arterial PH 2.9%, PH associated with interstitial lung disease 4.3%, PH associated with left ventricular disease 1.5%, and PH of unknown origin 2.9%. Further, there was a significant difference between EP and LP in the incidence of the right bundle branch block in standard electrocardiography, left atrial and right ventricular dimension, tricuspid annular plane systolic excursion, and Doppler-derived tricuspid lateral annular systolic velocity (S’) in echocardiography.
Echocardiography, particularly those evaluating the parameters included in the ESC/ERS guidelines of 2015, appears to be a useful tool in the detection of patients with a high PH probability. Additional tissue Doppler echocardiography seems to be a good option.
Keywords: echocardiography, mixed connective tissue disease, pulmonary hypertension, right heart catheterization, systemic sclerosis
1. Introduction
Systemic sclerosis (SSc) is a chronic autoimmune connective tissue disease. It is characterized by hardening of the skin caused by excessive fibrosis, which can also affect the internal organs and microcirculation disorders. The development of pulmonary arterial hypertension (PAH) during illness significantly worsens prognosis.[1,2] Similarly, in the course of mixed connective tissue disease (MCTD), the occurrence of pulmonary hypertension (PH) is responsible for increased mortality. For the diagnosis of MCTD, the presence of antibodies against U1 ribonucleoprotein (U1 RNP) is needed in combination with symptoms characteristic of systemic sclerosis, systemic lupus erythematosus, myositis or dermatomyositis, and/or rheumatoid arthritis. In both units, PH can develop on the basis of interstitial lung disease or as a result of isolated pulmonary arteriopathy. These are the 2 main causes of pre-capillary PH in connective tissue disease (CTD). Other etiopathogenetic factors include chronic thromboembolism and left ventricular dysfunction responsible for pre- and postcapillary PH, which are significantly lower in CTD.[1,3–5] The literature describes the prevalence of both CTD and PH in their course depending on the geographical location.[6] A wide range, from 5% to even 50% of SSc patients with PH and between 2% and 24% in MCTD, may be associated with different diagnostic criteria, with different methods of measurement [depending on whether only echocardiography or right heart catheterization (RHC) was performed], or depends on the selection of the studied population.[7–9] The purpose of this study was to evaluate the probability of PH in patients with SSc and MCTD based on echocardiographic studies according to the guidelines of the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) and assessment of its usefulness for detecting the increased likelihood of PH among patients at the Department of Rheumatology and Internal Medicine, Poznan University of Medical Science, with reference to literature data.
2. Material and methods
The study was based on a retrospective analysis of the history of the patients (ethical approval was not necessary) hospitalized at the Department of Rheumatology and Internal Medicine, Poznan University of Medical Science between 2005 and 2017. Diagnosis was performed or verified in case of SSc according to the ACR/EULAR criteria for 2013[10] and in case of MCTD based on the criteria by Alarcón-Segovia and Villareal[11] and/or Kasukawa et al.[12] The group consisted of 121 people, including 87 with the diagnosis of SSc and 34 with the diagnosis of MCTD. Finally, 83 patients were enrolled in the study: 69 SSc patients and 14 MCTD patients with complete data collection, including full echocardiography. In all cases, data from a detailed interview were noted (paying particular attention to the presence of limitations during physical activity - as symptomatic patients we included everyone with New York Heart Association class II and more, duration of the illness, current smoking, and family history of rheumatic diseases), along with those obtained from physical examination (in particular, data on the presence of telangiectasia and features of right ventricular dysfunction such as leg edema and enlarged liver), electrocardiogram (ECG) recording (including right bundle branch block, RBBB), the results of laboratory tests with particular emphasis on serological tests, analysis of forced vital capacity (FVC) and carbon monoxide diffusing capacity (DLCO), results of high-resolution computed tomography (HRCT) of lungs, if performed, and echocardiography test results and RHC results if performed. Transthoracic echocardiographic studies were performed using Vivid 7 machine (3.5 MHz), GE Vingmed Ultrasound AS, Norway at the Department of Cardiology, Poznan University of Medical Science. Echocardiographic data were used for assessing the likelihood of PH in accordance with the ESC/ERS guidelines for 2015.[13] Depending on the maximum velocity of tricuspid regurgitation and the presence of other echocardiographic indicators of PH, the patients were divided into 3 groups: low, intermediate, and high probability of PH (Table 1).
Table 1.
Echocardiographic probability of pulmonary hypertension (PH) in symptomatic patients with suspected pulmonary hypertension (according to ESC/ERS guidelines, 2015)[10].
