Aloe vera Is Effective and Safe in Short-term Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-analysis (original) (raw)
Abstract
Background/Aims
To evaluate the efficacy and safety of Aloe vera (AV) in patients with irritable bowel syndrome (IBS).
Methods
We searched the MEDLINE, EMBASE, and Cochrane databases for studies dated between 1st January 1960 and 30th December 2017. Eligible randomized controlled trials (RCTs) compared AV to placebo in patients with IBS. The primary outcome was standardized mean difference of the change in severity of IBS symptoms as measured by patient-rated scales. Secondary outcomes included response rate of IBS symptoms and adverse events. Heterogeneity among studies was assessed using Cochrane’s Q and _I_2 statistics.
Results
Three RCTs with a total of 151 patients with IBS were included. The meta-analysis showed a significant difference for patients with AV compared to those with placebo regarding improvement in IBS symptom score (standardized mean difference, 0.41; 95% CI, 0.07–0.75; P = 0.020). Using intention-to-treat analysis, the AV patients showed significantly better response rates of IBS symptoms compared to placebo (pooled risk ratio, 1.69; 95% CI, 1.05–2.73; P = 0.030). No adverse events related with AV were found in included studies. There was no significant heterogeneity of effects across studies (P = 0.900; _I_2 = 0%).
Conclusion
AV is effective and safe for the treatment of patients with IBS compared to placebo.
Keywords: Aloe, Irritable bowel syndrome, Meta-analysis, Review
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder that is diagnosed based on symptoms such as bowel habits change and abdominal discomfort without any organic cause. Multiple factors, including altered brain-gut interactions, visceral hypersensitivity, gut dysbiosis, increased intestinal permeability, and psychosocial factors contribute to the pathogenesis of IBS.1 In general, managing symptoms for patients with IBS involves life style modification, diet control, behavioral therapy, and medication.2 Complementary or alternative therapies can be considered for patients with IBS who have not responded fully to conventional therapies, although the efficacy of the treatment of IBS is still unclear in most cases.3
Aloe vera (AV) is an herbal medication used as a remedy for various diseases in traditional medicine. It has been shown to have hepato-protective, anti-inflammatory, and anti-ulcerative benefits.4 In particular, AV is commonly used as a strong laxative and as a substance to improve gastrointestinal motility.5 However, the efficacy of AV has not been fully demonstrated in the patients with IBS despite several randomized controlled trials (RCTs).6–8 The aim of this meta-analysis was to evaluate the efficacy and safety of AV in patients with IBS compared to placebo.
Materials and Methods
Search Strategy and Study Selection
This review and meta-analysis was based on the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRIS-MA) report guidelines.9 From 1st January 1960 to 30th December 2017, we conducted a search of the PubMed, EMBASE and Cochrane library databases for studies. The following search terms were used: (“irritable bowel syndrome” OR “IBS” OR “irritable colon”) and (“aloe vera” OR “aloe”). Two independent reviewers (S.W.H. and J.C.)searched and selected the articles. The studies that met all of the following criteria were included: (1) patient: adult patients with IBS; (2) intervention: AV group; (3) comparison: placebo group; (4) outcome: change of IBS symptoms and/or safety related to AV; and (5) study design: a prospective comparative study. All eligible studies compared AV to placebo in patients with IBS. Non-comparative studies, case reports, review articles, duplicated studies, abstracts, and pre-clinical studies were excluded from the meta-analysis.
Data Extraction and Quality Assessment
For eligible studies, data were independently extracted by 2 independent reviewers (S.W.H. and J.C.), and disagreements between the researchers were resolved through consensus. The following data were extracted for each study: author’s name; year of publication; regimen, formulation, and treatment period of AV; numbers of total patients and patients who responded; changes in IBS symptoms scored between before and after the treatment; and number of adverse events. The data were extracted based on the assessment point of time presented in the each study. If the data were incomplete or unclear, we contacted the author for further information. For assessment of the quality of the RCTs, we used the risk of bias tool developed by the Cochrane Group.10
Study Outcomes
Primary outcome was standardized mean difference (SMD) of the change in severity of IBS symptoms as measured by patient-rated scales. Secondary outcomes were the response rate of IBS symptoms and adverse events.
