Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review (original) (raw)
Abstract
Background
Phellodendri Cortex (PC) or Huang Bai. According to the scientific database of China Plant Species and Chinese pharmacopeia 2015 edition, PC has two main species which are Phellodendron amurense Rupr (PAR) or “Guan Huang bai” in Chinese and Phellodendron chinense Schneid (PCS) or “Chuan Huang bai” in Chinese. The crude drugs of PAR and PCS are also called Phellodendri amurensis cortex (PAC) and Phellodendri chinense cortex (PCC), respectively. The medicinal part of the plant is the dried trunk bark. PC has comprehensive therapeutic effects which include anti-inflammatory, antimicrobial, anticancer, hypotensive, antiarrhythmic, antioxidant, and antipyretic agents. The exact ingredients in PC and its species are not fully summarised.
Aim of the Study
This study was designed to review and evaluate the pharmacological actions of compounds and to explore the pharmacokinetic knowledge of PC and its species and to also identify the chemical compound(s) with a potential therapeutic effect on atopic dermatitis.
Methods
“Huang Bai” and its English, botanical, and pharmaceutical names were used as keywords to perform database search in Encyclopaedia of traditional Chinese Medicines, PubMed, EMBASE, MEDLINE, Science Direct, Scopus, Web of Science, and China Network Knowledge Infrastructure. The data selection criteria included all the studies that were related to the phytochemical, pharmacological, and pharmacokinetic perspectives of PC and its species or their active constituents. More importantly, the voucher number has been provided to ensure the genuine bark of PC used as the medicinal part in the studies.
Results
140 compounds were summarized from PC and its species: specifically, 18 compounds from PCC, 44 compounds from PCS, 34 compounds from PAC, and 84 compounds from PAR. Obacunone and obaculactone are probably responsible for antiatopic dermatitis effect. PC and its species possess a broad spectrum of pharmacological actions including anti-inflammatory effect, antibacterial effect, antiviral effect, antitumor effect, antigout effect, antiulcer effect, neuroprotective effect, and antiatopic dermatitis effect. PC could widely distribute in plasma, liver, spleen, kidney, and brain. Berberine may be responsible for the toxic effect on the susceptible users with hemolytic disease or in the peripartum and neonatal period.
Conclusions
The compounds of the crude bark of PC and its subspecies have showcased a wide range of pharmacological effects. Pharmacological efficacies of PC are supported by its diverse class of alkaloid, limonoid, phenolic acid, quinic acid, lignan, and flavonoid. Obacunone and obaculactone could be the bioactive compounds for atopic dermatitis management. PC and its subspecies are generally safe to use but extra care is required for certain conditions and group of people.
1. Introduction
Phellodendri Cortex (PC) is also known as “Huang bai” in Chinese and “Obaku” in Japanese. PC is a plant grown in China, Korea, Japan, Vietnam, and Far East of Russia. The earliest record of this plant was on “Shennong's Classic of Materia Medica” [1]. According to the scientific database of China Plant Species and Chinese pharmacopeia 2015 edition, PC has two main species which are Phellodendron amurense Rupr (PAR) or “Guan Huang bai” in Chinese and Phellodendron chinense Schneid (PCS) or “Chuan Huang bai” in Chinese. The crude drugs of PAR and PCS are called Phellodendri amurensis cortex (PAC) and Phellodendri chinense cortex (PCC), respectively. PAR and PCS are naturally grown in the Northeast and Southwest part of China, respectively [2]. According to the latest information from “information system of Chinese rare and endangered plants,” PAR is categorized as one of the second degrees of endangered plants. PAR and PCS could be interchangeably used in clinical application because both species contain similar chemical constituents [3]. According to the scientific database of China Plant Species and Chinese pharmacopeia 2015 edition, PC is categorized in the family of Phellodendron Rupr. The medicinal part of the plant is the dried trunk bark. PC had been viewed as one of the 50 fundamental herbs in Chinese herbalism. Traditionally, its medicinal part could exert therapeutic effects in various diseases such as meningitis, cirrhosis, dysentery, pneumonia, tuberculosis, etc. [4, 5]. Nowadays, PC has comprehensive therapeutic effects which include immune modulation, anti-inflammatory, antimicrobial, antibacterial, anticancer, hypotensive, antiarrhythmic, antioxidant, antigastric ulcer, and antipyretic agents, etc. [5]. According to the Clinical Chinese Materia Medica, 2006 edition, PC has a bitter flavor and cold nature and can enter the meridian of kidney, bladder, and large intestine. PC could clear heat, dry dampness, drain fire, eliminate steam, resolve toxin, and treat sores [6]. PC and its species contain various chemical derivatives. One of the important ones is alkaloids which contain berberine and jatrorrhizine. Both compounds were proven to be effective against some type of tumours, infections, neurological diseases [7]. To further acquire the knowledge on PC, a systematic review of its phytochemical, pharmacological, and pharmacokinetic properties is required. The aim of this study is to review and evaluate the pharmacological actions of compounds as well as to explore the pharmacokinetic knowledge of the PC and its species. The chemical compounds that exert therapeutic effect for atopic dermatitis are also desired.
2. Methods
Data were searched from the following databases until May 2018: Encyclopaedia of Traditional Chinese Medicines; PubMed; EMBASE; MEDLINE; ScienceDirect; SCOPUS; Web of Science; China Network Knowledge Infrastructure. The keywords used for the literature search included: Huang Bai and its English, botanical, and pharmaceutical names. The selection criteria included process controls of the herbal substances, reporting reference standards such as authentication of reference materials and profile chromatograms, and analytical procedures and validation data. Papers in English or Chinese language are included for this review. Scientific rigidity was determined by the chemical markers of herbs through the use of strict parameters in testing, quantitative, and qualitative measures of the bioactive components, such as high-performance liquid chromatography, fingerprint spectrum, correlations differentiation, and stability evaluation, reference standards, and toxicological assessments. Plant voucher specimens are a guarantee for traceability of the plant material and data verification for other researchers or commercial purposes [60]. The chemical formulas of the compounds of PC and its species were acquired from selected studies. Chemical structures and molecular weights were extracted from ChemDraw professional 170.
3. Results
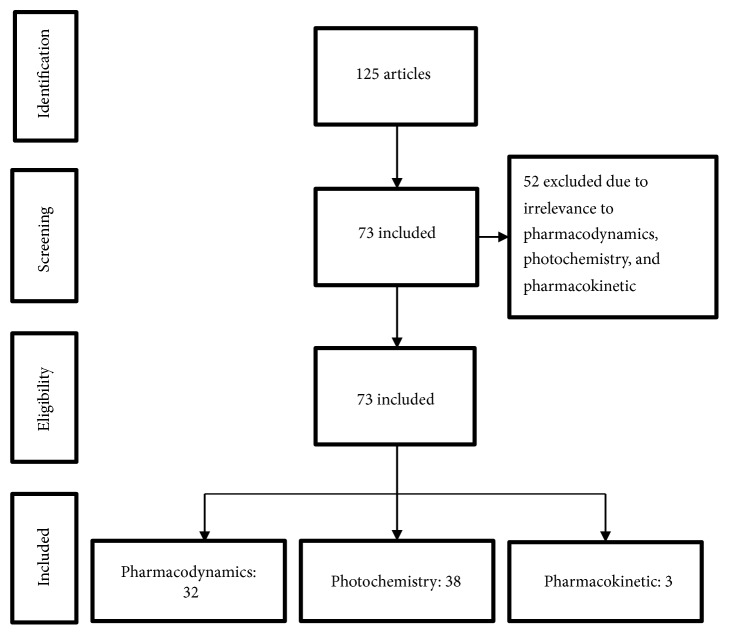
A total of 125 papers were identified through the literature search. Fifty-two papers were excluded based on the reasons for nonpharmacodynamic, nonphytochemistry, and nonpharmacological studies. 73 studies fitted the selection criteria. Among these articles, 32 studies are about pharmacodynamics, 38 studies are about phytochemistry, and 3 studies are about pharmacokinetics (Figure 1).
Figure 1.
PC study selection flowchart.
4. Bioactive Compounds
The crude barks of PC and its species contain alkaloids, isoquinoline alkaloid, limonoids, phenolic acid, quinic acid, lignans, and flavonoid, and so on. A summary of the bioactive compounds of the PC and its species reported in the included studies is presented in Table 1. Molecular formula, molecular weight, and chemical structure of the major constituted alkaloids of PC, including berberine, palmatine, and jatrorrhizine, are listed in Tables 2, 3, 4, and 5. They could exert a broad spectrum of pharmacological influence which contains antimicrobial, anti-inflammation, antitumor, antidepressant, and antiulcer effects [47]. Limonoids including limonin and obakunone play an important role in the anti-inflammatory effects of PC [11]. Phenolic acid or phenol carboxylic acids belong to aromatic acid compound substances which are characterized by a phenolic ring and an organic carboxylic acid function [61]. According to the description on Buchler quinine plant in Braunschweig, Germany, quinic acid is a natural sugary compound which can be found in multiple plants such as well-known coffee beans and tobacco leaves. Based on the description on PubChem, lignans affiliate a class of dibenzylbutane derivatives which exists in plants and in body fluids such as bile, serum, urine, etc. These compounds have anticancer potential. Quercetin belongs to flavonoids which can reduce coronary heart disease according to PubChem data. Two species of PC, PCS and PAR, can be differentiated based on the contents of the chemical compounds. Specifically, the 2005 edition of Chinese Medicinal encyclopedia described the PCS's minimal content of berberine hydrochloride and phellodendron hydrochloride to be 3.0% and 0.34%, respectively; the PAR's minimal content of berberine hydrochloride and palmatine hydrochloride is 0.60% and 0.30%, respectively [62].
Table 1.
Summary of chemical constituents isolated from PC and its different species (140 compounds).