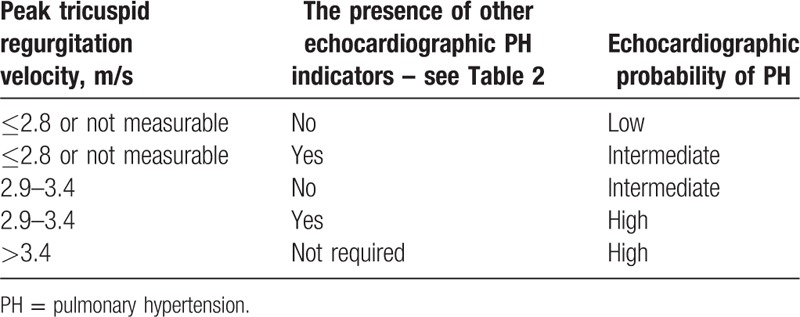
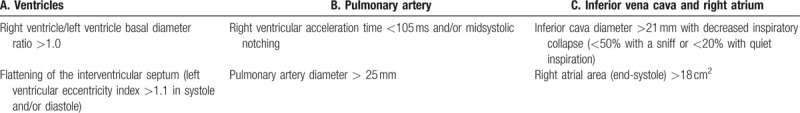
Other echocardiographic PH indicators were divided into 3 categories, from which at least 2 indicators from 2 different categories (A, B, or C) had to be present to change the echocardiographic probability of PH (Table 2).
Table 2.
Echocardiographic indicators suggesting the presence of pulmonary hypertension (PH), used for assessing the likelihood of PH in addition to tricuspid regurgitation velocity measurement in[10]Table 1.
Patients with intermediate and high probability were considered to be potential candidates for the treatment of specific PH. Pulmonary counseling was performed to exclude PH with pulmonary fibrosis. When the FVC was less than 70% of the expected value and significant changes were observed, being evidence of pulmonary fibrosis in the HRCT scans of the chest, PH association with interstitial lung disease was diagnosed. Thus, the RHC was canceled. In patients with no such association (when FVC > 70% of the predicted value and no significant pulmonary fibrosis in HRCT was observed), PH was considered to be arterial and standard RHC was performed to further qualify for a specific treatment program. In RHC, pre-capillary PH was diagnosed in patients with average resting pulmonary artery pressure ≥ 25 mm Hg and wedge pressure ≤ 15 mm Hg. Postcapillary PH was diagnosed in patients with average resting pulmonary artery pressure ≥ 25 mm Hg and wedge pressure > 15 mm Hg.
3. Statistical analysis
For the analyzed data, compatibility with the normal distribution using the Shapiro–Wilk/D’Agostino–Pearson test was checked. Parametric tests were used for parameters consistent with the normal distribution; otherwise, nonparametric tests were applied. Parameters such as age, left atrium diameter, right ventricle diameter, aorta diameter, peak tricuspid regurgitation velocity, inferior vena cava diameter, right atrial area, right ventricular outflow tract acceleration time (ACT), and Doppler-derived tricuspid lateral annular systolic velocity (S′) were described by arithmetic mean and standard deviation. Parameters such as duration of disease, left ventricle diameter, left ventricular posterior wall thickness, intraventricular septum thickness, pulmonary artery diameter, left ventricular eccentricity index, right ventricle/left ventricle basal diameter ratio, and tricuspid annular plane systolic excursion (TAPSE) were described by median and average deviation. For comparison between low-risk and elevated-risk patient groups, a Student t test for unrelated variables or the Mann–Whitney nonparametric test and the Chi-square test for nonmeasurable features were used. Further, P < .05 denoted statistical significance. The statistical package CSS Statistica v. 12.0 Stat Soft, Inc., Oklahoma was used in the calculations.
4. Results
Of the 121 SSc and MCTD patients, 83 patients (73 females – F, 10 males – M): 69 (61 F and 8 M) with SSc and 14 (12 F and 2 M) patients with MCTD were included in the statistical computation. The mean age in the study group was 56.6 ± 13.1 years. In SSc group, we found 58 patients classified as diffuse systemic sclerosis (dSSc) and 11 patients classified as limited systemic sclerosis (lSSc). In MCTD group, the most common manifestations were arthritis and lupus-like and scleroderma-like symptoms. After analyzing echocardiographic data, the likelihood of PH was defined as low in 67 patients, as intermediate in 9 patients, and as high in 7 patients. Because of the low abundance of the intermediate and the high subgroups of PH probability, these groups were pooled together for further calculations as a group of patients with elevated probability (EP) of PH compared with the group with low probability (LP) of PH. In the EP group of 16 patients, there were 15 patients with SSc and 1 patient with MCTD, respectively, that is, 21.7% and 7.1% of all patients diagnosed (Fig. 1).
