Diagnosis of Hodgkin Lymphoma in the Modern Era (original) (raw)
. Author manuscript; available in PMC: 2020 Jan 1.
Published in final edited form as: Br J Haematol. 2018 Nov 8;184(1):45–59. doi: 10.1111/bjh.15614
Summary
The Hodgkin lymphomas are a family of unique lymphoma subtypes, in which the nature of the neoplastic cell was enigmatic for many years. Much of the mystery has been solved, with all forms now considered to be of B-cell origin, in most cases of germinal centre derivation. Today we recognize Hodgkin lymphoma as an eponym that encompasses multiple entities. One of the unifying themes is the major contribution from the tumour microenvironment. Both the character of the neoplastic cells and the nature of the immune environment are critical to accurate diagnosis. Moreover, an understanding of the molecular alterations that characterize both the neoplastic cells and their microenvironment have led to therapeutic advances, targeting both neoplastic and reactive components. Other conditions may foster a similar inflammatory milieu and lead to lymphoproliferations that mimic the Hodgkin lymphomas. In this review we provide an update on the diagnostic features of the various subtypes and include additional information relevant for prognostic evaluation and investigation of potential therapeutic targets. Additionally, we also discuss those conditions that often cause confusion in diagnosis and need to be distinguished from the Hodgkin lymphomas.
Keywords: Classical Hodgkin lymphoma, Nodular lymphocyte predominant Hodgkin lymphoma, T-cell/ histiocyte-rich large B-cell lymphoma, grey zone lymphoma, Epstein Barr virus
Introduction
The term “Hodgkin’s disease”, now renamed as Hodgkin lymphoma, was first coined by Samuel Wilks, in recognition of the earlier report by Thomas Hodgkin from the Guy’s hospital in London (Hodgkin 1832). The name Hodgkin’s disease, popular for many years, reflected uncertainty regarding the cellular lineage of the Hodgkin/ Reed-Sternberg (HRS) cells and questions regarding their reactive or neoplastic nature. Today we accept Hodgkin lymphoma as a neoplasm derived from B cells, in which the unique cellular microenvironment plays an important role in accurate diagnosis and pathobiology. The diagnosis of Hodgkin lymphoma in the modern era relies on an appropriate clinical setting, and morphological and immunophenotypic assessment. In addition, advances in therapy have led to a greater appreciation of the importance of the microenvironment, and the identification of new therapeutic targets. We now view Hodgkin lymphoma as more than a single disease. The unique features of nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) have been recognized (Mason_, et al_ 1994). Moreover, nodular sclerosis classical Hodgkin lymphoma (NSCHL) exhibits major differences from mixed cellularity (MC) and lymphocyte depleted (LD) CHL, with the suggestion that they are separate entities as well. This review also will touch on diagnostic pitfalls, and conditions that mimic Hodgkin lymphoma.
Nodular lymphocyte predominant Hodgkin lymphoma
Clinical Features
NLPHL is relatively uncommon (5–10% of all Hodgkin lymphomas) and shows unique clinicopathological features compared to CHL. It has a peak incidence in the 4th decade, but also affects children. There is a male preponderance of 3:1. Most patients present with low stage disease (Stage I or II) and have a good prognosis. The most common clinical presentation is long standing isolated lymphadenopathy without systemic symptoms. NLPHL affects peripheral lymph node groups with general sparing of the mediastinum and axial lymph nodes. Mesenteric lymph node involvement can be seen but is very rare. Conversely, advanced stage disease has an aggressive clinical course, with a poor response to traditional CHL regimens; newer data point towards the efficacy of treatment regimens used for aggressive B-cell non-Hodgkin lymphomas (Fanale_, et al_ 2017, Xing_, et al_ 2014). The histological features and clinical presentation of advanced stage disease overlap with T-cell/ histiocyte-rich large B-cell lymphoma (THRLBCL), suggesting that they may represent a biological continuum (Hartmann_, et al_ 2013a).
Progressive transformation of germinal centres (PTGC) can occur in the same lymph node site as NLPHL, or may be found in uninvolved lymph nodes prior to a diagnosis of NLPHL, or following treatment (Ferry_, et al_ 1992). However, neither a definitive link between PTGC and NLPHL nor an elevated risk of progression to NLPHL has been identified.
Histology and immunophenotype
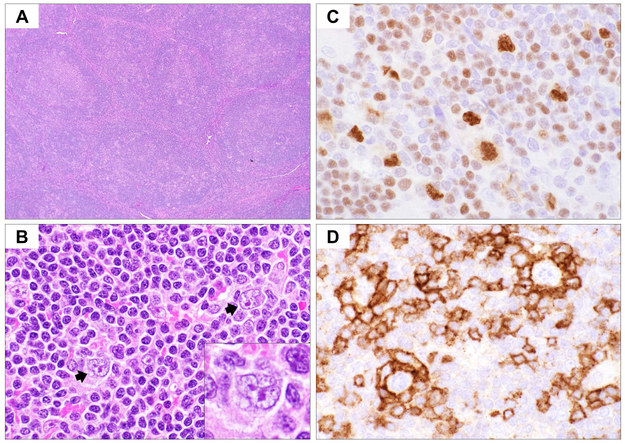
The neoplastic cells are termed lymphocyte predominant (LP) cells, replacing the older historical term of lymphocytic and histiocytic (L&H) cell, derived from the original Lukes and Butler category of lymphocytic and histiocytic predominant Hodgkin lymphoma. Due to the nuclear contour, these cells have also been referred to as “popcorn” cells. The infiltrate in NLPHL is generally vaguely nodular, but diffuse areas can be seen. (Fig. 1) Six immunoarchitectural patterns of NLPHL were described (Fan_, et al_ 2003). In the initial stages, the background is rich in small B cells, related to an origin of the process within lymphoid follicles (Patterns A and B). The LP cells are distributed within nodules in close association with small B cells and the follicular dendritic cell (FDC) meshwork. The small B cells have the phenotype of mantle zone B cells. As the disease progresses, the LP cells extend beyond this follicular environment (Pattern C). Eventually, more T cells are recruited into the infiltrate and T cells become predominant. (Pattern D). Over time, the FDC meshworks and nodular architecture may be both lost with resultant diffuse architecture (Pattern E), resembling THRLBCL. Pattern F, which is rare, has a disorganized B-cell rich background. A mixture of 2 or more patterns is commonly observed in a single biopsy. The variant patterns (Patterns C-F) are more often associated with disease recurrence, with the recurrences often resembling THRLBCL (Hartmann_, et al_ 2013b).
Fig 1.
Histological and immunophenotypic features of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL). (A) Haematoxylin and eosin stain at low magnification (x40) shows vaguely nodular architecture of NLPHL. (B) Scattered lymphocyte-predominant (LP) cells with polylobated (popcorn-like) nuclei are present in a background rich in small lymphocytes (x600). Also note the absence of other inflammatory cells. (C) Immunohistochemical (IHC) stain for OCT2 highlights the LP cells, usually with stronger expression than the background small B cells (x600). Also note the nuclear irregularity highlighted by OCT2 nuclear staining. (D) IHC for PD-1 shows small T cells forming tight rosettes surrounding the LP cells (x600).
The infiltrating T cells in NLPHL express a follicular T helper (TFH) cell phenotype, with positivity for CD4, PD1 (PDCD1) and CD57 (B3GAT1). The TFH cells are closely associated with LP cells with the formation of rosettes (Dorfman_, et al_ 2006, Nam-Cha_, et al_ 2008). Rarely, cytological atypia in the background T cells can lead to suspicion for a peripheral T-cell lymphoma (Sohani_, et al_ 2011), and the non-neoplastic T cells may show co-expression of CD4 and CD8 by flow cytometry (Rahemtullah_, et al_ 2006), which may lead to misdiagnosis as an abnormal T cell process.
LP cells show expression of CD45 and pan B cell markers (Table I). BCL6 is typically positive, which is characteristic of a germinal centre (GC) origin, although CD10, another GC marker, is negative (Anagnostopoulos_, et al_ 2000). Additionally, B cell transcription factors, PAX5, OCT1 (POU2F1) OCT2 (POU2F2), BOB.1 (POU2AF1) and PU.1 (SPI1) are positive, while often downregulated in CHL. The strong staining for OCT2 is especially useful in recognizing focal involvement. Immunoglobulin D (IgD) is expressed in the LP cells in some cases, which correlates with younger age group, male gender, cervical lymph node involvement and interfollicular distribution of LP cells (Prakash_, et al_ 2006, Untanu_, et al_ 2018). IgD-positive NLPHL appears to be linked to prior infection by Moraxella catarrhalis, a common bacterium in the upper respiratory tract that expresses an IgD-binding protein, thus activating IgD-expressing B cells (Hartmann et al 2015a).
Table I.
Commonly used immunophenotypic markers for the differential diagnosis of Hodgkin lymphomas.
| NLPHL | CHL | GZL | EBV+ DLBCL | ALCL | |
|---|---|---|---|---|---|
| CD30 | Negative (rare cases positive) | Positive, membrane and/or Golgi pattern | Positive | Frequently positive | Positive, membrane and Golgi pattern |
| CD15 | Negative (rare cases positive) | Positive, membrane and Golgi pattern | Positive in subset | Positive in subset | Negative |
| PAX5 | Positive | Positive, weak | Positive | Positive | Negative |
| CD20 | Positive | Negative or variable positive | Often positive | Positive | Negative |
| CD79a | Positive | Negative or weak | Often positive | Positive | Negative |
| OCT-2 | Positive, strong | Usually negative | Usually positive | Positive | Negative |
| BCL6 | Positive | Negative | Usually negative | Variable | Negative |
| IRF4/MUM1 | Usually negative | Positive | Positive | Positive | Positive |
| CD45 | Positive | Negative | Variable | Positive | Positive |
| T-cell markers | Negative | Negative (few cases positive) | Negative | Negative | Usually positive |
| Cytotoxic markers | Negative | Rare positive | Negative | Negative | Usually Positive |
| ALK | Negative | Negative | Negative | Negative | Positive in _ALK_-rearranged cases |
| EBV | Negative (rare cases positive) | Subset positive (usually MCCHL/LDCHL) | Usually negative | Positive | Negative |
CD30 (TNFRSF8) and CD15 (FUT4) are typically negative, although rare cases may express CD30, CD15 or both (Venkataraman_, et al_ 2011). However, CD30+ parafollicular immunoblasts are frequently present, which should not be misinterpreted as expression in the neoplastic cells and confused with CHL (Anagnostopoulos_, et al_ 2000). Only rare cases of NLPHL are positive for Epstein–Barr virus (EBV), a finding seen in both children and adults (Huppmann_, et al_ 2014).
