Intravenous immunoglobulin for the treatment of childhood encephalitis (original) (raw)
Abstract
Background
Encephalitis is a syndrome of neurological dysfunction due to inflammation of the brain parenchyma, caused by an infection or an exaggerated host immune response, or both. Attenuation of brain inflammation through modulation of the immune response could improve patient outcomes. Biological agents such as immunoglobulin that have both anti‐inflammatory and immunomodulatory properties may therefore be useful as adjunctive therapies for people with encephalitis.
Objectives
To assess the efficacy and safety of intravenous immunoglobulin (IVIG) as add‐on treatment for children with encephalitis.
Search methods
The Cochrane Multiple Sclerosis and Rare Diseases of the CNS group's Information Specialist searched the following databases up to 30 September 2016: CENTRAL, MEDLINE, Embase, CINAHL, ClinicalTrials.gov, and the WHO ICTRP Search Portal. In addition, two review authors searched Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index ‐ Science (CPCI‐S) (Web of Science Core Collection, Thomson Reuters) (1945 to January 2016), Global Health Library (Virtual Health Library), and Database of Abstracts of Reviews of Effects (DARE).
Selection criteria
Randomised controlled trials (RCTs) comparing IVIG in addition to standard care versus standard care alone or placebo.
Data collection and analysis
Two review authors independently selected articles for inclusion, extracted relevant data, and assessed quality of trials. We resolved disagreements by discussion among the review authors. Where possible, we contacted authors of included studies for additional information. We presented results as risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CI).
Main results
The search identified three RCTs with 138 participants. All three trials included only children with viral encephalitis, one of these included only children with Japanese encephalitis, a specific form of viral encephalitis. Only the trial of Japanese encephalitis (22 children) contributed to the primary outcome of this review and follow‐up in that study was for three to six months after hospital discharge. There was no follow‐up of participants in the other two studies. We identified one ongoing trial.
For the primary outcomes, the results showed no significant difference between IVIG and placebo when used in the treatment of children with Japanese encephalitis: significant disability (RR 0.75, 95% CI 0.22 to 2.60; P = 0.65) and serious adverse events (RR 1.00, 95% CI 0.07 to 14.05; P = 1.00).
For the secondary outcomes, the study of Japanese encephalitis showed no significant difference between IVIG and placebo when assessing significant disability at hospital discharge (RR 1.00, 95% CI 0.60 to 1.67). There was no significant difference (P = 0.53) in Glasgow Coma Score at discharge between IVIG (median score 14; range 3 to 15) and placebo (median 14 score; range 7 to 15) in the Japanese encephalitis study. The median length of hospital stay in the Japanese encephalitis study was similar for IVIG‐treated (median 13 days; range 9 to 21) and placebo‐treated (median 12 days; range 6 to 18) children (P = 0.59).
Pooled analysis of the results of the other two studies resulted in a significantly lower mean length of hospital stay (MD ‐4.54 days, 95% CI ‐7.47 to ‐1.61; P = 0.002), time to resolution of fever (MD ‐0.97 days, 95% CI ‐1.25 to ‐0.69; P < 0.00001), time to stop spasms (MD ‐1.49 days, 95% CI ‐1.97 to ‐1.01; P < 0.00001), time to regain consciousness (MD ‐1.10 days, 95% CI ‐1.48 to ‐0.72; P < 0.00001), and time to resolution of neuropathic symptoms (MD ‐3.20 days, 95% CI ‐3.34 to ‐3.06; P < 0.00001) in favour of IVIG when compared with standard care.
None of the included studies reported other outcomes of interest in this review including need for invasive ventilation, duration of invasive ventilation, cognitive impairment, poor adaptive functioning, quality of life, number of seizures, and new diagnosis of epilepsy.
The quality of evidence was very low for all outcomes of this review.
Authors' conclusions
The findings suggest a clinical benefit of adjunctive IVIG treatment for children with viral encephalitis for some clinical measures (i.e. mean length of hospital stay, time (days) to stop spasms, time to regain consciousness, and time to resolution of neuropathic symptoms and fever. For children with Japanese encephalitis, IVIG had a similar effect to placebo when assessing significant disability and serious adverse events.
Despite these findings, the risk of bias in the included studies and quality of the evidence make it impossible to reach any firm conclusions on the efficacy and safety of IVIG as add‐on treatment for children with encephalitis. Furthermore, the included studies involved only children with viral encephalitis, therefore findings of this review cannot be generalised to all forms of encephalitis. Future well‐designed RCTs are needed to assess the efficacy and safety of IVIG in the management of children with all forms of encephalitis. There is a need for internationally agreed core outcome measures for clinical trials in childhood encephalitis.
Plain language summary
An assessment of the effectiveness and safety of a treatment used for children with encephalitis
Background
At present, there is uncertainty among clinicians regarding the routine use of a treatment called intravenous immunoglobulin (IVIG) in the management of children with some forms of encephalitis (inflammation of the brain). This study is important because it is the first to evaluate through direct comparison whether adding IVIG to standard treatment has added beneficial effect in terms of clinical effectiveness and safety in the management of children with encephalitis.
Study characteristics
We searched medical databases for studies in which neither participants nor researchers were told which treatment was given (called a randomised double‐blind trial). The effectiveness and safety of IVIG were considered in terms of the occurrence of significant disability at six months after hospital discharge and the proportion of children experiencing at least one serious side effect.
Key results and quality of evidence
Up to 30 September 2016, only three studies comprising 138 children met the criteria to be included in this review. All three studies included only children with viral encephalitis. One study of Japanese encephalitis, a specific form of viral encephalitis, analysed both effectiveness and safety, and concluded that IVIG treatment had no additional beneficial effects when compared with placebo (pretend) treatment. The other two studies analysed other measurements, such as length of hospital stay, time to resolution of spasms, symptoms arising due to nerve damage, and time to regain consciousness and concluded that adding IVIG treatment was more effective than standard care alone when these outcomes were considered. The quality of the evidence was very low due to the small number of children and studies.
Conclusions
The quality of evidence in the included studies was very low, making it impossible to draw any firm and definite conclusions on the clinical efficacy and safety of IVIG treatment for children with encephalitis.
Furthermore, there was no information on funding while, for one study, the main authors' group was affiliated to the funding body: this is a well‐known potential source of conflict of interest and thus of bias.
Summary of findings
Summary of findings for the main comparison. Intravenous immunoglobulin with or without standard care compared with standard care alone or placebo for children with acute encephalitis.
| Intravenous immunoglobulin ± standard care compared with standard care alone or placebo for children with acute encephalitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: children with acute encephalitisSettings: secondary and tertiary careIntervention: intravenous immunoglobulinComparison: placebo or standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk (control) | Corresponding risk (intervention) | |||||
| Standard care or placebo | IVIG (± standard care) | |||||
| Significant disability assessed using Liverpool Outcome ScoreRR (M‐H, random‐effects analysis, 95% CI)Follow‐up: 3‐6 months | 364 per 1000 | 273 per 1000(80 to 945) | RR 0.75(0.22 to 2.60) | 22 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | Unclear risk of detection bias and high risk of publication bias. Small sample size. Study not powered on primary outcome. |
| ≥ 1 serious adverse eventRR (M‐H, random‐effects analysis, 95% CI) | 91 per 1000 | 91 per 1000(6 to 1000) | RR 1.00(0.07 to 14.05) | 22 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | Unclear risk of detection bias and high risk of publication bias. Small sample size. Study not powered on primary outcome. |
| Length of hospital stay(MD, random‐effects analysis, 95% CI) | The mean length of hospital stay was 13.26 | MD 4.54 lower (7.47 to 1.61 lower) | ‐ | 116 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,f,g,h,i | Unclear risk of selection bias in 1 study contributing to this outcome assessment. Unclear risk of allocation concealment, performance and detection bias in both studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVIG: intravenous immunoglobulin; M‐H: Mantel‐Haenszel; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. |
Background
Description of the condition
Encephalitis is a syndrome of neurological dysfunction that results from inflammation of the brain parenchyma. The worldwide annual incidence ranges from 3.5 to 7.4 per 100,000, rising to 16 per 100,000 in children, with the highest incidence in infants under one year of age (Thompson 2012). In England, the incidence of childhood encephalitis is 4.02 per 100,000 per year (Iro 2017). Encephalitis could result from an infection of the brain (infectious encephalitis) or from autoantibodies that affect the brain (immune‐mediated encephalitis), or both (Zuliani 2012).
Infections have been considered the major cause of encephalitis and more than 100 different causative pathogens have been recognised. Viruses are the most common pathogens known to cause encephalitis. However, a host of other pathogens including bacteria and protozoa have also been implicated.
Immune‐mediated disorders, such as acute disseminated encephalomyelitis, are now recognised to contribute to a significant proportion of cases where no infective cause is identified (Granerod 2010). More recently, several well‐characterised immunological syndromes that are mediated by antibodies against central nervous system surface proteins such as the N‐methyl‐D‐aspartate receptor and the voltage‐gated potassium channel‐complex and its associated proteins, have been identified in people with encephalitis (Hacohen 2013; Hacohen 2014), and these account for 4% and 7% of overall cases (Granerod 2010). However, despite routine investigations, no aetiology is found in up to 60% of cases of encephalitis (Davison 2003; Granerod 2010).
There is a high rate of mortality and morbidity from encephalitis, despite current standard of care. An exaggerated host immune response has been implicated in the pathogenesis of encephalitis and this has been shown to play a part in the disease pathogenesis (Cervantes‐Barragán 2012; de Aquino 2013; Lundberg 2008; Ramakrishna 2013).
While a mortality rate of up to 20% has been reported (Granerod 2010; Ramakrishna 2013), incomplete recovery after childhood encephalitis is common with persisting symptoms in up to 60% of affected children (Aygun 2001; Fowler 2010). One 12‐year prospective study of children with herpes simplex virus encephalitis demonstrated that neurological sequelae occurred in 63% of cases, including seizures in 44% and developmental delay in 25% (Fowler 2008). Long‐term complications such as severe physical impairment and behavioural, psychosocial, and educational difficulties have also been reported (Dowell 2000).
Encephalitis imposes a substantial economic and healthcare resource burden. Health, social, and economic costs are also extended where families are left bereaved or with a child who has sustained disability. One 12‐year US review reported an encephalitis‐associated hospitalisation rate of 6.9 per 100,000 people. The death rate in the same study was 5.8% of all hospitalisations (Vora 2014). In one ten year review of encephalitis admissions to paediatric intensive care unit (ICU) in the England and Wales, 80% of admitted children required invasive ventilation, 19% required cardiovascular support and 6% required renal dialysis and the mean length of stay on ICU was 4.6 days (UK Trialists). One US study reported approximately 19,000 hospitalisations (7.3 hospitalisations per 100,000 population) and 230,000 hospital days from encephalitis over a 10‐year period, with an estimated cost from encephalitis‐associated hospitalisations of USD28,000 leading to an annual national cost of USD650 million (Khetsuriani 2002).
Given this huge burden from encephalitis despite current standard of care, there is the need to identify other adjunctive treatment option that could be used in the management of children with encephalitis.
Irrespective of aetiology, the underlying pathogenesis in encephalitis is brain inflammation. The degree of brain inflammation seen in some forms of encephalitis correlates with clinical outcomes (Ramakrishna 2013). Attenuation of such inflammation either as a direct effect or through modulation of the immune response may therefore be key to improving clinical outcomes. Biological agents that possess anti‐inflammatory or immunomodulatory (or both) properties may therefore be useful as adjunctive therapies and could improve outcomes (Ramakrishna 2013; Rozenberg 2013). Intravenous immunoglobulin (IVIG) is one such biological agent.
Description of the intervention
The aim of this review was to assess the role of add‐on IVIG in the treatment of children with all forms of acute encephalitis. Intravenous aciclovir is used as first‐line treatment for children with infective encephalitis. In autoimmune encephalitis, corticosteroid therapy is sometimes used. Therefore, we considered both treatments in this review. Plasmapheresis is not universally available and was not considered in this review, likewise the use of experimental therapies.
Intravenous immunoglobulin
IVIG is a blood product made from pooled collections of human plasma collected from thousands of blood donors. It comes as a ready‐to‐use liquid formulation for intravenous administration. It has a half‐life of three to four weeks. IVIG is being used increasingly in the management of a wide range of neurological conditions and its efficacy has been established in a few of these (Hughes 2009). Licensed indications include as replacement therapy for people with primary and secondary antibody deficiency states, Kawasaki disease, haematological conditions (idiopathic immune thrombocytopenic purpura, B‐cell chronic lymphocytic leukaemia), and neurological conditions (myasthenia gravis, multifocal motor neuropathy, Guillain‐Barré syndrome, and chronic demyelinating polyneuropathy) (FDA 2013). IVIG is sometimes also used off‐label for the treatment of children with encephalitis.
The dose of IVIG for each indication varies. For primary and secondary antibody deficiency states, the starting dose is between 0.4 g/kg and 0.6 g/kg of bodyweight and needs to be adjusted based on clinical outcome. For neurological diseases, two doses of 2 g/kg of bodyweight over five days and given six weeks apart. For haematological conditions, a dose of 0.8 g/kg to 1 g/kg is used.
