Fish oil and depression: The skinny on fats (original) (raw)
. Author manuscript; available in PMC: 2018 Aug 12.
Published in final edited form as: J Integr Neurosci. 2017;16(Suppl 1):S115–S124. doi: 10.3233/JIN-170072
Abstract
Depression is the leading cause of disability worldwide, and even though many forms of therapy exist, about one third of patients treated with conventional antidepressants do not experience a response. For these reasons, new approaches to treat depression, including fish oil, are being investigated. Fish oil is known to have many beneficial side effects, and clinical trials demonstrate that supplementation with fish oil is beneficial in the management of depression. Fish oil contains omega-3 polyunsaturated fatty acids (PUFA), and there are several mechanisms by which PUFAs are thought to induce an antidepressant effect, including anti-inflammatory action and direct effects on membrane properties. This review will analyze and evaluate the clinical trials surrounding fish oil use in the treatment of depression, and will also review the likely sites of action of PUFAs at the cell membrane with special attention being placed on lipid rafts and G-proteins.
Keywords: G-protein, GPCR, lipid raft, depression, antidepressant, omega-3 fatty acids, cAMP
1. Introduction
According to the National Institute of Mental Health, about 16.1 million American adults (6.7% of adults) experienced one or more major depressive episodes in 2015 [63], and the Center of Disease Control reports that, based on notations made in the medical record, about 10.3% of all physician visits were in some way related to depression [42]. The economic burden of depression in the United States was estimated to be about $210.5 billion, and these costs were related to the direct treatment of depression, indirect costs of depression (i.e. missed work or decreased workplace productivity), and costs related to suicide [25]. The World Health Organization states that depression is the leading cause of disability, word-wide [67]. In 2001, it was stated that depression would be the second-leading cause of disability by 2020, but the time was reset to 2017 and being the leading cause rather than the second [66]. The severity of the physical symptoms and the health care costs beg the question, “why is there such poor control of such a major public health issue?” The answer is complex, and involves societal factors (inadequate funding for treatment both in the public setting and by insurance companies) and issues involving current drug therapy including the side effect profile of the medications, and the low rates of effectiveness for the treatments.
Current depression therapies center around the use of antidepressant medications, many of which have been available for decades. The first class of antidepressants available were the monoamine oxidase inhibitors (MAOI). Then, came the tricyclic antidepressants (TCA), and finally, the selective serotonin reuptake inhibitors (SSRI) and serotonin norepinephrine reuptake inhibitors (SNRI). These latter compounds are the most commonly used agents to treat depression, but this is due more to safety than therapeutic efficacy. Several electric stimulation approaches, including electroconvulsive therapy (ECT) and transcranial magnetic stimulation are also available, as is psychotherapy. Unfortunately, despite the extensive treatments available, nearly one third of patients remain unresponsive to any therapy.
For example, one study compared the effectiveness of cognitive therapy versus antidepressant medication versus placebo in moderate to severe cases of depression. Cognitive therapy and antidepressant therapy both demonstrated improved effectiveness measured by response and remission rates when compared to placebo. However, the response rates for the cognitive therapy and antidepressant therapy were both only 58%, while the remission rates were 40% for cognitive therapy and 46% for antidepressant medication [11]. In the STAR*D (Sequence Treated Alternatives to Relieve Depression) study, researchers evaluated the effectiveness of citalopram (an SSRI) by evaluating patients over 41 different clinical care settings. The patients were evaluated using measurement-based care, which means that the patients’ symptoms and side effects were evaluated at each visit. Then, the dosage of citalopram was adjusted accordingly using a universal treatment manual. The study demonstrated that the response rate for citalopram was 47% and the remission rate was either 28% or 33%, depending on the scale used to evaluate a patient’s symptoms. The average time to achieve response was 6 weeks, but a significant portion of the patients who experienced response or remission did so after 8 weeks of treatment. The average time to achieve remission was 7 weeks [62]. These studies exemplify the fact that the current first line therapies for MDD are not sufficient. In particular, the STAR*D trial alludes to the typical 6 to 12 week lag period of treatment before patients experience a true response, which is a very commonly reported disadvantage of SSRIs [18]. Other meta-analyses suggest that the lag period may be shorter, but this can be due to differences in response to the drug versus observable benefits of the drug [44,61]. Regardless, the current recommendations are to allow patients 4 weeks of treatment of an SSRI before augmenting the dosage or the medication in cases of lack of improvement of symptoms [26].