Recent studies show that LP cells do not express the programmed cell death ligand 1 or 2 (PD-L1 [CD274] /PD-L2 [PDCD1LG2]) (Fromm_, et al_ 2017), in contrast to HRS cells in CHL which often express PD-L1.
Genetics and molecular biology
The immunoglobulin (Ig) genes are clonally rearranged in LP cells with evidence of somatic hypermutation, consistent with a GC origin (Kuppers_, et al_ 1994, Marafioti_, et al_ 1997). The gene expression profile of microdissected LP cells points towards a late GC profile (Brune_, et al_ 2008). LP cells exhibit multiple chromosomal abnormalities, including rearrangements of BCL6 in about half of the cases (Franke_, et al_ 2002, Rudiger_, et al_ 2002, Wlodarska_, et al_ 2004).
Genomic studies have shown contradictory results regarding the relationship between NLPHL and THRLBCL. For example, earlier studies using comparative genomic hybridization (CGH) found a higher level of genomic imbalances by in NLPHL than in THRLBCL, suggesting they are cytogenetically distinct (Franke_, et al_ 2002). However, more recent studies using array CGH showed similarities between NLPHL and THRLBCL, in support of a common molecular pathogenesis (Hartmann_, et al_ 2015b). Notably, a familial risk for NLPHL has been shown in several studies, and in some instances both NLPHL and THRLBCL occurred in the same family (Saarinen_, et al_ 2011, Straus_, et al_ 2001)
Histological progression
A small percentage of NLPHL cases (3–14%) may progress to diffuse large B cell lymphoma (DLBCL) (Hansmann_, et al_ 1989). DLBCL may co-exist with NLPHL in a single site (Sundeen_, et al_ 1988), or occur later in the course of disease (Greiner_, et al_ 1996, Huang_, et al_ 2004). Studies of both components indicate that they are clonally related (Cotta_, et al_ 2011, Greiner_, et al_ 1996, Hartmann_, et al_ 2014). In general, the clinical presentation of DLBCL following NLPHL is similar to that of de novo DLBCL with a risk of both nodal and extranodal disease (Huang_, et al_ 2004), while focal DLBCL co-existing with NLPHL may have less clinical impact (Sundeen_, et al_ 1988).
A more complex issue is the association of NLPHL with THRLBCL. On a practical basis, according to the guidelines of the revised World Health Organization classification, a focal area of NLPHL with diffuse areas resembling THRLBCL is designated as NLPHL with areas of Pattern E (Swerdlow_, et al_ 2016). A recurrence with a purely diffuse pattern consistent with THRLBCL is designated as “THRLBCL-like transformation of NLPHL”, with indications that this progression is associated with an aggressive clinical course (Swerdlow_, et al_ 2016). Clinically, it is likely that advanced stage disease is the most crucial factor in therapeutic decision-making, irrespective of the exact histological pattern (Xing_, et al_ 2014). At this point a distinction between THRLBCL arising from NLPHL and de novo THRLBCL is still not possible (Pittaluga and Jaffe 2010).
Classical Hodgkin Lymphoma
Classical Hodgkin lymphomas (CHL) account for approximately 85% of all Hodgkin lymphoma cases. In CHL the HRS cells usually show a repressed B-cell programme and defective immunoglobulin expression. CHL also differs from NLPHL in the character of the microenvironment. Based on histological features, four different subtypes have been designated, which include nodular sclerosis CHL (NSCHL), mixed cellularity CHL (MCCHL), lymphocyte-depleted CHL (LDCHL) and lymphocyte-rich CHL (LRCHL). In clinical practice, it is ideal to subclassify CHL as one of the four subtypes. However, a precise sub-classification is not always possible, especially when the biopsy material is limited. In those cases, the diagnosis of CHL, not further subclassified, may be made, but it remains important to distinguish cases of CHL from NLPHL.
Epidemiology
Epidemiological studies suggest that at least some of the subtypes of CHL represent different entities (Cartwright and Watkins 2004, Mani and Jaffe 2009). NSCHL affects primarily young adults (ages 15–34 years) with a female predominance and is less common in the elderly. On the other hand, MCCHL and LDCHL exhibit a bimodal age distribution with one peak in the paediatric age group, and a second peak over 60 years. These differences also correlate with differences in socioeconomic status among the subtypes. NSCHL is associated with higher socioeconomic status (Clarke_, et al_ 2005), which has been postulated to be related to delayed exposure to common infections (Macmahon 1957). MCCHL and LDCHL are both more common in developing countries, and frequently associated with EBV infection. EBV is positive by immunohistochemical stains or in situ hybridization in HRS cells in 70–80% of MCCHL and LDCHL, but in only 10–25% of NSCHL (Gulley_, et al_ 1994, Karube_, et al_ 2013). When present in HRS cells, the virus typically displays a latency type II pattern, with expression of EBNA1, LMP1 and LMP2A. A history of infectious mononucleosis carries a small but significant risk of subsequent MCCHL (Hjalgrim_, et al_ 2003).
NSCHL is the most common subtype of CHL in the Western world, followed by MCCHL. There are fewer epidemiological data regarding LRCHL, which tends to occur in an older patient population, and is relatively uncommon (5% of cases). Earlier epidemiological studies showed a sustained increase in the incidence of NSCHL in Europe over the period from 1978 to 1997, while the incidence of other subtypes remained steady (Clavel_, et al_ 2006). However, a recent study has also shown a decline in the incidence of NSCHL of 6% per year since 2007 (Glaser_, et al_ 2015).
Clinical features
Most patients with CHL present with lymphadenopathy. The commonly involved nodal sites include cervical, mediastinal, supraclavicular and axillary, with some variation in site preference among different subtypes. (Laurent_, et al_ 2015). Classical studies indicated that the spread of disease followed the physiological direction of lymphatic flow (Smithers_, et al_ 1974); thus peripheral non-axial lymph node groups, such as mesenteric or epitrochlear lymph nodes, are rarely involved . Extranodal involvement usually arises from haematogenous dissemination, while primary extranodal disease is rare. The most commonly involved extranodal sites include lung, liver and bone (Musshoff 1971). Although sporadic studies, especially in the older literature, reported cutaneous involvement by CHL (Introcaso_, et al_ 2008), it is likely that these instances represent other entities, including EBV-positive mucocutaneous ulcer and primary cutaneous CD30-positive T-cell proliferative disease (lymphomatoid papulosis), both of which can resemble CHL (Dojcinov_, et al_ 2010, Eberle_, et al_ 2012). If cutaneous involvement by CHL is seen, it is usually by direct extension.
There are also some subtype-specific clinical features. NSCHL usually presents with mediastinal mass, occurring in 80% of patients. MCCHL typically presents with peripheral lymphadenopathy, and mediastinal involvement is uncommon. LDCHL is often associated with an aggressive clinical course, with widespread involvement including bone marrow at diagnosis, and more common B symptoms (Klimm_, et al_ 2011). In contrast, LRCHL often presents with early-stage disease, with rare B symptoms; the clinical features are similar to those of NLPHL, although late relapses are rare (Diehl_, et al_ 1999).
Histological features
The initial diagnosis of CHL should be made on an adequate tissue biopsy. A fine needle aspiration or even a needle core biopsy is inadequate, as the assessment of architecture is important for an accurate diagnosis. Like NLPHL, CHL is characterized by a paucity of neoplastic cells and a rich inflammatory background. Whereas the features of HRS cells are comparable among the histological subtypes, the microenvironment shows substantial differences.
Nodular Sclerosis Classical Hodgkin Lymphoma.
In NSCHL cellular nodules are surrounded by partial or complete collagen bands, containing variable numbers of HRS cells, including lacunar variants, in a mixed inflammatory background. The HRS cells often form cellular aggregates that may be associated with necrosis and histiocytic reaction. NSCHL has traditionally been graded into NS1 and NS2, based on the histological criteria from the British National Lymphoma Investigation (BNLI) (MacLennan_, et al_ 1989). The higher-grade NS2 lesions have more abundant HRS cells, frequent anaplastic-appearing HRS cells and areas of necrosis. In the original series NS2 was associated with poorer initial response to treatment and higher relapse rate (MacLennan_, et al_ 1989). However, the importance of grading is declining due to advances in therapy and is more relevant for cases with advanced clinical stage (van Spronsen_, et al_ 1997). Grading is currently not mandatory for clinical use.
Mixed Cellularity and Lymphocyte-Depleted Classical Hodgkin Lymphoma.
MCCHL and LDCHL share many clinical features and appear to represent part of a biological continuum. Both subtypes are often positive for EBV. They also both show association with human immunodeficiency virus infection (Biggar_, et al_ 2006, Shiels_, et al_ 2014). MCCHL is characterized by classic HRS cells in a rich inflammatory background, including eosinophils, neutrophils, histiocytes and plasma cells. Although fine interstitial fibrosis is common, a thick capsule or broad bands of fibrosis are usually absent. The cellular composition of LDCHL is like that of MCCHL, but in general there is a greater abundance of histiocytes and fewer reactive lymphocytes.
Lymphocyte-Rich Classical Hodgkin Lymphoma.
LRCHL is the last subtype that was defined and distinguished from NLPHL (Anagnostopoulos_, et al_ 2000, Diehl_, et al_ 1999). LRCHL bears some resemblance to NLPHL in that the background is also dominated by small B-lymphocytes that form nodules, often with regressed germinal centres in the periphery. Other inflammatory cells, such as eosinophils, are rare. Due to the histological resemblance, this subtype can easily be confused with NLPHL, and the demonstration of a classical HRS immunophenotype is essential in making the diagnosis. A minor subset has a more diffuse growth pattern.