| Compound derivatives | Chemical compounds | Methods | References | Original species |
|---|---|---|---|---|
| Alkaloid | Berberine | (i) UPLC-ESI-Q-TOF-MS | (i) [8] | (i) PAC |
| (ii) HPLC-DAD-ESI-MS2 | (ii) [9] | (ii) PCS and PAR | ||
| (iii) UPLC-Q/TOF-HDMS | (iii) [10] | (iii) PAC | ||
| (iv) HPLC-TLC- NMR-EI-MS | (iv) [11] | (iv) PAR | ||
| (v) HPLC-DAD-MS | (v) [12] | (v) PAR | ||
| (vi) N/A | (vi) [13] | (vi) PAC and PCS | ||
| 8,13-dioxo-14-butoxycanadine | CC | [14] | PCS | |
| Berberrubine | UPLC-ESI-Q-TOF-MS | [8] | PAC | |
| Berberastine | UPLC-Q/TOF-HDMS | [10] | PAC | |
| Bis-[4-(dimethylamino)phenyl]methanone | HPLC-ESI-MS/MS | [15] | PAR | |
| Brucine | CE | [16] | PCS | |
| Δ7-dehydrosophoramine | N/A | [17] | PAC | |
| Dihydrocyclobuxine-D | HPLC-ESI-MS/MS | [15] | PAR | |
| 3,4-dihydro-1-[(4-hydroxyphenyl)methyl]-7-methoxy-2-methyl-8-isoquinolinol | HPLC-ESI-MS/MS | [15] | PAR | |
| 3,4-dihydro-1-[(4-hydroxyphenyl)methyl]-7-methoxy-2-methyl-6-isoquinolinol | HPLC-ESI-MS/MS | [15] | PAR | |
| 7,8-dihydroxyrutaecarpine | HPLC-ESI-MS/MS | [15] | PAR | |
| 4-dimethylamino-4 -isopropylbenzene | HPLC-ESI-MS/MS | [15] | PAR | |
| Evodiamine | HPLC-ESI-MS/MS | [15] | PAR | |
| Palmatine | (i) UPLC-ESI-Q-TOF-MS | (i) [8] | (i) PAC | |
| (ii) HPLC-DAD-ESI-MS2 | (ii) [9] | (ii) PCS and PAR | ||
| (iii) UPLC-Q/TOF-HDMS | (iii) [10] | (iii) PCC | ||
| (iv) HPLC-DAD-MS | (iv) [12] | (iv) PAR | ||
| (v) N/A | (v) [18] | (v) PCS | ||
| Tetrahydropalmatine | UPLC-ESI-Q-TOF-MS | [8] | PAC | |
| Tetrahydroberberine | HPLC-ESI-MS/MS | [15] | PAR | |
| Phellodendrine | (i) UPLC-ESI-Q-TOF-MS | (i) [8] | (i) PAC | |
| (ii) HPLC-DAD-ESI-MS2 | (ii) [9] | (ii) PCS and PAR | ||
| (iii) UPLC-Q/TOF-HDMS | (iii) [10] | (iii) PAC | ||
| (iv) HPLC-DAD-MS | (iv) [12] | (iv) PAR | ||
| (v) N/A | (v) [18] | (v) PAC and PCS | ||
| Magnocurarine | (i) HPLC-ESI-MS/MS | (i) [15] | (i) PAR | |
| Magnoflorine | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| (iii) HPLC-DAD-MS | (iii) [12] | (iii) PAR | ||
| (iv) N/A | (iv) [19] | (iv) PCS | ||
| Jatrorrhizine or Neprotin, 2,9,10-Trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-3-ol | (i) UPLC-ESI-Q-TOF-MS | (i) [8] | (i) PAC | |
| (ii) HPLC-DAD-ESI-MS2 | (ii) [9] | (ii) PCS and PAR | ||
| (iii) HPLC-DAD-MS | (iii) [12] | (iii) PAR | ||
| (iv) N/A | (iv) [19] | (iv) PAC | ||
| 13-methoxyjatrorrhizine | HPLC-ESI-MS/MS | [15] | PAR | |
| _Ƴ_-Fagarine | CC | [20] | PAR | |
| Canthin-6-one | CC | [20] | PAR | |
| 4-methoxy-N-methyl-2-quinolone | CC | [20] | PAR | |
| Oxypalmatine | CC | [20] | PAR | |
| Candicine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Lotusine | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PAC | ||
| N-methylhigenamine-7-O-glucopyranoside | HPLC-ESI-MS/MS | [15] | PAR | |
| N-methylhigenamine-7-O-_β_-D-glucopyranoside | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| (−)-Oblongine | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| Isomer-of-berberine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Isomer-of-magnoflorine | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| Isomer-of-palmatine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Tetrahydroreticuline | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Tetrahydrojatrorrhizine | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| Menisperine | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| (iii) N/A | (iii) [19] | (iii) PAC | ||
| (+) N-methylcorydine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| N-methyl Tetrahydropalmatine | HPLC-ESI-MS/MS | [15] | PAR | |
| N-methylflindersine | HPLC-ESI-MS/MS | [15] | PAR | |
| Litcubine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Hydroxyl-palmatine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| 11-hydroxylpalmatine | HPLC-ESI-MS/MS | [15] | PAR | |
| 13-hydroxypalmatine | HPLC-ESI-MS/MS | [15] | PAR | |
| 7-hydroxy-8-methoxydedihydrorutaecarpine | HPLC-ESI-MS/MS | [15] | PAR | |
| Tetrahydropalmatine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Xanthoplanine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| N-methylphoebine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Columbamine | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| Dihydroxyl-jatrorrhizine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Epiberberine | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| (6aS)-1,2,10,11-tetramethoxy-6,6-dimethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de, g] quinolinium | UPLC-Q/TOF-HDMS | [10] | PCC | |
| Dasycarpamin | UPLC-Q/TOF-HDMS | [10] | PCC | |
| Pteleine | HPLC-UV | [21] | PAR | |
| (-)-(R)-platydesmin | HPLC-UV | [21] | PAR | |
| Noroxyhydrastinine | HPLC-UV | [21] | PAR | |
| Chilenine | HPLC-UV | [21] | PAR | |
| Rutecarpine | HPLC-ESI-MS/MS | [15] | PAR | |
| Skimmianine | HPLC-ESI-MS/MS | [15] | PAR | |
| Tembetarine | HPLC-ESI-MS/MS | [15] | PAR | |
| Tetramethyl-O-scutellarin | UPLC-Q/TOF-HDMS | [10] | PCC | |
| 5,8,13,13a-Tetrahydro-2,9,10,11-tetrahydroxy-3-methoxy-7-methyl-6H-dibenzo[a,g]quinolizinium | HPLC-ESI-MS/MS | [15] | PAR | |
| _Ƴ_-hydroxybutenolide derivatives II | UPLC-Q/TOF-HDMS | [10] | PCC | |
| Isoquinoline alkaloid | Armepavine | UPLC-ESI-Q-TOF-MS | [8] | PAC |
| Demethyleneberberine | UPLC-ESI-Q-TOF-MS | [8] | PAC | |
| 8-oxoberberine | HPLC-ESI-MS/MS | [15] | PAR | |
| 8-oxoepiberberine | HPLC-ESI-MS/MS | [15] | PAR | |
| 8-oxopalmatine | HPLC-ESI-MS/MS | [15] | PAR | |
| Oxyberberine | HPLC | [20] | PAR | |
| Oxypalmatine | HPLC | [20] | PAR | |
| Limonoid | Kihadanin B | N/A | [19] | PAC |
| Niloticin | N/A | [18] | PAC | |
| Niloticin acetate | N/A | [18] | PCS | |
| Obaculactone or limonin | (i) UPLC-ESI-Q-TOF-MS | (i) [8] | (i) PAC | |
| (ii) CC | (ii) [20] | (ii) PAR | ||
| (iii) UPLC-Q/TOF-HDMS | (iii) [10] | (iii) PCC | ||
| (iv) HPLC-TLC- NMR- EI-MS | (iv) [11] | (iv) PAC | ||
| (v) N/A | (v) [18] | (v) PAC | ||
| Derivative of obaculactone | UPLC-Q/TOF-HDMS | [10] | PCC | |
| Obacunone or Obacunoic acid | (i) CC | (i) [20] | (i) PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| (iii) HPLC-TLC- NMR- EI-MS | (iii) [11] | (iii) PAC | ||
| (iv) HPLC-DAD-ESI-MS2 | (iv) [9] | (iv) PAR | ||
| (v) N/A | (v) [18] | (v) PAC | ||
| 12_α_-hydroxylimonin | CC | [20] | PAR | |
| Piscidinol A | N/A | [18] | (i) PCS | |
| Rutaevin | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| Coniferin | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Vanilloloside | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| N-methyltetrahydrocolumbamine | UPLC-Q/TOF-HDMS | [10] | PCC | |
| N-acyl amines | Herculin | N/A | [19] | PAC |
| Phenolic acid | Ferulic acid | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR |
| (ii) HPLC-TLC- NMR- EI-MS | (ii) [11] | (ii) PAC | ||
| Methyl ferulate | CC | [4] | PCS | |
| Protocatechuic acid | CC | [4] | PCS | |
| Quinic acid | Quinic acid | UPLC-Q/TOF-HDMS | [10] | PAC |
| Neo-chlorogenic acid | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| 3-O-feruloylquinic acid | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PAC | ||
| 3-O-feruloylquinic acid glucoside | UPLC-Q/TOF-HDMS | [10] | PCC | |
| 4-O-feruloylquinic acid | HPLC | [22] | PCS | |
| 5-O-feruloylquinic acid | HPLC | [22] | PCS | |
| Chlorogenic acid | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PAC | ||
| (iii) HPLC | (iii) [23] | (iii) PAR | ||
| (iv) HPLC-DAD-MS | (iv) [12] | (iv) PAR | ||
| Methyl 3-_O_-feruloylquinate | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Methyl 5-O-feruloylquinate | NMR | [24] | PAR | |
| 3-Feruoyl-4-caffeoylquinic acid | HPLC-DAD-ESI-MS2 | [9] | PAR | |
| Sanleng acid | NMR | [25] | PAC | |
| Hydroxycinnamic acid | Caffeic Acid Methyl Ester | HPLC | [22] | PCS |
| Phytosterol | _β_-Sitosterol | N/A | [18] | PAC |
| Lignan | (+/-)-lyoniresinol | HPLC-DAD-ESI-MS2 | [9] | PAR |
| (+/-)-5,5′-dimethoxylariciresinol-4-_O_-glucoside | (i) HPLC-DAD-ESI-MS2 | (i) [9] | (i) PCS and PAR | |
| (ii) UPLC-Q/TOF-HDMS | (ii) [10] | (ii) PCC | ||
| Syringaresinol di-O-_β_-D-glucopyranoside | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| (-)-Syringaresinol | CC | [4] | PCS | |
| Flavonoid | Amurensin | N/A | [13] | PAC |
| Quercetin | HPLC-TLC- NMR- EI-MS | [11] | PAC | |
| Phellamurin | N/A | [18] | PAC | |
| Phellatin | N/A | [18] | PAC | |
| Phellavin | N/A | [18] | PAC | |
| Phellodendroside | N/A | [18] | PAC | |
| _β_-anhydronoricaritin | UV | [26] | PAR | |
| Icariside-1 | UV | [26] | PAR | |
| Phellamuretin | UV | [26] | PAR | |
| Phelloside | UV | [26] | PAR | |
| Dihydrophelloside | UV | [26] | PAR | |
| Isovaleric acid | UV | [26] | PAR | |
| Kaempferol | UV | [26] | PAR | |
| D-glucose | UV | [26] | PAR | |
| Rutaceae | Noricariside | N/A | [18] | PAC |
| Stigmastane | 7-Dehydrostigmasterol | N/A | [17] | PAC |
| Coumarin | 3-acetyl-3,4-dihydro-5,6-dimethoxy-1H-2-benzopyran-1-one | CC | [27] | PCS |
| Monosaccharide | Syringin | NMR | [25] | PAC |
| Daucosterol | NMR | [25] | PAC | |
| Paraben | N-propyl paraben | HPLC | [5] | PC |
| Phenolic lactone | Phellolactone | CC | [4] | PCS |
| Ferulate | N-butyl Ferulate | CC | [4] | PCS |
| Amurenlactone A | CC | [4] | PCS | |
| Amurenamide A | NMR | [25] | PAC | |
| Hydroxybenzaldehyde | 4-hydroxybenzaldehyde | CC | [4] | PCS |
| Phenolic aldehyde | Vanillin | CC | [4] | PCS |
| Glycoside | Sinapyl aldehyde-4-O-beta-D-glucopyranoside | NMR | [24] | PAR |
| 3,4,5-Trimetoxyphenol O-_β_-D-glucopyranoside | HPLC | [22] | PCS | |
| 2-methoxy-4-(2-propenyl)phenyl-beta-D-glucopyranoside | HPLC | [22] | PCS | |
| 2-(p-hydroxyphenyl)-ethanol-1-O-_β_-D-apiofuranosyl (1–6)-O-_β_-D-glucopyranoside | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| 2-(p-hydroxyphenyl) -ethanol-1-O-_β_-D-glucoside | UPLC-Q/TOF-HDMS | [10] | PCC | |
| 3, 5-dihydroxybenzoicacid-O-xylopyranosyl-glucopyranoside | HPLC-DAD-ESI-MS2 | [9] | PCS and PAR | |
| Phenolic glycoside | Tachioside | HPLC | [22] | PCS |
| Glucoside | Salidroside | HPLC | [22] | PCS |
| 4-hydroxybenzyl alcohol | HPLC | [22] | PCS | |
| Phenol | Methyl syringate | HPLC | [22] | PCS |
| Dehydrovomifoliol | (6S)-dehydrovomifoliol | HPLC | [22] | PCS |
| (6R,7aR)-epiloliolide | HPLC | [22] | PCS |
Table 2.