One additional drawback is that many of these drugs have significant side effect profiles. A better solution may be to look to other already commonly used supplements that may be beneficial in the treatment of depression.
An interesting possibility for depression therapy is fish oil, which contains several omega-3 polyunsaturated fatty acids (PUFA). For many years, the benefits of omega-3 fatty acids have been understood, since intervention for cardiovascular disease with these PUFAs caused the decreased production of VLDL. Furthermore, fish oil supplementation has also been shown to have antiplatelet activity, improve heart failure, improve vascular function in diabetics [5], decrease selected markers of oxidative stress [24], decrease osteoarthritis associated knee pain [45], improve outcomes in critically ill patients (especially acute lung injury/acute respiratory distress syndrome) [22], and improve clinical courses of several other conditions. Besides having a vast number of benefits, another advantage of fish oil supplementation is the virtually nonexistent side effect profile when the appropriate doses are administered. Due to the many benefits and few adverse effects, fish oil supplementation is used for many ailments, including psychiatric disorders.
In this review, we will attempt to discuss several clinical trials that have sought to test the effectiveness of omega-3 PUFAs in the treatment of depression, and we will review the possible molecular mechanisms involved in this process.
2. Clinical data
Current studies analyzing the potential for PUFAs in the management of depression have looked at eicosapentaenoic acid (EPA) and/or docosahexaenoic acid (DHA) as the forms of fatty acids. These are the so called “fish oils” because they are the omega-3 fatty acids that are readily found in fish oil supplements.
One meta-analysis examined 10 clinical trials and found that omega-3 fatty acids have a significant antidepressant effect in patients diagnosed with MDD or bipolar disorder. They also found that the dose of EPA administered did not have a significant impact on the rate of efficacy [32]. One study compared adding EPA to maintenance medication treatment for recurrent depression versus placebo. This found that EPA lowered depression rating scores by a mean reduction of 12.4, while placebo only resulted in a reduction of 1.6 based on the Hamilton Rating Scale for Depression (HRSD) [43]. A randomized control trial looked to determine the most effective dose of EPA by comparing 1, 2, and 4 grams per day (g_/day) versus placebo. The patients in this study had ongoing depression despite being given therapeutic doses of antidepressant therapy. Overall, this study found the highest response rates among the 1 g/_day of EPA [46]. On the other hand, another randomized control trial comparing EPA to placebo demonstrated a nonsignificant improvement in outcomes in the EPA group using the HRSD. The authors noted that the lack of significance could be due to the small sample size (57 participants) and low response rate [40].
Similar studies have been conducted analyzing the efficacy of DHA. One study compared three different doses of DHA, 1, 2, and 4 g_/day. The 1 g/_day of DHA group experienced the most benefit [38]. However, this study did not include a placebo group, so it is not possible to state that the DHA treatment resulted in an actual reduction of symptoms or if the response was due to the placebo. On the other hand, one placebo-controlled study showed that there is no statistical difference between DHA and placebo treatment, so the researchers concluded that DHA monotherapy does not have an impact on MDD [35]. It is noteworthy that these studies use different depression rating scales, so head to head comparison is not necessarily valid.
In addition to the above-mentioned studies, most studies surrounding fish oil use in depression have analyzed the usefulness of both EPA and DHA in the treatment of MDD. In one study, a combination of EPA and DHA was compared against placebo treatment. The EPA/DHA treated group experienced a greater reduction in the HRSD score when compared to placebo [57]. A similar study in pregnant patients compared omega-3 fatty acids with placebo. There was a statistically significant higher response rate in the treatment group versus the control group [58]. Another study compared the use of citalopram and PUFA versus citalopram and control (olive oil). The PUFA used in this study was a combination of EPA, DHA, and other omega-3 fatty acids. The study demonstrated significantly improved scores starting at week four of the trial [21].