Immunophenotype
Despite the variability in the histological appearance among the four subtypes, the malignant cells in CHL share a common phenotype, and immunohistochemical stains on paraffin sections are a useful tool. The most frequently used markers are CD30 and CD15. CD30 is expressed in nearly all cases, while CD15 is found in 75% to 85% of CHL cases. The strong expression of CD30 in HRS cells has led to the development of CD30-specific therapies, such as the antibody-drug conjugate Brentuximab Vedotin or reprogramed autologous T-cell therapies (Renner and Stenner 2018). IRF4/MUM1 is positive in nearly all cases but is less useful in discriminating CHL from other mimics, such as anaplastic large cell lymphoma.
In contrast to NLPHL, which often retains a B-cell phenotype, the HRS cells typically show repression of the B-cell programme with loss of B-cell antigen expression and immunoglobulin production (Tzankov_, et al_ 2003). The pan B-cell marker CD20 was traditionally reported negative in most cases, although recent experience indicates more frequent positivity, probably due to improvement in antigen retrieval techniques. Even so, CD20 is generally variable in a given case within the neoplastic population. CD79a is less often positive. The only B-cell-restricted antigen that is nearly always expressed in CHL is PAX5, which is useful to establish the B-cell identity of the tumour cells. The nuclear expression of PAX5 is usually weaker than that seen in the background reactive B cells, which is another diagnostically useful feature. Notably, the HRS cells in LRCHL more frequently express B-cell transcription factors and CD20 than the other CHL subtypes (Nam-Cha_, et al_ 2009).
T-cell markers, most commonly CD4 and CD2, are aberrantly expressed in the HRS cells in a small subset of NSCHL. Most T-cell antigen-positive cases are of the NS2 subtype and have a worse clinical outcome (Venkataraman_, et al_ 2013). The expression of cytotoxic molecules in HRS cells has also been linked to a poorer prognosis (Asano_, et al_ 2006).
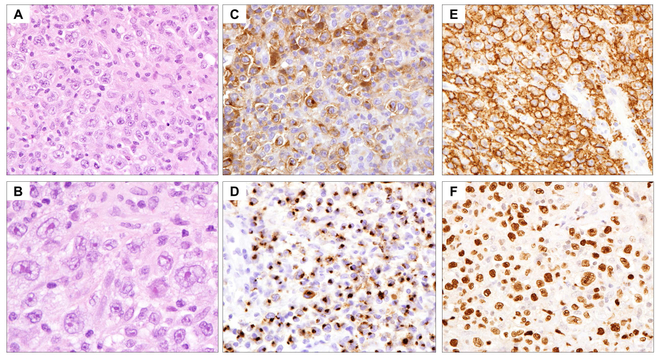
The immunophenotype aids the diagnosis and is also useful to investigate potential therapeutic targets. For example, PD-L1 and/or PD-L2 are often overexpressed in HRS cells through different mechanisms, including gene amplification, EBV infection and JAK-STAT pathway activation (Fig. 2A-B). PD-L1/2 is thought to play an important role in shaping the immunosuppressive microenvironment in CHL through suppression of T-cell functions (Carey_, et al_ 2017, Yamamoto_, et al_ 2008). Blockade of the PD-1 pathway has shown promising results in the treatment of relapsed or refractory CHL (Ansell_, et al_ 2015, Younes_, et al_ 2016). A recent clinical trial also indicated a strong correlation between the intensity of PD-L1 staining and the response to PD-L1/PD-1 inhibitors (Chen_, et al_ 2017).
Fig 2.
Patterns of PD-L1 in classical Hodgkin lymphoma. (A) Haematoxylin and eosin (H&E) stain shows a case of nodular sclerosis classical Hodgkin lymphoma (NSCHL) with clusters of Hodgkin/Reed-Sternberg (HRS) cells (x600). (B) Immunohistochemistry (IHC) highlights strong and uniform expression of PD-L1 in the HRS cells (x600). (C) H&E stain showing a different case of NSCHL with scattered HRS cells (arrows) (x600). (D) IHC demonstrating the expression of PD-L1 by stromal cells, while the HRS cells are negative (x600). The lace-like pattern surrounds the HRS cells.
The profile of the tumour microenvironment has also been suggested to have prognostic impact in CHL. For instance, the number of tumour-associated macrophages (TAM) was strongly associated with refractoriness to therapy, early relapse and shortened survival in adult CHL patients (Deau_, et al_ 2013, Steidl_, et al_ 2010a, Touati_, et al_ 2015, Tzankov_, et al_ 2010), although other studies have not confirmed the importance of TAMs (Cencini_, et al_ 2017, Gupta_, et al_ 2013). Interestingly, PD-L1 is also highly expressed in TAMs (Fig. 2C-D), which physically colocalize with PD-L1-expressing HRS cells in the microenvironmental niche (Carey_, et al_ 2017). The expression of PD-L1 in TAMs may also contribute to an immunosuppressive environment, and the intensity of PD-L1 staining in TAMs correlates with clinical response to the PD-1 blockade (Chen_, et al_ 2017). The contribution of TAM-supplied PD-L1 probably explains some of the tumour-promoting effects attributed to TAMs in some studies. Additionally, a high degree of tumour-infiltrating mast cells has also been reported to correlate with poorer outcome in MCCHL (Andersen_, et al_ 2016), and was associated with fibrosis in NSCHL, probably related to the production of pro-fibrotic cytokines, such as IL13 and TGF-β (TGFB1) (Nakayama_, et al_ 2016). The composition of the reactive T cells may impact the prognosis in CHL. Patients with increased FOXP3-positive regulatory T cells were found to have a better outcome in some studies (Greaves_, et al_ 2013), while opposite results have been shown in others (Schreck_, et al_ 2009).
The usefulness of flow cytometry in the diagnosis of CHL is limited, as the HRS cells generally escape detection by this technique. Several studies identified changes in the phenotype of CHL-infiltrating T lymphocytes, including bright expression of CD7 and CD45 (PTPRC), and an elevated CD4:CD8 ratio (Fromm_, et al_ 2010, Wu_, et al_ 2016). These features are not diagnostic, but the findings should prompt a more thorough histological investigation for CHL. Additionally, novel strategies have been recently developed in some laboratories to identify HRS cells by flow cytometry with high sensitivity and specificity, using a combination of antibodies reactive with antigens on HRS cells, including CD30, CD15, CD40, CD71 (TFRC) and CD95 (FAS) (Fromm and Wood 2012). However, these assays are currently not widely available in most clinical laboratories.
Molecular and cytogenetic studies
Molecular studies for B-cell clonality are generally not required to establish the diagnosis of CHL, as conventional polymerase chain reaction (PCR) analyses often give a false negative result, due to the scarcity of neoplastic cells. However, with single-cell isolation, clonal immunoglobulin gene rearrangement has been demonstrated (Kuppers_, et al_ 1994). The rearranged immunoglobulin genes harbour somatic hypermutations in the V regions, which supports the GC B cell derivation of HRS cells (Braeuninger_, et al_ 1997, Kanzler_, et al_ 1996). Interestingly, although HRS and LP cells both contain rearranged immunoglobulin genes, unlike LP cells, HRS cells lack immunoglobulin expression at both mRNA and protein levels, which has been attributed to several possible reasons, including crippling mutations in the rearranged immunoglobulin gene or its promoter region (Kanzler_, et al_ 1996, Theil_, et al_ 2001), downregulation of B cell transcription factors required for Ig expression, and hypermethylation in genes associated with the B-cell programmes. As damaging mutations in immunoglobulin genes and disruption of B-cell receptor signalling lead to apoptosis of normal GC cells, it was concluded that HRS cells are derived from pre-apoptotic GC B cells that are rescued from apoptosis by other transforming events (Kuppers 2012). Recent genetic studies have shown that HRS cells share a typical transcriptome pattern with a subset of GC cells that express CD30, suggesting their origin from these cells. Moreover, there is remarkable downregulation of genes involved in the regulation of genomic stability and cytokinesis in HRS cells, which may lead to their genomic instability and multinuclearity (Weniger_, et al_ 2018).
Cytogenetic and fluorescence in situ hybridisation studies show high prevalence of aneuploidy or hypertetraploidy, consistent with the multinucleation of HRS cells. Recent studies using array CGH to analyse copy number changes in microdissected HRS cells have consistently identified regions with recurrent gains or losses of chromosomal material, including gains in chromosomes 2p, 9p, 17q, 19q and 20q, and losses in 6q and 13q (Hartmann_, et al_ 2008, Steidl_, et al_ 2010b). Several genes involved in important biological functions localize in those regions, including JAK2, CD274 (PDL1) and PDCD1LG2 (PDL2) (all at 9p24), and genes involved in nuclear factor (NF)-κB signalling, such as REL (2p16), RELB (19q13), CD40 (20q13) and MAP3K14 (17q21). The amplification at 9p24.1 is critical in leading to amplification of CD274/PDCD1LG2 , specifically associated with both NSCHL and PMBL (Green_, et al_ 2010). In addition, amplification of JAK2 further induces PD-L1 protein expression.
Recent studies using next generation sequencing on microdissected CHL samples have further elucidated the genetic landscape of CHL. Recurrent mutations in the JAK-STAT pathway genes including STAT6, GNA13, XPO1 and ITPKB have been identified with high frequency (Tiacci_, et al_ 2018). By identifying STAT6 mutation, the most frequently mutated gene in CHL, in the circulation, it has been shown that measuring circulating tumour DNA can be used to monitor residual disease in CHL (Spina_, et al_ 2018). Furthermore, transcriptome analysis on CHL-derived cell lines and/or microdissected HRS cells have shown a gene expression profile distinct from NLPHL or other non-Hodgkin B-cell lymphomas, including down-regulation of B-cell receptor signalling and antibody responses, and upregulation of NF-κB and JAK-STAT signalling (Steidl_, et al_ 2012, Tiacci_, et al_ 2012).