Molecular formula, molecular weight and chemical structures of compounds derived from PCC species (18 compounds).
Table 3.
Molecular formula, molecular weight and chemical structures of compounds derived from PCS species (44 compounds).
| Compound derivatives | Compound | Molecular formula | Molecular weight | Chemical structures |
|---|---|---|---|---|
| Alkaloid | Berberine | C20H18NO4+ | 336.37 g/mol | 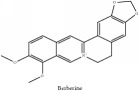 |
| 8,13-dioxo-14-butoxycanadine | C24H25NO7 | 439.46 g/mol | 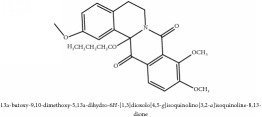 |
|
| Brucine | C23H26N2O4 | 394.47 g/mol | 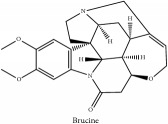 |
|
| Palmatine | C21H22NO4+ | 352.41 g/mol | 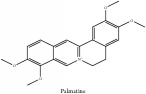 |
|
| Phellodendrine | C20H24NO4+ | 342.41 g/mol | 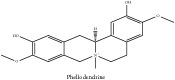 |
|
| Magnoflorine | C20H24NO4+ | 342.41 g/mol | 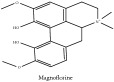 |
|
| Jatrorrhizine or Neprotin, 2,9,10-Trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-3-ol | C20H19NO4 | 337.38 g/mol | 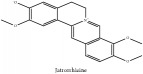 |
|
| Lotusine | C19H24NO3+ | 314.40 g/mol | 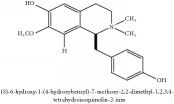 |
|
| (−)-Oblongine | C19H24NO3+ | 314.40 g/mol | 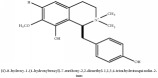 |
|
| Isomer-of-magnoflorine | N/A | |||
| Tetrahydrojatrorrhizine | C20H23NO4 | 341.41 g/mol | 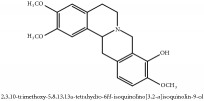 |
|
| Menisperine | C21H26NO4+ | 356.44 g/mol | 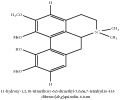 |
|
| Columbamine | C20H20NO4+ | 338.38 g/mol | 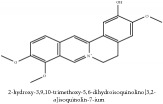 |
|
| Niloticin acetate | C32H50O4 | 498.75 g/mol | 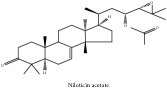 |
|
| Piscidinol A | C30H50O4 | 474.73 g/mol |  |
|
| Quinic acid | Chlorogenic acid | C16H18O9 | 354.31 g/mol | 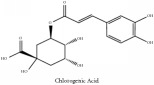 |
| Neo-chlorogenic acid | C16H18O9 | 354.31 g/mol | 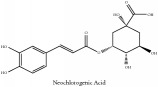 |
|
| 3-O-feruloylquinic acid | C17H20O9 | 368.34 g/mol | 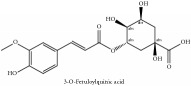 |
|
| 4-O-feruloylquinic acid | C17H20O9 | 368.34 g/mol | 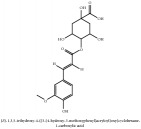 |
|
| 5-O-feruloylquinic acid | C17H20O9 | 368.34 g/mol | 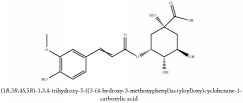 |
|
| Hydroxycinnamic acid | Caffeic Acid Methyl Ester | C10H10O4 | 194.19 g/mol | 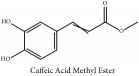 |
| Phenolic acid | Ferulic acid | C10H10O4 | 194.19 g/mol | 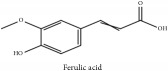 |
| Methyl ferulate | C11H12O4 | 208.21 g/mol | 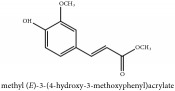 |
|
| Protocatechuic acid | C7H6O4 | 154.12 g/mol | 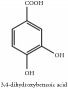 |
|
| Lignan | (+/-)-5,5′-dimethoxylariciresinol-4-_O_-glucoside | C28H38O13 | 582.6 g/mol | 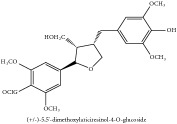 |
| Syringaresinol di-O-_β_-D-glucopyranoside | C33H44O18 | 728.70 g/mol | 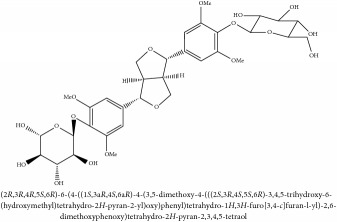 |
|
| (-)-syringaresinol | C22H26O8 | 418.44 g/mol | 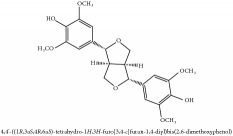 |
|
| Coumarin | 3-acetyl-3,4-dihydro-5,6-dimethoxy-1H-2-benzopyran-1-one | C13H14O5 | 250.25 g/mol | 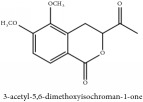 |
| Paraben | N-propyl paraben | C10H12O3 | 180.20 g/mol | 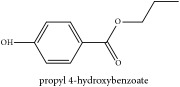 |
| Phenolic lactone | Phellolactone | C13H14O8 | 298.25 g/mol | 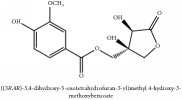 |
| Ferulate | N-butyl Ferulate | C14H18O4 | 250.29 g/mol | 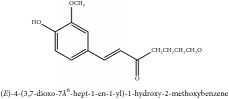 |
| Amurenlactone A | C17H20O9 | 368.34 g/mol | 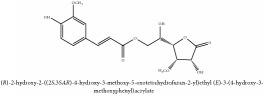 |
|
| Hydroxybenzaldehyde | 4-hydroxybenzaldehyde | C7H8O2 | 124.14 g/mol | 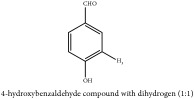 |
| Phenolic aldehyde | Vanillin | C8H8O3 | 152.15 g/mol | 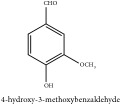 |
| Glycoside | 3,4,5-trimetoxyphenol O-_β_-D-glucopyranoside | C9H11O3 | 167.18 g/mol | 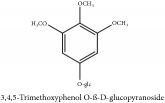 |
| 2-methoxy-4-(2-propenyl)phenyl-beta-D-glucopyranoside | C16H22O7 | 326.35 g/mol | 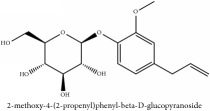 |
|
| 2-(p-hydroxyphenyl)-ethanol-1-O-_β_-D-apiofuranosyl (1–6)-O-_β_-D-glucopyranoside | C9H12O | 136.19 g/mol | 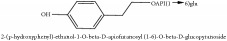 |
|
| 3, 5-dihydroxybenzoicacid-O-xylopyranosyl-glucopyranoside | C8H8O3 | 152.15 g/mol | 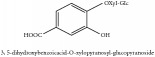 |
|
| Phenolic glycoside | Tachioside | C12H16O8 | 288.25 g/mol | 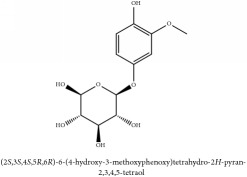 |
| Glucoside | Salidroside | C14H20O7 | 300.31 g/mol | 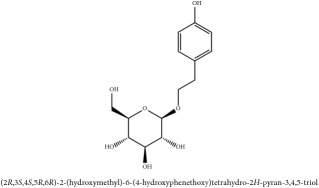 |
| 4-Hydroxybenzyl alcohol | C7H8O2 | 124.14 g/mol | 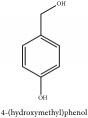 |
|
| Phenol | Methyl Syringate | C10H12O5 | 212.20 g/mol | 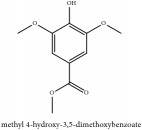 |
| Dehydrovomifoliol | (6S)-dehydrovomifoliol | C13H18O3 | 222.28 g/mol | 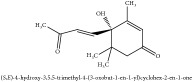 |
| (6R,7aR)-epiloliolide | C11H16O3 | 196.25 g/mol | 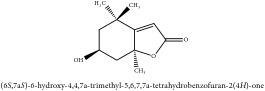 |
Table 4.
Molecular formula, molecular weight and chemical structures of compounds derived from PAC species (34 compounds).
Table 5.
Molecular formula, molecular weight and chemical structures of compounds derived from PAR species (84 compounds).