IVIG treatment is safe; however, adverse events such as chills, headache, fever, vomiting, allergic reaction, nausea, arthralgia, low blood pressure, and low back pain may occur. Rarely, sudden fall in blood pressure, anaphylactic shock, and thromboembolic reactions could occur. Cases of reversible aseptic meningitis and isolated cases of haemolytic anaemia have been observed as well as acute renal failure. Since IVIG is a blood product, there is the risk of transmission of infectious agents such as HIV and viral hepatitis by contaminated products (Looney 2006).
Aciclovir
Aciclovir is widely used in the treatment of herpesvirus infections, particularly herpes simplex and varicella‐zoster virus. It is a synthetic nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 and 2 as well as varicella‐zoster virus (Elion 1983). Aciclovir is available in oral, topical, and intravenous forms. It is poorly water soluble and has poor oral bioavailability hence intravenous administration is necessary if high concentrations are required, such as in serious infections. The intravenous form is used in the treatment of herpes simplex and varicella‐zoster virus encephalitis and the dose is based on weight and varies with age:
- neonate (20 mg/kg);
- child one to three months (10 mg/kg to 20 mg/kg);
- child three months to 12 years (500 mg/m2 every eight hours);
- child 12 years to 18 years (10 mg/kg every eight hours).
Duration of treatment for encephalitis is 14 to 21 days (BNF Publications).
Adverse effects such as nausea, vomiting, diarrhoea, abdominal pain, hepatitis, and renal dysfunction have been reported with aciclovir use. Interactions have also been reported with ciclosporin (increased risk of toxicity), mycophenolate (increased plasma concentration of aciclovir and inactive metabolite of mycophenolate), probenecid (reduced excretion of aciclovir and increased plasma concentration), tacrolimus (increased risk of nephrotoxicity), and theophylline (increase plasma concentration of theophylline) (BNF Publications).
Corticosteroids
The role of corticosteroid in the treatment of encephalitis is not yet established. Although not routinely used in herpes simplex virus encephalitis (HSVE), corticosteroids are often used in people with HSVE with marked cerebral oedema, brain shift, or raised intracranial pressure. Information from experimental animal research (Sellner 2005; Thompson 2000), and from clinical observations (Kamei 2005; Lizarraga 2013; Ramos‐Estebanez 2014), indicate a substantial benefit in outcomes for people with HSVE treated with adjuvant dexamethasone. Corticosteroids are potent anti‐inflammatory agents. Their clinical use along with antiviral therapy has been advocated in people with HSVE and cerebral oedema where they reduce brain swelling. Results of a prospective randomised controlled trial (RCT) on the use of adjunctive corticosteroid therapy in HSVE are awaited (Martinez‐Torres 2008).
How the intervention might work
Intravenous immunoglobulin
IVIG has multiple actions which may operate in concert with each other. For a particular disease, there may be one predominant mechanism of action depending on the underlying disease pathogenesis. The most relevant actions of IVIG include the following:
- inhibition of complement binding and prevention of membrane attack complex formation (Basta 1996; Basta 2003; Lutz 1996; Mollnes 1995);
- neutralisation of certain pathogenic cytokines (Crow 2007; Stangel 1997);
- regulation of autoantibodies or cytokines by anti‐idiotypic or anticytokine antibodies (Dietrich 1990; Rossi 1988);
- blockade of Fc gamma receptors on macrophages (Dalakas 2004; Kazatchkine 2001);
- modulation of T‐cell function and antigen recognition (Caramello 2006; Dalakas 1998; Ramakrishna 2013; Tha‐In 2008; Trinath 2013).
Additional actions include the effect of IVIG on superantigens and enhancement of remyelination (Dalakas 1998). Antiviral functions of IVIG and its potential to inhibit viral infection has been demonstrated in vitro (Frenzel 2012; Kishimoto 2004; Krause 2002).
Aciclovir
The inhibitory activity of aciclovir is highly selective due to its affinity for the enzyme thymidine kinase encoded by herpes simplex and varicella‐zoster virus. This viral enzyme converts aciclovir into aciclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, aciclovir triphosphate stops replication of herpes viral DNA. This is accomplished in three ways:
- competitive inhibition of viral DNA polymerase;
- incorporation into and termination of the growing viral DNA chain; and
- inactivation of the viral DNA polymerase (Elion 1983; Elion 1993; Kerpel‐Fronius 1983).
Corticosteroids
Corticosteroids are powerful endogenous immunosuppressors, especially for the innate immune response and the subsequent inflammatory reaction (Esposito 2012; McKay 1999). Experimental data indicate that they may attenuate central nervous system damage by reducing cytokine and prostaglandin production, and limiting the nitric oxide concentration induced by the increased expression of immunological nitric oxide synthase (McKay 1999; Meyding‐Lamadé 2002). Dexamethasone represses lipopolysaccharide‐induced nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) activation in the brain (Glezer 2003); cortisol can abolish stimulated interleukin (IL)‐1β; and tumour necrosis factor (TNF)‐α gene expression in microglial cells. Ultimately, the effects of corticosteroids lead to a decrease in proinflammatory signal transduction pathways and gene expression, which is an essential endogenic mechanism to avoid exaggerated responses during immunogenic challenges (Sergerie 2007).
Why it is important to do this review
There remains significant mortality and morbidity from childhood encephalitis despite standard treatment. Effective immunomodulatory strategies could improve outcomes in terms of reduce mortality and morbidity. Several previous reports point to a possible beneficial effect of IVIG in different forms of encephalitis (Caramello 2006; Hacohen 2013; Titulaer 2013; Wang 2006). In one paediatric cohort study, IVIG was used in only 35% (17/48) of participants presenting with probable autoimmune encephalopathy (Dalakas 1998; Hacohen 2013); whilst in one prospective surveillance study, only 15% (6/40) of children with acute disseminated encephalomyelitis received IVIG (Absoud 2013; Marchioni 2013).
However, there remains a lack of consensus amongst clinicians on the use of IVIG in childhood encephalitis and practice varies widely. This review therefore aims to provide a detailed analysis of existing data on the use of IVIG in the treatment of children with encephalitis and may provide additional supportive evidence to inform on its routine use in clinical practice.
Objectives
To assess the efficacy and safety of intravenous immunoglobulin (IVIG) as add‐on treatment for children with encephalitis.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs and excluded uncontrolled or non‐randomised trials.
Types of participants
Inclusion criteria
Children aged six weeks to 17 years with a clinical diagnosis of acute (symptoms present within 24 hours prior to hospitalisation) or subacute (symptoms present between 24 hours and four weeks prior to hospitalisation) encephalitis as defined by the trial investigators. Since there is the likelihood of variation in how encephalitis is diagnosed, where the eligibility of participants in an identified study (in terms of the diagnosis of encephalitis) was in doubt, we planned to apply predefined diagnostic criteria adapted from the Consensus Statement of the International Encephalitis Consortium (Venkatesan 2013) to ascertain eligibility. We planned to agree a diagnosis of encephalitis if the following features were present.
- Altered mental state (reduced or altered conscious level, irritability, altered personality or behaviour, lethargy), and any two of the following:
- brain imaging evidence consistent with encephalitis or immune‐mediated encephalopathy that appears acute in onset;
- cerebrospinal fluid (CSF) pleocytosis: CSF white blood count of 5 cells/mm3 or greater;
- presence of autoantibodies such as N‐methyl D‐aspartate receptor antibodies, and voltage‐gated potassium channel antibodies, in CSF or blood (or both);
- generalised or partial seizures not fully attributable to a pre‐existing seizure disorder;
- new‐onset focal neurological signs (including movement disorders);
- abnormality on electroencephalogram (EEG) or cerebral function analysis monitor that is consistent with encephalitis and not attributable to another cause;
- fever 38 ºC or greater within 72 hours before or after presentation to hospital.
Exclusion criteria
Children with chronic encephalitis (i.e. where presenting symptoms had lasted longer than four weeks).
Children with known hypersensitivity to IVIG.
Types of interventions
- For experimental trials, the intervention group was IVIG (regardless of dose, time of commencement, and duration of treatment) used either alone or in combination with standard care. The control group was standard care alone or in combination with placebo. Standard care was defined as intravenous aciclovir or corticosteroid therapy (methylprednisolone, prednisone, prednisolone, or dexamethasone), or both.
- For cohort and case‐control studies, intervention was any treatment regimen that included IVIG while the control group was any treatment regimen without IVIG.
Types of outcome measures
Primary outcomes
- Proportion of participants with significant disability (= 'poor outcome') at six months after treatment.
- Significant disability using any validated disability assessment scale such as (but not limited to) the Glasgow Outcome Scale Extended (score of 2 or less) (Beers 2012), Liverpool Outcome Score (score of 2 or less) (Lewthwaite 2010), or the modified Rankin Scale (score of 4 or more) (van Swieten 1988).
- Proportion of participants with at least one serious adverse event as defined in the trial.
Where the definition of a serious adverse event was not clearly defined, we used the definition from the International Conference on Harmonisation (ICH) Harmonised Tripartite Guideline (ICH 1994), which defines a serious adverse event as any adverse event that results in any of the following outcomes: is life‐threatening, results in death, requires prolongation of hospital stay, results in persistent or significant disability/incapacity, congenital abnormality. Mortality was reported separately.
Secondary outcomes
Short term (during hospital admission)
- Proportion of participants with significant disability at discharge.
- Glasgow Outcome Score at discharge (either as continuous or categorised data, as reported in the trial).
- Length of hospital stay (either as continuous or categorised data, as reported in the trial).
- Proportion of participants requiring invasive ventilation and duration (either as continuous or categorised data, as reported in the trial).
- Time to fever resolution (either as continuous or categorised data, as reported in the trial).
- Time to stop spasms (either as continuous or categorised data, as reported in the trial).
- Time to regain consciousness (either as continuous or categorised data, as reported in the trial).
- Time to resolution of neuropathic symptoms (either as continuous or categorised data, as reported in the trial).
Long term (at six months' postdischarge from hospital)
- Any cognitive impairment as defined in the trial (binary: yes/no), major cognitive disability as defined by in the trial (binary: yes/no), and mean cognitive scores using any validated age‐appropriate psychometric instrument.
- Bayley Scales of Infant Development (Bayley 2006).
- Wechsler Preschool and Primary Scale of Intelligence (Wechsler 2002).
- Wechsler Intelligence Scale for Children (Wechsler 2003).
- Poor adaptive functioning as defined in the trial (binary: yes/no) or adaptive behaviour scores less than two standard deviations from the mean, using any validated age‐appropriate scale.
- Vineland Adaptive Behaviour Scales (Sparrow 1986; Sparrow 1993).
- Adaptive Behaviour Assessment System (Harrison 2003).
- Behavior Assessment System for Children Parent Rating Scales (Reynolds 2004).
- Quality of life assessment scores obtained using any validated age‐appropriate tool.
- Pediatric Quality of Life Inventory (PedsQL) (Varni 1999).
- Health‐related quality of life (Matza 2004).
- Number of seizures per participant and the proportion of participants with new diagnosis of epilepsy.
Search methods for identification of studies
Electronic searches
We conducted a systematic search without language restrictions to identify all relevant published and unpublished RCTs using the optimally sensitive strategy developed for Cochrane for the identification of RCTs (Lefebvre 2011). The Trials Search Co‐ordinator of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group ran the initial searches for all prospectively registered and ongoing trials in the following databases:
- Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 9);
- MEDLINE (PubMed) (1966 to 30 September 2016);
- Embase (Embase.com) (1974 to 30 September 2016);
- Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1981 to 30 September 2016);
- ClinicalTrials.gov (clinicaltrials.gov);
- World Health Organization (WHO) International Clinical Trials Registry Portal (ICTRP) search portal (apps.who.int/trialsearch/).
The keywords used to search for trials for this review are listed in Appendix 1.
In addition, two review authors (MI, NGM) searched the Global Health Library (Virtual Health Library) (www.globalhealthlibrary.net/php/index.php) and Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐Science (CPCI‐S) (Web of Science) (1945 to date) using a combination of free‐text and MeSH terms to describe the population and intervention as illustrated in Appendix 1. We applied the search filter 'Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); PubMed format' to MEDLINE and adaptations of it to the other databases except CENTRAL (Higgins 2011). We applied no language or date limits to our search. We identified non‐RCTs using the same search strategy, without the RCT/controlled clinical trial filters.
Searching other resources
We screened reference lists of included studies and related reviews for additional trials. In addition, we contacted experts working in this field to enquire about any ongoing trials or unpublished data.
Data collection and analysis
Selection of studies
Two review authors (MI, NGM) independently assessed the eligibility of studies to be included in this review using an eligibility form. We performed screening at two levels. Level one screening involved a broad screen of title or abstracts (or both), as available. In all instances where the title or abstract (or both) suggested that the report related to a trial reporting on the use of IVIG in the treatment of encephalitis in a group of children, we retrieved the full‐text article for further assessment. Level two screening involved a comprehensive assessment of the full text of retrieved articles to ascertain eligibility. We compared multiple reports of the same study, and used the most comprehensive report. We linked multiple publications together as companion reports, but excluded true duplicates. We documented the exclusion of any study from the review, and have described the reasons for exclusion in the Characteristics of excluded studies table. We resolved any disagreements by discussion between the two review authors (MI and NGM) without the need to consult the other coauthors (MA, AJP).