These genetic alterations and the consequential dysregulation probably play pivotal roles in the pathogenesis of CHL, and tremendous effort has been made to develop new therapies that target these key pathways (Mottok and Steidl 2018). For example, immune checkpoint inhibitors targeting the PD-L1/PD-1 axis, as described earlier, have proven effective in the treatment of CHL. JAK inhibitors, such as ruxolitinib, have also been actively tested in clinical trials (Van Den Neste_, et al_ 2018). Bortezomib, a proteasome inhibitor, has also been added to combination regimens for relapsed or refractory CHL with the aim to downregulate the NF-κB signalling pathways, although early results have not been successful (Balzarotti_, et al_ 2016, Mendler_, et al_ 2008).
Differential diagnosis
Although most cases of CHL can be diagnosed based on morphological and immunophenotypic features, the distinction from other types of non-Hodgkin B-cell or T-cell lymphomas and a variety of EBV-associated lymphoproliferations can prove difficult. Here we discuss a few common conditions that are also common problems in a haematopathology consultation service.
B-cell lymphoma unclassifiable, with features intermediate between DLBCL and CHL (Grey zone lymphoma). As its name suggests, Grey zone lymphoma (GZL) shows features of both large B-cell lymphoma and CHL or, more specifically, primary mediastinal large B-cell lymphoma (PMBL) and NSCHL. A close relationship between NSCHL and PMBL had been suspected for some years, as these tumours can present as composite lymphoma, or sequentially in the same patient. They also show similarities in their gene expression and genetic profiles (Rosenwald_, et al_ 2003, Savage_, et al_ 2003). Thus, mediastinal GZL (MGZL) was proposed as a missing link, representing a tumour with transitional features (Traverse-Glehen_, et al_ 2005).
The tumour cells in MGZL resemble both PMBL (with a sheet-like growth pattern) and NSCHL (with pleomorphic large cells in a fibrotic stroma). Unlike CHL, there is usually a sparse inflammatory infiltrate and tumour cell density is consistently high (Fig. 3). There is often an asynchrony between morphology and immunophenotype. For example, the neoplastic cells may closely resemble HRS cells, but show a preserved B-cell programme, with strong and uniform expression of CD20 and CD79a. However, uniform expression of CD20 in a tumour that is otherwise typical for NSCHL should not lead to a diagnosis of MGZL. Conversely, MGZL may show a histological appearance suggestive of PMBL, but with strong CD30 or CD15 expression and loss of B-cell antigens (Traverse-Glehen_, et al_ 2005). Notably, genetic studies have identified chromosomal aberrations that are shared with both PMBL and NSCHL, with gains at 2p16 (REL locus) and 9p24.1 (loci of JAK2, CD274 and PDCD1LG2) (Eberle_, et al_ 2011). The gains at 9p24.1 are associated with expression of PD-L1, correlating with a response to checkpoint inhibitors in some patients with GZL (Melani_, et al_ 2017). The absence of EBV in nearly all cases helps in the differential diagnosis with EBV-positive DLBCL (see below).
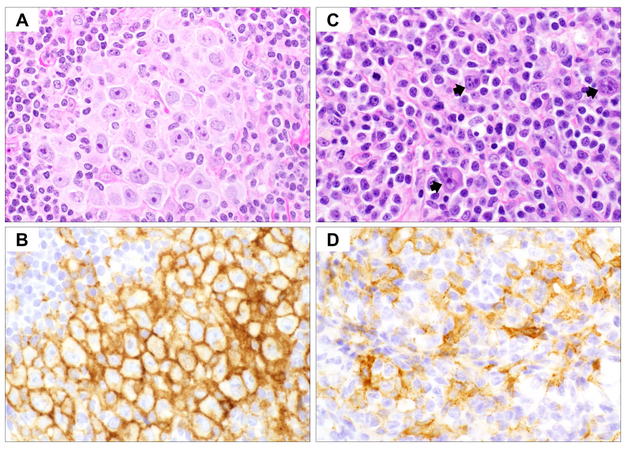
Fig 3.
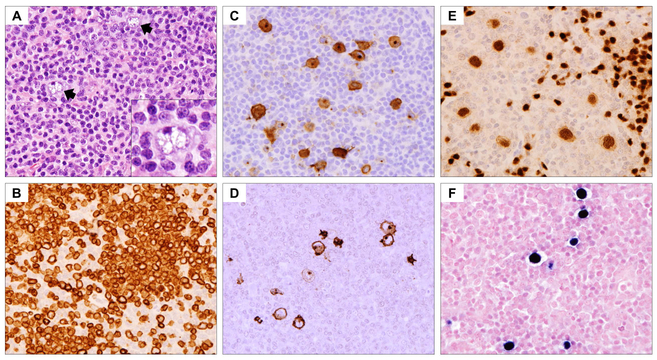
Mediastinal Grey zone lymphoma (GZL). (A-B) Low and high magnification haematoxylin and eosin stains demonstrate cohesive sheets of large atypical neoplastic cells with variation in size and morphology (A: x400; B: x600). A subset of cells exhibits Hodgkin/Reed-Sternberg cell-like features with irregular nuclei and prominent nucleoli. (C-F) Immunohistochemical studies (x400) show typical immunophenotype of classical Hodgkin lymphoma in the atypical cells with expression of CD30 (C) and CD15 (D), but the atypical cells retain strong expression of B-cell markers CD20 (E) and OCT2 (F). Also note marked nuclear variability and range in cell size and shape.
Clinically, GZL presents most commonly in young male patients aged 20 to 40 years. Patients usually present with a bulky mediastinal mass, causing local symptoms. The disease can spread to supraclavicular lymph nodes, lung, liver, spleen and bone marrow, while involvement of other extranodal sites is rare (Eberle_, et al_ 2011, Pilichowska_, et al_ 2017). Patients with GZL have a worse outcome compared with that of either CHL or PMBL (Evens_, et al_ 2015, Sarkozy_, et al_ 2017, Wilson_, et al_ 2014). Thus, the distinction of GZL from CHL or PMBL is important, as it has been recommended that these patients be treated with dose-intensive regimens (Kritharis_, et al_ 2016, Sarkozy_, et al_ 2017, Wilson_, et al_ 2014).
EBV-positive DLBCL, not otherwise specified.
This disease was formerly designated as EBV-positive DLBCL of the elderly, but was later found to also sporadically occur in young patients (Nicolae_, et al_ 2015). A subset of cases shows overlapping histological features with CHL, with variable numbers of large transformed HRS-like cells and a polymorphic reactive component. The histological features may resemble those of THRLBCL, but the presence of EBV helps in confirming the diagnosis (Lim_, et al_ 2002). Clinically, both nodal and extranodal sites can be involved, although the disease is predominantly nodal in young patients. The most commonly involved extranodal sites are the lungs and gastrointestinal tract. The prognosis also differs significantly between older and young patients, in that it is highly aggressive in elderly patients, with a median survival of about 2 years, much worse than CHL or EBV-negative DLBCL (Asano_, et al_ 2009). Younger patients appear to have an excellent prognosis (Nicolae_, et al_ 2015).
Immunophenotypically, the neoplastic cells have a preserved B-cell programme, and often show an activated B-cell phenotype with expression of IRF4/MUM1. CD30 is frequently positive, while CD15 is sometimes co-expressed. EBV-positive atypical cells can be demonstrated by in situ hybridization of EBV-encoded RNAs (EBER), and the EBV-infected cells usually exhibit type II or type III EBV latency, expressing LMP1 with or without EBNA2. The association with EBV is believed to be secondary to age-related immunosenescence. However, even in younger patients, a tolerogenic immune environment characterized by high expression of PD-L1 and IDO (IDO1) in tumour cells and/or the histiocytic/dendritic cell microenvironment can often be found (Nicolae_, et al_ 2015).
EBV-associated B-cell lymphoproliferations.
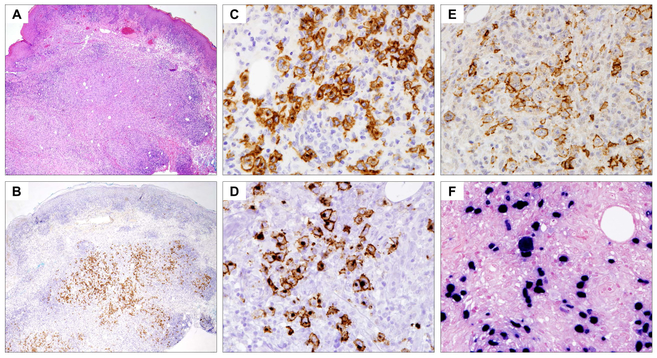
A variety of other EBV-associated B-cell lymphoproliferations of varied malignant potential may mimic CHL, and include EBV-positive mucocutaneous ulcer and polymorphic B-cell lymphoproliferative disorders (B-LPD) (Dojcinov_, et al_ 2011, Natkunam_, et al_ 2017). EBV-positive mucocutaneous ulcer is a newly recognized EBV-associated B-cell proliferation, often with Hodgkin-like features, occurring in patients with age-related or iatrogenic immunosuppression (Dojcinov_, et al_ 2010). It is characterized by localized involvement in cutaneous or mucosal sites and an indolent clinical course, generally with good response to conservative management. Patients usually present with sharply circumscribed, often painful, ulcerative lesions in the oral mucosa, skin or gastrointestinal tract. The presence of lymph node involvement should suggest an alternative diagnosis. Histologically, there is usually a dense polymorphic infiltrate in the ulcer base, consisting of a variable number of plasma cells, eosinophils, histiocytes and large transformed cells resembling HRS cells (Fig. 4). At the base of the lesion there is a band-like infiltrate of mature T cells, contributing to the sharp demarcation of the process from the underlying soft tissue. The large transformed cells are B cells with variable expression of CD20 and strong CD30; CD15 is expressed in about half of the cases. Both EBER and LMP1 are expressed. In immunocompetent elderly individuals, spontaneous regression may be seen. For most patients receiving therapeutic immunosuppression, the lesions usually respond to reduction in immunosuppression, with rituximab used in some cases (Hart_, et al_ 2014). Spread beyond the local site is rare, and the presence of nodal involvement should suggest another EBV-related lesion.
Fig 4.