| Compound derivatives | Compound | Molecular formula | Molecular weight | Chemical structures |
|---|---|---|---|---|
| Alkaloid | Berberine | C20H18NO4+ | 336.37 g/mol | 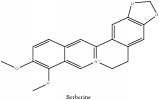 |
| Bis-[4-(dimethylamino)phenyl]methanone | C17H20N2O | 268.36 g/mol | 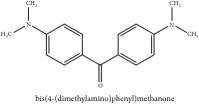 |
|
| Dihydrocyclobuxine-D | C25H44N2O | 388.64 g/mol | 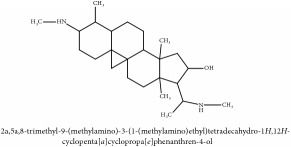 |
|
| 3,4-Dihydro-1-[(4-hydroxyphenyl)methyl]-7-methoxy-2-methyl-8-isoquinolinol | C20H24NO4+ | 342.41 g/mol | 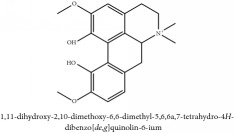 |
|
| 3,4-Dihydro-1-[(4-hydroxyphenyl)methyl]-7-methoxy-2-methyl-6-isoquinolinol | C20H17NO5 | 351.36 g/mol | 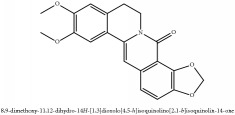 |
|
| 7,8-Dihydroxyrutaecarpine | C18H13N3O3 | 319.32 g/mol | 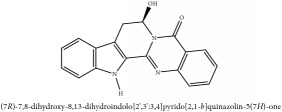 |
|
| 4-Dimethylamino-4 -isopropylbenzene | C18H22NO+ | 268.38 g/mol | 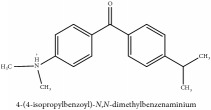 |
|
| Evodiamine | C19H17N3O | 303.37 g/mol | 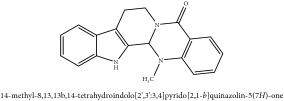 |
|
| Palmatine | C21H22NO4+ | 352.41 g/mol | 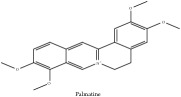 |
|
| Pteleine | C13H13NO3 | 231.25 g/mol | 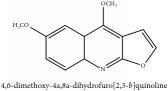 |
|
| (-)-(R)-platydesmin | C15H19NO3 | 261.32 g/mol | 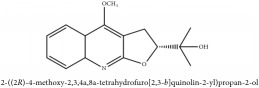 |
|
| Noroxyhydrastinine | C10H9NO3 | 191.19 g/mol | 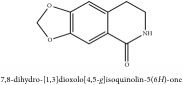 |
|
| Chilenine | C19H15NO7 | 369.33 g/mol | 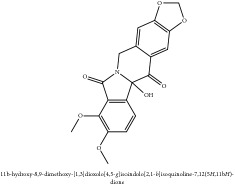 |
|
| Phellodendrine | C20H24NO4+ | 342.41 g/mol | 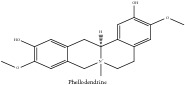 |
|
| Magnocurarine | C19H24NO3+ | 314.40 g/mol | 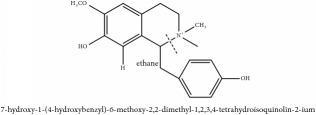 |
|
| Magnoflorine | C20H24NO4+ | 342.41 g/mol | 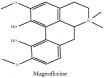 |
|
| Jatrorrhizine or Neprotin, 2,9,10-Trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-3-ol | C20H19NO4+ | 337.38 g/mol | 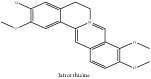 |
|
| 13-Methoxyjatrorrhizine | C21H22NO5+ | 368.41 g/mol | 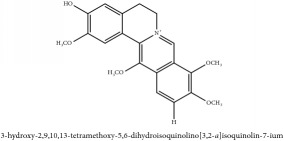 |
|
| _Ƴ_-Fagarine | C13H11NO3 | 229.24 g/mol | 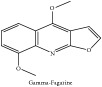 |
|
| Canthin-6-one | C14H8N2O | 220.23 g/mol | 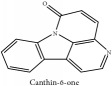 |
|
| 4-Methoxy-N-methyl-2-quinolone | C11H11NO2 | 189.21 g/mol | 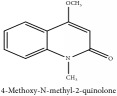 |
|
| Candicine | C10H16NO+ | 166.24 g/mol | 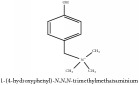 |
|
| Lotusine | C19H24NO3+ | 314.40 g/mol | 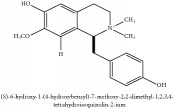 |
|
| N-Methylhigenamine-7-O-glucopyranoside | C17H20N2O | 268.36 g/mol | 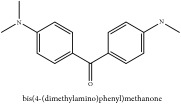 |
|
| N-methylhigenamine-7-O-_β_-D-glucopyranoside | N/A | N/A | 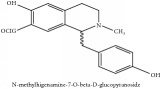 |
|
| (−)-Oblongine | C19H24NO3+ | 314.40 g/mol | 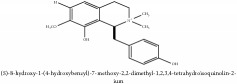 |
|
| Isomer-of-berberine | N/A | |||
| Isomer-of-magnoflorine | ||||
| Isomer-of-palmatine | ||||
| Tetrahydroreticuline | C19H22NO4+ | 328.39 g/mol | 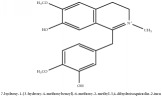 |
|
| Tetrahydrojatrorrhizine | C20H23NO4 | 341.41 g/mol | 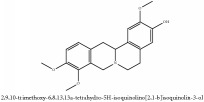 |
|
| Menisperine | C21H26NO4+ | 356.44 g/mol | 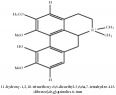 |
|
| (+) N-methylcorydine | C21H26NO4+ | 356.44 g/mol | 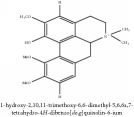 |
|
| N-Methyl Tetrahydropalmatine | C22H28NO4+ | 370.47 g/mol | 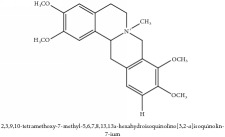 |
|
| N-Methylflindersine | C15H15NO2 | 241.29 g/mol | 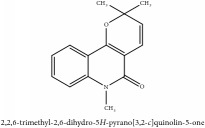 |
|
| Litcubine | C19H22NO4+ | 328.39 g/mol | 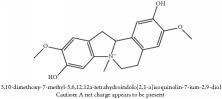 |
|
| Hydroxyl-palmatine | C22H22O5 | 366.41 g/mol | 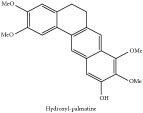 |
|
| 11-Hydroxylpalmatine | C21H22NO5+ | 368.41 g/mol | 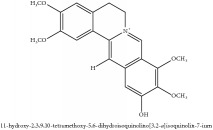 |
|
| 13-Hydroxypalmatine | C21H22NO5+ | 368.41 g/mol | 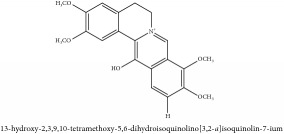 |
|
| 7-Hydroxy-8-methoxydedihydrorutaecarpine | N/A | |||
| Tetrahydropalmatine | C21H25NO4 | 355.43 g/mol | 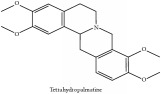 |
|
| 5,8,13,13a-Tetrahydro-2,9,10,11-tetrahydroxy-3-methoxy-7-methyl-6H-dibenzo[a,g]quinolizinium | C21H20NO6+ | 382.39 g/mol | 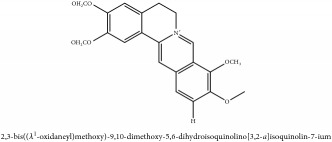 |
|
| Tetrahydroberberine | C20H21NO4 | 339.39 g/mol | 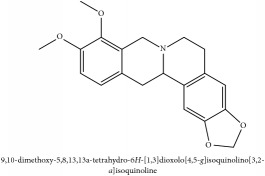 |
|
| Xanthoplanine | C21H26NO4+ | 356.44 g/mol | 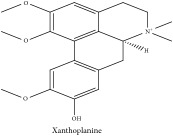 |
|
| N-methylphoebine | C22H26NO5+ | 384.45 g/mol | 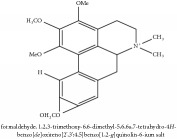 |
|
| Columbamine | C20H20NO4+ | 338.38 g/mol | 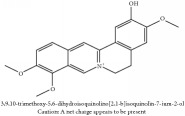 |
|
| Dihydroxyl-jatrorrhizine | N/A | |||
| Epiberberine | C20H18NO4+ | 336.37 g/mol | 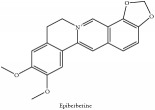 |
|
| Rutecarpine | C18H13N3O | 287.32 g/mol | 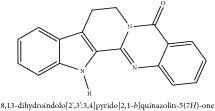 |
|
| Skimmianine | C14H14NO4+ | 260.27 g/mol | 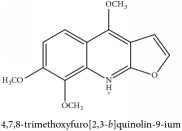 |
|
| Tembetarine | C21H22NO4+ | 352.41 g/mol | 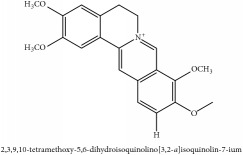 |
|
| 8-Oxoberberine | C20H17NO5 | 351.36 g/mol |  |
|
| 8-Oxoepiberberine | C20H17NO5 | 351.36 g/mol |  |
|
| 8-Oxopalmatine | C21H21NO5 | 367.40 g/mol | 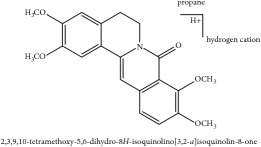 |
|
| Oxyberberine | C20H17NO5 | 351.36 g/mol | 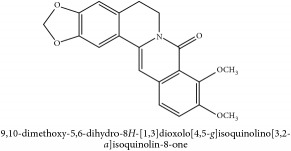 |
|
| Oxypalmatine | C21H21NO5 | 367.40 g/mol | 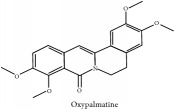 |
|
| Limonoid | Obaculactone or limonin | C26H30O8 | 470.52 g/mol | 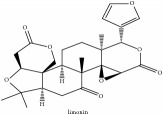 |
| Obacunone or Obacunoic acid | C26H30O7 | 454.52 g/mol | 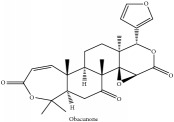 |
|
| 12_α_-hydroxylimonin | C26H30O9 | 470.52 g/mol | 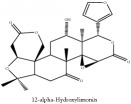 |
|
| Rutaevin | C26H30O9 | 486.52 g/mol | 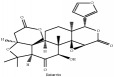 |
|
| Coniferin | C16H22O8 | 342.34 g/mol | 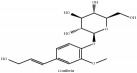 |
|
| Vanilloloside | C14H20O8 | 316.31 g/mol | 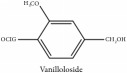 |
|
| Phenolic acid | 2-(p-hydroxyphenyl)-ethanol-1-O-_β_-D-apiofuranosyl (1–6)-O-_β_-D-glucopyranoside | C9H12O | 136.19 g/mol | 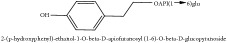 |
| 3, 5-dihydroxybenzoicacid-O-xylopyranosyl-glucopyranoside | C8H8O3 | 152.15 g/mol | 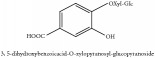 |
|
| Ferulic acid | C10H10O4 | 194.19 g/mol | 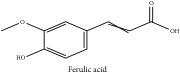 |
|
| Quinic acid | Neochlorogenic acid | C16H18O9 | 354.31 g/mol | 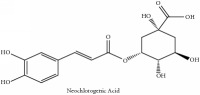 |
| 3-O-feruloyl quinic acid or 5-O-feruloyl quinic acid | C17H20O9 | 368.34 g/mol | 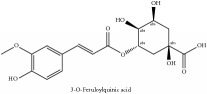 |
|
| Chlorogenic acid | C16H18O9 | 354.31 g/mol | 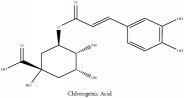 |
|
| Methyl 3-_O_-feruloylquinate | C18H22O9 | 382.37 g/mol | 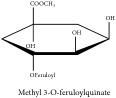 |
|
| Methyl 5-O-feruloylquinate | C8H13O6 | 205.19 g/mol | 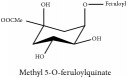 |
|
| 3-Feruoyl-4-caffeoylquinic acid | C26H26O12 | 530.48 g/mol | 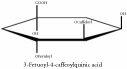 |
|
| Lignan | (+/-)-lyoniresinol | C22H28O8 | 420.46 g/mol | 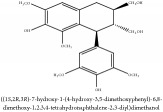 |
| (+/-)-5,5′-dimethoxylariciresinol-4-_O_-glucoside | C28H38O13 | 582.6 g/mol | 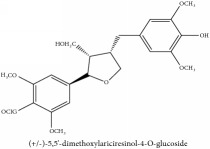 |
|
| Syringaresinol di-O-_β_-D-glucopyranoside | C33H44O18 | 728.70 g/mol | 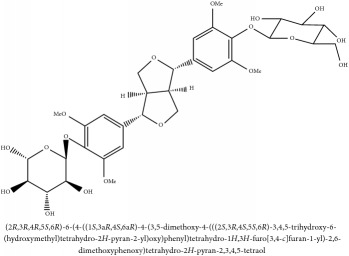 |
|
| Flavonoid | _β_-anhydronoricaritin | N/A | ||
| Icariside-1 | C26H28O11 | 516.50 g/mol | 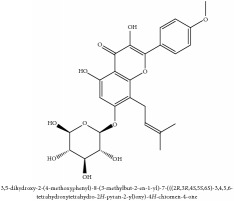 |
|
| Phellamuretin | C20H20O6 | 356.37 g/mol | 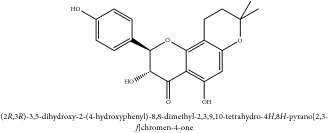 |
|
| Phelloside | C31H40CrO17 | 736.64 g/mol | 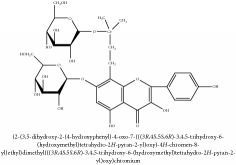 |
|
| Dihydrophelloside | C31H44CrO17 | 740.67 g/mol | 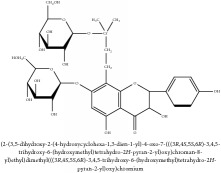 |
|
| Isovaleric acid | C5H10O2 | 102.13 g/mol | 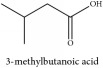 |
|
| Kaempferol | C15H10O6 | 286.24 g/mol | 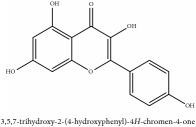 |
|
| D-glucose | C6H12O6 | 180.16 g/mol | 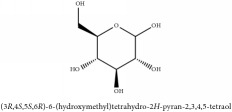 |
|
| Paraben | N-propyl paraben | C10H12O3 | 180.20 g/mol | 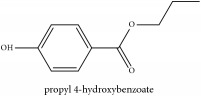 |
| Glycoside | Sinapyl aldehyde-4-O-beta-D-glucopyranoside | C13H14O6 | 266.25 g/mol | 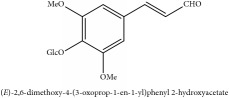 |
5. Pharmacology
5.1. Anti-Inflammatory Effects
The PAR extract could efficiently adjust lipopolysaccharide (LPS)-induced release of nitric oxide (NO) and inducible nitric oxide sythase (iNOS) production in microglia of both BV2 cells and mice. Besides, PAR extract could also attenuate the LPS-stimulated release of tumor necrosis factor-α (TNF-α) and interleukin 1_β_ (IL-1_β_) from microglia. More importantly, the latter mechanism is more significant than the previous one in terms of IL-1_β_ release. The inhibition of NO suggested that the extract of PAR probably could affect NO-induced neuronal cell death [28]. Another study had also vindicated the anti-inflammatory effect of PC extract on ear swelling model of mice. Magnoflorine and phellodenrine belong to alkaloids isolated from PC that are the effective compounds against oxazolone-induced contact-delayed type hypersensitivity (DTH) reaction induced by picryl chloride. Another mechanism in this study is that PC could reduce myeloperoxidase (MPO) activity to its utmost by restraining leukocyte mobility and/or a secretory activity by phellodendron. In contrast, PC extract exerts no effect on phospholipase A2 (PLA2) activity and less effect in arachidonic acid (AA) induced swelling [29]. PC showcased the inhibitory effect on the build-up of NO in LPS-stimulated macrophage Raw 264.7 cells and inhibited iNOS expression. In contrast, PC has no effect on cyclooxygenase-2 (COX-2) expression in LPS-induced RAW 264.7 cells [30]. PCC and PAC could not only shrink the size of edema, but also reduce the activity of MPO and the content of reactive oxygen species (ROS) caused by 12-O-tetradecanoyl-phorbol-13-acetate (TPA). They can also restrain the levels of TNF-α, Il-1_β_, IL-6, and COX-2 in mice treated by TPA. Remarkably, there are a number of chemical compounds in these two species of PC including berberine, palmatine, and phellodendrine. And they are viewed as the anti-inflammatory active candidates. In addition, they may jointly take effect in this regard [3]. The nonalkaloid PAR extract suppressed NO production; besides, limonin and obakunone significantly downregulated NO production and iNOS gene expression via an nuclear factor-_κ_B (NF-_κ_B)-mediated pathway [11]. PC could reverse the airway inflammation by reducing the infiltration of inflammatory cells and releasing of inflammatory mediators into the affected lung and airways. This could vindicate its applications on the infectious pulmonary diseases [31].