Figure 1.
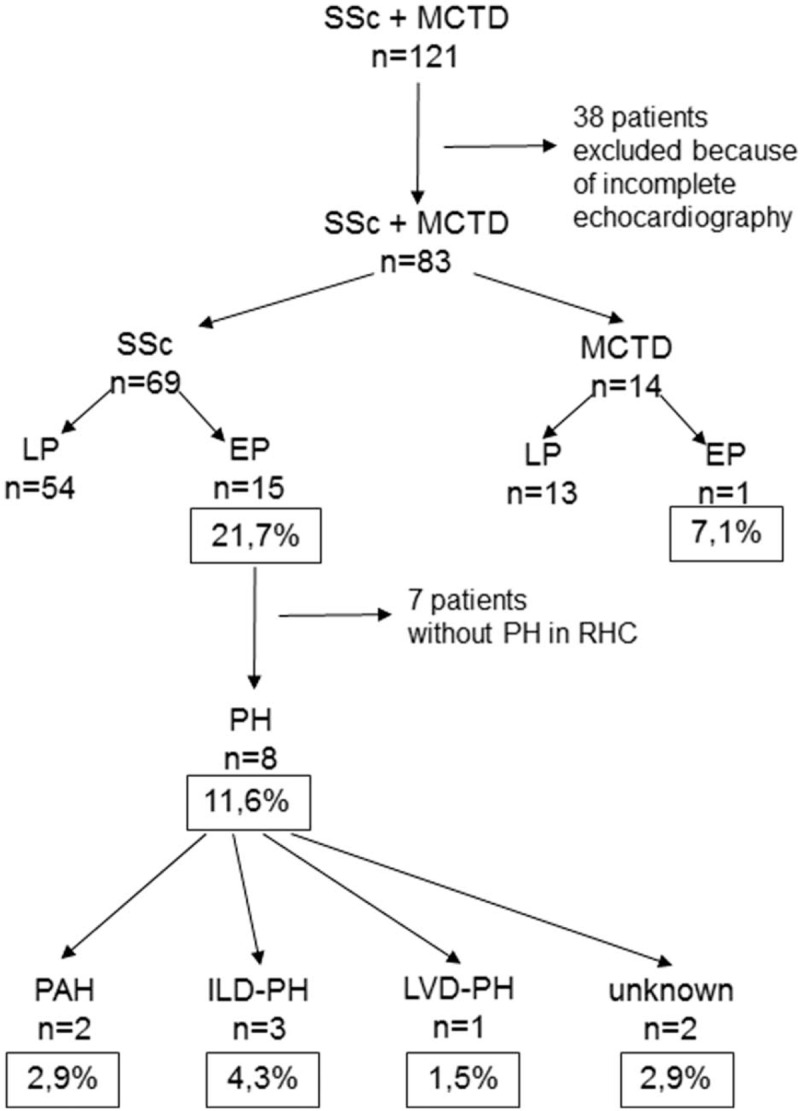
Incidence of PH in patients with SSc and MCTD. EP = elevated probability of PH, LP = low probability of PH.
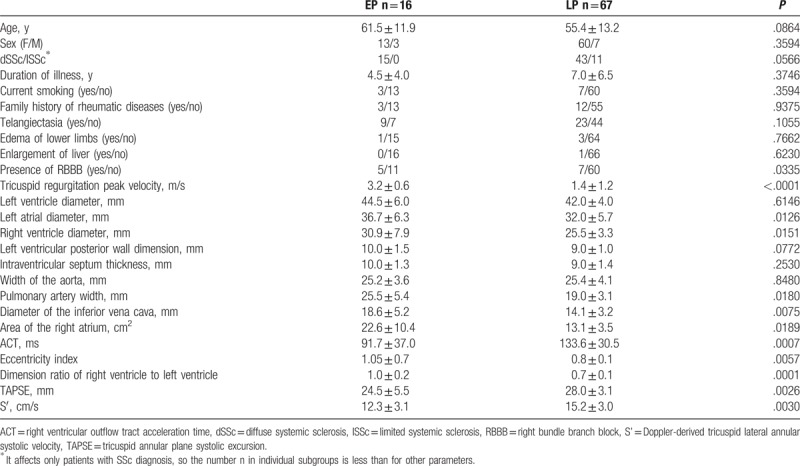
EP and LP groups did not differ statistically in terms of age, sex, duration of illness, history of smoking, or family history of rheumatic diseases. There were no differences between the groups for telangiectasia or right ventricular dysfunction (edemas of lower limbs and liver enlargement). There was a significant difference between the groups in the prevalence of RBBB in standard ECG and the size of the left atrium and right ventricular echocardiography, TAPSE, and tissue Doppler echocardiography S’. The details of the relationships described above are summarized in Table 3.