Furthermore, there are studies that compare EPA and DHA. In one study, Su and colleagues showed that EPA is superior to DHA in clinical antidepressant efficacy [60]. Another study evaluated the ability of EPA and DHA to impede the incidence of depression in patients receiving interferon-alpha (IFN-α) for Hepatitis C treatment. This found that EPA significantly decreased the incidence of depression, while DHA did not when compared to placebo. However, EPA and DHA were noted to significantly delay the onset of depression even though the DHA did not impact the incidence [59]. Additionally, one report analyzed the results of two seemingly contradictory studies and concluded that EPA may have more benefits than DHA in the treatment of MDD [37]. Thus, EPA is suggested to be more efficacious than DHA therapy. Finally, one randomized control trial comparing EPA to DHA to placebo found that neither EPA nor DHA treatment was better than placebo for treatment of depression [39]. This last result is very surprising because the study had a bigger sample size than many of the previously mentioned studies, but at the same time, this study had a high dropout rate. Originally, the study included 196 participants, and only 154 completed the study. Therefore, the results showing no benefit to either EPA or DHA as compared to placebo could be due to attrition bias.
Overall, many of the clinical trials that examine the therapeutic efficacy of omega-3 PUFAs in the management of depression show a benefit of adding fish oil. Also, most of the studies report no negative outcomes of the PUFAs in terms of side effects. The variable results regarding the efficacy are most likely due to the small sample size used in almost all the trials and the high rates of dropout from study participation. Additionally, there is not a consistent set up regarding the use of the PUFAs in terms of them being used as a monotherapy or in conjunction with other antidepressants. Theoretically, conjunction therapy would be the most beneficial, especially because the mechanism of action of PUFAs may be independent of the monoamine system (see below). Even though it is difficult to compare amongst the various studies, it is fairly clear that treatment with some form of fish oil does result in improved clinical outcomes, and since there are additional side effects to the cardiovascular system it would be beneficial to begin depressed patients on a fish oil supplement, especially if they have been resistant to standard therapy.
3. Mechanism of action
There are two main schools of thought when it comes to the mechanism of action the PUFAs utilize to achieve an antidepressant response. The first is that the PUFAs are able to exert an anti-inflammatory response on neural cells, which results in an antidepressant effect. The second school of thought is that the PUFAs cause membrane modification either by a direct interaction with the plasma membrane or via a modification of the G-protein, G_α_ s. There is no reason that these must be mutually exclusive.
While not proven causative, depressed patients and animal models of depression show decreased neurogenesis [3,28], and one of the causes for this may be pro-inflammatory cytokines, such as interleukin-1-β (IL-1_β_) [23,30,31]. Several studies have shown that there are increased levels of IL-1_β_ in the peripheral blood and CSF of depressed individuals [7,27,34,36,41,47,52]. Besides IL-1_β_, other inflammatory markers have also been noted to be involved in the pathogenesis of depression such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [52]. Fish oils are relevant to this discussion because one study demonstrated that EPA and DHA, along with sertraline (SSRI) and venlafaxine (SNRI), were able to reverse the effects of IL-1_β_ at human hippocampal cells in vitro [4]. Similar findings have also been shown in clinical trials using patients treated with IFN-α to combat hepatitis C infections. The patients were given EPA and DHA to prevent IFN-_α_-induced depression, and the results demonstrated that EPA was able to decrease, significantly, the incidence of depression [59]. Another study found that a subset of patients with high inflammation profiles were more responsive to EPA as compared to placebo, lending evidence to the anti-inflammatory mechanism of action [48]. Additionally, one study revealed that DHA inhibits oxidative reactions and pro-inflammatory responses in microglia [33], and this finding was consistent with several previous studies that showed DHA is anti-inflammatory and antioxidative [10,14,29,51]. Therefore, based on these studies, one suggestion for the therapeutic mechanism of fish oil in the treatment of depression is that the PUFAs exert an anti-inflammatory effect on neural tissue.