5.2. Antimicrobic Effects
For the antibacterial effect, the study showed both aqueous and ethanol extracts of PAR exerted an intermediate antimicrobial effect. Besides, PAR extracts had a slightly better effect on gram-positive bacteria than gram-negative one because of different sensitivity. The most sensitive bacteria is Streptococcus pyogenes [33]. For the bacteria in the oral cavity, PC could have a strong inhibitory effect on Porphyromonas gingivalis; moderate inhibitory effect on Streptococcus mutans; partial effect on Streptococcus sanguis; no effect on Streptococcus mitis [34]. Propionibacterium acnes which are the culprit for acne is active to PC which is one of the crude herbs in the clinical trial and the second best herb in terms of antiacne activity in this study [39]; Mycoplasma hominis which causes infections on humans genital tracts and respiratory tracts is susceptive to PC, and the susceptibility rate is 93% [35]. Salmonellosis which is responsible for food poisoning is vulnerable to PC extract because it can lower the IgG levels and induce TNF-α expression in RAW264.7 cells [36]. In case of PAR, berberine could restrain the bacterial adhesion of Staphylococcus aureus and intracellular invasion into human gingival fibroblasts [37]. Moreover, berberine could attenuate the aminoglycoside resistance of P. aeruginosa, A. xylosoxidans, and B. cepacia in the MexXY-dependent manner. It also inhibits MexXY- or MexVW-mediated resistance of P. aeruginosa mutants, synergistically restrains MexXY-mediated gentamicin resistance in P. aeruginosa mutants, and enhances the synergistic effect of piperacillin and amikacin in multimedication resistant P. aeruginosa strains. The extract of PCS significantly downregulated minimal inhibitory concentrations (MICs) of amikacin and gentamicin in the two multimedication resistant P. aeruginosa strains [38]. PC showed its potential on the inhibition of Propionibacterium acnes strains. Its MIC50 and MIC90 were 24 _μ_g/ml and 190 _μ_g/ml, respectively [39]. For fungal infections, the monomers of PC showed antifungal activity through compromising the integrity of fungal cell wall and cell membrane and increasing the expressions of energy metabolic genes. Therefore the life expectancy of Microsporum Canis is shortened. Furthermore, the mingling use of palmatine hydrochloride and berberine hydrochloride could effectively treat Microsporum Canis induced dermatomycosis in rabbit [40]. For virus infections, the ethanol extract of PAR exerted moderate effect against Herpes Simplex Virus by either interrupting virion envelope structures or disguising as indispensable viral compounds for absorption or infiltration of host cells [33]. Another study had proved that PAR has a broad spectrum of functions against virus-like VSV-GFP, PR8-GFP, NDV-GFP, HSV-GSP, H3-GFP, and EV-71 in vitro and also has effects on different strains of influenza A such as H1N1, H5N2, H7N3, and H9N2 in vivo mice model [41].
5.3. Antitumor Effects
There are 3 compounds in PCS that have been found to resist three types of tumours which included leukemic cell lines K562 and HEL, breast cancer cell line MDA, and prostate cancer cell line PC3. The compound [(21S, 23R) epoxy-24-hydroxy-21β, 25-diethoxy] tirucalla-7-en-3-one has a relatively strong effect as Adriamycin against four tumors with the measurement of IC50; toonaciliatin K and piscidinol A have a comparably moderate effect in this regard [42]. There are 9 most effective compounds of PC for prostate cancer, namely, magnoflorine-O-glucuronide, (p-hydroxybenzyl)-6, 7-dihydroxy-N-methyl tetrahydro iso-quinoline-7-O-p-D-glucopyranosid, magnoflorine, menisperine-O-glucuronide, menisperine, berberine, Jatrorrhizine, obaculactone, and obacunone [43]. Polysaccharides from an aqueous extract of PCS act on cell-mediated stimulation and humoral immunity instead of tumor cell inhibition to exert tumoricidal activity. Specifically, some polysaccharides stimulate macrophages and NK cells via _β_-glucan-binding lectin site of complement receptor type 3 [44].
5.4. Antigout Effects
Si-Miao-Wan (SMW) formula had been proved to be effective for gout and gouty arthritis. In this formula, PC is the monarch herb which is the core ingredient to guide the other three herbs indicating that the alkaloids and organic acids in PC are the potential compounds for SMW. Alkaloids include candicine, oblongine, phellodendrine, tembetarine, magnoflorine, lotusine, n-methylterahydrocolumbamine, menisperine, noroxyhydrastinine, demethyleneberberine, tetrahydropalmatine, oxyberberine, armepavine, oxypalmatine, columbamine, jatrorrhizine, thalifendine, berberrubine, n-methyl canadine, palmatine, berberine, obaculactone, obacunone, and amurenlaetone B, and organic acids include neochlorogenic acid, chlorogenic acid, cryptogenic acid, cryptochlorogenin acid, caffeoyl-CH2-O-quinic acid, 3-O-feruloylqinic acid, ferulic acid, and sanleng acid [45]. Er-Miao-Wan formula is a modified version of SMW, which had been elucidated for its chief ingredient PCS which exerts potent hypouricemic effect [46].
5.5. Antiulcer Effects
Peptic ulcers are associated with psychological stress and mental illness. The middle dosage of PC extract could significantly reduce the levels of serotonin in the brain and noradrenaline in the adrenal gland. Both serotonin and noradrenaline take effect in the mental depression. Besides, for the molecule in PC, berberine has the ability to inhibit monoamine oxidase-A and modulate the brain noradrenaline, serotonin, and dopamine levels [47]. Another study revealed that PC could protect the gastric mucosa by reinforcing the gastric mucosal barrier through endogenous sulfhydryl compounds and diethyldithiocarbamate-sensitive compounds [48].
5.6. Antioxidant Effects
The antioxidant activity of PAR is proportional to its extract's concentration. In another aspect, the ethanol extract exhibited a better antioxidant effect because of its high concentration of phenolics and flavonoids than aqueous extract [33]. Phellodendrine from PC could play an antioxidant role by modulating the AKT/NF-_κ_B pathway in the zebrafish embryo. Besides, phellodendrine could undo the expression of AKT and NF-_κ_B, IKK, and COX-2 [49].
5.7. Sun Screening Effects
The sun screening effects of PC have been demonstrated through an experiment which was designed for sun screening effect of 50% alcohol extracts of 100 Chinese herbal medicines. The study showed PC could absorb 91.8% of ultraviolet-C, 79.1% of ultraviolet-B, and 50.7% of ultraviolet-A. It is regarded as highly effective for sun screening if the absorption rate of ultraviolet is higher than 90%. Therefore, PC could be a strong candidate for sunproof of ultraviolet-C [50]. Also, PC could also improve skin oxidative lesion induced by ultraviolet radiation via decreasing lipid peroxidation and increasing antioxidant enzymes activities [51].
5.8. Other Effects
PC stimulates longitudinal bone growth and chondrocyte proliferation via upregulating bone morphogenetic protein-2 (BMP-2) and insulin-like growth factor (IGF-1) expression in the growth cartilage [52]. Besides, PC could activate the fibrinogen system to take the hemostatic effect. This validated charcoaled PC could stop bleeding [53]. The extract of PC also shows neuroprotective effect through adjusting the PC-12 cell apoptosis which was induced by 1-methyl-4-phenylpyridinium (MPP+) and hindered the release of cytochrome C into the cytosol [54]. PAR could ease the symptoms of atopic dermatitis by decreasing the numbers of mast cells, serum levels of TNF-α, and INF- γ and the expression levels of cytokines [56]. PAR could delay or even prevent the progression of diabetic nephropathy by correcting the high blood sugar state, antioxidant enzyme system, and kidney malfunction and reversing histopathological changes inflicted by diabetes on kidney [57]. To further elaborate its mechanism on the compound level, berberine could attenuate the renal malfunction by inhibiting renal aldose reductase and decreasing oxidative stress [58]. Magnoflorine and phellodendrine could inhibit the immune response by suppressing local graft versus host reaction and induction phase of picryl chloride-induced delayed-type hypersensitivity [59]. To counter asthma attack, the extract of PCS inhibits tracheal smooth muscle contraction induced by high K+. Meanwhile, it could also block tracheal smooth muscle concentration induced by Nifedipine [6]. The pharmacological activities of PC and its related derivatives have been listed in Table 6.
Table 6.