Table 3.
Comparison of the elevated probability (EP) group of pulmonary hypertension (PH) and the low probability group (LP) of PH.
Of the 16 patients with EP, 10 with SSc underwent RHC. Of the remaining 6 who did not undergo RHC, 3 did not consent to this study (including 2 SSc patients and 1 MCTD patient), 2 patients with SSc were diagnosed with PH due to pulmonary fibrosis (ILD-PH), and in 1 patient with SSc, PH was associated with left ventricular dysfunction (LVD-PH) (EF 15% in echocardiography and dilated cardiomyopathy). Of the patients who underwent RHC, PH was finally confirmed in 3 patients, and specific treatment was begun in 2 patients (the third after a detailed analysis was disqualified from treatment because of interstitial lung disease). Further, PH in RHC was not confirmed in 7 SSc patients. Thus, from the group of 15 patients with EP in the echocardiography study, 8 patients remained; they were diagnosed in RHC with PH, or for various reasons, were excluded from the RHC and PH was assumed. This group represented 11.6% of the analyzed SSc patients. Unfortunately, the only patient with MCTD and elevated PH risk in echocardiography did not agree to undergo RHC.
5. Discussion
PH development both in the SSc and in the MCTD course worsens the prognosis and causes increased mortality. Therefore, it is important to detect this complication early and, if possible, to use appropriate treatment. The literature suggests that the prevalence of PH in CTD is more frequent than that of idiopathic PH; hence, it is important to examine rheumatoid patients in this respect.[14] It is important to identify patients at risk for PH based on noninvasive testing. There are several screenings in use. The most common published work appears to be the 2009 ESC/ERS guideline, which relies on clinical symptoms (dyspnea and syncope) and selected echocardiographic parameters [tricuspid regurgitation peak velocity (TRV) or right ventricular diameter].[15] Unfortunately, these are insufficient to detect all cases of PH because the clinical symptoms may be poorly expressed or nonspecific.[16] The algorithm proposed in 2012 by the Australian Scleroderma Interest Group (ASIG) seems to be a better solution.[17] It is based primarily on respiratory function tests and the concentration of N-terminal pro B-type natriuretic peptide (NT-proBNP), in addition to selected echocardiographic parameters or pulmonary HRCT. In 2013, another algorithm for detecting patients to whom HRC should be performed[18] was proposed based on DETECT study (which refers to evidence-based detection of PAH in systemic sclerosis). This is a 2-step algorithm. In the first place, the author described the patients who should have undergone echocardiography. FVC/DLCO values, presence of telangiectasia and anti-centromere antibodies, NT-proBNP and uric acid concentrations, and deviation of the heart axis to the right in the ECG were considered. In the case of a positive evaluation, echocardiography was performed, considering TRV and right atrial area. In the study by Hao et al,[16] the ESC/ERS algorithm of 2009 had a smaller sensitivity, specificity, and negative predictive value than the ASIG and DETECT algorithms do. In our work, we decided to evaluate the probability of PH in symptomatic patients using only echocardiography, but we increased the number of evaluated parameters according to the ESC/ERS guidelines of 2015 for PH recognition.[13] None of the works published thus far have used these criteria. In addition, the strength of our project was the fact that all echocardiographic studies throughout the assessed period were performed by the same experienced cardiologist. Thereby, we had measurements that were not included in the ASIG or DETECT algorithm and are now included in the ESC/ERS guidelines (e.g., left ventricular eccentricity index). We have obtained similar results to this described by Walker et al,[19] from the EUSTAR group, based on only an echocardiographic study, 22.3% for dSSc and 20.5% for lSSc, noting that there was no statistically significant difference in PAH frequencies between patients with dSSc and lSSc. Launay et al[20] also obtained a similar result: 18.3% in echocardiography, and after RHC, the frequency of PH was determined to be 16.2%, irrespective of the SSc form. RHC is the gold standard in PH diagnosis. Unfortunately, not all our patients managed to do it. However, excluding from the group of EP, patients who had undergone RHC with results excluding PH, we could estimate the prevalence of PH in our SSc patients to be 11.6%. Avouac et al[7] in their RHC-based study, evaluated the prevalence of PH to be 7%. Similarly, Iudici et al[6] reported 6.5%. However, these authors cite the fact that some differences in the prevalence of hypertension can be observed, depending on the geographical location. Similarly, Walker et al[21] from the EUSTAR group reported differences depending on the geographical location, with more severe organ dysfunction in the SSc course in eastern European patients. According to some authors, attention should be paid to patients with limit values of resting mean pulmonary arterial pressure between 21 and 24 mm Hg. After analyzing the DETECT data, it was found that 15% of the patients had this outcome.