Another possibility for the mechanism of action of fish oil in the treatment of depression is modification of neurotransmitter signaling. There are two ways by which the PUFAs may be affecting the membrane: 1) direct modification of plasma membrane signaling domains or 2) modification of G-protein within those signaling domains.
Before findings surrounding the mechanism are discussed, a brief background regarding structural aspects of the plasma membrane is warranted. There are specific, highly organized regions of the cell membrane known as lipid rafts, which are rich in cholesterol, sphingolipids, cytoskeletal proteins, and an array of signaling molecules including heterotrimeric G-protein subunits and second messenger molecules [1]. The grouping of G-proteins within lipid rafts results in alterations to neurotransmitter signaling. The most likely mechanism for G-protein localization to lipid rafts is that the G_α_ subunit undergoes fatty acylation (palmitoylation and/or myristoylation), and these modifications effectively target the G-proteins to the lipid raft [1].
Postmortem brain samples showed that G_α_ s localizes to the lipid rafts in depressed subjects who completed suicide [12]. When the G_α_ s subunit is located in the lipid raft, it is unable to form a functional complex with adenylyl cyclase therein, resulting in dampened cAMP signaling [2]. Recent PET imaging data demonstrate diminished cAMP in the brains of depressed subjects, resolving to normal levels following successful treatment [19].
Furthermore, there is evidence that suggests that the lipid raft itself may play a role in antidepressant effectiveness because antidepressant and antipsychotic drugs have been shown to accumulate in lipid rafts [15]. Another study showed that escitalopram accumulates within lipid rafts, but the nonantidepressant R enantiomer does not [16]. Since escitalopram and R-citalopram have equal lipophilicity, there is likely a lipid raft protein that acts as a binding site for antidepressants. This study also revealed that antidepressant accumulation in rafts is a slow process mirroring the time course for the invitro effects of these drugs [9,16,69]. Similarly, published findings analyzing the components of raft and non-raft membrane samples show that DHA is present in both [54]. DHA’s preference to localize into non-raft membrane samples might create a DHA-rich domain capable of altering conformation of both membrane domains and signaling proteins [64]. In such circumstance, PUFAs could affect neurotransmitter signaling and second messengers. One group demonstrated that DHA incorporates into specific regions to avoid cholesterol interactions [55].
In addition, PUFAs may act indirectly at the plasma membrane by modifying G-proteins. As stated above, G-proteins undergo fatty acylation which targets the proteins to the lipid rafts. When fatty acylation is modified, G-protein association with the membrane, as well as the interaction of components within the heterotrimer, is altered. This modifies downstream signaling. Furthermore, PUFA modifies acylation of certain small G proteins, preventing their association with lipid rafts. For example, palmitic acid on the GTPase, Fyn, was replaced by acylation with EPA and/or arachidonic acid. This study also raised the possibility that the PUFAs are affecting the overall lipid raft structure. They noted that caveolin, which is a protein localized to lipid rafts, was not displaced from the rafts in response to the PUFAs. As a result, they concluded that the dislocation of Fyn is due to the direct effect of the PUFAs inhibiting Fyn palmitoylation [65]. Similar studies demonstrated that PUFA treatment resulted in displacement of various proteins (including Lck, LAT, etc) from lipid rafts [56,68]. While this study did not examine heterotrimeric G proteins, a similar effect of PUFA is certainly possible. Furthermore, PUFAs are able to affect fatty acylation of several signaling proteins [8,9,17,20,49].