Data extraction and management
Two review authors (MI and NGM) extracted the data from studies independently using a standardised data extraction sheet, accompanied by a data extraction guideline that was developed after piloting the data extraction form. The data extraction form included information on outcomes as listed in the primary and secondary objectives of the review as well as data related to the study characteristics, participant characteristics, interventions and comparators, follow‐up details, declaration of interest for the primary investigators, and source of funding for each study. Where the relevant data were unclear or missing, we contacted the publication author to clarify this or provide the missing data. We resolved disagreements by discussion between two review authors (MI and NGM). We managed and analysed all data using Review Manager 5 software (RevMan 2014).
Assessment of risk of bias in included studies
Experimental studies
Randomised controlled trials
Two review authors (MI and NGM) assessed the risk of bias in the included trials using Cochrane's tool for assessing risk of bias to evaluate the internal validity of the design and conduct of included studies (Higgins 2011). This tool describes a number of domains to be judged on the adequacy of each study: selection bias (sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors for each outcome separately), attrition bias (incomplete outcome data for each outcome separately), reporting bias (selective outcome reporting), and other bias.
To classify the methodological quality of studies, we considered bias for selection, detection, and attrition. We judged a study as having a high risk of attrition bias based on either the reasons for missing data or the difference between the percentage of participants missing in the control and treatment groups. We used the summary quality assessment at the analysis stage as a means of interpreting the results. For each domain and for the summary assessment, we assigned the risk of bias categories as: low risk of bias, plausible bias unlikely to seriously alter the results; unclear risk of bias, plausible bias that raises some doubt about the results; and high risk of bias, plausible bias that seriously weakens confidence in the results (Higgins 2011). We rated a study as 'low risk of bias' when none of the three domains was affected, 'high risk of bias' when at least one of the three domains was affected and 'unclear risk of bias' in all other cases. We considered the risk of bias in included studies when we interpreted the review's results. We compared the outcomes listed in the study protocol, where this was accessible in the public domain, with the outcomes reported in the published manuscript. There was no disagreement in assessing the risk of bias between review authors.
Controlled clinical trials, case‐control studies, and cohort studies
We did not include case‐control or cohort studies in this review. We planned to evaluate the quality of case‐control and cohort studies (prospective and retrospective) using the appropriate Newcastle‐Ottawa Scales and considering the risk of bias in the following domains: selection: case and control (case‐control studies) or exposed and non‐exposed (cohort studies), comparability, and assessment of exposure (case‐ control studies) or outcome (cohort studies) (Appendix 2; Appendix 3). To assess the methodological quality of non‐RCTs, we planned to consider the risk of bias in all three domains listed above and to rate a study as 'high risk of bias' if a least one of the three domains was affected and 'low risk of bias' where none of the domains was affected and 'unclear risk of bias' in all other cases. We planned to assess the quality of non‐randomised studies in relation to the presence of potential confounders that could make interpretation of the results difficult.
Criteria for assessing quality of safety data for all studies
We assessed the risk of bias for serious adverse events by considering specific factors that may have had a large influence on the adverse events data. We evaluated methods for monitoring and detecting a serious adverse event for each study as below.
- Did the researchers actively monitor for serious adverse events (low risk of bias), or did they simply provide spontaneous reporting of serious adverse events that arose (high risk of bias)?
- Did the authors define serious adverse events according to an accepted international classification and report the number of serious adverse events?
Measures of treatment effect
We carried out statistical analyses using Review Manager 5 software (RevMan 2014). For dichotomous data (e.g. mortality), we calculated the risk ratio (RR) with 95% confidence intervals (CIs) to express the effect size. We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) and additional harmful outcome (NNTH). However, this was not calculated because the findings of the meta‐analysis for mortality did not show a statistically significant reduction or increase in risk difference (RD) between the study groups. Only one of the included studies reported on mortality as a binary outcome (number of deaths/total number of participants in each group), with no survival data presented. Therefore, we were unable to analyse mortality as a hazard ratio as planned. For continuous data (e.g. length of hospital stay), we calculated mean differences (MD) with 95% CIs. For both individual and pooled data, we reported effect estimates from both continuous and dichotomous data as P values and 95% CI.
Unit of analysis issues
In all studies, we used the individual participant as the unit of analysis. We found no cross‐over trials.
Dealing with missing data
Where contact details were available, we contacted the main authors to obtain additional data not reported in the published articles. We planned to perform an intention‐to‐treat analysis if this was necessary and possible.
Assessment of heterogeneity
We tested the results from the studies for heterogeneity using the Chi2 test (Higgins 2003). We assessed statistical heterogeneity by visually inspecting forest plots and by using the Chi2 test for heterogeneity (with a P < 0.10 for significance). We used the I2 statistic as a measure of inconsistency across studies. We evaluated the degree of heterogeneity according to the following I2 thresholds:
- 0% to 40%: might not be important;
- 30% to 60%: may represent moderate heterogeneity;
- 50% to 90%: may represent substantial heterogeneity;
- 75% to 100%: considerable heterogeneity.
If substantial statistical heterogeneity was found to be present according to the Chi2 test for heterogeneity or I2 statistic, we performed meta‐analysis with a random‐effects model rather than a fixed‐effect model.
Assessment of reporting biases
The process of reporting bias is described in the Assessment of risk of bias in included studies section. We assessed studies for reporting bias by analysing inclusion and exclusion criteria and blinding of participants and observers. We also analysed primary and secondary outcomes for any reporting biases. We planned to assess reporting bias by visually inspecting the funnel plot to detect the presence of asymmetry if more than 10 studies were found. We did not perform this since the number of studies included did not reach the prespecified threshold and as such the power of the test was too low.
Data synthesis
Meta‐analysis
When more than one study reported continuous or dichotomous data, we pooled the results. We pooled estimates using a fixed‐effect model meta‐analysis where trials were sufficiently homogenous (I2 < 50%). In contrast, we used a random‐effects model where there was sufficient heterogeneity to suggest that treatment effects might differ between trials (I2 > 50%). If data for an outcome were reported using different measurement scales or defined threshold values that could not be adjusted to a uniform scale/statistic (e.g. mean and median for length of hospital stay), we performed a pooled analysis of only those data that had been reported in a similar way and a qualitative description of the results of the other studies not included in the meta‐analysis in the text. Where trials reported binary outcomes such as disability ('poor outcome'), cognitive impairment, or major cognitive disability using different measurement scales or threshold value definitions, or both, we performed only a qualitative description of results. For studies with multiple intervention groups, we performed separate meta‐analyses synthesising each of the different comparisons of interventions for each of the different outcomes.
Subgroup analysis and investigation of heterogeneity
Since clinical outcomes for encephalitis may vary depending on factors such as aetiology, baseline immune status, dose, and timing of IVIG administration as well as timing of standard therapy given, we planned to compare the following possible subgroups:
- different encephalitis types (infective, immune mediated and unknown aetiology);
- primary or secondary immunodeficiency versus immunocompetent people;
- low dose IVIG (less than 1 g/kg) versus high dose (1 g/kg or greater);
- early (within five days of hospital admission) versus late (beyond five days of hospital admission) IVIG treatment;
- early (within 48 hours of admission) versus late (beyond 48 hours of admission) institution of standard therapy.
However, because of limited available data subgroup analyses were not performed.
Sensitivity analysis
We planned to perform sensitivity analysis to assess whether the effect of IVIG varied with the exclusion of studies rated as high or unclear risk of bias. If the results observed remain unchanged, we planned to consider the evidence to be robust. We did not perform a sensitivity analysis because the risk of bias among the included studies was highly diverse and none of the included studies was of adequate methodological quality based on our prespecified definition.
'Summary of findings' table
We created a 'Summary of findings' table using the following prespecified outcomes:
- significant disability;
- at least one serious adverse event;
- length of hospital stay.
We used the five (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro 2014). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and we made comments to aid reader's understanding of the review where necessary.
We planned to include the following prespecified outcomes in the 'Summary of findings' table:
- need for and duration of invasive ventilation;
- cognition;
- poor adaptive function;
- quality of life;
- new‐onset epilepsy.
However, none of the included studies reported on these outcomes.
Results
Description of studies
Results of the search
The process of study identification and selection is shown in Figure 1. The search strategy retrieved 822 titles (MEDLINE (160), Embase (433), the Cochrane Library (11), CINAHL (57) Clinicaltrials.gov (one), Web of Science (23), Global Health Library (135), and DARE (two). One additional record was retrieved through Google search. Of the 681 articles remaining after removal of duplicates, 668 were excluded following level one screening because the titles/abstracts of the articles did not suggest that the report related to a trial reporting on the use of IVIG in the treatment of encephalitis in a child or group of children. Therefore, 13 articles had the potential to be included in this review. We retrieved the full article for all 13 papers and after reading the full text, considered three articles of three trials to be eligible for inclusion. We found one ongoing trial in the UK of IVIG in childhood encephalitis but this was not included since results of the study are not yet available (NCT02308982). One trial is awaiting classification (Fan 2010). This is a study of high‐dose immunoglobulin in severe viral encephalitis and included both children and adults. The findings were presented as aggregate data and the results for children only were not presented separately. We attempted to contact the authors for this study but no contact details have been found so far. We contacted the main author of one of the included studies (Rayamajhi 2015), but we were unable to obtain the information required.
1.
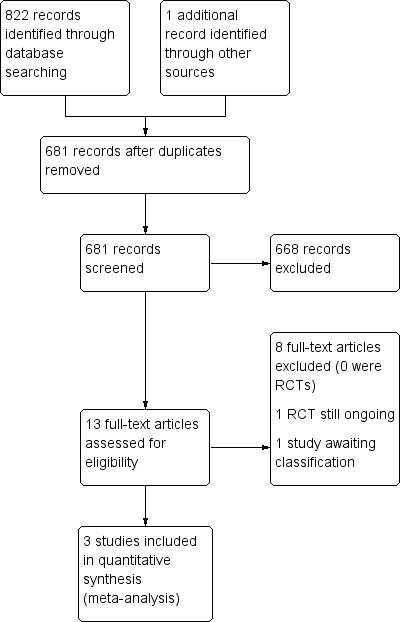
Study flow diagram. RCT: randomised controlled trial.
Included studies
Three studies met the criteria to be included. See Characteristics of included studies table.
Chen 2006 was an RCT of IVIG in addition to standard treatment in children with viral encephalitis. Between February 2000 to October 2004, 62 children aged seven months to 12 years with a diagnosis of viral encephalitis defined according to the guidelines and symptoms described in "Applied Pediatrics" sixth edition were enrolled and randomly split into an observation group (32 children) and a control group (30 children). The proportion of enrolled boys was 58.1%. The mean (± standard deviation (SD) ages for children in the observation were 6.16 ± 2.38 years and for children in the control group were 5.88 ± 2.46 years. Baseline characteristics between both groups (gender, age, clinical symptoms and signs, severity of disease, and duration of hospital stay) did not differ significantly. Both groups underwent conventional treatment which included fever and spasm reductions, reduction of intracranial pressure, maintenance of hydration, electrolyte, and acid‐base balance, application of hormones and antivirals, prevention and treatment of infections, and upkeep of morale. In addition, the observation group received IVIG 400 mg/kg/day for three to five days. Outcome measures included time taken for fever reduction, length of seizures, time taken to regain consciousness, and duration of stay in hospital. The authors concluded that children who received IVIG treatment had significantly quicker recovery time in terms of days to reduction of fever, regaining of consciousness level, and resolution of spasms. Additionally, the authors reported a significantly shorter duration of hospital stay in the observation compared with the control group.
Rayamajhi 2015 was a pilot feasibility RCT conducted at two centres in Nepal from May to June 2009. Children were aged one to 14 years with clinically diagnosed acute encephalitis syndrome using predefined diagnostic criteria (history of fever that lasted less than 14 days, altered consciousness defined as Glasgow Coma Score less than 15 with or without a history of seizures, and consistent CSF findings, i.e. white cell count less than 1000 cells/mm3 with no organisms on Gram stain and CSF:plasma glucose ratio greater than 40%) and suspected to have Japanese encephalitis. Children were excluded if they had a single seizure lasting less than 15 minutes with full recovery of consciousness within 60 minutes (as these were assumed to have had a febrile convulsion); positive blood slide or rapid antigen test for Plasmodium falciparum parasites, clinical or laboratory (or both) features suggestive of bacterial meningitis where antibiotic treatment had already been commenced (for referred children); and a GCS less than 3 (out of 15) and were receiving artificial ventilation without signs of spontaneous respiration, and with absent oculocephalic reflex (as prior studies have shown an extremely low chance of meaningful recovery). Ethical approval and informed consent were obtained. Enrolled children were randomly allocated to receive either IVIG 400 mg/kg/day or an equivalent volume of 0.9% saline (placebo) for five days. Randomisation was stratified by centre using a variable block size of four, six, or eight, selected on a random basis. Participants were assessed at discharge and after three to six months. The reported primary objective was to assess the feasibility of a multicentre placebo‐controlled RCT of IVIG in a resource‐poor setting. Reported secondary outcomes were death or neurological sequelae at discharge or follow‐up, and adverse events. In addition, anti‐Japanese encephalitis virus (anti‐JEV) antibody titres were compared between children treated and not treated with IVIG. A total of 23 children were screened, 22 met the eligibility criteria and were enrolled. One child was not enrolled due to meeting an exclusion criterion. The proportion of enrolled boys was 54.5%. With the exception of CSF protein levels which were significantly higher in the IVIG group, baseline characteristics of the two study groups were comparable; the median age for both groups did not differ statistically and was five years for the IVIG group and seven years for the placebo (saline) group. No protocol breaches were reported. The authors reported that a trial of IVIG in Japanese encephalitis in Nepal is feasible and that IVIG may augment the development of neutralising antibodies in JEV‐positive people.