Epstein–Barr virus (EBV)-positive mucocutaneous ulcer, skin biopsy (x20). (A) Haematoxylin and eosin stain demonstrates a dense dermal infiltrate extending to subcutis. (B) Immunohistochemistry (IHC) for CD30 highlights the atypical cells, showing a localized distribution confined to the subcutis (x20). (C-E) IHC studies at higher magnification (x400) showing that the atypical cells express CD30 (C), CD15 (D) and CD20 (E). (F) In-situ hybridization for EBV-encoded RNAs (EBER, at x400) shows that the atypical cells are uniformly positive for EBV. Note the variation in size of the EBV-positive cells, which is characteristic of EBV-associated B-cell lymphoproliferative disorders.
Polymorphic B-LPDs are morphologically mass-forming lesions that contain atypical B cells, including some that may resemble HRS cells. In the post-transplant setting, they are designated as polymorphic post-transplant lymphoproliferative disorders (PTLD), but polymorphic PTLD-like lesions can be seen in other, mainly iatrogenic, immunodeficiency settings (Natkunam_, et al_ 2018). They may present in nodal or extranodal sites. Most polymorphic B-LPDs contain a mixed infiltrate of B cells with a full range of maturation from small and medium-sized B cells to immunoblasts and mature plasma cells. A subset of cases may contain HRS-like cells, but in contrast to CHL, EBER is positive in the full morphological spectrum, and not confined to the HRS-like cells. The distinction of polymorphic PTLD with HRS-like cells from true CHL-type PTLD (considered a form of monomorphic PTLD) is important, as the clinical management will differ (Dierickx_, et al_ 2015). Cases of polymorphic B-LPD occurring in patients receiving long term therapy with methotrexate provide a particular diagnostic challenge and may closely mimic CHL (Kamel_, et al_ 1996). In this setting a complete clinical history is essential, as withdrawal of immunosuppressive therapy will often lead to clinical regression (Natkunam_, et al_ 2017).
Anaplastic large cell lymphoma.
Anaplastic large cell lymphoma (ALCL) is a T-cell lymphoma characterized by strong CD30 expression. It may or may not be associated with ALK rearrangement. Clinically, most patients present with advanced stage, with peripheral or abdominal lymphadenopathy, often associated with involvement of extranodal tissue (less common in ALK-negative cases) and bone marrow. Some patients may have a leukaemic presentation.
Histologically, ALCL shows a broad morphological spectrum, but the neoplastic cells typically show horseshoe-shaped nuclei (“hallmark” cells). The neoplastic cells may range from small to very large, and only rarely should be confused with HRS cells. The tumour cells often grow in cohesive sheets, which may mimic the syncytial variant of NSCHL (Vassallo_, et al_ 2006). Involvement of lymph node sinuses is another typical feature of ALCL, and is rarely seen in CHL. Immunophenotypically, CD30 is strongly positive, except in the small cell variant, while the expression of CD15 is rare. The majority of ALCLs express one or more T-cell antigens (CD2, CD5 or CD4, while CD3 is often negative), whereas a subset may show a so-called null-cell phenotype, with loss of all T-cell markers. In general, the expression of B-cell markers, such as PAX5, or less commonly CD20, and CD15 suggest the diagnosis of CHL. A panel of immunohistochemical stains including EMA, CD45 and cytotoxic molecules may also aid in the differential diagnosis. In difficult cases, proof of T-cell clonality by PCR may help to rule out CHL, while finding clonal immunoglobulin gene rearrangement favours Hodgkin lymphoma.
Peripheral T-cell lymphomas with “Hodgkin-like” cells.
Peripheral T-cell lymphomas (PTCLs) encompass a functionally and morphologically diverse group of lymphomas. A subset of PTCLs, especially those with a follicular helper T-cell phenotype such as angioimmunoblastic T-cell lymphoma, may contain HRS-like cells of B-cell lineage that are EBV-positive (Eladl_, et al_ 2017, Moroch_, et al_ 2012, Nicolae_, et al_ 2013) (Fig. 5). The HRS-like cells are positive for PAX5 or CD20, and often express both CD30 and CD15. As atypia in the background T-cell population may be minimal, misdiagnosis as CHL may occur. In rare cases of PTCL the neoplastic cells themselves may express both CD30 and CD15, mimicking HRS cells and suggesting a diagnosis of CHL (Barry_, et al_ 2003). In most cases, tumour cell density is high and the neoplastic cells are often cohesive, resembling ALCL. HRS-like cells with expression of both CD30 and CD15 have also been reported in primary cutaneous CD30-positive T-cell LPDs, and in instances of lymph node involvement can provide a diagnostic challenge to the unwary (Eberle_, et al_ 2012).
Fig 5.
Peripheral T-cell lymphomas with “Hodgkin-like” cells. (A) Haematoxylin and eosin stain (x400) demonstrates an atypical lymphoid infiltrate composed of small-to-medium-sized lymphoid cells and scattered large “Hodgkin-like” cells (arrows and inset). (B) Immunohistochemistry (IHC) shows expression of CD3 in the small-to-medium-sized atypical T cells, also highlighting the cytological atypia (x400). (C-F) IHC and in situ hybridization studies (x400) showing that the large “Hodgkin-like” cells are positive for CD30 (C), CD15 (D), PAX5 (E) and EBV (F).
Conclusion
A variety of benign and malignant lymphoid proliferations can display histological features resembling Hodgkin lymphoma. All of these Hodgkin mimics emphasize the importance of a complete clinical history and an adequate excisional biopsy for proper diagnosis. In challenging cases expert consultation should be sought, and appropriate immunophenotypic and molecular studies may be required for resolution of the diagnosis. Communication between the treating physician and the pathologist is critical, both in terms of establishing an initial diagnosis, but also for patient management. With the continued expansion and availability of targeted agents, detailed pathological characterization of the neoplastic cells and their microenvironment will be paramount.
Acknowledgments
This work was supported by the Intramural Research Budget of the Center for Cancer Research, National Cancer Institute.
Footnotes
The authors have no conflict of interest to declare.
References
- Anagnostopoulos I, Hansmann ML, Franssila K, Harris M, Harris NL, Jaffe ES, Han J, van Krieken JM, Poppema S, Marafioti T, Franklin J, Sextro M, Diehl V & Stein H (2000) European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood, 96, 1889–1899. [PubMed] [Google Scholar]
- Andersen MD, Kamper P, Nielsen PS, Bendix K, Riber-Hansen R, Steiniche T, Hamilton-Dutoit S, Clausen M & d’Amore F (2016) Tumour-associated mast cells in classical Hodgkin’s lymphoma: correlation with histological subtype, other tumour-infiltrating inflammatory cell subsets and outcome. European Journal of Haematology, 96, 252–259. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA & Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New England Journal of Medicine, 372, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano N, Oshiro A, Matsuo K, Kagami Y, Ishida F, Suzuki R, Kinoshita T, Shimoyama Y, Tamaru J, Yoshino T, Kitamura K, Fukutani H, Morishima Y & Nakamura S (2006) Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin’s lymphoma: a clinicopathologic study. Journal of Clinical Oncology, 24, 4626–4633. [DOI] [PubMed] [Google Scholar]
- Asano N, Yamamoto K, Tamaru J, Oyama T, Ishida F, Ohshima K, Yoshino T, Nakamura N, Mori S, Yoshie O, Shimoyama Y, Morishima Y, Kinoshita T & Nakamura S (2009) Age-related Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood, 113, 2629–2636. [DOI] [PubMed] [Google Scholar]
- Balzarotti M, Brusamolino E, Angelucci E, Carella AM, Vitolo U, Russo E, Congiu A, Gotti M, Massidda S, Botto B, Annechini G, Spina M, Re A, Zilioli VR, Merli F, Salvi F, Stelitano C, Bonfichi M, Rodari M, Murru R, Magagnoli M, Anastasia A, Mazza R, Giordano L & Santoro A (2016) B-IGEV (bortezomib plus IGEV) versus IGEV before high-dose chemotherapy followed by autologous stem cell transplantation in relapsed or refractory Hodgkin lymphoma: a randomized, phase II trial of the Fondazione Italiana Linfomi (FIL). Leukemia and Lymphoma, 57, 2375–2381. [DOI] [PubMed] [Google Scholar]
- Barry TS, Jaffe ES, Sorbara L, Raffeld M & Pittaluga S (2003) Peripheral T-cell lymphomas expressing CD30 and CD15. American Journal of Surgical Pathology, 27, 1513–1522. [DOI] [PubMed] [Google Scholar]
- Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R & Engels EA (2006) Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood, 108, 3786–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeuninger A, Kuppers R, Strickler JG, Wacker HH, Rajewsky K & Hansmann ML (1997) Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proceedings of the National Academy of Sciences of the United States of America, 94, 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune V, Tiacci E, Pfeil I, Doring C, Eckerle S, van Noesel CJ, Klapper W, Falini B, von Heydebreck A, Metzler D, Brauninger A, Hansmann ML & Kuppers R (2008) Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. Journal of Experimental Medicine, 205, 2251–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, Hu X, Redd R, Freeman GJ, Neuberg D, Hodi FS, Liu XS, Shipp MA & Rodig SJ (2017) Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood, 130, 2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright RA & Watkins G (2004) Epidemiology of Hodgkin’s disease: a review. Hematological Oncology, 22, 11–26. [DOI] [PubMed] [Google Scholar]