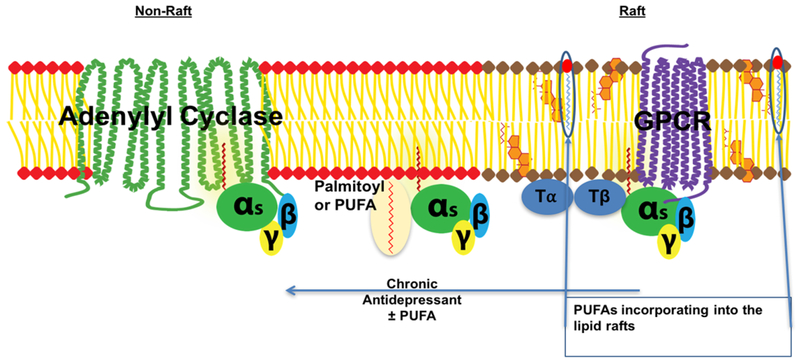
As stated above, antidepressant treatment results in the translocation of G_α_ s out of lipid rafts, and G_α_ s is then able to activate adenylyl cyclase more efficiently resulting in increased levels of cAMP [1,2,13,69]. Treatment with omega-3 fatty acids might cause antidepressant effects due to omega-3 fatty acids association with rafts, modifying raft structure, and/or releasing raft-associated proteins into nonraft membrane sections [53] (Figure 1). One study discovered that PUFA treatment facilitated the coupling between the estrogen GPCR, GPER1, G_α_ s, and adenylyl cyclase [6]. As mentioned above, there are also studies that show EPA and DHA’s ability to target rafts directly [50]. Lastly, it is important to also remember that PUFAs do regulate the palmitoylation of several different proteins, and it is very reasonable to suggest that G_α_ s is included in this group. If the G_α_ s is modified this may facilitate translocation from lipid raft and increased functional complexes with adenylyl cyclase.
Fig. 1.
This scheme depicts the antidepressant-induced translocation of G_α_ s (depicted as the heterotrimer with β and γ subunits) out of lipid rafts to non-raft sections of the membrane. The center of the image demonstrates the possibility of PUFA directly modifying G_α_ s via acylation. Also, PUFA has the ability to directly incorporate into the rafts, depicted image right.
T_α/T_β = tubulin; GPCR = G protein coupled receptor.
4. Conclusion
This review has attempted to analyze the clinical trials that have been conducted with respect to PUFA antidepressant properties and to posit a mechanism for the antidepressant actions of PUFA. The findings of the trials suggest that fish oil supplementation is beneficial in the treatment of depression when compared with placebo, and the data suggest that the best outcomes occur when the fish oil is used as an adjunct to standard antidepressant therapies. It is difficult, however, to compare clinical trials due, to the differences in experimental design and methodology. Additionally, this review has suggested the three major ideas involved in regard to the mechanism of action of the PUFA: 1) anti-inflammatory action 2) direct membrane modification and 3) indirect membrane modification via direct modification of signaling proteins. There are data to support all three of these mechanisms, and we conclude it is likely that interplay exits amongst them. Fortunately, methodology development in neuroscience is progressing at a rapid pace, suggesting that answers may soon be forthcoming.
Acknowledgements
This work was supported by USPHS grant R01AT009169 and VA Merit Award BX001149.
Abbreviations
cAMP
Cyclic adenosine monophosphate
DHA
Docosahexaenoic acid
ECT
Electroconvulsive therapy
EPA
Eicosapentaenoic acid
g_/_day
Grams per day
GPCR
G-protein coupled receptor
GTP
Guanosine triphosphate
HRSD
Hamilton rating scale for depression
IFN-α
Interferon-alpha
IFN-γ
Interferon-gamma
IL-1_β_
Interleukin-1-beta
LAT
Linker for activation of T cells
MAOI
Monoamine oxidase inhibitor
PET
Positron emission tomography
PUFA
Polyunsaturated fatty acids
SNRI
Serotonin norepinephrine reuptake inhibitor
STAR*D
Sequence treated alternatives to relieve depression
TCA
Tricyclic antidepressant
TNF-α
Tumor necrosis factor-alpha
References
- [1].Allen JA, Halverson-Tamboli RA and Rasenick MM, Lipid raft microdomains and neurotransmitter signalling, Nat Rev Neurosci 8 (2007), 128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- [2].Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL and Rasenick MM, Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling, Mol Pharmacol 76 (2009), 1082–1093. doi: 10.1124/mol.109.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, Mann JJ and Arango V, Antidepressants increase neural progenitor cells in the human hippocampus, Neuropsychopharmacology 34 (2009), 2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borsini A, Alboni S, Horowitz MA, Tojo LM, Cannazza G, Su KP, Pariante CM and Zunszain PA, Rescue of IL-1_β_-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants, Brain Behav Immun 65 (2017), 230–238. doi: 10.1016/j.bbi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brinson BE and Miller S, Fish oil: What is the role in cardiovascular health?, J Pharm Pract 25 (2012), 69–74. doi: 10.1177/0897190011406983. [DOI] [PubMed] [Google Scholar]