Wu 2014 was an RCT comparing the clinical efficacy of gamma globulin in the treatment of hand, foot, and mouth disease (HFMD) complicated by viral encephalitis in children admitted to a general hospital in China between January 2009 and January 2013. The diagnosis of HFMD was based on published definition guidelines by the Chinese Department of Ministry of Health. Ethical approval and consent were obtained. Eighty children (aged one to six years) were enrolled and randomly assigned to three groups: experimental group one (Ex1; 27 children), experimental group two (Ex2; 26 children), and control group (CG; 27 children); according to a random‐sampling table. There was no statistically significant difference between the groups in age, gender, and duration of illness. The CG was given conventional comprehensive treatment including dehydration, reduction of intracranial pressure, nutritional support, anti‐infection treatment, keeping the skin clean, and general precautions to prevent infection. Children in Ex1, were given intravenous injection of human blood gamma globulin at 400 mg/kg/day for three consecutive days, in addition to the comprehensive treatment. Children in Ex2 received a spray of human interferon alfa‐2h, one or two sprays per time, three times per day on three consecutive days, in addition to the comprehensive treatment. Children with underlying immune problems and who were unconscious were excluded. The outcomes measured included time for fever to subside, convulsions to be under control, rashes to subside, neuropathic symptoms to subside, and mean stay in hospital. Adverse events occurring in children in the two experimental groups were compared. The authors concluded that the treatment of children with HFMD complicated by encephalitis with gamma globulin and human interferon can relieve clinical symptoms, improve prognosis, and reduce sequelae.
Excluded studies
We excluded eight studies at level two screening due to their study design. More details about excluded studies are detailed in the Characteristics of excluded studies table.
Ongoing studies
We identified one ongoing multicentre, double‐blind, placebo‐controlled RCT of IVIG in childhood encephalitis (NCT02308982). In the trial, 308 children aged six weeks to 16 years with encephalitis are planned to be recruited and randomised 1:1 to receive two doses (1 g/kg/dose) of either IVIG or matching placebo in addition to standard treatment. Follow‐up is for 12 months' post randomisation and the primary outcome will be assessed using the Glasgow Outcome Score Extended ‐ Pediatric version at 12 months' post randomisation.
Risk of bias in included studies
We extracted information from the published papers and by contacting the primary authors of the included papers, where this was possible. Overall results of all the risk of bias assessments are summarised in the Figure 2 and Figure 3 and 'Risk of bias' table. We resolved disagreements between the two review authors.
2.
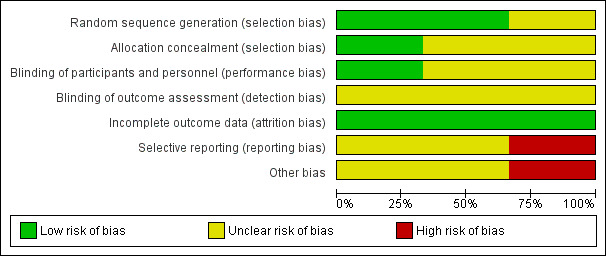
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
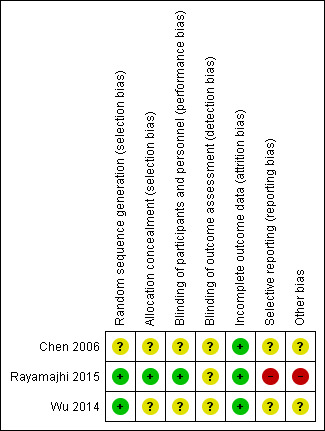
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All three studies were described as RCTs (Chen 2006; Rayamajhi 2015; Wu 2014). However only two of these described the method of randomisation used, which we judged appropriate (Rayamajhi 2015; Wu 2014). We judged the risk of selection bias for both studies as low. We judged the risk of selection bias for Chen 2006 as unclear since the exact method of randomisation was not reported.
Allocation concealment
Only one study reported the method of allocation concealment (Rayamajhi 2015). The randomisation code for each participant in the study was kept in a sealed envelope and was opened by independent investigators. Technicians who were not otherwise connected to the study opened the envelopes containing participant allocation, drew up the study drug, and covered the syringe in opaque tape (because of the subtle differences in colour and viscosity of IVIG and 0.9% saline) before giving it to the nurses for administration. It was unclear whether masking was also applied to the infusion equipment. In addition, empty vials of the study drug were concealed in paper bags. We judged this to be adequate and therefore gave a grade of low risk of bias. We gave two studies a grade of unclear risk of bias for method of allocation concealment since this information was not provided (Chen 2006; Wu 2014).
Blinding
Rayamajhi 2015 was described as a double‐blind study. All investigators, care providers, and participants were blind to the treatment allocation. However, it is unclear whether the investigators who performed the outcome assessment were also blind to the treatment allocation. Therefore, we rated this study at low risk for performance bias and unclear risk for detection bias. Neither of the other two studies gave information on blinding and we judged the risk of performance and detection bias for both studies to be unclear (Chen 2006; Wu 2014).
Incomplete outcome data
Only one study followed up participants beyond the hospitalisation period (Rayamajhi 2015). The number of participants lost to follow‐up in each group was carefully reported in using a flow chart and this was equal for both study groups. Fewer participants in the IVIG group compared with the placebo group were included in the assessment of the additional outcomes of the study: difference in anti‐JEV neutralising antibody titres and cytokine abundance and the reason for missing data was clearly provided. Therefore, we rated the risk of attrition bias for Rayamajhi 2015 as low. Review of data presented in Chen 2006 and Wu 2014 did not suggest any loss to follow‐up at the time of discharge, thus we rated the risk of attrition bias for both studies as low.
Selective reporting
All three studies identified clinically relevant outcomes (Chen 2006; Rayamajhi 2015; Wu 2014). However, only one study reported on outcomes beyond the hospitalisation period (Rayamajhi 2015). Only one study reported on neurological outcomes (Rayamajhi 2015), which are of crucial clinical relevance in encephalitis with only one study using a validated assessment scale for assessment of this outcome (Rayamajhi 2015). We identified the protocol for only one of the included studies (Rayamajhi 2015) and some a priori identified endpoints were not reported. Details of participant death were also only provided for the IVIG and not the placebo group. Therefore, we rated the risk of reporting bias as high. The protocols for the other included studies were not publicly available thus we were unable to check whether all predefined outcomes were reported (Chen 2006; Wu 2014). Therefore, we rated the risk of reporting bias for the two studies as unclear.
Other potential sources of bias
The sample size for Rayamajhi 2015 was calculated to detect differences in the study groups for a tertiary outcome. Therefore, it is unclear whether the study was adequately powered to detect any differences between the study groups for either the primary or secondary outcomes. There was some discrepancy in the reported recruitment period. Key information such as the split in the number of participants admitted to ICU was not reported, this information is important since admission to ICU is a surrogate for disease severity. There was also an imbalance with the number of participants in both study groups who received corticosteroid therapy, which is a potential confounder and this was not considered in the analysis. The main authors' group provided funding for the study hence conflict of interests could not be excluded. Therefore, we rated Rayamajhi 2015 as having high risk for other bias. The sample sizes for Chen 2006 and Wu 2014 were small and information on sample size calculation for both studies was not provided. Therefore, it is unclear whether these studies were sufficiently powered to detect differences between the study groups. Also, no funding information was provided for both studies. Specific information on the eligibility criteria used to identify participants in Wu 2014 was not provided which makes it impossible to ascertain how well the study population truly represents children with viral encephalitis. Therefore, we judged the risk of other bias for both studies as unclear (Chen 2006; Wu 2014). Due to the limited number of included studies (fewer than 10), we were unable to investigate potential publication bias using a funnel plot.
Risk of bias for adverse events
Based on our predefined criteria, we rated Rayamajhi 2015 as having a low risk of bias for adverse events and the other two studies as having unclear risk of bias (Chen 2006; Wu 2014). Further information is provided in Table 2.
1. Risk of bias (adverse events).
| Study | Risk of bias | Did the researchers actively monitor for AEs or did they simply provide spontaneous reporting of AEs that arose? | Risk of bias | Did the authors define SAEs according to an accepted international classification and report the number of SAEs? |
|---|---|---|---|---|
| Chen 2006 | Unclear | Information not provided. | Unclear | Information not provided. |
| Rayamajhi 2015 | Low | The authors reported that participants were monitored for AEs from first day of commencing treatment until death or discharge | Low | AEs graded using WHO recommendations and reported to an independent data safety monitoring committee. Information recorded on standardised proformas. |
| Wu 2014 | Unclear | Information not provided. | Unclear | Information not provided. |
Effects of interventions
See: Table 1
Primary outcomes
Proportion of participants with significant disability (= 'poor outcome') assessed at six months after treatment
Only one study reported proportion of participants with significant disability (Rayamajhi 2015). Rayamajhi 2015 assessed disability at three to six months' follow‐up using a validated assessment tool, the Liverpool Outcome Score. Based on the predefined definitions for this review (significant disability = Liverpool Outcome Score of 2 or less), 3/11 (27.3%) participants in the IVIG group versus 4/11 (36.4%) participants in the placebo group had significant disability at three to six months (RR 0.75, 95% CI 0.22 to 2.60; P = 0.65) (Analysis 1.1).
1.1. Analysis.
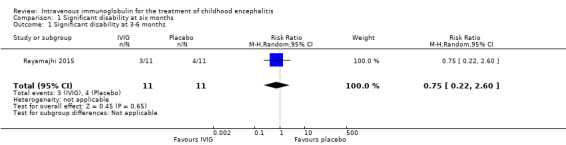
Comparison 1 Significant disability at six months, Outcome 1 Significant disability at 3‐6 months.
Proportion of participants with at least one serious adverse event
All three studies reported on adverse events. Only one study provided information on the criteria used to categorise adverse events where adverse events were graded using the WHO recommendations (Rayamajhi 2015). Reported serious adverse events included death, melaena, and hypotension. For the other included studies for which a clear definition of serious adverse event was not provided (Chen 2006; Wu 2014), we used the ICH Tripartite Guideline to determine which reported adverse events were serious, based on available information reported in the paper. Adverse events reported by Chen 2006 included flushing and itchy skin while Wu 2014 reported on nausea, palpitations, and chest tightness. We did not consider any of these adverse events to meet the serious adverse event criteria. Therefore, information on serious adverse events was only available from one study (Rayamajhi 2015). The number of participants experiencing at least one significant adverse event was 1/11 in the IVIG group and 1/11 in the placebo group (RR 1.00, 95% CI 0.07 to 14.05; P = 1.00) (Analysis 2.1). In Rayamajhi 2015, the proportion of deaths in the IVIG group was 1/11 versus 2/11 in the placebo group (RR 0.50, 95% CI 0.05 to 4.75; P = 0.55) (Analysis 2.2). Rayamajhi 2015 reported no significant difference in the number of participants experiencing either hypotension or melaena in either group (RR 1.00, 95% CI 0.07 to 14.05; P = 1.00) (Analysis 2.3; Analysis 2.4).
2.1. Analysis.
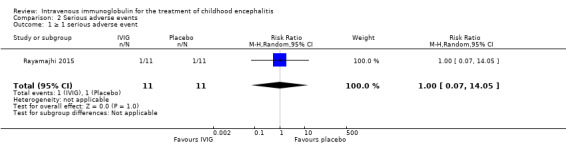
Comparison 2 Serious adverse events, Outcome 1 ≥ 1 serious adverse event.
2.2. Analysis.
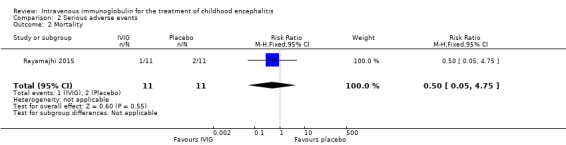
Comparison 2 Serious adverse events, Outcome 2 Mortality.
2.3. Analysis.
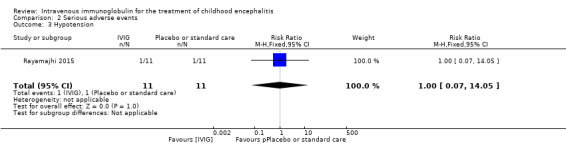
Comparison 2 Serious adverse events, Outcome 3 Hypotension.