Pharmacological activities of PC and processed PC.
| Pharmacological Activity | Tested Substance | In vivo/ In vitro | Model or sample | Active concentration | Administration (In vivo) | References |
|---|---|---|---|---|---|---|
| Anti-inflammatory effect | PAR extract with voucher specimens | In vitro | BV2 cells (Mouse microglial cell line) | 100 _μ_g/ml | [28] | |
| PCC extract | In vivo, in vitro | 12-_O_-Tetradecanoylphorbol-13-acetate-induced mouse ear edema | (i) TPA and AA tests: 0.5 mg/ear(ii) TPA multiple application: 1 mg/ear(iii) DTH test: 1 mg/ear | Topical application | [29] | |
| PCC extract with voucher specimens | In vivo, in vitro | lipopolysaccharides-induced systemic inflammation mice model and macrophage RAW 264.7 cells | 1,10,100 _μ_g/mL | Oral administration | [30] | |
| Ethanol extract of PCC with voucher specimens | In vivo | 12-O-tetradecanoyl-phorbol-13-acetate- induced ear edema in mice | 200-400 mg/kg | Administered intragastrically | [3] | |
| PAR methanol extract with voucher specimens | In vitro, in vivo | ICR mice and male Wistar rats | IC50:Methanol extract 20.9 ± 3.8 _μ_g/mL;Non-alkaloids22.0 _μ_g/mL;Limonin 15.8 ± 5.2 _μ_m;Obakunone2.6 ± 1.1 _μ_m | Oral administration | [11] | |
| PCC methanol extract with voucher specimens | In vivo | Lipopolysaccharides- induced acute airway inflammation on a mouse model | 100, 200 and 400 mg/kg | Administrated by gavage | [31] | |
| Demethyleneberberine | In vivo | Acute colitis mice model | 150,300 mg/kg | Oral administration | [32] | |
| Anti-bacterial effect | Ethanol Extract of PAR;Aqueous extract of PAR | In vitro | Enterococcus faecium, Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa | MIC and MBC: 3.676 mg/ml and 7.353 mg/ml for ethanol extract;6.25 mg/ml and 50 mg/ml for aqueous extract | [33] | |
| PCC extract with voucher specimens | In vitro | Streptococcus. mitis,Streptococcus. sanguis,Streptococcus. mutans,Streptococcus. gingivalis | 2.5 g/ml | [34] | ||
| PCC extract | In vitro | Mycoplasma hominis | 0.24-250 mg/ml | [35] | ||
| Aqueous extract of PCC | In vivo, In vitro | S. Typhimurium 21 infected mouse model | 2.5 or 5 mg/day | Administrated by gavage | [36] | |
| Berberine in PAR | In vitro | Staphylococcus aureus | 32 to 128 _μ_g/mL | [37] | ||
| Berberine in PCS | In vitro | P. aeruginosa | 100 ml | [38] | ||
| PCC | In vitro | Propionibacterium acnes | MIC50%: 24 _μ_g/mLMIC90%:190 _μ_g/mL | [39] | ||
| Anti-fungal effect | Berberine hydrochloride, palmatine hydrochloride | In vitro, In vivo | Microsporum Canis –induced dermatitis in rabbits | MICs 1 mg/ml | Administered through the sterile pipette tip | [40] |
| Anti-viral effect | Ethanol extract of PAR;Aqueous extract of PAR | In vitro | African green monkey kidney cells | 6.73 ± 0.87 mg/ml for aqueous extract;4.26 ± 0.59 mg/ml for ethanol extract | [33] | |
| Aqueous extract of PAR with voucher specimens | In vitro, in vivo | H1N1, H5N2, H7N3 or H9N2 infected BALB/c mice | 0.8 _μ_g/g in a total volume of 200 _μ_l at 1, 3 and 5 days before infection | Oral administration | [41] | |
| Anti-tumor effect | PCS extract with voucher specimens | In vitro | (i) Leukemic cell lines K562(ii) Leukemic cell lines HEL(iii) Breast cancer cell line MDA(iv) Prostate cancer cell line PC3 | (i) IC50 of Compound 1: 7.66 ±2.08;(ii) IC50 of compound 3: 14.30 ± 1.93;(iii) IC50 of compound 4: 11.81 ± 2.79 | [42] | |
| PCC extract | In vivo, in vitro | Prostate cancer cell infested Male BALB/c-nude mice model | 1.6 g/kg per day for 28 days | Administered intragastrically | [43] | |
| Aqueous extract of PCS with voucher specimens | In vitro In vivo | Sarcoma 180 ascites cells implanted Mice model | 2 mg/100g;5 mg/100g;10 mg/100g | Injected intraperitoneally daily for 10 days | [44] | |
| Anti-gout effect | Compounds of Si-Miao-Wan (containing PCC) | In vivo | Male Sprague-Dawley rats | 1.0 ml/100 g | Oral administration | [45] |
| PCS extract with voucher specimens | In vivo | Uricase inhibitor potassium oxonate induced male ICR mice model | 480 mg/kg | Oral or intraperitoneal administration | [46] | |
| Anti-ulcer effect | Ethanol extract of PCC with voucher specimens | In vivo In vitro | Acetic acid-induced chronic gastric ulcers on Sprague Dawley rats model | 30 mg/kg/day | Administered intragastrically once a day for seven days. | [47] |
| Aqueous extract of PCC | In vivo | Ethanol-induced gastric lesions on Male Wistar rats model | 100 mg/kg | Oral administration | [48] | |
| Anti-oxidant effect | Ethanol extract of PAR;Aqueous extract of PAR | In vitro | For anti-microbial Enterococcus faecium, Staphylococcus aureus, Streptococcus pyogenes,Escherichia coli, Klebsiella pneumonia, and Pseudomonas aeruginosa;Anti-herpes simplex virus tested on African green monkey kidney cells | 25 mg/ml | [33] | |
| Phellodendrine isolated from PCC extract | In vivo | AAPH-induced oxidative stress on zebrafish embryo model | 200 _μ_g/mL | Waterborne exposure | [49] | |
| Sun screening effect | PCC extract + 50% ethanol | In vitro | PC extract+50% ethanol | 0.5 mg/ml | [50] | |
| PCC extract | In vivo | UVB lamp inflicted skin lesions on the dorsal of the rats model | 200, 400 or 800 mg/kg, once daily for 11 days | Oral administration | [51] | |
| Bone-growth effect | PCC extract | In vivo | 72 intact 21-day-old female Sprague–Dawley rats | 100 and 300 mg/kg | Oral administration | [52] |
| Hemostatic effect | PCC Carbonisatus-carbon dots | In vivo In vitro | Mouse tail amputation and liver scratch on male Kunming mice models | 5, 2 and 1 mg_/_kg | Subcutaneous administration | [53] |
| Neuroprotective effect | PCC extract with voucher specimens | In vitro | PC-12 cells | 10 and 30 _μ_g/mL | [54] | |
| PCC and PAC extract with voucher specimens | In vitro | PC-12 cells | 0.1 and 1 g/ml for 2 hours | [55] | ||
| Counter-atopic dermatitis effect | Salt processed PAC extract with voucher specimens | In vivo | 2,4-dinitrochlorobenzene induced skin lesions on the NC/Nga mice model | 200 _μ_l | Topical administration | [56] |
| Counter-diabetic nephropathy effect | PAR aqueous extract | In vivo | Streptozotocin-induced diabetes on male Sprague-Dawley rats model | 379 mg/kg | Oral administration | [57] |
| Berberine | In vivo | Streptozotocin-induced diabetes on male Wistar rats model | 200 mg/kg once a day for 12 weeks | Oral intubation | [58] | |
| Immunity suppressing effect | Phellodendrine and cyclophosphamide isolated from PCC in saline water | In vivo | ddY mice, BALB/c mice, and Hartlay guinea pigs | (i) 0.1 ml/10 g for mice(ii) 1 ml for guinea pigs | Administered intraperitoneally | [59] |
| Anti-asthmatic effect | n-butyl alcohol extract of PCS with voucher specimens | In vivo | BALB/c mice asthmatic model induced by saline solution | The IC50 of n-butyl alcohol extract of PCS was 12.2 ± 1.3 _μ_g/mL | intranasally | [6] |
6. Pharmacokinetics
According to Chinese Pharmacopeia (Edition 2015), phellodendrine has been used as one of the evaluating indexes of PC. Phellodendrine could be rapidly absorbed in tissues such as plasma, liver, spleen, kidney, and brain. Besides, kidney is the major distribution tissue and the target organ of phellodendrine. Furthermore, the experimental study on animal suggested that this constituent has no long-term build-up effect on the tissues. Intriguingly, the extract of phellodendrine could be found in the animal brain tissue indicating that this constituent may penetrate the common medication's biggest hurdle: blood-brain barrier. The maximum concentration time is at 5 minutes after intravenous administration. The elimination half-life is no longer than 2 hours [63]. Another constituent from PC is magnoflorine which shows low bioavailability and high absorption and elimination rates after oral and intravenous administration of this constituent in pure compound form. While its bioactivity can be dramatically increased, absorption and elimination rates can be significantly decreased after oral administration of the PC decoction. Similarly, with oral administration of mixture, magnoflorine (40 mg/kg) and berberine (696.4 mg/kg, the equivalent dosage in PC decoction), the bioavailability and absorption and elimination rates have a similar trend. It suggested berberine plays an important role in the drug-drug interaction with magnoflorine in the PC decoction. On the other hand, these findings also warn us that the mingling use of berberine and magnoflorine possibly increases the risk of toxicity which possibly gives some support to the theory that herbal medicine may achieve better therapeutic effects and fewer side effects [64]. When it comes to the different type of processed PC, they have different kinds of effects. For the raw PC, it could downregulate CYP1A2 and activate CYP3A4. As for rice-wine and salt-water processed PC, they can alter the activities of cytochrome P450. Also, rice-wine processed PC alone can counter the inhibitory effect of CYP1A2 and promote the induction of CYP3A4 [65].
6.1. Toxicity and Contraindication
Several studies have been conducted regarding the toxicology of PC applications. So far, no conclusive result has been reached due to the controversial results from different studies. The common allegation for PC application is neonatal jaundice and kernicterus. Due to this concern, it even causes the complete ban on the use of related herbs including PC in Singapore since 1978. Besides, according to the latest Singaporean official regulations for health supplements guideline, PC is still on the list of prohibited or restricted ingredients. However, according to a cohort study, under the guidance of Chinese medicine practitioners, the application of PC's berberine is clinically safe even in patients who have hematological diseases with profound cytopenia and multiple comorbidities. Despite these, some precautionary measures such as bilirubin and hemoglobin monitoring are still required for the patients who have underlining hemolytic disease. On the other hand, the restriction for PC is necessary for the users in their peripartum and neonatal period due to the concern of its aggravation risk for neonatal jaundice and kernicterus [66].
6.2. Processing, Differentiation, and Authentication
Traditional Chinese medicine (TCM) processing or preparation or “Pao Zhi” in Chinese is a unique technique and process in TCM. Pao Zhi is a technique to turn the raw herbs into decoction pieces. This technique must be performed under the guidance of TCM's theory to satisfy the different requirements of medicinal materials and special production processes. The quality of Pao Zhi directly affects the therapeutic effects of herbal medicine [67]. It has a time-honored history because as early as 5 B.C. during the Jin dynasty, “Leigong Treatise on the Preparation” was composed as a book for Pao Zhi. It systematically summarized the herbal processing techniques until the Jin dynasty. Intriguingly, it stated that the right way of using the bark of PC is to remove the coarse bark [68]. In the Song dynasty, Pao Zhi had regulated a mandatory process for Chinese medicinal product [67].
In terms of PC's processing, TCM doctors in history emphasized the importance of PC's processing and recorded 16 kinds of methods for PC's processing in the books. Nowadays, the most common types of processing are raw PC, PC fried with salt, PC fried with wine, PC fried with honey, and fried-to-charcoal PC [69]. However, it still lacks official standard when it comes to quantity and quality of the processed PC and its subspecies; some of the existing pioneer studies could explain this ambiguous and abstract concept in a scientific way. Besides, for PC's quality control, the most practical approach is to use thin layer chromatography (TLC) for qualitative and high-performance liquid chromatography (HPLC) for quantitative measurement [70]. According to the results of thin layer chromatography, water percentage in the raw PC is less than 10.0%, PC fried with salt is less than 8.0%, PC fried with wine is less than 7.0%, and PC fried with honey is less than 8.0%. Total ash content in the raw PC is less than 8.0%; acid insoluble ash content is less than 0.8%; PC fried with salt is less than 8.0%; acid insoluble ash content is less than 0.6%; PC fried with wine is less than 8.0%; acid insoluble ash content is less than 0.8%; PC fried with honey is less than 8.0%; acid insoluble ash content is less than 0.4%. For alcohol-soluble extract, the raw PC is more than 16.0%, PC fried with salt is more than 20.0%, PC fried with wine is more than 16.0%, and PC fried with honey is more than 22.0%. For percentage of phellodendrine, the raw PC is more than 0.41%, and berberine is more than 3.92%; PC fried with salt is more than 0.36%, and berberine is more than 3.89%; PC fried with honey is more than 0.37%, and berberine is more than 3.90% [69].