- [6].Cao W, Ma Z, Rasenick MM, Yeh S and Yu J, N-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth, PLoS One 7 (2012), e52838. doi: 10.1371/journal.pone.0052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Capuron L and Miller AH, Cytokines and psychopathology: Lessons from interferon-alpha, Biol Psychiatry 56 (2004), 819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [8].Chini B and Parenti M, G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there?, J Mol Endocrinol 32 (2004), 325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- [9].Czysz AH and Rasenick MM, G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids, CNS Neurol Disord Drug Targets 12 (2013), 466–473. doi: 10.2174/1871527311312040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S and Layé S, Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization, J Neurochem 105 (2008), 296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- [11].DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O’Reardon JP, Lovett ML, Gladis MM, Brown LL and Gallop R, Cognitive therapy vs medications in the treatment of moderate to severe depression, Arch Gen Psychiatry 62 (2005), 409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- [12].Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN and Rasenick MM, Postmortem brain tissue of depressed suicides reveals increased Gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase, J Neurosci 28 (2008), 3042–3050. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Donati RJ and Rasenick MM, Chronic antidepressant treatment prevents accumulation of gsalpha in cholesterolrich, cytoskeletal-associated, plasma membrane domains (lipid rafts), Neuropsychopharmacology 30 (2005), 1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- [14].Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BH and Langmann T, Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration, J Neurochem 110 (2009), 1863–1875. doi: 10.1111/j.1471-4159.2009.06286.x. [DOI] [PubMed] [Google Scholar]
- [15].Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, Lambert JJ, Zieglgänsberger W, Holsboer F and Rupprecht R, Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains, J Neurosci 25 (2005), 10198–10206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erb SJ, Schappi JM and Rasenick MM, Antidepressants Accumulate in Lipid Rafts Independent of Monoamine Transporters to Modulate Redistribution of the G Protein, G_α_s, J Biol Chem 291 (2016), 19725–19733. doi: 10.1074/jbc.M116.727263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Escribá PV, Wedegaertner PB, Goñi FM and Vögler O, Lipid-protein interactions in GPCR-associated signaling, Biochim Biophys Acta 1768 (2007), 836–852. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [18].Frazer A and Benmansour S, Delayed pharmacological effects of antidepressants, Mol Psychiatry 7(Suppl. 1) (2002), S23–S28. doi: 10.1038/sj.mp.4001015. [DOI] [PubMed] [Google Scholar]
- [19].Fujita M, Richards EM, Niciu MJ, Ionescu DF, Zoghbi SS, Hong J, Telu S, Hines CS, Pike VW, Zarate CA and Innis RB, cAMP signaling in brain is decreased in unmedicated depressed patients and increased by treatment with a selective serotonin reuptake inhibitor, Mol Psychiatry 22 (2017), 754–759. doi: 10.1038/mp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fukata Y and Fukata M, Protein palmitoylation in neuronal development and synaptic plasticity, Nat Rev Neurosci 11 (2010), 161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- [21].Gertsik L, Poland RE, Bresee C and Rapaport MH, Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder, J Clin Psychopharmacol 32 (2012), 61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Glenn JO and Wischmeyer PE, Enteral fish oil in critical illness: Perspectives and systematic review, Curr Opin Clin Nutr Metab Care 17 (2014), 116–123. doi: 10.1097/MCO.0000000000000039. [DOI] [PubMed] [Google Scholar]