Statistical Methods
This meta-analysis was based on the Cochrane handbook for systematic review.11 Dichotomous outcomes were calculated with risk ratio (RR) and 95% confidence interval (CI). Continuous outcomes with different units of measurement were analyzed with SMD and 95% CI. Intention-to-treat analysis included the study outcomes occurring during RCTs, regardless of whether the patients were taking the treatment to which they had been randomized. Per-protocol analysis included all outcomes from the patients who successfully adhered to the protocol during the trials. Subgroup analysis was also performed to evaluate the effect of AV according to the treatment period (1 month vs over 3 months). Heterogeneity among the studies was estimated using Cochrane’s Q test and _I_2 statistics. A random-effects model was applied using the Mantel-Haenszel and inverse-variance methods for binary and continuous outcomes, respectively. If substantial heterogeneity was identified, the possible clinical causes were assessed. Publication bias was estimated by analyzing the asymmetry of a funnel plot, which was not applicable if fewer than 10 studies were included. Statistical analyzes were performed using Review Manager (RevMan) version 5.3. A _P_-value < 0.05 was considered to be statistically significant.
Results
Results of Search
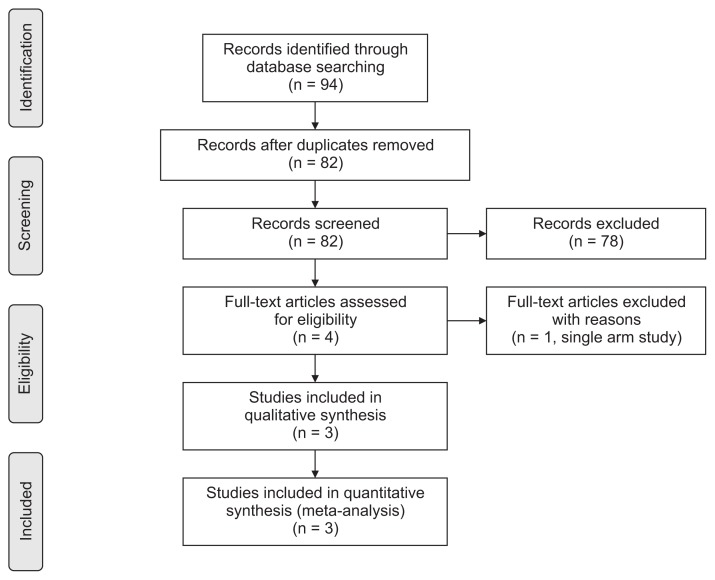
A total of 94 articles were identified through a combination of search terms. Figure 1 shows the flowchart of the study selection strategy. After removing 12 duplicates, 78 studies were excluded in the screening process based on titles and abstracts. Finally, 3 prospective RCTs were included in the meta-analysis after excluding a single-arm study.6–8 Because the numeric outcomes could not be extracted from 1 study included in this meta-analysis, we collected additional unpublished data from the corresponding author.7
Figure 1.
Flow chart of the study selection.
Characteristics of Included Studies
This meta-analysis included 3 prospective RCTs from 151 study participants (76 in the AV group and 75 in the placebo group). Among them, 2 studies6,8 were conducted via a parallel design, and the other7 was a cross-over study. The characteristics and major outcomes of the included studies are summarized in Table.
Table.