Previously, the differentiation of PCS and PAR is based on the experiences of the herbal professionals to distinguish the minor differences from their appearances in naked eyes and microscope. Due to the increasing mixing applications and counterfeits, more efficient and accurate approaches are required. It is noticeable that the mass fractions of obaculactone and obacunone have a plunging order among raw PC, PC fried with wine, and PC fried with salt. Therefore, it is reasonable to deduce limonin and obacunone have been undergoing a series of chemical reactions during herbal processing. For differentiation of PCS and PAR, the mass fractions of limonin and obacunone have a significant difference between PCS and PAR. The mass fraction of obaculactone and obacunone in PAR is 10 times higher than in PCS. As a result, limonin and obacunone could be utilized as the differential targeted chemical constituents for PC's differentiation [71]. HPLC method demonstrates that PC and charcoaled PC have a significant disparity in terms of characteristic chromatograms and chemical constituents. The peak numbers and proportions of characteristic chromatograms are reducing with the increasing temperature of charcoaled PC. On the other hand, due to the heating effect, berberine hydrochloride will transfer into berberrubine by losing one methyl. As a result, berberrubine is vindicated to be another targeted chemical constituent for charred PC identification [72]. For authentication of PC and its subspecies, berberine hydrochloride could be used as another targeted chemical constituent except for limonin and obacunone as mentioned above [73].
7. Discussion
This review work has illustrated the diverse bioactive properties of PC and its species associated with active pharmacological actions in vitro and in vivo. Its clinical applications which are demonstrated in these experimental studies indicated the potency of the bioactive compounds and its pharmacological effects.
The diverse derivatives are the backbone for the pharmaceutical efficacies of PC and its species. The alkaloids play a very significant role in this regard not only because they account for a great proportion of constituents in the whole herb, but also because these constituents are relatively well-studied compared to other constituents in other derivatives. Berberine, magnoflorine, palmatine, and phellodendrine are viewed as the anti-inflammatory active candidates in experimental studies. Besides, palmatine and berberine could exert antimicrobial effects. Berberine also has the ability to inhibit monoamine oxidase-A and modulate the brain noradrenaline, serotonin, and dopamine for antiulcer efficacy. The nonalkaloid in PAR extract suppressed NO production; besides, limonin and obakunone significantly downregulated NO production and iNOS gene expression via an NF-κ_B-mediated pathway. [(21S, 23R) Epoxy-24-hydroxy-21_β, 25-diethoxy] tirucalla-7-en-3-one, toonaciliatin K, and piscidinol have tumor-shrinking effects on four types of tumors. Polysaccharides from an aqueous extract of PCS act on cell-mediated stimulation and humoral immunity to exert tumoricidal activity.
Phellodendrine and magnoflorine have well absorption rate in the animal organs which could indicate their safety for human trials. However, berberine could interact with magnoflorine or other constituents which could further increase the tissue absorption rates. This should be noticed by other clinical trials or advanced studies to avoid adverse effects. It is also noticeable that different processing techniques or “Pao Zhi” could render the herb with different kind of therapeutic effects. For the raw PC, it could downregulate CYP1A2 and activate CYP3 A4. As for rice-wine and salt-water processed PC, they can alter the activities of cytochrome P450. Also, for rice-wine processed PC alone, it can counter the inhibitory effect of CYP1A2 and promote the induction of CYP3A4. For toxicology, there is no affirmative conclusion in terms of PC and its species' absolute clinical safety. Therefore, safety precautionary measures are still required for vulnerable groups of people. For the processed PC and its species, berberrubine, limonin, and obacunone became targeted chemical constituents for authentication of charred PC, PC, and its subspecies. Furthermore, obacunone and obaculactone are probably responsible for antiatopic dermatitis effect [56].
8. Conclusions
In summary, the compounds of the crude bark of PC and its species have showcased a wide range of pharmacological effects. Pharmacological efficacies of PC are supported by its diverse class of alkaloids, limonoids, phenolic acid, quinic acid, lignans, and flavonoid. Through this review, in total, 140 chemical compounds had been summarized. Among these compounds are 18 compounds from PCC, 44 compounds from PCS, 34 compounds from PAC, and 84 compounds from PAR. However, more studies are still needed to demonstrate more knowledge to allow a better understanding of this herb and its species.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Dong Y. Q., Hong H. X., Zhong G. Y. Research status and problems of phellodendron quality. Research and Practice of Chinese Medicines. 2007;3 [Google Scholar]
- 2.Zheng Y., Hui R. H., Hou D. Y. Studies on flavones and anti-oxidation effect of phellodendron. Journal of Anshan Normal University. 2010 [Google Scholar]
- 3.Xian Y.-F., Mao Q.-Q., Ip S.-P., Lin Z.-X., Che C.-T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. Journal of Ethnopharmacology. 2011;137(3):1425–1430. doi: 10.1016/j.jep.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Zhang W., Qi H., Shi Y. Phenolic constituents of Phellodendron chinense bark. Canadian Journal of Chemistry. 2009;87(9):1218–1221. doi: 10.1139/V09-112. [DOI] [Google Scholar]
- 5.Ryuk J. A., Zheng M. S., Lee M. Y., et al. Discrimination of Phellodendron amurense and P. chinense based on DNA analysis and the simultaneous analysis of alkaloids. Archives of Pharmacal Research. 2012;35(6):1045–1054. doi: 10.1007/s12272-012-0612-y. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q.-J., Chen W., Dan H., et al. Cortex phellodendri extract relaxes airway smooth muscle. Evidence-Based Complementary and Alternative Medicine. 2016;2016:9. doi: 10.1155/2016/8703239.8703239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X., Sun L., Li D., You G., Wang M., Ren X. Quality evaluation of phellodendri chinensis cortex by fingerprint chemical pattern recognition. Molecules. 2018;23(9, article 2307) doi: 10.3390/molecules23092307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Yan G., Zhang A., et al. Rapid discovery and global characterization of chemical constituents and rats metabolites of Phellodendri amurensis cortex by ultra-performance liquid chromatography-electrospray ionization/quadrupole-time-of-flight mass spectrometry coupled with pattern recognition approach. Analyst. 2013;138(11):p. 3303. doi: 10.1039/c3an36902a. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y. M., Su G. H., Sze S. C.-W., Ye W., Tong Y. Quality assessment of Cortex phellodendri by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. Biomedical Chromatography. 2010;24(4):438–453. doi: 10.1002/bmc.1311. [DOI] [PubMed] [Google Scholar]
- 10.Sun H., Wang H., Zhang A., et al. Chemical discrimination of cortex Phellodendri amurensis and cortex Phellodendri chinensis by multivariate analysis approach. Pharmacognosy Magazine. 2016;12(45):41–49. doi: 10.4103/0973-1296.176023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii A., Okuyama T., Wakame K., Okumura T., Ikeya Y., Nishizawa M. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. Journal of Natural Medicines. 2017;71(4):745–756. doi: 10.1007/s11418-017-1107-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z., Liu H., Zhang B., Liao Y., Zhang Y., Zhang Z. Quantitative and chemical fingerprint analysis for quality evaluation of the dried bark of wild Phellodendron amurense Rupr. based on HPLC-DAD-MS combined with chemometrics methods. Analytical Methods. 2015;7(5):2041–2049. doi: 10.1039/C4AY02827A. [DOI] [Google Scholar]
- 13.Zhou J., Xie G., Yan X. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities. 1st. Vol. 1. Isolated Compounds A-C; 2011. [Google Scholar]
- 14.Li X., Liang J., Lu X., Tao L. A new protoberberine from the bark of Phellodendron chinense. Chemistry of Natural Compounds. 2011;47(5):770–772. doi: 10.1007/s10600-011-0054-7. [DOI] [Google Scholar]
- 15.Xian X., Sun B., Ye X., Zhang G., Hou P., Gao H. Identification and analysis of alkaloids in cortex Phellodendron amurense by high-performance liquid chromatography with electrospray ionization mass spectrometry coupled with photodiode array detection. Journal of Separation Science. 2014;37(13):1533–1545. doi: 10.1002/jssc.201400012. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y.-M., Sheu S.-J., Chiou S.-H., Chang H.-C., Chen Y.-P. A comparative study on commercial samples of Phellodendri cortex. Planta Medica. 1993;59(6):557–561. doi: 10.1055/s-2006-959761. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J., Xie G., Yan X. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications. Isolated Compounds D-G, vol. 2, 2011.