- [23].Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T and Yirmiya R, Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression, Mol Psychiatry 13 (2008), 717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- [24].Gray P, Chappell A, Jenkinson AM, Thies F and Gray SR, Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise, Int J Sport Nutr Exerc Metab 24 (2014), 206–214. doi: 10.1123/ijsnem.2013-0081. [DOI] [PubMed] [Google Scholar]
- [25].Greenberg PE, Fournier AA, Sisitsky T, Pike CT and Kessler RC, The economic burden of adults with major depressive disorder in the United States (2005 and 2010), J Clin Psychiatry 76 (2005), 155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- [26].Hirsch M and Birnbaum RJ, Selective serotonin reuptake inhibitors: Pharmacology, administration, and side effects, in: UpToDate, UpToDate, Roy-Byrne PP and Solomon D, eds, 2017. [Google Scholar]
- [27].Howren MB, Lamkin DM and Suls J, Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis, Psychosom Med 71 (2009), 171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- [28].Kempermann G, Krebs J and Fabel K, The contribution of failing adult hippocampal neurogenesis to psychiatric disorders, Curr Opin Psychiatry 21 (2008), 290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- [29].Komatsu W, Ishihara K, Murata M, Saito H and Shinohara K, Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress, Free Radic Biol Med 34 (2003), 1006–1016. doi: 10.1016/S0891-5849(03)00027-3. [DOI] [PubMed] [Google Scholar]
- [30].Koo JW and Duman RS, IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress, Proc Natl Acad Sci USA 105 (2008), 751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T and Suzuki T, Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice, Synapse 64 (2010), 721–728. [DOI] [PubMed] [Google Scholar]
- [32].Lin PY and Su KP, A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids, J Clin Psychiatry 68 (2007), 1056–1061. doi: 10.4088/JCP.v68n0712. [DOI] [PubMed] [Google Scholar]
- [33].Lu DY, Tsao YY, Leung YM and Su KP, Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for _ω_−3 fatty acids, Neuropsychopharmacology 35 (2010), 2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B and Maj M, The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression, Metab Brain Dis 24 (2009), 27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- [35].Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF and Puryear LJ, A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression, Am J Psychiatry 160 (2003), 996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- [36].Miller AH, Maletic V and Raison CL, Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression, Biol Psychiatry 65 (2009), 732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mischoulon D, The impact of omega-3 fatty acids on depressive disorders and suicidality: Can we reconcile 2 studies with seemingly contradictory results?, J Clin Psychiatry 72 (2011), 1574–1576. doi: 10.4088/JCP.11com07463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, Papakostas GI, Dording CM, Sonawalla SB, Nierenberg AA, Alpert JE and Fava M, A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder, Eur Neuropsychopharmacol 18 (2008), 639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- [39].Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA and Hyman Rapaport M, A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression, J Clin Psychiatry 76 (2015), 54–61. doi: 10.4088/JCP.14m08986. [DOI] [PubMed] [Google Scholar]
- [40].Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, Smith J, Beaumont EC, Dahan LE, Alpert JE, Nierenberg AA and Fava M, A double-blind, randomized controlled trial of ethyleicosapentaenoate for major depressive disorder, J Clin Psychiatry 70 (2009), 1636–1644. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, Fallgatter AJ and Riederer P, Consensus paper of the WFSBP task force on biological markers: Biological markers in depression, World J Biol Psychiatry 8 (2007), 141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- [42].National Center for Health Statistics: Depression, Centers for Disease Control and Prevention, 2016, https://www.cdc.gov/nchs/fastats/depression.htm.