Characteristics and Major Outcomes of the Included Studies
| Author | Year | Design | Region | Arms | No. of patients | Age (mean ± SD) | Regimen | Diagnostic criteria | Assessment point of time | Adverse event | Outcomes | Type of outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Davis | 2006 | Parallel/RCT | UK | Aloe vera | 31 | NR | 50 mL × 4 times/day per os | Rome II | 1 and 3 months | None | Response ratea | Dic |
| Placebo | 27 | NR | Mean change in IBS score | Con | ||||||||
| Hutchings | 2011 | Cross-over/RCT | UK | Aloe vera/Placebo | 55 | 46.0 ± 13.6 | 60 mL × 2 times/day per os | Rome II | 5 months | NR | GSRSEQ5D | ConCon |
| Placebo/Aloe vera | 55 | 47.0 ± 13.7 | SF-12IBSQOL | ConCon | ||||||||
| Størsrud | 2015 | Parallel/RCT | Sweden | Aloe vera extract (AVH200) | 33 | 43.9 ± 13.3 | 1tab × 2 times/day per os | Rome III | 4 weeks | None | Response rateb | Dic |
| Placebo | 35 | 44.2 ± 14.5 | IBS-SSSHAD | ConCon |
Quality Assessment
The risk of bias for each RCT is shown in Figure 2. The method of randomization and allocation concealment was clearly described in these studies,6,8 but the other7 was not. In addition, Hutchings et al7 reported incomplete outcomes and showed a high drop-out rate. The relatively short washout period may have created a potential risk of carry-over effect in this study.
Figure 2.

Evaluation of risk of bias. Green for low risk of bias, yellow for unclear risk of bias and red for high risk of bias. This figure indicates the risk of bias of each domain in each study.
Mean Differences in Irritable Bowel Syndrome Symptoms Scores
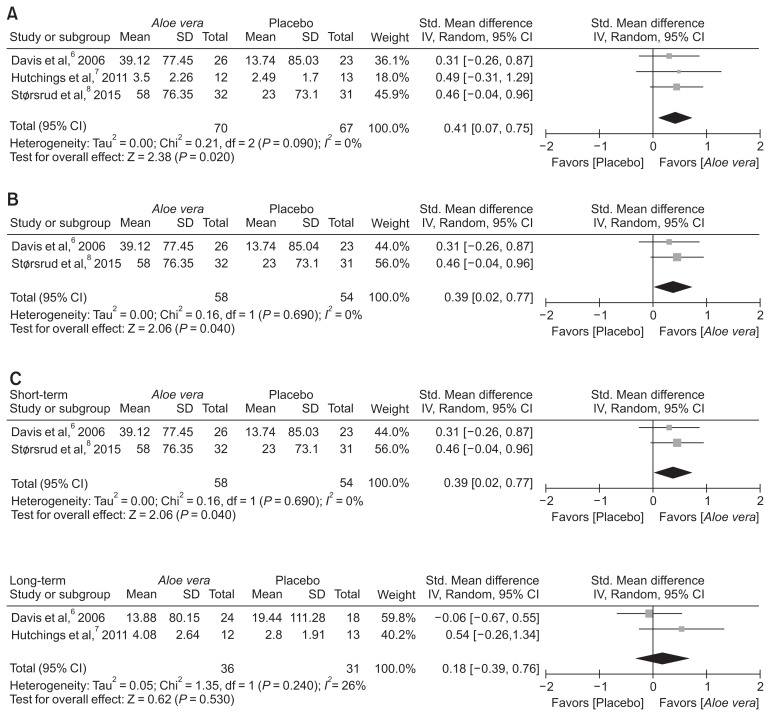
Data from the IBS symptom scores before and after treatments were retrieved on different scales from the included RCTs. As in the study by Hutching et al,7 unreported data were included in this meta-analysis. Moreover, data associated with the second treatment period were excluded from this meta-analysis because of concerns about the carry-over effect. The study by Hutching et al7 showed a high drop-out rate, which made the result of the analysis unreliable. For this reason, intention-to-treat analysis was not performed. Meta-analysis showed a significantly greater difference for AV compared to placebo in terms of improvement of IBS symptom score (SMD, 0.41; 95% CI, 0.07–0.75; P = 0.020) (Fig. 3A). There was no significant heterogeneity of effect among these included studies (P = 0.900; _I_2 = 0%). Except for the study by Hutching et al,7 AV also showed a significant improvement of IBS symptoms score compared to placebo (SMD, 0.39; 95% CI, 0.02–0.77; P = 0.040) (Fig. 3B).
Figure 3.
Standardized mean differences in improvement of irritable bowel syndrome symptom scores. Comparison of mean differences in improvement of irritable bowel syndrome scores between Aloe vera and placebo groups, (A) the forest plots for all included studies, (B) high quality studies, and (C) subgroup analysis according to the treatment period: short-term (1 month) vs long-term (over 3 months) period. IV, inverse variance.