[22] This condition was considered to be transient between the norm and PH, and such patients required particularly careful observation.[23] In our group, we recorded 3 such patients, which constituted 4.35% of all the SSc patients studied. PH, also in CTD, may have different causes, and its etiology is important for further therapy.[24] Specific treatment is provided for patients with PAH, while the remaining causes of PH are an exclusion factor.[25] In our study population, 2 patients were diagnosed with PAH, accounting for 2.9% of all the SSc patients analyzed. These patients have been included in the PAH-specific treatment program in cooperation with Cardiology Clinic. It is worth noting that in the 2 patients who did not consent to RHC and belonged to the EP group, no changes in lung function and no fibrotic changes were observed in the HRCT of the chest. One might assume that in their case, we also dealt with the vascular etiology of PH. Hence, the prevalence of PAH in the surveyed population was estimated to be 2.9% to 5.8%. In addition, 3 patients reported ILD-PH, which was 4.3%, and 1 patient was diagnosed with LVD-PH, which was 1.5%. PAH seemed to be the main cause of PH in SSc. This confirmed the findings of the DETECT study, where 19% of the patients were PAH affected, while the prevalences of ILD-PH and LVD-PH were 6%.[18] In the study by García Hernández et al,[26] the PAH rate in the population of 184 patients with SSc was estimated to be 13.6%. An Australian study of a large population of 1579 patients evaluated the prevalence of PAH to be 8.4%.[27] In the previously cited works, Iudici et al[6] reported the prevalence of individual groups to be PAH 3.7%, ILD-PH 1.4%, and LVD-PH 1.3%, while Avouaci et al[7] reported the prevalences to be 3.4%, 1.6%, and 2%, respectively. The prevalence of PAH at our center was relatively low, but it is important to remember that this condition is one of the leading causes of death in SSc. Its course is insidious, and it is often diagnosed in at a later stage, and patients with PAH associated with SSc are significantly less likely to respond to therapy than patients with idiopathic PAH.[1,28] This is explained by the fact that in cases of autoimmune diseases, with persistent chronic inflammatory processes, there is much more serious vascular remodeling than in other cases of PAH.[1] A separate issue is the prevalence of PH in MCTD. Our MCTD patient with EP of PH, as in the case of the 2 SSc patients, showed no changes in pulmonary function tests or fibrotic lesions in the HRCT of the chest, hence, the high probability of vascular etiology. As for the prevalence of PH in MCTD, Gunnarsson et al,[8] after analyzing the literature, selected 4 publications and estimated it to be 19% to 24%. In their own observational studies, this frequency was set to be 2.0% to 3.4%.[8] MCTD is placed second after SSc as the cause of PH in CTD.[29] However, the course of the disease is milder than that of SSc. This may be confirmed by the work of Sobanski et al,[30] wherein it was shown that in patients with PH associated with CTD, the presence of U1 RNP antibodies correlates with reduced mortality. It is important to determine whether SSc or MCTD patients are likely to develop PH based on clinical judgment. After analyzing our 2 groups EP and LP, selected on the basis of echocardiography, we found that they did not differ in terms of gender, age, or duration of illness. Similar observations were made by Kumar et al.[31] Further, in the work of Iudici et al,[6] sex, age, and duration of the disease were not related to the occurrence of PH. This is an important diagnostic observation: we should remember that PH may occur in younger patients with CTD, even in the early stages of the disease. Similarly, current smoking habit or family history of rheumatic diseases did not show a statistically significant difference between the 2 groups in our work, although it is reported in the literature on the adverse effect of cigarette smoking on complications of SSc associated with the cardiovascular, respiratory, and digestive system.[32] Among the symptoms in the study, we assessed the presence of telangiectasia and the features of right ventricular dysfunction. There were no statistically significant differences in these parameters between the 2 studied groups, although telangiectasia was described in the literature as a potential clinical marker of PH.[33] As for the SSc subtypes, there was no significant difference in the predisposition to PH between patients with dSSc and lSSc, which was previously described as a disadvantage of lSSc.[34] At present, more authors are inclined toward the same findings as our proposal.[6,20,26,31,35] In additional studies, we observed a statistically significant difference in the prevalence of RBBB in resting 12-lead ECG, predominantly of EP patients. Previously, the utility of simple noninvasive diagnostic tests, such as ECG, in the detection of patients with high PH risk has been reported. However, until now, the features evaluated were primarily the deviation of the heart axis to the right or features of the right ventricular overload.