2.4. Analysis.
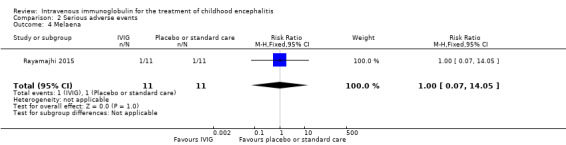
Comparison 2 Serious adverse events, Outcome 4 Melaena.
Secondary outcomes
Proportion of participants with significant disability at discharge
Only one of the included studies assessed disability at the time of discharge and reported 8/11 participants in each group as having significant disability at discharge (RR 1.00, 95% CI 0.60 to 1.67; P = 1.00). (Analysis 3.1) (Rayamajhi 2015).
3.1. Analysis.
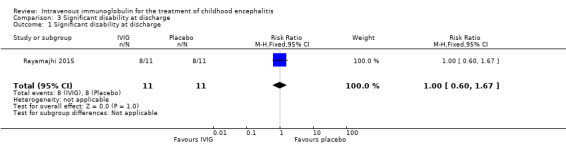
Comparison 3 Significant disability at discharge, Outcome 1 Significant disability at discharge.
Glasgow Coma Score at discharge
Only one included study assessed Glasgow Coma Score at discharge and reported a no significant difference between the IVIG and placebo groups (IVIG: median score 14, range 3 to 15; placebo: median score 14, range 7‐15; P = 0.53) (Rayamajhi 2015).
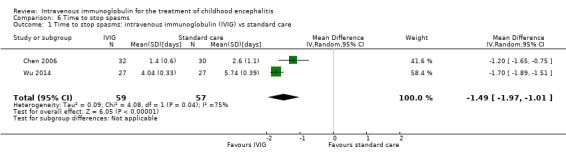
Length of hospital stay
All three studies reported length of hospital stay. Two studies reported length of stay as mean and SDs (Chen 2006; Wu 2014), while one study reported length of hospital stay as median and range (Rayamajhi 2015). Pooled analysis of two studies found a significant difference in favour of the IVIG group (MD ‐4.54 days, 95% CI ‐7.47 to ‐1.61; P = 0.002) (Analysis 4.1) (Chen 2006; Wu 2014). Rayamajhi 2015 found no significant difference between groups (IVIG: median 13 days; range 9 to 21; placebo: median 12 days; range 6 to 18; P value 0.59). Wu 2014 also compared mean length of stay between participants receiving IVIG or interferon in addition to standard care and reported a significant difference in favour of IVIG (MD ‐0.57, 95% CI ‐0.99 to ‐0.15; P = 0.008) (Analysis 4.2).
4.1. Analysis.
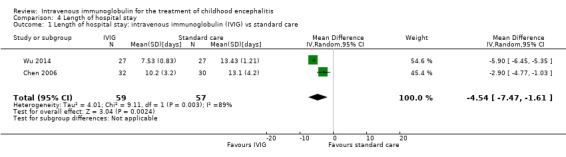
Comparison 4 Length of hospital stay, Outcome 1 Length of hospital stay: intravenous immunoglobulin (IVIG) vs standard care.
4.2. Analysis.
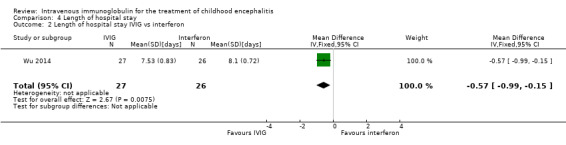
Comparison 4 Length of hospital stay, Outcome 2 Length of hospital stay IVIG vs interferon.
Proportion of participants requiring invasive ventilation and duration
None of the included studies reported the proportion of participants requiring invasive ventilation and duration of ventilation.
Time to fever resolution
Two of the included studies reported on days to fever resolution as mean and SDs (Chen 2006; Wu 2014). Pooled analysis showed a significant difference in favour of IVIG (MD ‐0.97 days, 95% CI ‐1.25 to ‐0.69; P < 0.00001) (Analysis 5.1). Wu 2014 also compared days to fever resolution between participants receiving IVIG or interferon in addition to standard care and reported a statistically significant difference in favour of IVIG (MD ‐0.27 days, 95% CI ‐0.42 to ‐0.12; P = 0.0006) (Analysis 5.2).
5.1. Analysis.
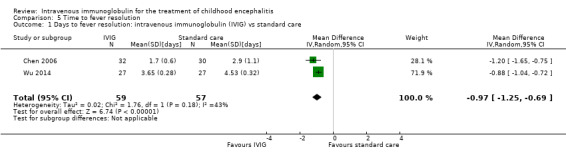
Comparison 5 Time to fever resolution, Outcome 1 Days to fever resolution: intravenous immunoglobulin (IVIG) vs standard care.
5.2. Analysis.
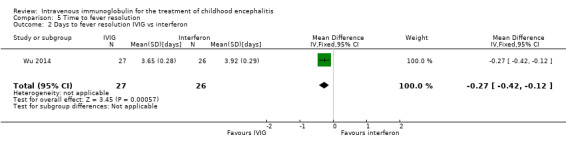
Comparison 5 Time to fever resolution, Outcome 2 Days to fever resolution IVIG vs interferon.
Time to stop spasms
Two of the included studies reported on days to stop convulsions/spasms as mean and SDs (Chen 2006; Wu 2014). Pooled analysis showed a significant difference in favour of IVIG (MD ‐1.49 days, 95% CI ‐1.97 to ‐1.01; P < 0.00001) (Analysis 6.1). Wu 2014 also compared time to stop spasms between the IVIG and interferon group and reported no significant difference between groups (MD ‐0.17, 95% CI ‐0.34 to 0.00; P = 0.06) (Analysis 6.2).
6.1. Analysis.
Comparison 6 Time to stop spasms, Outcome 1 Time to stop spasms: intravenous immunoglobulin (IVIG) vs standard care.
6.2. Analysis.
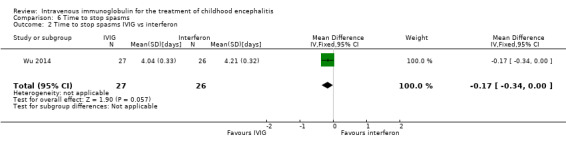
Comparison 6 Time to stop spasms, Outcome 2 Time to stop spasms IVIG vs interferon.
Time to regain consciousness
Only one study reported time to regain consciousness as mean and SDs and found a statistically significant difference in favour of the IVIG group (MD ‐1.10, 95% CI ‐1.48 to ‐0.72; P < 0.00001) (Analysis 7.1) (Chen 2006).
7.1. Analysis.
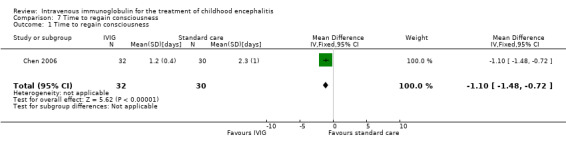
Comparison 7 Time to regain consciousness, Outcome 1 Time to regain consciousness.
Time to resolution of neuropathic symptoms
Wu 2014 reported time to resolution of neuropathic symptoms as mean and SDs and found a statistically significant difference in favour of the IVIG group when compared with standard care (MD ‐3.20 days, 95% CI ‐3.34 to ‐3.06; P < 0.00001) (Analysis 8.1). There was a statistically significant difference in favour of the IVIG group when compared with interferon (MD ‐0.49 days, 95% CI ‐0.70 to ‐0.28; P < 0.00001) (Analysis 8.2).
8.1. Analysis.
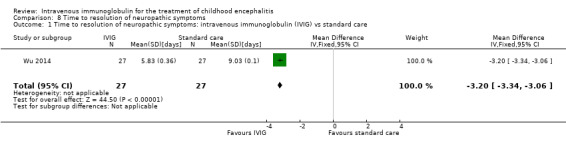
Comparison 8 Time to resolution of neuropathic symptoms, Outcome 1 Time to resolution of neuropathic symptoms: intravenous immunoglobulin (IVIG) vs standard care.
8.2. Analysis.
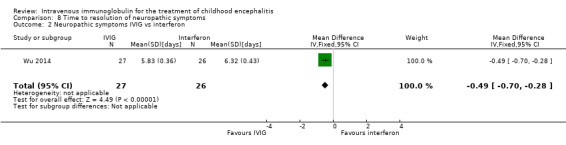
Comparison 8 Time to resolution of neuropathic symptoms, Outcome 2 Neuropathic symptoms IVIG vs interferon.
Cognitive impairment
None of the included studies reported cognitive impairment.
Poor adaptive functioning
None of the included studies reported poor adaptive functioning.
Quality of life assessment
None of the included studies reported quality of life.
Number of seizures and proportion of participants with new diagnosis of epilepsy
None of the included studies reported number of seizures and proportion of participants with new diagnosis of epilepsy.
Discussion
Summary of main results
The purpose of this review was to evaluate whether treatment with IVIG in addition to standard care for children with encephalitis was different in terms of efficacy and safety when compared with standard care alone or placebo. We did this by performing a systematic review of RCTs reporting head‐to‐head comparison of these treatments. Three RCTs involving 138 participants (70 participants treated with IVIG either alone or in addition to standard care and 68 participants treated with either standard care or placebo) were included and contributed to this review data. However, not all the included studies contributed to the data for the different outcomes assessed with only 22 participants (11 participants in each group) from one study of Japanese encephalitis (Rayamajhi 2015) contributing to the primary outcomes of this review. Only one study followed up participants beyond hospital discharge, for three to six months (Rayamajhi 2015).
The main conclusion based on the findings of one study in children with Japanese encephalitis was that IVIG treatment did not offer additional benefit over placebo when clinical efficacy (proportion of participants with significant disability) and safety (proportion of participants with at least one serious adverse event) were considered. Similarly, the median length of hospital stay and Glasgow Coma Score at the time of hospital discharge were similar for children with Japanese encephalitis who received IVIG treatment and those who received placebo. Based on the findings of two other studies of viral encephalitis, adjunctive IVIG treatment was more effective than standard care alone in reducing the mean length of hospital stay, time to resolution of spasms, neuropathic symptom, time to regain consciousness, and time to resolution of fever. Methodological issues in the included studies have been highlighted.
The quality of evidence was judged as very low (Table 1).
Overall completeness and applicability of evidence
The studies included in this review were heterogeneous in terms of the type of encephalitis that participants had. None of the studies included participants with non‐viral or immune‐mediated forms of encephalitis. The dose of IVIG used in all three studies was similar.
The objective of this review was achieved allowing comparison between IVIG and standard care or placebo in terms of the main clinical efficacy and safety outcomes; however, only one study contributed data to the primary outcome analysis. For this review, we selected outcome measures that are thought to be clinically relevant in encephalitis after consulting with specialists in the field. Several predefined secondary outcomes such as cognitive functioning, adaptive functioning, and quality of life were not reported in the included studies which impaired comparison of these outcomes. Assessment of long‐term outcomes is extremely important in the management of people with encephalitis since up to 60% of affected people can experience long‐term morbidity. Participants were not followed up in two of the included studies (Chen 2006; Wu 2014), and follow‐up was short (three to six months) in the only study where participants were followed up (Rayamajhi 2015).
The main limitations of the findings of this review included the small number of identified studies contributing to the meta‐analysis, the small sample size in each of the included studies, the short follow‐up period, and the inherent methodological limitations in each of the included studies which might have a role in the final assessment of efficacy and safety of IVIG treatment. In addition, the quality of evidence was very low for the prespecified outcomes reported in the included studies (Table 1). Furthermore, there was heterogeneity in terms of the type of encephalitis studied and the eligibility criteria used limits generalisability of the findings. All included studies were conducted in lower middle‐income countries: Nepal (Rayamajhi 2015) and China (Chen 2006; Wu 2014), which further limits the extent to which the findings could be applied to other settings such as high middle‐income countries. Therefore, it is not possible to draw any definite conclusions on the safety and efficacy of IVIG in the treatment of childhood encephalitis from these data. This highlights the need for a well‐designed RCT of a large sample size in which participants are followed up for a longer time and clinically relevant outcomes such as adaptive and cognitive functioning are assessed. The identified ongoing trial looks promising and could be key to addressing the objectives of this review (NCT02308982).
Quality of the evidence
We included three RCTs but not all contributed to the analyses for the different outcomes with only one study contributing data for the primary outcome. Where two studies reported on the same efficacy outcome, there was no heterogeneity of results. The body of evidence was judged to be of very low quality (Table 1).
Potential biases in the review process
The review authors declared no conflicts of interest in this review. We undertook an extensive and comprehensive search to minimise bias in the review process. The trial search strategy and contacts initiated with the main investigators suggested the likelihood that all relevant studies were identified and as far as possible, all relevant data were obtained. Two review authors independently screened trials identified by the search, extracted the data, and assessed the quality of included trials to minimise potential biases.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first meta‐analysis of IVIG treatment for childhood encephalitis published. One recently published systematic review of IVIG use in paediatric neurology and neurodevelopmental disorders concluded that it is possible that IVIG improves recovery in acute disseminated encephalomyelitis and that IVIG with or without additional immunotherapy is associated with significant or full recovery in N‐methyl‐D‐aspartate receptor antibody encephalitis (Gadian 2017). Of note, the conclusions of the review were based on qualitative synthesis without fully considering the potential influences of bias on the internal validity of the results.