Subgroup analysis was conducted to identify the efficacy of AV according to the treatment period (1 month vs over 3 months). In a short-term treatment for 1 month, AV showed a significantly favorable effect on improvement of IBS symptom score (SMD, 0.39; 95% CI, 0.03–0.77; P = 0.040), but not in a long-term treatment over 3 months (SMD, 0.18; 95% CI, −0.39–0.76; P = 0.530) compared to placebo (Fig. 3C).
Response Rates
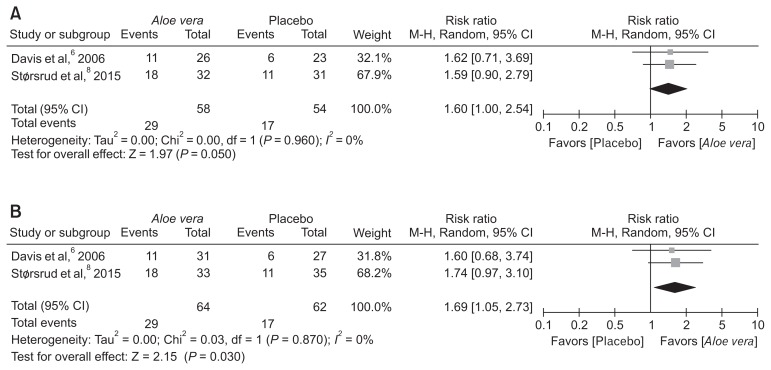
Two of the 3 included RCTs reported response rates, both intention-to-treat and per-protocol analysis were applied. In this meta-analysis, there was a higher response rate in the AV group compared to the placebo group (per-protocol analysis; pooled RR, 1.60; 95% CI, 1.00–2.54; P = 0.050) (Fig. 4A), (intention-to-treat analysis; pooped RR, 1.69; 95% CI, 1.05–2.73; P = 0.030) (Fig. 4B). There was no heterogeneity among included studies (P = 0.960; _I_2 = 0%).
Figure 4.
Response rates. Comparison of response rates between Aloe vera and placebo groups using (A) per-protocol and (B) intention-to-treat analysis. M-H, Mantel-Haenszel.
Adverse Events
No significant difference in number of adverse events between the AV and placebo groups was reported in the studies. The follow-up duration ranged from 1 to 5 months (Table).
Discussion
In this meta-analysis of 3 prospective RCTs regarding the efficacy of AV for the treatment of IBS, AV showed a significant improvement in IBS symptom scores and response rates compared to placebo. There was no significant heterogeneity among these RCTs. All of the included studies showed a favorable tendency toward the AV group in spite of no statistical significance due to the small number of enrolled patients in each study. This meta-analysis is the first to demonstrate the therapeutic efficacy of AV for controlling symptoms in patients with IBS.
AV is a plant belonging to the family Liliaceae and is commonly used to treat and prevent various diseases in traditional medicine.12 Barbaloin, one of the major components in AV, plays a critical role as a laxative.13,14 It is metabolized to aloe-emodine-9-anthrone (AE-anthrone) by intestinal bacteria.15 AE-anthrone enhances intestinal motility and increases paracellular permeability by inhibiting Na+/ K+-adenosine triphosphatase at the colonic mucosa.14 In addition, it affects the release of prostaglandin-like materials and stimulates mucus secretions in the colon.16 As a result, AV can increase the amount of water in the intestinal lumen and has a laxative effect in humans.14 Therefore, AV may be a potential therapeutic agent in patients with constipation-predominant IBS or functional constipation.
The number of mast cells in the colonic tissue in patients with IBS is significantly higher compared to healthy controls.17 In addition, pro-inflammatory cytokine levels in peripheral blood increased in a subset of patients with IBS.18 These findings suggest that a low-grade inflammatory response in the colonic mucosa results in increased intestinal permeability and symptoms in patients with IBS. Previous studies reported that AV has an anti-inflammatory action on various medical conditions, such as a skin wounds, peptic ulcers, and colitis.19 AV administration healed a gastric ulcer by reducing leukocyte adherence in post-capillary venules and serum TNF-alpha level, and elevating serum interleukin-10 level in rats.4 In a prospective RCT, administration of AV reduced the clinical and histological disease activity in patients with ulcerative colitis.20 Thus, the anti-inflammatory action of AV may be a possible mechanism of action for the treatment of IBS.