[18,36] SSc conduction disorders are primarily associated with fibrosis within the cardiac conduction system. In our work, we observed that RBBB may also be associated with PH. The results of our echocardiographic studies, which are not included in the criteria for PH probability estimation, have been interesting and have significantly differed between the study groups. These include the left atrial diameter, right ventricle diameter, TAPSE, and S’. Right ventricular enlargement in patients with PH is quite obvious. Left atrium enlargement, however, is a surprise, as it was previously described mainly in the course of LVD-PH.[37,38] Meune et al,[39] who examined a group of 100 patients with SSc without PH, concluded that left atrial enlargement and left and right ventricular dysfunction are common in SSc and are related to the primary myocardial process. Single publications also describe the thickening of the posterior wall of the left ventricle and the interventricular septum in PH.[40] These changes may suggest that PH also leads to remodeling not only of the right ventricle or atrium but also of the whole heart already altered by the underlying disease. Significant differences between the EP and LP groups also involved TAPSE. This parameter was not included in the 2015 ESC/ERS guidelines, although for many years, it has been identified as one of the main factors monitoring the effectiveness of PH treatment.[41] In the present study, the echocardiographic study included tissue Doppler echocardiography, with a particular emphasis on S’. In this regard, we also observed significant differences between the groups studied, to the disadvantage of the EP group. The results are consistent with the results of Gopal et al,[42] who described in their work a negative correlation between pulmonary vascular resistance in RHC and TAPSE and S’. Tissue Doppler ultrasound echocardiography is an excellent tool for evaluating right ventricular function, and it is important to extend diagnostics for its implementation, with particular regard to S’, particularly as it is easy to evaluate and the results of this noninvasive study correlate with RHC parameters, which remain the gold standard in PH recognition.
6. Research limitations
The primary limitation of the study appears to be a small group of patients from a statistical standpoint. However, both SSc and MCTD are not common diseases, and the data used in this study come from only 1 center. Therefore, from a clinical point of view, the studied group is quite numerous. In addition, not all patients had echocardiography that met the expectations of this study. Our study is a retrospective study, covering material from 2005. At that time, all measurements that were subsequently included in the ESC/ERS 2015 criteria were not taken. Therefore, it was possible to use the collected data only from those patients who had full echocardiography. Hence, of 121 patients, only 83 patients finally qualified for the final analysis. Further, the fact that not all patients with established intermediate or high probability of PH in echocardiography have the RHC performed. Unfortunately, despite our best efforts, we failed to convince all the patients and they did not consent to the study. In addition, in some patients who were known to be excluded from specific treatment because the cause of PH was interstitial lung disease or left ventricular dysfunction, RHC was withdrawn because the best clinical practice was to not expose these patients to additional invasive testing, which would not change the treatment anyway.
7. Conclusion
The prevalence of PH in our group was estimated at 11.6% among patients with SSc and 7.1% among patients with MCTD.
Echocardiographic studies, particularly those evaluating the parameters included in the ESC/ERS guidelines of 2015, are useful tools for assessing the increased likelihood of PH. An important issue is the careful observation of “gray zone” patients with a limit value of the mean resting pulmonary artery pressure in RHC between 21 and 24 mm Hg. Patients from the group with the EP of PH did not differ in gender, duration of illness, telangiectasia, and signs of right ventricular dysfunction. It also obliges us to diagnose for PH in younger patients and in the early stages of illness, as well as in patients with poorly defined symptoms in the physical examination. Our study shows a significant decrease in S’ in patients with PH, which may suggest the advantage of taking this parameter into account and using tissue Doppler echocardiography when estimating the probability of PH.
Author contributions
Conceptualization: Karolina Niklas.
Data curation: Karolina Niklas, Tatiana Mularek-Kubzdela.
Formal analysis: Karolina Niklas, Arkadiusz Niklas.
Investigation: Karolina Niklas.
Methodology: Karolina Niklas, Arkadiusz Niklas, Tatiana Mularek-Kubzdela.
Project administration: Karolina Niklas.
Resources: Karolina Niklas.
Software: Arkadiusz Niklas.
Supervision: Tatiana Mularek-Kubzdela, Mariusz Puszczewicz.
Writing – original draft: Karolina Niklas.
Writing – review & editing: Arkadiusz Niklas, Tatiana Mularek-Kubzdela, Mariusz Puszczewicz.