Authors' conclusions
Implications for practice.
Evidence of very low quality suggests a beneficial effect of intravenous immunoglobulin (IVIG) in reducing length of hospital stay, time to resolution of fever, spasms, neuropathic symptoms, and time to regain consciousness and a lack of benefit in terms of mortality and significant disability. However, due to the limitations of the included studies, we are unable to draw definite conclusions from the presented evidence on the safety and efficacy of IVIG treatment for childhood encephalitis.
Implications for research.
Outcomes for childhood encephalitis are poor despite currently available treatment, which highlights the need to identify other treatment options. There is increasing evidence of a beneficial role of IVIG in the treatment of encephalitis, yet robust trials assessing this are lacking. Therefore, well‐designed randomised controlled trials are needed to investigate the role of IVIG in all forms of encephalitis and to provide evidence to inform clinical practice on the management of children with encephalitis. Future trials should include a clear objective definition of encephalitis, which is aligned with the published consensus definition for encephalitis (Venkatesan 2013) to ensure a homogenous population. There remains the need for internationally agreed core outcome measures for clinical trials in childhood encephalitis. For future trials, consideration should be given to clinically relevant outcomes including disability, cognitive functioning, and adaptive skills and, where possible, these should be assessed using validated tools. Long‐term follow‐up (beyond the hospitalisation period) needs to be assessed. Consideration should be given to a large sample size with statistical power to detect clinically significant differences in these outcomes and longer‐term follow‐up. Results of an ongoing trial will be crucial in addressing this key question (NCT02308982).
Acknowledgements
The authors would like to thank Nia Wyn Roberts of the Bodleian Health Care Libraries, University of Oxford, Oxford (UK) who generated the search terms and contributed to the electronic searches section of the protocol. We would also like to thank Liliana Coco, Cinzia Del Giovane, Dr Weinstick, Bianca Weinstock‐Guttman, and Antonietta Citterio for their helpful comments and suggestions, and Andrea Fittipaldo for her help with the review search.
Appendices
Appendix 1. Search strategies
| Source | Search strategy |
|---|---|
| CENTRAL (the Cochrane Library) (2016, Issue 9) | #1 encephalitis:ti,ab,kw (Word variations have been searched) #2 meningoencephalitis:ti,ab,kw (Word variations have been searched) #3 ((brain or cerebral) near/2 (infection* or infectious or inflamm* or swell*)):ti,ab,kw (Word variations have been searched) #4 #1 or #2 or #3 #5 MeSH descriptor: [Immunoglobulins, Intravenous] explode all trees #6 ((intravenous or intra‐venous) near/3 immunoglobulin*):ti,ab,kw (Word variations have been searched) #7 ivig or igiv:ti,ab,kw (Word variations have been searched) #8 immunoglobulin* or immunotherap*:ti (Word variations have been searched) #9 #5 or #6 or #7 or #8 #10 #4 and #9 |
| MEDLINE (PubMed) (1966 to 30 September 2016) | 1 exp Encephalitis/2 encephalitis.ti,ab.3 meningoencephalitis.ti,ab.4 ((brain or cerebral) adj2 (infection* or infectious orinflamm* or swell*)).ti,ab.5 1 or 2 or 3 or 46 adolescent/ or exp child/ or infant/ or infant, newborn/7 (child* or preschool* or pre‐school* or schoolchild* orinfant* or infancy or baby or babies or toddler* or teen*or adolescen* or youth*).ti,ab.8 6 or 79 Immunoglobulins, Intravenous/10 ((intravenous or intra‐venous) adj3immunoglobulin*).ti,ab.11 (ivig or igiv).ti,ab.12 (immunoglobulin* or immunotherap*).ti.13 9 or 10 or 11 or 1214 5 and 8 and 1315 limit 14 to "reviews (maximizes specificity)"16 randomised controlled trial.pt.17 controlled clinical trial.pt.18 randomized.ab.19 placebo.ab.20 drug therapy.fs.21 randomly.ab.22 trial.ab.23 groups.ab.24 16 or 17 or 18 or 19 or 20 or 21 or 22or 2325 exp animals/ not humans.sh.26 24 not 2527 14 and 26 |
| Embase (embase.com) (1974 to 30 September 2016) | 1 exp *Encephalitis/2 encephalitis.ti,ab.3 meningoencephalitis.ti,ab.4 ((brain or cerebral) adj2 (infection* or infectious orinflamm* or swell*)).ti,ab.5 1 or 2 or 3 or 46 adolescent/ or child/ or boy/ or girl/ or infant/ or preschoolchild/ or school child/ or toddler/7 (child* or preschool* or pre‐school* or schoolchild* orinfant* or infancy or baby or babies or toddler* or teen* oradolescen* or youth*).ti,ab.8 6 or 79 exp *immunoglobulin/ and intravenous drug administration/10 exp *immunoglobulin/iv [Intravenous Drug Administration]11 ((intravenous or intra‐venous) adj3 immunoglobulin*).ti,ab.12 (ivig or igiv).ti,ab.13 (immunoglobulin* or immunotherap*).ti.14 9 or 10 or 11 or 12 or 1315 5 and 8 and 1416 limit 15 to "reviews (maximizes specificity)"17 randomised controlled trial/18 controlled clinical trial/19 single blind procedure/ or double blind procedure/20 crossover procedure/21 random*.tw.22 placebo*.tw.23 ((singl* or doubl*) adj (blind* or mask*)).tw.24 (crossover or cross over or factorial* or latin square).tw.25 (assign* or allocat* or volunteer*).tw.26 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 |
| CINAHL (EBSCO host) (1981 to 30 September 2016) | S1 AB randomised controlled trial or controlled clinical trial or randomized or placebo or drug therapy or randomly or trial or groupsS2 AB inflamm* or swell* or infection* or infectious or Immunoglobulins, Intravenous or intravenous or intra‐venous or immunoglobulin* or ivig or igiv or immunoglobulin* or immunotherap*S3 MH Encephalitis OR AB ( encephalitis or meningoencephalitis )S4 S2 OR S3S5 S1 AND S4S6 AB randomised controlled trial or controlled clinical trial or randomized or placebo or drug therapy or randomly or trial or groupsLimiters ‐ Age Groups: Infant, Newborn: birth‐1 month, Infant: 1‐23 months, Child, Preschool: 2‐5 years, Child: 6‐12 years, Adolescent: 13‐18 years |
| ClinicalTrials.gov(www.clinicaltrials.gov) | (encephalitis OR meningoencephalitis) AND (immunoglobulin OR immunoglobulins OR ivig OR igiv OR immunotherapy) |
| ICTRP Search Portal (apps.who.int/trialsearch) | (encephalitis OR meningoencephalitis) AND (immunoglobulin OR immunoglobulins OR ivig OR igiv OR immunotherapy) |
| Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐ Science (CPCI‐S) (Web of Science Core Collection, Thomson Reuters) (1945 to 30 September 2016) | # 1 TOPIC: (encephalitis OR meningoencephalitis) OR TOPIC: (((brain or cerebral) NEAR/2 (infection* or infectious or inflamm* or swell*)))# 2 TOPIC: (child* or preschool* or pre‐school* or schoolchild* or infant* or infancy or baby or babies or toddler* or teen* or adolescen* or youth*)# 3 TOPIC: (((intravenous or intra‐venous) NEAR/3 immunoglobulin*)) OR TOPIC: (ivig or igiv) OR TITLE: (immunoglobulin* or immunotherap*)# 4 #3 AND #2 AND #1# 5 TOPIC: (random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)# 6 #5 AND #4 |
| Global Health Library (Virtual Health Library) (www.globalhealthlibrary.net/php/index.php) | (encephalitis OR meningoencephalitis) AND (immunoglobulin OR immunoglobulins OR ivig OR igiv OR immunotherapy) |
| Database of Abstracts of Reviews of Effects (DARE) | (encephalitis OR meningoencephalitis) AND (immunoglobulin OR immunoglobulins OR ivig OR igiv OR immunotherapy) |
Appendix 2. Newcastle Ottawa Scale (cohort studies)
Note: a study can be awarded a maximum of one star for each numbered item within the 'Selection' and 'Outcome' categories. A maximum of two stars can be given for 'Comparability.'
Selection
1. Representativeness of the exposed cohort:
a) Truly representative (one star)
b) Somewhat representative (one star)
c) Selected group
d) No description of the derivation of the cohort
2. Selection of the non‐exposed cohort:
a) Drawn from the same community as the exposed cohort (one star)
b) Drawn from a different source
c) No description of the derivation of the non‐exposed cohort
3. Ascertainment of exposure:
a) Secure record (e.g. surgical records) (one star)
b) Structured interview (one star)
c) Written self‐report
d) No description
e) Other
4. Demonstration that outcome of interest was not present at start of study:
a) Yes (one star)
b) No
Comparability
1. Comparability of cohorts on the basis of the design or analysis:
a) The study controls for age, sex, and marital status (one star)
b) Study controls for any additional factors (list) (one star)
c) Cohorts are not comparable on the basis of the design or analysis controlled for confounders
Outcome
1. Assessment of outcome:
a) Independent blind assessment (one star)
b) Record linkage (one star)
c) Self‐report
d) No description
e) Other
2. Was follow‐up long enough for outcomes to occur:
a) Yes (one star)
b) No
Indicate the median duration of follow‐up and a brief rationale for the assessment above:____________________
3. Adequacy of follow‐up of cohorts:
a) Complete follow‐up ‐ all subjects accounted for (one star)
b) Subjects lost to follow‐up unlikely to introduce bias ‐ number lost less than or equal to 20% or description of those lost suggested no different from those followed (one star)
c) Follow up rate less than 80% and no description of those lost
d) No statement
Appendix 3. Newcastle Ottawa Scale (case‐control studies)
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
Selection
1. Is the case definition adequate?
a) Yes, with independent validation (one star)
b) Yes, e.g. record linkage or based on self‐reports
c) No description
2. Representativeness of the cases:
a) Consecutive or obviously representative series of cases (one star)
b) Potential for selection biases or not stated
3. Selection of controls:
a) Community controls (one star)
b) Hospital controls
c) No description
4. Definition of controls:
a) No history of disease (endpoint) (one star)
b) No description of source
Comparability
1. Comparability of cases and controls on the basis of the design or analysis
a) Study controls for _______________ (select the most important factor) (one star)
b) Study controls for any additional factor (one star) (This criterion could be modified to indicate specific control for a second important factor)
Exposure
1. Ascertainment of exposure:
a) Secure record (e.g. surgical records) (one star)
b) Structured interview where blind to case/control status (one star)
c) Interview not blinded to case/control status
d) Written self‐report or medical record only
e) No description
2. Same method of ascertainment for cases and controls:
a) Yes (one star)
b) No
3. Non‐response rate:
a) Same rate for both groups (one star)
b) Non‐respondents described
c) Rate different and no designation
Data and analyses
Comparison 1. Significant disability at six months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Significant disability at 3‐6 months | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.22, 2.60] |
Comparison 2. Serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥ 1 serious adverse event | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.05] |
| 2 Mortality | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 3 Hypotension | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 4 Melaena | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
Comparison 3. Significant disability at discharge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Significant disability at discharge | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.60, 1.67] |
Comparison 4. Length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay: intravenous immunoglobulin (IVIG) vs standard care | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐4.54 [‐7.47, ‐1.61] |
| 2 Length of hospital stay IVIG vs interferon | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐0.99, ‐0.15] |
Comparison 5. Time to fever resolution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days to fever resolution: intravenous immunoglobulin (IVIG) vs standard care | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.25, ‐0.69] |
| 2 Days to fever resolution IVIG vs interferon | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.42, ‐0.12] |
Comparison 6. Time to stop spasms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to stop spasms: intravenous immunoglobulin (IVIG) vs standard care | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐1.97, ‐1.01] |
| 2 Time to stop spasms IVIG vs interferon | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.34, 0.00] |
Comparison 7. Time to regain consciousness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to regain consciousness | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.48, ‐0.72] |
Comparison 8. Time to resolution of neuropathic symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of neuropathic symptoms: intravenous immunoglobulin (IVIG) vs standard care | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐3.34, ‐3.06] |
| 2 Neuropathic symptoms IVIG vs interferon | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.70, ‐0.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2006.
| Methods | Randomised controlled trial.Conducted in paediatric hospital in China.62 children enrolled (observation group = 32, control group = 30).Randomly split into study groupsDose of IVIG/placebo used: 400 mg/kg/day for 3‐5 days.Outcome assessment done during period of hospitalisation. | |
|---|---|---|
| Participants | Inclusion criteria Aged 8 months to 12 years. Diagnosis of viral encephalitis as described in "Applied Pediatrics" 6th edition. Exclusion criteriaNot mentioned. | |
| Interventions | Control group: conventional treatment including fever and spasm reduction, reduction of intracranial pressure, maintenance of hydration, electrolyte and acid‐base balance, antiviral treatment, treatment of infections.Observation group: conventional treatment as above + IVIG 400 mg/kg/day continuously for 3‐5 days. | |
| Outcomes | Time for fever reduction.Time taken to stop further spasms.Time taken to regain consciousness.Duration of hospital stay. | |
| Notes | No information on ethical approval or informed consent.No information provided on funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation but method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts and all participants were included in outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available in public domain. |
| Other bias | Unclear risk | Small sample size and no sample size calculation so unclear if sample size large enough to detect significant differences between study groups.No information on funding provided. |
Rayamajhi 2015.