In subgroup analysis according to the treatment period, AV treatment showed a favorable efficacy for the improvement of IBS symptom scores at 1 month, but not over 3 months, compared to placebo. The incremental SMD in the improvement of IBS symptom scores by AV against placebo was 0.39 and 0.18 at 1 and over 3 months, respectively. It suggests the possibility of AV tachyphylaxis in the treatment of IBS. However, the possible mechanism of the progressive decrease in therapeutic response to AV in patients with IBS remains unclear. In the study by Davis et al,6 the dropout rate of placebo group at 3 months was higher compared to the AV group (33.3% vs 22.6%). The relatively high drop-out rate of placebo group may result from the lack of efficacy, and the selection bias may underestimate the long-term efficacy of AV in patients with IBS in the study. Thus, further prospective RCTs are needed to determine the therapeutic efficacy of AV on the long-term treatment of IBS.
In this meta-analysis, serious adverse events were not reported. However, the long-term safety or rare adverse events of AV could not be demonstrated in this meta-analysis from the 3 prospective RCTs of 151 study participants conducted within 5 months. AV was classified as a group 2B material by the International Agency for Research on Cancer, suggesting that AV is possibly carcinogenic to humans, because there is sufficient evidence in experimental animals for the carcinogenicity of whole leaf extract of AV.21 In a 2-year study, oral administration of AV significantly increased the incidence of colorectal adenoma and carcinoma compared to placebo in male and female rats.22 Moreover, Modi and Hussan23 reported a case of melanosis coli after the long-term oral administration of AV. To date, there is insufficient evidence for the carcinogenicity and long-term safety of AV in humans, especially patients with IBS who require long-term or repeated treatments due to the chronic, relapsing nature of the disease. Thus, a long-term prospective RCT is needed to determine the long-term safety as well as efficacy of AV in patients with IBS.
There are several limitations in this study. First, only 3 prospective RCTs were included in the meta-analysis. In addition, the randomization and allocation concealment method could not be found in the study by Hutching et al,7 suggesting that it had a high possibility of bias in the study design. Second, the therapeutic efficacy of AV in patients with IBS could not be evaluated according to the subtype of IBS because only the study by Davis et al6 performed a subgroup analysis according the IBS subtypes among these studies included in the meta-analysis. In the study, AV showed an improvement of the symptom scores for patients with diarrhea-predominant or mixed type IBS, especially in terms of the sub-scores of pain and bowel habit satisfaction. Recently, probiotics originated from AV leaf such as Lactobacillus brevis strains selectively inhibited the growth of harmful enteropathogens in the gut.12 In a placebo-controlled trial, the frequencies of watery feces and abdominal pain in patients with IBS were significantly reduced by L. brevis KB290.24 These findings suggest that the efficacy of AV on diarrhea-predominant or mixed type IBS may be explained by the probiotic effects of AV. Further studies are needed to evaluate the therapeutic efficacy and potential action mechanism of AV in patients with IBS based on the subtype.
In conclusion, AV was effective for the treatment IBS compared to placebo in this meta-analysis of RCTs. The short-term use of AV may be safe in patients with IBS.
Acknowledgements
The authors sincerely thank Myoung-jin Jang, the Medical Research Collaborating Center, and Seoul National University Hospital for their contributions to this article.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Seung Wook Hong, Jaeyoung Chun, and Sunmin Park contributed to the literature search, study selection and data extraction; Seung Wook Hong performed statistical analysis and wrote the manuscript; Jaeyoung Chun revised the manuscript; and Sunmin Park, Hyun Jung Lee, Jong Pil Im, and Joo Sung Kim contributed to study design and revised the manuscript.