Footnotes
Abbreviations: ACT = right ventricular outflow tract acceleration time, ASIG = Australian Scleroderma Interest Group, CTD = connective tissue disease, DLCO = carbon monoxide diffusing capacity, dSSc = diffuse systemic sclerosis, ECG = electrocardiogram, ESC/ERS = European Society of Cardiology/European Respiratory Society, FVC = forced vital capacity, HRCT = high-resolution computed tomography, ILD = interstitial lung disease, lSSc = limited systemic sclerosis, LVD = left ventricular dysfunction, MCTD = mixed connective tissue disease, NT-proBNP = N-terminal pro B-type natriuretic peptide, PAH = pulmonary arterial hypertension, PH = pulmonary hypertension, RBBB = right bundle branch block, RHC = right heart catheterization, S’ = Doppler-derived tricuspid lateral annular systolic velocity, SSc = systemic sclerosis, TAPSE = tricuspid annular plane systolic excursion, TRV = tricuspid regurgitation peak velocity, U1 RNP = U1 ribonucleoprotein.
Funding/support: This study was not supported by any funding.
None of the authors declare conflict of interest.
The authors of this work have nothing to disclose.
References
- [1].Gashouta MA, Humbert M, Hassoun PM. Update in systemic sclerosis-associated pulmonary arterial hypertension. Presse Med 2014;43:e293–304. [DOI] [PubMed] [Google Scholar]
- [2].Skride A, Sablinskis K, Avidan Y, et al. Pulmonary arterial hypertension associated with connective tissue disease: insights from Latvian PAH registry. Eur J Intern Med 2017;40:e13–4. [DOI] [PubMed] [Google Scholar]
- [3].Cao Z, Mathai SC, Hummers LK, et al. Exhaled nitric oxide in pulmonary arterial hypertension associated with systemic sclerosis. Pulm Circ 2016;6:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gunther S, Jaïs X, Maitre S, et al. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum 2012;64:2995–3005. [DOI] [PubMed] [Google Scholar]
- [5].Zakir RM, Berkowitz RL, Saric M, et al. A comparison of nesiritide vs. epoprostenol in a patient with precapillary pulmonary hypertension due to scleroderma complicated by postcapillary pulmonary hypertension. Congest Heart Fail 2005;11:331–4. [DOI] [PubMed] [Google Scholar]
- [6].Iudici M, Codullo V, Giuggioli D, et al. Pulmonary hypertension in systemic sclerosis: prevalence, incidence and predictive factors in a large multicentric Italian cohort. Clin Exp Rheumatol 2013;31(2 suppl 76):31–6. [PubMed] [Google Scholar]
- [7].Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 2010;37:2290–8. [DOI] [PubMed] [Google Scholar]
- [8].Gunnarsson R, Andreassen AK, Molberg Ø, et al. Prevalence of pulmonary hypertension in an unselected, mixed connective tissue disease cohort: results of a nationwide, Norwegian cross-sectional multicentre study and review of current literature. Rheumatology (Oxford) 2013;52:1208–13. [DOI] [PubMed] [Google Scholar]
- [9].Ciurzyński M, Bienias P, Irzyk K, et al. Usefulness of echocardiography in the identification of an excessive increase in pulmonary arterial pressure in patients with systemic sclerosis. Kardiol Pol 2011;69:9–15. [PubMed] [Google Scholar]
- [10].van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alarcón-Segovia DA, Villarreal M. Kasukawa R, Sharp GC. Classification and diagnostic criteria for mixed connective tissue disease. Mixed Connective Tissue Disease and Anti-nuclear Antibodies. Amsterdam: Elsvier Science; 1987. 33–40. [Google Scholar]
- [12].Kasukawa R, Tojo T, Miyawaki S. Kasukawa R, Sharp GC, et al. Prelimary diagnostic criteria for classification of mixed connective tissue disease. Mixed Connective Tissue Disease and Anti-nuclear Antibodies. Amsterdam: Elsevier Science; 1987. 41–7. [Google Scholar]
- [13].Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Kardiol Pol 2015;73:1127–206. [DOI] [PubMed] [Google Scholar]
- [14].Yang X, Mardekian J, Sanders KN, et al. Prevalence of pulmonary arterial hypertension in patients with connective tissue diseases: a systematic review of the literature. Clin Rheumatol 2013;32:1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–537. [DOI] [PubMed] [Google Scholar]
- [16].Hao Y, Thakkar V, Stevens W, et al. A comparison of the predictive accuracy of three screening models for pulmonary arterial hypertension in systemic sclerosis. Arthritis Res Ther 2015;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thakkar V, Stevens MW, Prior D, et al. N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study. Arthritis Res Ther 2012;14:R143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Launay D, Mouthon L, Hachulla E, et al. Prevalence and characteristics of moderate to severe pulmonary hypertension in systemic sclerosis with and without interstitial lung disease. J Rheumatol 2007;34:1005–11. [PubMed] [Google Scholar]