- Cencini E, Fabbri A, Rigacci L, Lazzi S, Gini G, Cox MC, Mancuso S, Abruzzese E, Kovalchuk S, Goteri G, Di Napoli A, Bono R, Fratoni S, Di Lollo S, Bosi A, Leoncini L & Bocchia M (2017) Evaluation of the prognostic role of tumour-associated macrophages in newly diagnosed classical Hodgkin lymphoma and correlation with early FDG-PET assessment. Hematological Oncology, 35, 69–78. [DOI] [PubMed] [Google Scholar]
- Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Zhang Y, Ricart AD, Balakumaran A, Moskowitz CH & Keynote (2017) Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of Clinical Oncology, 35, 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CA, Glaser SL, Keegan TH & Stroup A (2005) Neighborhood socioeconomic status and Hodgkin’s lymphoma incidence in California. Cancer Epidemiology, Biomarkers and Prevention, 14, 1441–1447. [DOI] [PubMed] [Google Scholar]
- Clavel J, Steliarova-Foucher E, Berger C, Danon S & Valerianova Z (2006) Hodgkin’s disease incidence and survival in European children and adolescents (1978–1997): report from the Automated Cancer Information System project. European Journal of Cancer, 42, 2037–2049. [DOI] [PubMed] [Google Scholar]
- Cotta CV, Coleman JF, Li S & Hsi ED (2011) Nodular lymphocyte predominant Hodgkin lymphoma and diffuse large B-cell lymphoma: a study of six cases concurrently involving the same site. Histopathology, 59, 1194–1203. [DOI] [PubMed] [Google Scholar]
- Deau B, Bachy E, Ribrag V, Delarue R, Rubio MT, Bosq J, Varet B, Brousse N, Hermine O & Canioni D (2013) Macrophage, mast cell and T lymphocyte infiltrations are independent predictive biomarkers of primary refractoriness or early relapse in classical Hodgkin lymphoma. Leukemia and Lymphoma, 54, 41–45. [DOI] [PubMed] [Google Scholar]
- Diehl V, Sextro M, Franklin J, Hansmann ML, Harris N, Jaffe E, Poppema S, Harris M, Franssila K, van Krieken J, Marafioti T, Anagnostopoulos I & Stein H (1999) Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. Journal of Clinical Oncology, 17, 776–783. [DOI] [PubMed] [Google Scholar]
- Dierickx D, Tousseyn T & Gheysens O (2015) How I treat posttransplant lymphoproliferative disorders. Blood, 126, 2274–2283. [DOI] [PubMed] [Google Scholar]
- Dojcinov SD, Venkataraman G, Pittaluga S, Wlodarska I, Schrager JA, Raffeld M, Hills RK & Jaffe ES (2011) Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood, 117, 4726–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S & Jaffe ES (2010) EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. American Journal of Surgical Pathology, 34, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman DM, Brown JA, Shahsafaei A & Freeman GJ (2006) Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. American Journal of Surgical Pathology, 30, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle FC, Salaverria I, Steidl C, Summers TA Jr., Pittaluga S, Neriah SB, Rodriguez-Canales J, Xi L, Ylaya K, Liewehr D, Dunleavy K, Wilson WH, Hewitt SM, Raffeld M, Gascoyne RD, Siebert R & Jaffe ES (2011) Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Modern Pathology, 24, 1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle FC, Song JY, Xi L, Raffeld M, Harris NL, Wilson WH, Pittaluga S & Jaffe ES (2012) Nodal involvement by cutaneous CD30-positive T-cell lymphoma mimicking classical Hodgkin lymphoma. Am J Surg Pathol, 36, 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladl AE, Satou A, Elsayed AA, Suzuki Y, Kato S, Asano N & Nakamura S (2017) Clinicopathological Study of 30 Cases of Peripheral T-cell Lymphoma with Hodgkin and Reed-Sternberg-like B-cells from Japan. American Journal of Surgical Pathology, 41, 506–516. [DOI] [PubMed] [Google Scholar]
- Evens AM, Kanakry JA, Sehn LH, Kritharis A, Feldman T, Kroll A, Gascoyne RD, Abramson JS, Petrich AM, Hernandez-Ilizaliturri FJ, Al-Mansour Z, Adeimy C, Hemminger J, Bartlett NL, Mato A, Caimi PF, Advani RH, Klein AK, Nabhan C, Smith SM, Fabregas JC, Lossos IS, Press OW, Fenske TS, Friedberg JW, Vose JM & Blum KA (2015) Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. American Journal of Hematology, 90, 778–783. [DOI] [PubMed] [Google Scholar]
- Fan Z, Natkunam Y, Bair E, Tibshirani R & Warnke RA (2003) Characterization of variant patterns of nodular lymphocyte predominant hodgkin lymphoma with immunohistologic and clinical correlation. American Journal of Surgical Pathology, 27, 1346–1356. [DOI] [PubMed] [Google Scholar]
- Fanale MA, Cheah CY, Rich A, Medeiros LJ, Lai CM, Oki Y, Romaguera JE, Fayad LE, Hagemeister FB, Samaniego F, Rodriguez MA, Neelapu SS, Lee HJ, Nastoupil L, Fowler NH, Turturro F, Westin JR, Wang ML, McLaughlin P, Pinnix CC, Milgrom SA, Dabaja B, Horowitz SB & Younes A (2017) Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood, 130, 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry JA, Zukerberg LR & Harris NL (1992) Florid progressive transformation of germinal centers. A syndrome affecting young men, without early progression to nodular lymphocyte predominance Hodgkin’s disease. American Journal of Surgical Pathology, 16, 252–258. [PubMed] [Google Scholar]
- Franke S, Wlodarska I, Maes B, Vandenberghe P, Achten R, Hagemeijer A & De Wolf-Peeters C (2002) Comparative genomic hybridization pattern distinguishes T-cell/histiocyte-rich B-cell lymphoma from nodular lymphocyte predominance Hodgkin’s lymphoma. American Journal of Pathology, 161, 1861–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm JR & Wood BL (2012) Strategies for immunophenotyping and purifying classical Hodgkin lymphoma cells from lymph nodes by flow cytometry and flow cytometric cell sorting. Methods, 57, 368–375. [DOI] [PubMed] [Google Scholar]
- Fromm JR, Thomas A & Wood BL (2010) Increased expression of T cell antigens on T cells in classical Hodgkin lymphoma. Cytometry. Part B: Clinical Cytometry, 78, 387–388. [DOI] [PubMed] [Google Scholar]
- Fromm JR, Thomas A & Wood BL (2017) Characterization and Purification of Neoplastic Cells of Nodular Lymphocyte Predominant Hodgkin Lymphoma from Lymph Nodes by Flow Cytometry and Flow Cytometric Cell Sorting. American Journal of Pathology, 187, 304–317. [DOI] [PubMed] [Google Scholar]
- Glaser SL, Clarke CA, Keegan TH, Chang ET & Weisenburger DD (2015) Time Trends in Rates of Hodgkin Lymphoma Histologic Subtypes: True Incidence Changes or Evolving Diagnostic Practice? Cancer Epidemiology, Biomarkers and Prevention, 24, 1474–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves P, Clear A, Coutinho R, Wilson A, Matthews J, Owen A, Shanyinde M, Lister TA, Calaminici M & Gribben JG (2013) Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. Journal of Clinical Oncology, 31, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL & Shipp MA (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood, 116, 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner TC, Gascoyne RD, Anderson ME, Kingma DW, Adomat SA, Said J & Jaffe ES (1996) Nodular lymphocyte-predominant Hodgkin’s disease associated with large-cell lymphoma: analysis of Ig gene rearrangements by V-J polymerase chain reaction. Blood, 88, 657–666. [PubMed] [Google Scholar]
- Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C, Dunn CD, Craig FE, Williams JW Jr. & Banks PM (1994) Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood, 83, 1595–1602. [PubMed] [Google Scholar]
- Gupta S, Yeh S, Chami R, Punnett A & Chung C (2013) The prognostic impact of tumour-associated macrophages and Reed-Sternberg cells in paediatric Hodgkin lymphoma. European Journal of Cancer, 49, 3255–3261. [DOI] [PubMed] [Google Scholar]
- Hansmann ML, Stein H, Fellbaum C, Hui PK, Parwaresch MR & Lennert K (1989) Nodular paragranuloma can transform into high-grade malignant lymphoma of B type. Human Pathology, 20, 1169–1175. [DOI] [PubMed] [Google Scholar]
- Hart M, Thakral B, Yohe S, Balfour HH Jr., Singh C, Spears M & McKenna RW (2014) EBV-positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. American Journal of Surgical Pathology, 38, 1522–1529. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Martin-Subero JI, Gesk S, Husken J, Giefing M, Nagel I, Riemke J, Chott A, Klapper W, Parrens M, Merlio JP, Kuppers R, Brauninger A, Siebert R & Hansmann ML (2008) Detection of genomic imbalances in microdissected Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma by array-based comparative genomic hybridization. Haematologica, 93, 1318–1326. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Doring C, Jakobus C, Rengstl B, Newrzela S, Tousseyn T, Sagaert X, Ponzoni M, Facchetti F, de Wolf-Peeters C, Steidl C, Gascoyne R, Kuppers R & Hansmann ML (2013a) Nodular lymphocyte predominant hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma--endpoints of a spectrum of one disease? PloS One, 8, e78812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S, Eichenauer DA, Plutschow A, Mottok A, Bob R, Koch K, Bernd HW, Cogliatti S, Hummel M, Feller AC, Ott G, Moller P, Rosenwald A, Stein H, Hansmann ML, Engert A & Klapper W (2013b) The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood, 122, 4246–4252; quiz 4292. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Eray M, Doring C, Lehtinen T, Brunnberg U, Kujala P, Vornanen M & Hansmann ML (2014) Diffuse large B cell lymphoma derived from nodular lymphocyte predominant Hodgkin lymphoma presents with variable histopathology. BMC Cancer, 14, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S, Thurner L, Kemele M, Regitz E, Fadle N, Preuss K, Bohle R, Vornanen M, von Muller L, Kempf VA., Kuppers, R., Hansmann, M., Pfreundcshnuh, M. (2015a) Involvement of Moraxella Catarrhalis in the Pathogenesis of Hodgkin Lymphoma (Nodular Lymphocye Predominant Type, IGD-Positive). Hematological Oncology, 33, 2. [Google Scholar]
- Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, Madsen M, Hartmann S, Doring C, Vucic E, Chan FC, Ennishi D, Tousseyn T, de Wolf-Peeters C, Perner S, Wlodarska I, Steidl C, Gascoyne RD & Hansmann ML (2015b) Array comparative genomic hybridization reveals similarities between nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. British Journal of Haematology, 169, 415–422. [DOI] [PubMed] [Google Scholar]
- Rosdahl N, Konradsen HB, Storm HH & Melbye M (2003) Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. New England Journal of Medicine, 349, 1324–1332. [DOI] [PubMed] [Google Scholar]
- Hodgkin T (1832) On some Morbid Appearances of the Absorbent Glands and Spleen. Medico-Chirurgical Transactions, 17, 68–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JZ, Weisenburger DD, Vose JM, Greiner TC, Aoun P, Chan WC, Lynch JC, Bierman PJ & Armitage JO (2004) Diffuse large B-cell lymphoma arising in nodular lymphocyte predominant Hodgkin lymphoma: A report of 21 cases from the Nebraska Lymphoma Study Group. Leukemia and Lymphoma, 45, 1551–1557. [DOI] [PubMed] [Google Scholar]
- Huppmann AR, Nicolae A, Slack GW, Pittaluga S, Davies-Hill T, Ferry JA, Harris NL, Jaffe ES & Hasserjian RP (2014) EBV may be expressed in the LP cells of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) in both children and adults. American Journal of Surgical Pathology, 38, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introcaso CE, Kantor J, Porter DL & Junkins-Hopkins JM (2008) Cutaneous Hodgkin’s disease. Journal of the American Academy of Dermatology, 58, 295–298. [DOI] [PubMed] [Google Scholar]
- Kamel OW, Weiss LM, van de Rijn M, Colby TV, Kingma DW & Jaffe ES (1996) Hodgkin’s disease and lymphoproliferations resembling Hodgkin’s disease in patients receiving long-term low-dose methotrexate therapy. American Journal of Surgical Pathology, 20, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Kanzler H, Kuppers R, Hansmann ML & Rajewsky K (1996) Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. Journal of Experimental Medicine, 184, 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube K, Niino D, Kimura Y & Ohshima K (2013) Classical Hodgkin lymphoma, lymphocyte depleted type: clinicopathological analysis and prognostic comparison with other types of classical Hodgkin lymphoma. Pathology, Research and Practice, 209, 201–207. [DOI] [PubMed] [Google Scholar]
- Klimm B, Franklin J, Stein H, Eichenauer DA, Haverkamp H, Diehl V, Fuchs M, Borchmann P & Engert A (2011) Lymphocyte-depleted classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin study group. Journal of Clinical Oncology, 29, 3914–3920. [DOI] [PubMed] [Google Scholar]
- Kritharis A, Pilichowska M & Evens AM (2016) How I manage patients with grey zone lymphoma. British Journal of Haematology, 174, 345–350. [DOI] [PubMed] [Google Scholar]
- Kuppers R (2012) New insights in the biology of Hodgkin lymphoma. Hematology: The Education Program of the American Society of Hematology, 2012, 328–334. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R & Hansmann ML (1994) Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proceedings of the National Academy of Sciences of the United States of America, 91, 10962–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Do C, Gourraud PA, de Paiva GR, Valmary S & Brousset P (2015) Prevalence of Common Non-Hodgkin Lymphomas and Subtypes of Hodgkin Lymphoma by Nodal Site of Involvement: A Systematic Retrospective Review of 938 Cases. Medicine (Baltimore), 94, e987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MS, Beaty M, Sorbara L, Cheng RZ, Pittaluga S, Raffeld M & Jaffe ES (2002) T-cell/histiocyte-rich large B-cell lymphoma: a heterogeneous entity with derivation from germinal center B cells. Am J Surg Pathol, 26, 1458–1466. [DOI] [PubMed] [Google Scholar]
- MacLennan KA, Bennett MH, Tu A, Hudson BV, Easterling MJ, Hudson GV & Jelliffe AM (1989) Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin’s disease. A study of 1659 patients. Cancer, 64, 1686–1693. [DOI] [PubMed] [Google Scholar]
- Macmahon B (1957) Epidemiological evidence of the nature of Hodgkin’s disease. Cancer, 10, 1045–1054. [DOI] [PubMed] [Google Scholar]
- Mani H & Jaffe ES (2009) Hodgkin lymphoma: an update on its biology with new insights into classification. Clinical Lymphoma & Myeloma, 9, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Falini B, Delsol G, Isaacson PG, Pileri S & Stein H (1997) Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. New England Journal of Medicine, 337, 453–458. [DOI] [PubMed] [Google Scholar]
- Mason DY, Banks PM, Chan J, Cleary ML, Delsol G, de Wolf Peeters C, Falini B, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES, Knowles DM, Miiller-Hermelink HK, Pileri S, Ralfkiaer E, Stein H & Warnke R (1994) Nodular lymphocyte predominance Hodgkin’s disease. A distinct clinicopathological entity. American Journal of Surgical Pathology, 18, 526–530. [DOI] [PubMed] [Google Scholar]
- Melani C, Major A, Schowinsky J, Roschewski M, Pittaluga S, Jaffe ES, Pack SD, Abdullaev Z, Ahlman MA, Kwak JJ, Morgan R, Rabinovitch R, Pan Z, Haverkos BM, Gutman JA, Pollyea DA, Smith CA, Wilson WH & Kamdar M (2017) PD-1 Blockade in Mediastinal Gray-Zone Lymphoma. N Engl J Med, 377, 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler JH, Kelly J, Voci S, Marquis D, Rich L, Rossi RM, Bernstein SH, Jordan CT, Liesveld J, Fisher RI & Friedberg JW (2008) Bortezomib and gemcitabine in relapsed or refractory Hodgkin’s lymphoma. Annals of Oncology, 19, 1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroch J, Copie-Bergman C, de Leval L, Plonquet A, Martin-Garcia N, Delfau-Larue MH, Molinier-Frenkel V, Belhadj K, Haioun C, Audouin J, Swerdlow SH, Marafioti T & Gaulard P (2012) Follicular Peripheral T-cell Lymphoma Expands the Spectrum of Classical Hodgkin Lymphoma Mimics. American Journal of Surgical Pathology, 36, 1636–1646. [DOI] [PubMed] [Google Scholar]
- Mottok A & Steidl C (2018) Biology of classical Hodgkin lymphoma: implications for prognosis and novel therapies. Blood, 131, 1654–1665. [DOI] [PubMed] [Google Scholar]
- Musshoff K (1971) Prognostic and therapeutic implications of staging in extranodal Hodgkin’s disease. Cancer Research, 31, 1814–1827. [PubMed] [Google Scholar]
- Nakayama S, Yokote T, Hiraoka N, Nishiwaki U, Hanafusa T, Nishimura Y & Tsuji M (2016) Role of mast cells in fibrosis of classical Hodgkin lymphoma. International Journal of Immunopathology and Pharmacology, 29, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam-Cha SH, Roncador G, Sanchez-Verde L, Montes-Moreno S, Acevedo A, Dominguez-Franjo P & Piris MA (2008) PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. American Journal of Surgical Pathology, 32, 1252–1257. [DOI] [PubMed] [Google Scholar]
- Nam-Cha SH, Montes-Moreno S, Salcedo MT, Sanjuan J, Garcia JF & Piris MA (2009) Lymphocyte-rich classical Hodgkin’s lymphoma: distinctive tumor and microenvironment markers. Modern Pathology, 22, 1006–1015. [DOI] [PubMed] [Google Scholar]
- Natkunam Y, Goodlad JR, Chadburn A, de Jong D, Gratzinger D, Chan JK, Said J & Jaffe ES (2017) EBV-Positive B-Cell Proliferations of Varied Malignant Potential: 2015 SH/EAHP Workshop Report-Part 1. American Journal of Clinical Pathology, 147, 129–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Gratzinger D, Chadburn A, Goodlad JR, Chan JKC, Said J, Jaffe ES & de Jong D (2018) Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood. In press. DOI 10.1182/blood-2018-04-842559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolae A, Pittaluga S, Venkataraman G, Vijnovich-Baron A, Xi L, Raffeld M & Jaffe ES (2013) Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. American Journal of Surgical Pathology, 37, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolae A, Pittaluga S, Abdullah S, Steinberg SM, Pham TA, Davies-Hill T, Xi L, Raffeld M & Jaffe ES (2015) EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood, 126, 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, Sehn LH, Feldman T, Abramson JS, Kritharis A, Hernandez-Ilizaliturri FJ, Lossos IS, Press OW, Fenske TS, Friedberg JW, Vose JM, Blum KA, Jagadeesh D, Woda B, Gupta GK, Gascoyne RD, Jaffe ES & Evens AM (2017) Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv, 1, 2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga S & Jaffe ES (2010) T-cell/histiocyte-rich large B-cell lymphoma. Haematologica, 95, 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Fountaine T, Raffeld M, Jaffe ES & Pittaluga S (2006) IgD Positive L&H Cells Identify a Unique Subset of Nodular Lymphocyte Predominant Hodgkin Lymphoma. American Journal of Surgical Pathology, 30, 585–592. [DOI] [PubMed] [Google Scholar]
- Rahemtullah A, Reichard KK, Preffer FI, Harris NL & Hasserjian RP (2006) A double-positive CD4+CD8+ T-cell population is commonly found in nodular lymphocyte predominant Hodgkin lymphoma. American Journal of Clinical Pathology, 126, 805–814. [DOI] [PubMed] [Google Scholar]
- Renner C & Stenner F (2018) Cancer Immunotherapy and the Immune Response in Hodgkin Lymphoma. Frontiers in Oncology, 8, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD & Staudt LM (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. Journal of Experimental Medicine, 198, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger T, Gascoyne RD, Jaffe ES, de Jong D, Delabie J, De Wolf-Peeters C, Poppema S, Xerri L, Gisselbrecht C, Wiedenmann S & Muller-Hermelink HK (2002) Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Annals of Oncology, 13 Suppl 1, 44–51. [DOI] [PubMed] [Google Scholar]