- [43].Nemets B, Stahl Z and Belmaker RH, Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder, Am J Psychiatry 159 (2002), 477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- [44].Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF and Fava M, Timing of onset of antidepressant response with fluoxetine treatment, Am J Psychiatry 157 (2000), 1423–1428. doi: 10.1176/appi.ajp.157.9.1423. [DOI] [PubMed] [Google Scholar]
- [45].Peanpadungrat P, Efficacy and safety of fish oil in treatment of knee osteoarthritis, J Med Assoc Thai 98(Suppl. 3) (2015), S110–S114. [PubMed] [Google Scholar]
- [46].Peet M and Horrobin DF, A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs, Arch Gen Psychiatry 59 (2002), 913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- [47].Raison CL, Capuron L and Miller AH, Cytokines sing the blues: Inflammation and the pathogenesis of depression, Trends Immunol 27 (2006), 24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R and Mischoulon D, Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study, Mol Psychiatry 21 (2016), 71–79. doi: 10.1038/mp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, Richter DW, Jahn R and Ponimaskin E, Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling, Mol Pharmacol 72 (2007), 502–513. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- [50].Rockett BD, Franklin A, Harris M, Teague H, Rockett A and Shaikh SR, Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells, J Nutr 141 (2011), 1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Saw CL, Huang Y and Kong AN, Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid, Biochem Pharmacol 79 (2010), 421–430. doi: 10.1016/j.bcp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- [52].Schiepers OJ, Wichers MC and Maes M, Cytokines and major depression, Prog Neuropsychopharmacol Biol Psychiatry 29 (2005), 201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [53].Schley PD, Brindley DN and Field CJ, (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells, J Nutr 137 (2007), 548–553. [DOI] [PubMed] [Google Scholar]
- [54].Shaikh SR, Kinnun JJ, Leng X, Williams JA and Wassall SR, How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems, Biochim Biophys Acta 1848 (2015), 211–219. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- [55].Shaikh SR, Rockett BD, Salameh M and Carraway K, Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells, J Nutr 139 (2009), 1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- [56].Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinger H and Waldhäusl W, Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains, J Cell Biol 143 (1998), 637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Su KP, Huang SY, Chiu CC and Shen WW, Omega-3 fatty acids in major depressive disorder. A preliminary doubleblind, placebo-controlled trial, Eur Neuropsychopharmacol 13 (2003), 267–271. doi: 10.1016/S0924-977X(03)00032-4. [DOI] [PubMed] [Google Scholar]
- [58].Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC and Pariante CM, Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial, J Clin Psychiatry 69 (2008), 644–651. doi: 10.4088/JCP.v69n0418. [DOI] [PubMed] [Google Scholar]
- [59].Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, Chang HC and Pariante CM, Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial, Biol Psychiatry 76 (2014), 559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- [60].Su KP, Yang HT, Chang JP, Shih YH, Guu TW, Kumaran SS, Gałecki P, Walczewska A and Pariante CM, Eicosapentaenoic and docosahexaenoic acids have different effects on peripheral phospholipase A2 gene expressions in acute depressed patients, Prog Neuropsychopharmacol Biol Psychiatry 80 (2018), 227–233. doi: 10.1016/j.pnpbp.2017.06.020. [DOI] [PubMed] [Google Scholar]
- [61].Taylor MJ, Freemantle N, Geddes JR and Bhagwagar Z, Early onset of selective serotonin reuptake inhibitor antidepressant action: Systematic review and meta-analysis, Arch Gen Psychiatry 63 (2006), 1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M and Team SDS, Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice, Am J Psychiatry 163 (2006), 28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- [63].U.S. Department of Health and Human Services, National Institute of Mental Health, Major Depression Among Adults, https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml.
- [64].Wassall SR and Stillwell W, Docosahexaenoic acid domains: The ultimate non-raft membrane domain, Chem Phys Lipids 153 (2008), 57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- [65].Webb Y, Hermida-Matsumoto L and Resh MD, Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids, J Biol Chem 275 (2000), 261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- [66].World Health Organization, The World Health Report: 2001: Mental Health: New Understanding, New Hope, WHO, Geneva, 2001. [Google Scholar]
- [67].World Health Organization, “Depression: Let’s talk” says WHO, as depression tops list of causes of ill health, 2017. [Google Scholar]
- [68].Zeyda M, Staffler G, Horejsi V, Waldhausl W and Stulnig TM, LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids, J Biol Chem 277 (2002), 28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- [69].Zhang L and Rasenick MM, Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: A 5-hydroxytryptamine transporter-independent action of this antidepressant compound, J Pharmacol Exp Ther 332 (2010), 977–984. doi: 10.1124/jpet.109.162644. [DOI] [PMC free article] [PubMed] [Google Scholar]