- [21].Walker UA, Tyndall A, Czirják L, et al. EUSTAR co-authors. Geographical variation of disease manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research (EUSTAR) group database. Ann Rheum Dis 2009;68:856–62. [DOI] [PubMed] [Google Scholar]
- [22].Visovatti SH, Distler O, Coghlan JG, et al. Borderline pulmonary arterial pressure in systemic sclerosis patients: a post-hoc analysis of the DETECT study. Arthritis Res Ther 2014;16:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kovacs G, Olschewski H. Borderline pulmonary pressures in scleroderma: a ‘pre-pulmonary arterial hypertension’ condition? Arthritis Res Ther 2015;17:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Giordano N, Montella A, Corallo C, et al. Pulmonary hypertension: a correct diagnosis for a suitable therapy in scleroderma patients. Clin Exp Rheumatol 2015;33(4 suppl 91):S182–189. [PubMed] [Google Scholar]
- [25].Hinchcliff M, Fischer A, Schiopu E, et al. PHAROS Investigators. Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol 2011;38:2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].García Hernández FJ, Castillo Palma MJ, Montero Mateos E, et al. Screening of pulmonary hypertension in a Spanish cohort of patients with systemic sclerosis. Med Clin (Barc) 2016;146:1–7. [DOI] [PubMed] [Google Scholar]
- [27].Morrisroe K, Huq M, Stevens W, et al. Australian Scleroderma Interest Group (ASIG). Risk factors for development of pulmonary arterial hypertension in Australian systemic sclerosis patients: results from a large multicenter cohort study. BMC Pulm Med 2016;16:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013;144:1346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Coghlan JG, Galiè N, Barberà JA, et al. AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017;76:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sobanski V, Giovannelli J, Lynch BM, et al. Characteristics and survival of anti-U1 RNP antibody-positive patients with connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheumatol 2016;68:484–93. [DOI] [PubMed] [Google Scholar]
- [31].Kumar U, Ramteke R, Yadav R, et al. Prevalence and predictors of pulmonary artery hypertension in systemic sclerosis. J Assoc Physicians India 2008;56:413–7. [PubMed] [Google Scholar]
- [32].Leask A. When there's smoke there's...scleroderma: evidence that patients with scleroderma should stop smoking. J Cell Commun Signal 2011;5:67–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shah AA, Wigley FM, Hummers LK. Telangiectases in scleroderma: a potential clinical marker of pulmonary arterial hypertension. J Rheumatol 2010;37:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chang B, Schachna L, White B, et al. Natural history of mild-moderate pulmonary hypertension and the risk factors for severe pulmonary hypertension in scleroderma. J Rheumatol 2006;33:269–74. [PubMed] [Google Scholar]
- [35].Vandecasteele EH, De Pauw M, Brusselle G, et al. The heart and pulmonary arterial hypertension in systemic sclerosis. Acta Clin Belg 2016;71:1–8. [DOI] [PubMed] [Google Scholar]
- [36].Kovacs G, Avian A, Foris V, et al. Use of ECG and other simple non-invasive tools to assess pulmonary hypertension. PLoS One 2016;11:e0168706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Katikireddy CK, Singh M, Muhyieddeen K, et al. Left atrial area and right ventricle dimensions in non-gated axial chest CT can differentiate pulmonary hypertension due to left heart disease from other causes. J Cardiovasc Comput Tomogr 2016;10:246–50. [DOI] [PubMed] [Google Scholar]
- [38].Jivraj K, Bedayat A, Sung YK, et al. Left atrium maximal axial cross-sectional area is a specific computed tomographic imaging biomarker of world health organization group 2 pulmonary hypertension. J Thorac Imaging 2017;32:121–6. [DOI] [PubMed] [Google Scholar]
- [39].Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008;58:1803–9. [DOI] [PubMed] [Google Scholar]
- [40].Karoli NA, Rebrov AP. Pulmonary hypertension, involvement of the right and left cardiac parts in patients with ankylosing spondylarthritis. Klin Med (Mosk) 2004;82:31–4. [PubMed] [Google Scholar]
- [41].Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–41. [DOI] [PubMed] [Google Scholar]
- [42].Gopal DM, Doldt B, Finch K, et al. Relation of novel echocardiographic measures to invasive hemodynamic assessment in scleroderma-associated pulmonary arterial hypertension. Arthritis Care Res (Hoboken) 2014;66:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]