- Saarinen S, Aavikko M, Aittomaki K, Launonen V, Lehtonen R, Franssila K, Lehtonen HJ, Kaasinen E, Broderick P, Tarkkanen J, Bain BJ, Bauduer F, Unal A, Swerdlow AJ, Cooke R, Makinen MJ, Houlston R, Vahteristo P & Aaltonen LA (2011) Exome sequencing reveals germline NPAT mutation as a candidate risk factor for Hodgkin lymphoma. Blood, 118, 493–498. [DOI] [PubMed] [Google Scholar]
- Sarkozy C, Molina T, Ghesquieres H, Michallet AS, Dupuis J, Damotte D, Morsschauser F, Parrens M, Martin L, Dartigues P, Stamatoullas A, Hirsch P, Fabiani B, Bouabdallah K, da Silva MG, Maerevoet M, Laurent C, Coiffier B, Salles G & Traverse-Glehen A (2017) Mediastinal gray zone lymphoma: clinico-pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica, 102, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, Kurtin P, Dal Cin P, Ladd C, Feuerhake F, Aguiar RC, Li S, Salles G, Berger F, Jing W, Pinkus GS, Habermann T, Dalla-Favera R, Harris NL, Aster JC, Golub TR & Shipp MA (2003) The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood, 102, 3871–3879. [DOI] [PubMed] [Google Scholar]
- Schreck S, Friebel D, Buettner M, Distel L, Grabenbauer G, Young LS & Niedobitek G (2009) Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematological Oncology, 27, 31–39. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Koritzinsky EH, Clarke CA, Suneja G, Morton LM & Engels EA (2014) Prevalence of HIV Infection among U.S. Hodgkin lymphoma cases. Cancer Epidemiology, Biomarkers and Prevention, 23, 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers DW, Lillicrap SC & Barnes A (1974) Patterns of lymph node involvement in relation to hypotheses about the modes of spread of Hodgkin’s disease. Cancer, 34, 1779–1786. [DOI] [PubMed] [Google Scholar]
- Sohani AR, Jaffe ES, Harris NL, Ferry JA, Pittaluga S & Hasserjian RP (2011) Nodular lymphocyte-predominant hodgkin lymphoma with atypical T cells: a morphologic variant mimicking peripheral T-cell lymphoma. American Journal of Surgical Pathology, 35, 1666–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina V, Bruscaggin A, Cuccaro A, Martini M, Di Trani M, Forestieri G, Manzoni M, Condoluci A, Arribas A, Terzi-Di-Bergamo L, Locatelli SL, Cupelli E, Ceriani L, Moccia AA, Stathis A, Nassi L, Deambrogi C, Diop F, Guidetti F, Cocomazzi A, Annunziata S, Rufini V, Giordano A, Neri A, Boldorini R, Gerber B, Bertoni F, Ghielmini M, Stussi G, Santoro A, Cavalli F, Zucca E, Larocca LM, Gaidano G, Hohaus S, Carlo-Stella C & Rossi D (2018) Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood, 131, 2413–2425. [DOI] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC & Gascoyne RD (2010a) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. New England Journal of Medicine, 362, 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl C, Telenius A, Shah SP, Farinha P, Barclay L, Boyle M, Connors JM, Horsman DE & Gascoyne RD (2010b) Genome-wide copy number analysis of Hodgkin Reed-Sternberg cells identifies recurrent imbalances with correlations to treatment outcome. Blood, 116, 418–427. [DOI] [PubMed] [Google Scholar]
- Steidl C, Diepstra A, Lee T, Chan FC, Farinha P, Tan K, Telenius A, Barclay L, Shah SP, Connors JM, van den Berg A & Gascoyne RD (2012) Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood, 120, 3530–3540. [DOI] [PubMed] [Google Scholar]
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, Fischer RE, Jackson CM, Lin AY, Baumler C, Siegert E, Marx A, Vaishnaw AK, Grodzicky T, Fleisher TA & Lenardo MJ (2001) The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood, 98, 194–200. [DOI] [PubMed] [Google Scholar]
- Sundeen JT, Cossman J & Jaffe ES (1988) Lymphocyte predominant Hodgkin’s disease nodular subtype with coexistent “large cell lymphoma”. Histological progression or composite malignancy? American Journal of Surgical Pathology, 12, 599–606. [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD & Jaffe ES (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127, 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil J, Laumen H, Marafioti T, Hummel M, Lenz G, Wirth T & Stein H (2001) Defective octamer-dependent transcription is responsible for silenced immunoglobulin transcription in Reed-Sternberg cells. Blood, 97, 3191–3196. [DOI] [PubMed] [Google Scholar]
- Tiacci E, Doring C, Brune V, van Noesel CJ, Klapper W, Mechtersheimer G, Falini B, Kuppers R & Hansmann ML (2012) Analyzing primary Hodgkin and Reed-Sternberg cells to capture the molecular and cellular pathogenesis of classical Hodgkin lymphoma. Blood, 120, 4609–4620. [DOI] [PubMed] [Google Scholar]
- Tiacci E, Ladewig E, Schiavoni G, Penson A, Fortini E, Pettirossi V, Wang Y, Rosseto A, Venanzi A, Vlasevska S, Pacini R, Piattoni S, Tabarrini A, Pucciarini A, Bigerna B, Santi A, Gianni AM, Viviani S, Cabras A, Ascani S, Crescenzi B, Mecucci C, Pasqualucci L, Rabadan R & Falini B (2018) Pervasive mutations of JAK-STAT pathway genes in classical Hodgkin lymphoma. Blood, 131, 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati M, Delage-Corre M, Monteil J, Abraham J, Moreau S, Remenieras L, Gourin MP, Dmytruk N, Olivrie A, Turlure P, Girault S, Labrousse F, Preux PM, Jaccard A & Bordessoule D (2015) CD68-positive tumor-associated macrophages predict unfavorable treatment outcomes in classical Hodgkin lymphoma in correlation with interim fluorodeoxyglucose-positron emission tomography assessment. Leukemia and Lymphoma, 56, 332–341. [DOI] [PubMed] [Google Scholar]
- Traverse-Glehen A, Pittaluga S, Gaulard P, Sorbara L, Alonso MA, Raffeld M & Jaffe ES (2005) Mediastinal gray zone lymphoma - The missing link between classic Hodgkin’s lymphoma and mediastinal large B-cell lymphoma. American Journal of Surgical Pathology, 29, 1411–1421. [DOI] [PubMed] [Google Scholar]
- Tzankov A, Zimpfer A, Pehrs AC, Lugli A, Went P, Maurer R, Pileri S & Dirnhofer S (2003) Expression of B-cell markers in classical hodgkin lymphoma: a tissue microarray analysis of 330 cases. Modern Pathology, 16, 1141–1147. [DOI] [PubMed] [Google Scholar]
- Tzankov A, Matter MS & Dirnhofer S (2010) Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma. Pathobiology, 77, 301–308. [DOI] [PubMed] [Google Scholar]
- Untanu RV, Back J, Appel B, Pei Q, Chen L, Buxton A, Hodgson DC, Ehrlich PF, Constine LS, Schwartz CL & Hutchison RE (2018) Variant histology, IgD and CD30 expression in low-risk pediatric nodular lymphocyte predominant Hodgkin lymphoma: A report from the Children’s Oncology Group. Pediatric Blood & Cancer, 65, e26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Neste E, Andre M, Gastinne T, Stamatoullas A, Haioun C, Belhabri A, Reman O, Casasnovas O, Ghesquieres H, Verhoef G, Claessen MJ, Poirel HA, Copin MC, Dubois R, Vandenberghe P, Stoian IA, Cottereau AS, Bailly S, Knoops L & Morschhauser F (2018) A phase II study of the oral JAK1/JAK2 inhibitor ruxolitinib in advanced relapsed/refractory Hodgkin lymphoma. Haematologica, 103, 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen DJ, Vrints LW, Hofstra G, Crommelin MA, Coebergh JW & Breed WP (1997) Disappearance of prognostic significance of histopathological grading of nodular sclerosing Hodgkin’s disease for unselected patients, 1972–92. British Journal of Haematology, 96, 322–327. [DOI] [PubMed] [Google Scholar]
- Vassallo J, Lamant L, Brugieres L, Gaillard F, Campo E, Brousset P & Delsol G (2006) ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin’s lymphoma: report of 10 cases. American Journal of Surgical Pathology, 30, 223–229. [DOI] [PubMed] [Google Scholar]
- Venkataraman G, Raffeld M, Pittaluga S & Jaffe ES (2011) CD15-expressing nodular lymphocyte-predominant Hodgkin lymphoma. Histopathology, 58, 803–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman G, Song JY, Tzankov A, Dirnhofer S, Heinze G, Kohl M, Traverse-Glehen A, Eberle FC, Hanson JC, Raffeld MA, Pittaluga S & Jaffe ES (2013) Aberrant T-cell antigen expression in classical Hodgkin lymphoma is associated with decreased event-free survival and overall survival. Blood, 121, 1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger MA, Tiacci E, Schneider S, Arnolds J, Ruschenbaum S, Duppach J, Seifert M, Doring C, Hansmann ML & Kuppers R (2018) Human CD30+ B cells represent a unique subset related to Hodgkin lymphoma cells. Journal of Clinical Investigation. 128, 2996–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Pittaluga S, Nicolae A, Camphausen K, Shovlin M, Steinberg SM, Roschewski M, Staudt LM, Jaffe ES & Dunleavy K (2014) A prospective study of mediastinal gray-zone lymphoma. Blood, 124, 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska I, Stul M, De Wolf-Peeters C & Hagemeijer A (2004) Heterogeneity of BCL6 rearrangements in nodular lymphocyte predominant Hodgkin’s lymphoma. Haematologica, 89, 965–972. [PubMed] [Google Scholar]
- Wu D, Thomas A & Fromm JR (2016) Reactive T cells by flow cytometry distinguish Hodgkin lymphomas from T cell/histiocyte-rich large B cell lymphoma. Cytometry. Part B: Clinical Cytometry, 90, 424–432. [DOI] [PubMed] [Google Scholar]
- Xing KH, Connors JM, Lai A, Al-Mansour M, Sehn LH, Villa D, Klasa R, Shenkier T, Gascoyne RD, Skinnider B & Savage KJ (2014) Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood, 123, 3567–3573. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T & Uchiyama T (2008) PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood, 111, 3220–3224. [DOI] [PubMed] [Google Scholar]
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB, Collins G, Savage KJ, Trneny M, Kato K, Farsaci B, Parker SM, Rodig S, Roemer MGM, Ligon AH & Engert A (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncology, 17, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]