References
- 1.Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20:12144–12160. doi: 10.3748/wjg.v20.i34.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 3.Hussain Z, Quigley EM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:465–471. doi: 10.1111/j.1365-2036.2006.02776.x. [DOI] [PubMed] [Google Scholar]
- 4.Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12:2034–2039. doi: 10.3748/wjg.v12.i13.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werawatganon D, Rakananurak N, Sallapant S, et al. Aloe vera attenuated gastric injury on indomethacin-induced gastropathy in rats. World J Gastroenterol. 2014;20:18330–18337. doi: 10.3748/wjg.v20.i48.18330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis K, Philpott S, Kumar D, Mendall M. Randomised double-blind placebo-controlled trial of Aloe vera for irritable bowel syndrome. Int J Clin Pract. 2006;60:1080–1086. doi: 10.1111/j.1742-1241.2006.00980.x. [DOI] [PubMed] [Google Scholar]
- 7.Hutchings HA, Wareham K, Baxter JN, et al. A randomised, crossover, placebo-controlled study of Aloe vera in patients with irritable bowel syndrome: effects on patient quality of life. ISRN Gastroenterol. 2011;2011 doi: 10.5402/2011/206103. 206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Størsrud S, Pontén I, Simrén M. A pilot study of the effect of aloe barbadensis mill. extract (AVH200(R)) in patients with irritable bowel syndrome: a randomized, double-blind, placebo-controlled study. J Gastrointestin Liver Dis. 2015;24:275–280. doi: 10.15403/jgld.2014.1121.243.sst. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011) The Cochrane Collaboraton; 2011. [Google Scholar]
- 12.Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J Tradit Complement Med. 2015;5:21–26. doi: 10.1016/j.jtcme.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelton RM. Aloe vera. Its chemical and therapeutic properties. Int J Dermatol. 1991;30:679–683. doi: 10.1111/j.1365-4362.1991.tb02607.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishii Y, Tanizawa H, Takino Y. Studies of aloe. V. Mechanism of cathartic effect. (4) Biol Pharm Bull. 1994;17:651–653. doi: 10.1248/bpb.17.651. [DOI] [PubMed] [Google Scholar]
- 15.Che QM, Akao T, Hattori M, Kobashi K, Namba T. Isolation of a human intestinal bacterium capable of transforming barbaloin to aloeemodin anthrone. Planta Med. 1991;57:15–19. doi: 10.1055/s-2006-960007. [DOI] [PubMed] [Google Scholar]
- 16.Capasso F, Mascolo N, Autore G, Duraccio MR. Effect of indomethacin on aloin and 1,8 dioxianthraquinone-induced production of prostaglandins in rat isolated colon. Prostaglandins. 1983;26:557–562. doi: 10.1016/0090-6980(83)90193-4. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 18.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Akaberi M, Sobhani Z, Javadi B, Sahebkar A, Emami SA. Therapeutic effects of Aloe spp. in traditional and modern medicine: a review. Biomed Pharmacother. 2016;84:759–772. doi: 10.1016/j.biopha.2016.09.096. [DOI] [PubMed] [Google Scholar]
- 20.Langmead L, Feakins RM, Goldthorpe S, et al. Randomized, double-blind, placebo-controlled trial of oral Aloe vera gel for active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:739–747. doi: 10.1111/j.1365-2036.2004.01902.x. [DOI] [PubMed] [Google Scholar]
- 21.IARC. Aloe vera. (IAC Monographs).Some drugs and herbal products. 2016;108:37–71. [PMC free article] [PubMed] [Google Scholar]
- 22.Yokohira M, Matsuda Y, Suzuki S, et al. Equivocal colonic carcinogenicity of Aloe arborescens Miller var. natalensis berger at high-dose level in a Wistar Hannover rat 2-y study. J Food Sci. 2009;74:T24–T30. doi: 10.1111/j.1750-3841.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 23.Modi RM, Hussan H. Melanosis coli after long-term ingestion of cape aloe. ACG Case Rep J. 2016;3:e157. doi: 10.14309/crj.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami K, Habukawa C, Nobuta Y, Moriguchi N, Takemura T. The effect of Lactobacillus brevis KB290 against irritable bowel syndrome: a placebo-controlled double-blind crossover trial. Biopsychosoc Med. 2012;6:16. doi: 10.1186/1751-0759-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]