| Methods | Pilot randomised, double‐blind placebo‐controlled trial.Recruitment from 2 centres in Nepal: Kanthi Children's Hospital in Kathmandu (tertiary) and the Paediatric Department at BP Koirala Institute of Health Sciences in Dharan, Nepal (large regional hospital).23 children screened, 1 excluded due to meeting an exclusion criterion.22 children randomised.Treatment duration: 5 days.Follow‐up: assessed at discharge and at 3‐6 months. | |
|---|---|---|
| Participants | Inclusion criteriaAged 1‐14 years with a clinical diagnosis of acute encephalitis syndrome (history of fever < 14 days, altered consciousness; GCS < 15, with or without history of seizures, and consistent CSF findings (white cell count < 1000 cells/mm3 with no organisms on Gram stain and CSF:plasma glucose ratio > 40%). Exclusion criteria Presumed diagnosis of febrile convulsion (single seizure lasting < 15 minutes with full recovery of consciousness within 60 minutes). Positive blood slide or rapid antigen for Plasmodium falciparum. Clinical/laboratory features suggestive of bacterial meningitis where antibiotics had already been commenced. GCS < 3 and receiving artificial ventilation without signs of spontaneous respiration and with absent oculocephalic reflex. | |
| Interventions | IVIG 400 mg/kg/day for 5 days.Equivalent volume of 0.9% normal saline for 5 days. | |
| Outcomes | Primary outcomeAssessment of study feasibility. Secondary and other outcomes Death or neurological sequelae at discharge or follow‐up. Adverse events. Other outcomesDifference in anti‐JEV antibody titres between children treated and not treated with IVIG. | |
| Notes | Protocol approved by Nepal Health Research Council.Ethical approval obtained from the ethics committees of BP Koirala Institute of Health Sciences, Kanti Children's Hospital, and the Liverpool School of Tropical Medicine.Written informed consent obtained.Study was funded by the Liverpool Brain Infections Group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Randomisation code concealed and opened by independent investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and care providers blind to study drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawals provided, numbers lost to follow‐up similar in each group. |
| Selective reporting (reporting bias) | High risk | Primary outcome in published paper was different from that stated in the published protocol in ClinicalTrials.gov.Authors have not reported on prespecified adverse events such as infusion site reaction, diarrhoea, rise in blood pressure, and change in urinary output.Details of participant death provided for treatment group but not placebo group. |
| Other bias | High risk | Small sample size, calculation based on determining difference in study groups for a tertiary outcome.Authors' group affiliated to funding body.Information on the split in the number of participants in both study groups admitted to the intensive care unit not provided.Imbalance in number of participants who received corticosteroid therapy.Recruitment period unclear, authors reported 2 different recruitment periods: May‐June 2009 and May‐September 2009. |
Wu 2014.
| Methods | Randomised controlled trial.Conducted in general hospital in China.80 children enrolled (control group = 27, experimental group 1 = 27, experimental group 2 = 26).Allocation using random sampling table.Dose of IVIG/placebo: 400 mg/kg/day for 3 consecutive days.Outcome assessment during period of hospitalisation. | |
|---|---|---|
| Participants | Inclusion criteriaAged 1‐6 years. Diagnosis of hand, foot, and mouth (based on the Chinese Department of Ministry of Health published guidelines) complicated by encephalitis. Exclusion criteriaChildren with underlying immune problems and who were unconscious. | |
| Interventions | Control group: conventional comprehensive treatment including dehydration, reduction of intracranial pressure, nutritional support, anti‐infection treatment, keeping the skin clean, and general precautions to prevent infection.Experimental group 1: intravenous injection of human blood gamma globulin*, 400 mg/kg a day for 3 consecutive days in addition to the comprehensive treatment.Experimental group 2: spray of human interferon alfa‐2h, 1 or 2 sprays per time, 3 times/day on 3 consecutive days. | |
| Outcomes | Time for fever to subside.Time to control convulsions.Time to resolution of rashes.Time for neuropathic symptoms to subside.Mean stay in hospital.Adverse events. | |
| Notes | Ethical approval obtained.Informed consent obtained.No information provided on funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation using random sampling table. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts and all participants included in outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available in public domain. |
| Other bias | Unclear risk | Small sample size and no sample size calculation so unclear if sample size large enough to detect significant differences between study groups.No information on funding provided.Diagnostic criteria used for encephalitis not mentioned in paper. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhatt 2012 | Not study design of interest. Quasi‐controlled trial of IVIG compared with standard therapy in children with acute encephalitis syndrome complicated by myocarditis. Not randomised study. |
| Erol 2013 | Not study design of interest. Case series of children with acute disseminated encephalomyelitis. Not randomised study, no control group. |
| Gelli 1974 | Not study of interest. Single case report. |
| Girija 2010 | Not study design of interest. Case series of children with acute disseminated encephalomyelitis. Not randomised study, no control group. |
| İncecik 2013 | Not study design of interest. Case series of children with acute disseminated encephalomyelitis. Not randomised study, no control group. |
| Růžek 2013 | Not study design of interest. Single case report. |
| Samileh 2007 | Not study design of interest. Case series of children with acute disseminated encephalomyelitis. Not randomised study, no control group. |
| Unay 2004 | Not study of interest. Single case report. |
Characteristics of studies awaiting assessment [ordered by study ID]
Fan 2010.
| Methods | Random allocation. |
|---|---|
| Participants | 72 cases of children and adults. |
| Interventions | IVIG 400 mg/kg/dose for 5 consecutive days + standard care vs standard care alone. |
| Outcomes | Length of hospital stay, time to reduce fever, time to reduce twitching, time to restore consciousness, time to recover from paralysis, time to improvement in mental state. |
| Notes | Results presented as aggregate data and results for children aged < 17 years were not presented separately. We tried to contact the authors but no contact details found to date. |
Characteristics of ongoing studies [ordered by study ID]
NCT02308982.
| Trial name or title | Investigating the Role of Early Intravenous Immunoglobulin Treatment for Children with Encephalitis (IgNiTE). |
|---|---|
| Methods | Multicentre, double‐blind, placebo‐controlled, randomised controlled trial of IVIG versus placebo in addition to standard care. |
| Participants | 308 children with encephalitis, aged 6 weeks to 16 years. |
| Interventions | IVIG 1 g/kg/dose + standard care vs placebo + standard care for 2 days. |
| Outcomes | Primary outcome: good recovery (score ≤ 2) on the Glasgow Outcome Score Extended (Pediatric version). |
| Starting date | January 2016. |
| Contact information | mildred.iro@paediatrics.ox.ac.uk. |
| Trial name | |
| Methods | |
| Outcomes | |
| Notes |
Differences between protocol and review
In addition to the outcomes specified in the protocol for this review, we included the following clinically relevant outcomes:
- time to fever resolution;
- time to stop spasms;
- time to regain consciousness;
- time to resolution of neuropathic symptoms.
We added 'Other bias' to the Risk of bias table, which covered bias such as funding.
Contributions of authors
Protocol design: MAI.
Protocol review and approval: NGM, MA, and AJP.
Selection of studies for inclusion, data extraction, and assessment of risk of bias: MAI; verified by NGM.
Data analysis: MAI.
Initial draft and subsequent revisions to incorporate feedback and suggestions from other authors: MAI.
Sources of support
Internal sources
- None, Other.
No source of support received
External sources
- None, Other.
Declarations of interest
MAI: none.
NGM: none.
MA: none.
AJP: none.
New
References
References to studies included in this review
Chen 2006 {published data only}
- Chen D‐M, Li S‐N. The clinical observation of high dose immunoglobulin for children viral encephalitis. Chinese Journal of Primary Medicine and Pharmacy 2006;13(5):816‐7. [Google Scholar]
Rayamajhi 2015 {published data only}
- Rayamajhi A, Nightingale S, Bhatta NK, Singh R, Ledger E, Bista KP, et al. A preliminary randomized double blind placebo‐controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal. PloS One 2015;10(4):e0122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu H, Wan Z, Liu L, Qiu L, Xiuling D. Comparison of the clinical efficacy of immunoglobulin and interferon in the treatment of hand foot and mouth disease complicated with viral encephalitis. Chinese Journal of Primary Medicine and Pharmacy 2014;21:3127‐8. [Google Scholar]
References to studies excluded from this review
Bhatt 2012 {published and unpublished data}
- Bhatt GC, Sankar J, Kushwaha KP. Use of intravenous immunoglobulin compared with standard therapy is associated with improved clinical outcomes in children with acute encephalitis syndrome complicated by myocarditis. Pediatric Cardiology 2012;33(8):1370‐6. [DOI] [PubMed] [Google Scholar]
Erol 2013 {published data only}
- Erol I, Ozkale Y, Alkan O, Alehan F. Acute disseminated encephalomyelitis in children and adolescents: a single center experience. Pediatric Neurology 2013;49(4):266‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelli 1974 {published data only}
- Gelli GP, Arioni G, Laurenti J. Preliminary results of the use of human anti‐measles immunoglobulin in measles encephalitis. Minerva Pediatrica 1974;26(26):1291‐4. [PubMed] [Google Scholar]
Girija 2010 {published data only}
- Girija A, RamachandranNair R, Rafeequ M, Manoj P. Multimodal treatment of acute disseminated encephalomyelitis in children. Journal of Pediatric Neurology 2010;8(4):359‐65. [Google Scholar]
İncecik 2013 {published data only}
- İncecik F, Hergüner M, Altunbaşak Ş. Acute disseminated encephalomyelitis: an evaluation of 15 cases in childhood. Turkish Journal of Pediatrics 2013;55(3):253‐9. [PubMed] [Google Scholar]
Růžek 2013 {published data only}
- Růžek D, Dobler G, Niller HH. May early intervention with high dose intravenous immunoglobulin pose a potentially successful treatment for severe cases of tick‐borne encephalitis?. BMC Infectious Diseases 2013;13(306):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samileh 2007 {published data only}
- Samileh N, Hassan T. Acute disseminated encephalomyelitis in children. A descriptive study in Tehran, Iran. Saudi Medical Journal 2007;28(3):396‐9. [PubMed] [Google Scholar]
Unay 2004 {published data only}
- Unay B, Sarici SU, Bulakbaşi N, Akin R, Gökçay E. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis associated with hepatitis A infection. Pediatrics International 2004;46(2):171‐3. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fan 2010 {published data only}
- Fan J‐L. The clinical observation of the high dose intravenous immunoglobulin for severe viral encephalitis. Internal Medicine of China 2010;02:118‐9. [Google Scholar]
References to ongoing studies
NCT02308982 {unpublished data only}
- NCT02308982. Investigating the role of early intravenous immunoglobulin treatment for children with encephalitis [A phase III multi‐centre randomised, double blind, placebo controlled trial to assess the role of intravenous immunoglobulin in the management of children with encephalitis]. clinicaltrials.gov/ct2/show/record/NCT02308982 Date first received; 1 December 2014.