- 18.Zhou J., Xie G., Yan X. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications. 1st. Vol. 4. Isolated Compounds N-S; 2011. [Google Scholar]
- 19.Zhou J., Xie G., Yan X. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications. 1st. Vol. 3. Isolated Compounds H-M; 2011. [Google Scholar]
- 20.Min Y. D., Kwon H. C., Yang M. C., et al. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Archives of Pharmacal Research. 2007;30(1):58–63. doi: 10.1007/BF02977779. [DOI] [PubMed] [Google Scholar]
- 21.Li C. D., Liu Y., Shi Y. P. Simultaneous determination of six alkaloids in Phellodendron amurense by high-performance liquid chromatography. Acta Chromatographica. 2013;25(2):275–285. doi: 10.1556/AChrom.25.2013.2.5. [DOI] [Google Scholar]
- 22.Li Y. P., Li D. D., Ding L. Q., Qiu F. Non-alkoloids components from Phellodendri Cortex. Chinese Traditional and Herbal Drugs. 2016;47(15):2621–2626. doi: 10.7501/j.issn.0253-2670.2016.15.007. [DOI] [Google Scholar]
- 23.Zhang Z., Zhang Y., Zhang Z., et al. Comparative analysis of DNA barcoding and HPLC Fingerprint to trace species of phellodendri cortex, an Important Traditional Chinese Medicine from multiple sources. Biological & Pharmaceutical Bulletin. 2016;39(8):1325–1330. doi: 10.1248/bpb.b16-00210. [DOI] [PubMed] [Google Scholar]
- 24.Ida Y., Satoh Y., Ohtsuka M., Nagasao M., Shoji J. Phenolic constituents of phellodendron amurense bark. Phytochemistry. 1993;35(1):209–215. doi: 10.1016/S0031-9422(00)90536-3. [DOI] [Google Scholar]
- 25.Zhou H., Wang D., Cui Z. Ferulates, amurenlactone A and amurenamide A from traditional Chinese medicine cortex Phellodendri Amurensis. Journal of Asian Natural Products Research. 2008;10(5):409–413. doi: 10.1080/10286020801966534. [DOI] [PubMed] [Google Scholar]
- 26.Shevchuk O. I., Maksyutina N. P., Litvinenko V. I. Flavonoids of Phellodendron sachalinense and Ph. amurense. Chemistry of Natural Compounds. 1968;4(2):66–70. doi: 10.1007/BF00568013. [DOI] [Google Scholar]
- 27.Cui W., Tian J., Ma Z., Guo Y., Wang J., Li X. A new isocoumarin from bark of Pellodendoron chinense. Natural Product Research. 2003;17(6):427–429. doi: 10.1080/14786410310001617695. [DOI] [PubMed] [Google Scholar]
- 28.Park Y.-k., Chung Y. S., Kim Y. S., Kwon O. Y., Joh T. H. Inhibition of gene expression and production of iNOS and TNF-α in LPS-stimulated microglia by methanol extract of Phellodendri cortex. International Immunopharmacology. 2007;7(7):955–962. doi: 10.1016/j.intimp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Cuellar M. J., Giner R. M., Recio M. C., Manez S., Rios J. L. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72(3):221–229. doi: 10.1016/S0367-326X(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y. Y., Kim M. H., Han J. M., et al. The anti-inflammatory potential of cortex phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. International Immunopharmacology. 2014;19(2):214–220. doi: 10.1016/j.intimp.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Mao Y.-F., Li Y.-Q., Zong L., You X.-M., Lin F.-Q., Jiang L. Methanol extract of Phellodendri cortex alleviates lipopolysaccharide-induced acute airway inflammation in mice. Immunopharmacology and Immunotoxicology. 2010;32(1):110–115. doi: 10.3109/08923970903193325. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Li R., Shi M., et al. Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-κB signaling and T-helper cell homeostasis. Inflammation Research. 2017;66(2):187–196. doi: 10.1007/s00011-016-1005-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang W., Zu Y., Fu Y., et al. In vitro antioxidant, antimicrobial and anti-herpes simplex virus type 1 activity of Phellodendron amurense Rupr. American Journal of Chinese Medicine. 2009;37(1):195–203. doi: 10.1142/s0192415x09006655. [DOI] [PubMed] [Google Scholar]
- 34.Wong R. W. K., Hägg U., Samaranayake L., Yuen M. K. Z., Seneviratne C. J., Kao R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. International Journal of Oral and Maxillofacial Surgery. 2010;39(6):599–605. doi: 10.1016/j.ijom.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che Y., Mao S., Jiao W., Fu Z. Susceptibilities of Mycoplasma hominis to herbs. American Journal of Chinese Medicine. 2005;33(2):191–196. doi: 10.1142/S0192415X05002862. [DOI] [PubMed] [Google Scholar]
- 36.Yin M. C., Chang C. H., Su C. H., Yu B., Hsu Y. M. Pteris multifida, Cortex phellodendri, and probiotics attenuated inflammatory status and immunity in mice with a Salmonella enterica serovar Typhimurium infection. Bioscience, Biotechnology, and Biochemistry. 2018:1–12. doi: 10.1080/09168451.2018.1447356. [DOI] [PubMed] [Google Scholar]
- 37.Yu H.-H., Kim K.-J., Cha J.-D., et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. Journal of Medicinal Food. 2005;8(4):454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 38.Morita Y., Nakashima K., Nishino K., et al. Berberine is a novel type efflux inhibitor which attenuates the MexXY-mediated aminoglycoside resistance in Pseudomonas aeruginosa. Frontiers in Microbiology. 2016;7, article 1223 doi: 10.3389/fmicb.2016.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higaki S., Nakamura M., Morohashi M., Hasegawa Y., Yamagishi T. Activity of eleven Kampo formulations and eight Kampo crude drugs against Propionibacterium acnes isolated from acne patients: retrospective evaluation in 1990 and 1995. The Journal of Dermatology. 1996;23(12):871–875. doi: 10.1111/j.1346-8138.1996.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 40.Xiao C., Ji Q., Wei Q., Liu Y., Bao G. Antifungal activity of berberine hydrochloride and palmatine hydrochloride against Microsporum canis -induced dermatitis in rabbits and underlying mechanism. BMC Complementary and Alternative Medicine. 2015;15, article 177 doi: 10.1186/s12906-015-0680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Weeratunga P., Kim M. S., et al. Inhibitory effects of an aqueous extract from Cortex Phellodendri on the growth and replication of broad-spectrum of viruses in vitro and in vivo. BMC Complementary and Alternative Medicine. 2016;16, article 265 doi: 10.1186/s12906-016-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan C., Zhang Y.-D., Wang X.-H., et al. Tirucallane-type triterpenoids from the fruits of Phellodendron chinense Schneid and their cytotoxic activities. Fitoterapia. 2016;113:132–138. doi: 10.1016/j.fitote.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Zhang A., Wang M., et al. Screening the active compounds of Phellodendri Amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Scientific Reports. 2017;7, article 46234 doi: 10.1038/srep46234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S.-D., Lai Y.-S., Kim C.-H. Immunopontentiating and antitumor activities of the purified polysaccharides from Phellodendron chinese SCHNEID. Life Sciences. 2004;75(25):2621–2632. doi: 10.1016/j.lfs.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Lu J.-J., Hu X.-W., Li P., Chen J. Global identification of chemical constituents and rat metabolites of Si-Miao-Wan by liquid chromatography-electrospray ionization/quadrupole time-of-flight mass spectrometry. Chinese Journal of Natural Medicines. 2017;15(7):550–560. doi: 10.1016/S1875-5364(17)30082-1. [DOI] [PubMed] [Google Scholar]
- 46.Kong L. D., Yang C., Ge F., Wang H. D., Guo Y. S. A Chinese herbal medicine Ermiao wan reduces serum uric acid level and inhibits liver xanthine dehydrogenase and xanthine oxidase in mice. Journal of Ethnopharmacology. 2004;93(2-3):325–330. doi: 10.1016/j.jep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Wang X., Zhu X., et al. Gastroprotective effect of alkaloids from cortex phellodendri on gastric ulcers in rats through neurohumoral regulation. Planta Medica. 2017;83(3-4):277–284. doi: 10.1055/s-0042-114044. [DOI] [PubMed] [Google Scholar]
- 48.Takase H., Inoue O., Saito Y., Yumioka E., Suzuki A. Roles of sulfhydryl compounds in the gastric mucosal protection of the herb drugs composing oren-gedoku-to (a traditional herbal medicine) The Japanese Journal of Pharmacology. 1991;56(4):433–439. doi: 10.1254/jjp.56.433. [DOI] [PubMed] [Google Scholar]
- 49.Li L., Huang T., Tian C., et al. The defensive effect of phellodendrine against AAPH-induced oxidative stress through regulating the AKT/NF-κB pathway in zebrafish embryos. Life Sciences. 2016;157:97–106. doi: 10.1016/j.lfs.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 50.Cheng S. Y., Luo D. X., He Q. J., Li Z. H. Determination of the Sun-screening Effect for 50% Alcohol Extracts of 100 Chinese Herbal Medicine Most in Use. China Academic Journal Electronic Publishing House; 2005. [Google Scholar]
- 51.Yan H., Sun X., Sun S., et al. Anti-ultraviolet radiation effects of Coptis chinensis and Phellodendron amurense glycans by immunomodulating and inhibiting oxidative injury. International Journal of Biological Macromolecules. 2011;48(5):720–725. doi: 10.1016/j.ijbiomac.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Lee S. H., Lee H. J., Lee S. H., et al. Effects of Huang Bai (Phellodendri Cortex) on bone growth and pubertal development in adolescent female rats. Chinese Medicine. 2018;13(3) doi: 10.1186/s13020-017-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X., Wang Y., Yan X., et al. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine. 2018;13(4):391–405. doi: 10.2217/nnm-2017-0297. [DOI] [PubMed] [Google Scholar]
- 54.Jung H. W., Jin G.-Z., Kim S. Y., Kim Y. S., Park Y.-K. Neuroprotective effect of methanol extract of Phellodendri Cortex against 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12 cells. Cell Biology International. 2009;33(9):957–963. doi: 10.1016/j.cellbi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Xian Y.-F., Lin Z.-X., Ip S.-P., Su Z.-R., Chen J.-N., Lai X.-P. Comparison the neuropreotective effect of Cortex Phellodendri chinensis and Cortex Phellodendri amurensis against beta-amyloid-induced neurotoxicity in PC12 cells. Phytomedicine. 2013;20(2):187–193. doi: 10.1016/j.phymed.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 56.Park S., Kim D. S., Kang S., Shin B. K. Synergistic topical application of salt-processed Phellodendron amurense and Sanguisorba officinalis Linne alleviates atopic dermatitis symptoms by reducing levels of immunoglobulin E and pro-inflammatory cytokines in NC/Nga mice. Molecular Medicine Reports. 2015;12(5):7657–7664. doi: 10.3892/mmr.2015.4348. [DOI] [PubMed] [Google Scholar]
- 57.Kim H.-J., Kong M.-K., Kim Y.-C. Beneficial effects of Phellodendri Cortex extract on hyperglycemia and diabetic nephropathy in streptozotocin-induced diabetic rats. Journal of Biochemistry and Molecular Biology. 2008;41(10):710–715. doi: 10.5483/bmbrep.2008.41.10.710. [DOI] [PubMed] [Google Scholar]
- 58.Liu W., Liu P., Tao S., et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Archives of Biochemistry and Biophysics. 2008;475(2):128–134. doi: 10.1016/j.abb.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Mori H., Fuchigami M., Inoue N., Nagai H., Koda A., Nishioka I. Principle of the bark of Phellodendron amurense to suppress the cellular immune response. Planta Medica. 1994;60(5):445–449. doi: 10.1055/s-2006-959529. [DOI] [PubMed] [Google Scholar]
- 60.Kreiner J., Pang E., Lenon G. B., Yang A. W. Saposhnikoviae divaricata: a phytochemical, pharmacological, and pharmacokinetic review. Chinese Journal of Natural Medicines. 2017;15(4):255–264. doi: 10.1016/S1875-5364(17)30042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heleno S. A., Martins A., Queiroz M. J., Ferreira I. C. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chemistry. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 62.Shan H. Q., Duan L. S., Qi X., Yang W. Quality control of preparations with phellodendron chinense schneid or phellodendron amurense rupr in three editions of Chinese pharmacopoeia. Chinese Pharmaceutical Affairs. 2016 [Google Scholar]
- 63.Li Y., Liu X., Wang H., et al. Pharmacokinetic studies of phellodendrine in rat plasma and tissues after intravenous administration using ultra-high performance liquid chromatography–tandem mass spectrometry. Journal of Chromatography B. 2016;1029-1030:95–101. doi: 10.1016/j.jchromb.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Tian X., Li Z., Lin Y., Chen M., Pan G., Huang C. Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS. Analytical and Bioanalytical Chemistry. 2014;406(3):841–849. doi: 10.1007/s00216-013-7530-9. [DOI] [PubMed] [Google Scholar]
- 65.Liu P.-P., Jia T.-Z., Xu S., Zhang F. Application of cocktail probe drugs for detecting influences of raw and processed phellodendri cortex on cytochrome P450 isoforms. Journal of Chinese Medicinal Materials. 2015;38(10):2065–2069. [PubMed] [Google Scholar]
- 66.Linn Y.-C., Lu J., Lim L.-C., et al. Berberine-induced haemolysis revisited: safety of Rhizoma coptidis and Cortex phellodendri in chronic haematological diseases. Phytotherapy Research. 2012;26(5):682–686. doi: 10.1002/ptr.3617. [DOI] [PubMed] [Google Scholar]
- 67.Yao H. W., Liu Y. Study of the processing of Chinese herbal medicine: status quo and development strategy. China Pharm, 2008.
- 68.Jin M. Capital Food Medicine. 2018. Discussion on the easily confusing species Huang Bai and Guan Huangbai in the adjustment. [Google Scholar]
- 69.Wu Q., Ju C. G., Ai X., Zhang F. Study on the quality standards of cork and its different processed products from different places of purchase. Asia-Pacific Traditional Medicine. 2017 [Google Scholar]
- 70.Zhou S., Liu Y. G., Zhang G. X., Chen Z. L. Research Progress on Chemical Composition and Quality Control of Corktree. China Pharm; 2012. [Google Scholar]
- 71.Zhang Z. L., Liu D. H., Huang Y. C., Wei G. Determination of limonin and obacunone in Phellodendri Amurensis Cortex, Phellodendri Chinensis Cortex and their processed products by HPLC. Chinese Traditional Patent Medicine. 2011 [Google Scholar]
- 72.Su H., Yue L., Liu Y., et al. Comparison on HPLC specific chromatograms between phellodendri Chinensis cortex and charred phellodendri Chinensis cortex decoction pieces. Chinese Journal of Experimental Traditional Medical Formulae. 2017 [Google Scholar]
- 73.Liang F. L., Zhao H. Z., Lian Y. C., Huang Z. G. Rapid distinguishment and identification of cortex phellodendri chinense and cortex phellodendri amurensis based on TLC. Journal of Zhaoqing University. 2017 [Google Scholar]