Additional references
Absoud 2013
- Absoud M, Lim MJ, Chong WK, Goede C, Foster K, Gunny R, et al. Paediatric acquired demyelinating syndromes: incidence, clinical and magnetic resonance imaging features. Multiple Sclerosis 2013;19(1):76‐86. [PUBMED: PMID: 22516794] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aygun 2001
- Aygun A, Kabakus N, Celik I, Turgut M, Yoldas T, Gök U, et al. Long term neurological outcome of acute encephalitis. Journal of Tropical Pediatrics 2001;47(4):243‐7. [PUBMED: PMID 111523767] [DOI] [PubMed] [Google Scholar]
Basta 1996
- Basta M, Illa I, Dalakas M C. Increased in vitro uptake of the complement C3b in the serum of patients with Guillain‐Barré syndrome, myasthenia gravis and dermatomyositis. Journal of Neuroimmunology 1996;71:227‐9. [DOI] [PubMed] [Google Scholar]
Basta 2003
- Basta M, Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al. F(ab)'2‐mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nature Medicine 2003;9(4):431‐8. [DOI] [PubMed] [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. Technical Manual. 3rd Edition. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Beers 2012
- Beers SR, Wisniewski SR, Garcia‐Filion P, Tian Y, Hahner T, Berger RP, et al. Validity of a pediatric version of the Glasgow Outcome Scale ‐ Extended. Journal of Neurotrauma 2012;10(6):1126‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF Publications
- British National Formulary Publications. British National Formulary for Children (BNF for Children). Chapter 5.3.2: Herpesvirus infections; page 326. London (UK): British Medical Association, the Royal Pharmaceutical Society, the Royal College of Paediatrics and Child Health, the Neonatal and Paediatric Pharmacists Group, 2014‐2015. [Google Scholar]
Caramello 2006
- Caramello P, Canta F, Balbiano R, Lipani F, Ariaudo S, Agostini M, et al. Role of intravenous immunoglobulin administration in Japanese encephalitis. Clinical Infectious Diseases 2006;43(12):1620‐1. [PUBMED: PMID 17109300] [DOI] [PubMed] [Google Scholar]
Cervantes‐Barragán 2012
- Cervantes‐Barragán L, Firner S, Bechmann I, Waisman A, Lahl K, Sparwasser T, et al. Regulatory T cells selectively preserve immune privilege of self‐antigens during viral central nervous system infection. Journal of Immunology (Baltimore, Md. : 1950) 2012;188(8):3678‐85. [PUBMED: PMID: 22407917] [DOI] [PubMed] [Google Scholar]
Crow 2007
- Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. A role for IL‐1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg?. Blood 2007;109:155‐8. [DOI] [PubMed] [Google Scholar]
Dalakas 1998
- Dalakas M. Mechanism of action of intravenous immunoglobulin and therapeutic considerations in the treatment of autoimmune neurologic diseases. Neurology 1998;51(6 Suppl 5):2‐8. [PUBMED: PMID 9851723] [DOI] [PubMed] [Google Scholar]
Dalakas 2004
- Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence‐based indications and safety profile. Pharmacology & Therapeutics 2004;102:177‐93. [DOI] [PubMed] [Google Scholar]
Davison 2003
- Davison KL, Crowcroft NS, Ramsay ME, Brown DW, Andrews NJ. Viral encephalitis in England, 1989‐1998: what did we miss?. Emerging Infectious Diseases 2003;9(2):234‐40. [PUBMED: PMID 12603996] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Aquino 2013
- Aquino MT, Puntambekar SS, Savarin C, Bergmann CC, Phares TW, Hinton DR, et al. Role of CD25(+) CD4(+) T cells in acute and persistent coronavirus infection of the central nervous system. Virology 2013;447:112‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietrich 1990
- Dietrich G, Kazatchkine MD. Normal immunoglobulin G (IgG) for therapeutic use (intravenous Ig) contain antiidiotypic specificities against an immunodominant, disease‐associated, cross‐reactive idiotype of human anti‐thyroglobulin autoantibodies. Journal of Clinical Investigation 1990;85:620‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowell 2000
- Dowell E, Easton A, Solomon T. The consequences of encephalitis. Report of a Postal Survey (Year 2000 survey) carried out by the Encephalitis Support Group. www.encephalitis.info/images/iPdf/Research2/CONSEQUENCES1.pdf (accessed 10 April 2014).
Elion 1983
- Elion GB. The biochemistry and mechanism of action of acyclovir. Journal of Antimicrobial Chemotherapy 1983;12 Suppl B:9‐17. [DOI] [PubMed] [Google Scholar]
Elion 1993
- Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. Journal of Medical Virology 1993;Suppl 1:2‐6. [DOI] [PubMed] [Google Scholar]
Esposito 2012
- Esposito S, Picciolli I, Semino M, Principi N. Steroids and childhood encephalitis. Pediatric Infectious Disease Journal 2012;31:759‐60. [DOI] [PubMed] [Google Scholar]
FDA 2013
- US Food, Drug Administration. Immune globulin intravenous (IGIV) indications. Page last updated: 6 October 2013. www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm133691.htm (accessed 15 May 2014).
Fowler 2010
- Fowler A, Stödberg T, Eriksson M, Wickström R. Long‐term outcomes of acute encephalitis in childhood. Pediatrics 2010;126(4):828‐35. [PUBMED: PMID 20876179] [DOI] [PubMed] [Google Scholar]
Fowler 2008
- Fowler A, Stödberg T, Eriksson M, Wickström R. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. European Journal of Paediatric Neurology: EJPN 2008;12:484‐90. [DOI] [PubMed] [Google Scholar]
Frenzel 2012
- Frenzel K, Ganepola S, Michel D, Thiel E, Krüger DH, Uharek L, et al. Antiviral function and efficacy of polyvalent immunoglobulin products against CMV isolates in different human cell lines. Medical Microbiology and Immunology 2012;201:277‐86. [DOI] [PubMed] [Google Scholar]
Gadian 2017
- Gadian J, Kirk E, Holliday K, Lim M, Absoud M. Systematic review of immunoglobulin use in paediatric neurological and neurodevelopmental disorders. Developmental Medicine and Child Neurology 2017;59(2):133‐44. [DOI] [PubMed] [Google Scholar]
Glezer 2003
- Glezer I, Munhoz CD, Kawamoto EM, Marcourakis T, Avellar MC, Scavone C. MK‐801 and 7‐Ni attenuate the activation of brain NF‐kappa B induced by LPS. Neuropharmacology 2003;45:1120‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEprofiler (GRADEpro). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Granerod 2010
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population‐based prospective study. Lancet Infectious Diseases 2010;10(12):835‐44. [PUBMED: PMID: 20952256] [DOI] [PubMed] [Google Scholar]
Hacohen 2013
- Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, Cross H, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. Journal of Neurology, Neurosurgery, and Psychiatry 2013;84(7):748‐55. [PUBMED: 23175854] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacohen 2014
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, et al. N‐methyl‐D‐aspartate receptor antibodies in post‐herpes simplex virus encephalitis neurological relapse. Movement Disorders 2014;29:90‐6. [DOI] [PubMed] [Google Scholar]
Harrison 2003
- Harrison PL, Oakland T. ABAS®‐II Adaptive Behavior Assessment System®. 2nd Edition. San Antonio (TX): Harcourt Assessment, 2003. [Google Scholar]
Higgins 2003
- Higgins T, Thompson G, Deeks J, Altman D. Measuring inconsistencies in meta‐analysis. BMJ 2003;327(414):557‐60. [PUBMED: PMCID: PMC192859; PUBMED: PMID: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hughes 2009
- Hughes RA, Dalakas MC, Cornblath DR, Latov N, Weksler ME, Relkin N. Clinical applications of intravenous immunoglobulins in neurology. Clinical and Experimental Immunology 2009;158(Suppl 1):34‐42. [PUBMED: PMID: 19883422] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1994
- International Conference on Harmonisation. ICH harmonised tripartite guideline clinical safety data management: definitions and standards for expedited reporting E2A. Current step 4 version. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. 27 October 1994. www.ich.org/fileadmin/Public\_Web\_Site/ICH\_Products/Guidelines/Efficacy/E2A/Step4/E2A\_Guideline.pdf (accessed prior to 25 September 2017).
Iro 2017
- Iro MA, Sadarangani M, Goldacre R, Nickless A, Pollard AJ, Goldacre MJ. 30‐year trends in admission rates for encephalitis in children in England and effect of improved diagnostics and measles‐mumps‐rubella vaccination: a population‐based observational study. Lancet Infectious Diseases 2017;17(4):422‐30. [DOI] [PubMed] [Google Scholar]
Kamei 2005
- Kamei S, Sekizawa T, Shiota H, Mizutani T, Itoyama Y, Takasu T, et al. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76:1544‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazatchkine 2001
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. New England Journal of Medicine 2001;345:747‐55. [DOI] [PubMed] [Google Scholar]
Kerpel‐Fronius 1983
- Kerpel‐Fronius S, Nyerges G, Mészner Z, Eckhardt S, Walker E, Bridgen D. Intravenous acyclovir (ACV, Zovirax) therapy of varicella zoster infections in immunologically endangered patients. Orvosi Hetilap 1983;124(48):2913‐8. [PubMed] [Google Scholar]
Khetsuriani 2002
- Khetsuriani N, Holman C, Anderson L. Burden of encephalitis‐associated hospitalizations in the United States, 1988‐1997. Clinical Infectious Diseases 2002;35(2):175‐82. [PUBMED: PMID: 12087524] [DOI] [PubMed] [Google Scholar]
Kishimoto 2004
- Kishimoto C, Hiraoka Y, Takada H. Effects of immunoglobulin upon murine myocarditis caused by influenza A virus: superiority of intact type to F(ab')2 type. Journal of Cardiovascular Pharmacology 2004;43(1):61‐7. [DOI] [PubMed] [Google Scholar]
Krause 2002
- Krause I, Wu R, Sherer Y, Patanik M, Peter J, Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations, a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Medicine (Oxford, England) 2002;12:133‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lewthwaite 2010
- Lewthwaite P, Begum A, Ooi M, Faragher B, Lai BF, Sandaradura I, et al. Disability after encephalitis: development and validation of a new outcome score. Bulletin of the World Health Organization 2010; Vol. 88, issue 8:584‐92. [DOI] [PMC free article] [PubMed]
Lizarraga 2013
- Lizarraga KJ, Alexandre LC, Ramos‐Estebanez C, Merenda A. Are steroids a beneficial adjunctive therapy in the immunosuppressed patient with herpes simplex virus encephalitis?. Case Reports in Neurology 2013;5:52‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Looney 2006
- Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG). Best Practice & Research. Clinical Haematology 2006;19:3‐25. [DOI] [PubMed] [Google Scholar]
Lundberg 2008
- Lundberg P, Ramakrishna C, Brown J, Tyszka J, Hamamura M, Hinton D, et al. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. Journal of Virology 2008;82:7078‐88. [PUBMED: PMCID: PMC2446972; PUBMED: PMID: 18480436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutz 1996
- Lutz HU, Stammler P, Jelezarova E, Nater M, Späth PJ. High doses of immunoglobulin G attenuate immune aggregate‐mediated complement activation by enhancing physiologic cleavage of C3b in C3bn‐IgG complexes. Blood 1996;88:184‐93. [PUBMED: PMID: 8704173] [PubMed] [Google Scholar]
Marchioni 2013
- Marchioni E, Ravaglia S, Montomoli C, Tavazzi E, Minoli L, Baldanti F, et al. Postinfectious neurologic syndromes: a prospective cohort study. Neurology 2013;80(10):882‐9. [PUBMED: PMID: 23325908] [DOI] [PubMed] [Google Scholar]
Martinez‐Torres 2008
- Martinez‐Torres F, Menon S, Pritsch M, Victor N, Jenetzky E, Jensen K, et al. Protocol for German trial of Acyclovir and Corticosteroids in Herpes‐simplex‐virus‐Encephalitis (GACHE): a multicenter, multinational, randomized, double‐blind, placebo‐controlled German, Austrian and Dutch trial [ISRCTN45122933]. BMC Neurology 2008;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matza 2004
- Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK. Assessment of health‐related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value in Health 2004;7:79‐92. [DOI] [PubMed] [Google Scholar]
McKay 1999
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor‐kappa B and steroid receptor‐signaling pathways. Endocrine Reviews 1999;20(4):435‐59. [DOI] [PubMed] [Google Scholar]
Meyding‐Lamadé 2002
- Meyding‐Lamadé U, Seyfer S, Haas J, Dvorak F, Kehm R, Lamadé W, et al. Experimental herpes simplex virus encephalitis: inhibition of the expression of inducible nitric oxide synthase in mouse brain tissue. Neuroscience Letters 2002;318:21‐4. [DOI] [PubMed] [Google Scholar]
Mollnes 1995
- Mollnes TE, Høgåsen K, Hoaas BF, Michaelsen TE, Garred P, Harboe M. Inhibition of complement‐mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its C1 binding properties. Scandinavian Journal of Immunology 1995;41:449‐56. [PUBMED: PMID:7725063] [DOI] [PubMed] [Google Scholar]
Ramakrishna 2013
- Ramakrishna C, Openshaw H, Cantin EM. The case for immunomodulatory approaches in treating HSV encephalitis. Future Virology 2013;8(3):259‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos‐Estebanez 2014
- Ramos‐Estebanez C, Lizarraga KJ, Merenda A. A systematic review on the role of adjunctive corticosteroids in herpes simplex virus encephalitis: is timing critical for safety and efficacy?. Antiviral Therapy 2014;19:133‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2004
- Reynolds CR, Kamphaus RW. Assessment of effective intervention. Behavior Assessment System for Children (BASC‐2). 2nd Edition. Vol. 32, Crowley (TX): AGS Publishing, 2004:121‐4. [Google Scholar]
Rossi 1988
- Rossi F, Sultan Y, Kazatchkine MD. Anti‐idiotypes against autoantibodies and alloantibodies to VIII:C (anti‐haemophilic factor) are present in therapeutic polyspecific normal immunoglobulins. Clinical and Experimental Immunology 1988;74:311‐6. [PMC free article] [PubMed] [Google Scholar]
Rozenberg 2013
- Rozenberg F. Acute viral encephalitis. Handbook of Clinical Neurology / Edited by P.J. Vinken and G.W. Bruyn 2013;112:1171‐81. [DOI] [PubMed] [Google Scholar]
Sellner 2005
- Sellner J, Dvorak F, Zhou Y, Haas J, Kehm R, Wildemann B, et al. Acute and long‐term alteration of chemokine mRNA expression after anti‐viral and anti‐inflammatory treatment in herpes simplex virus encephalitis. Neuroscience Letters 2005;374:197‐202. [DOI] [PubMed] [Google Scholar]
Sergerie 2007
- Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. Journal of Infectious Diseases 2007;195:817‐25. [DOI] [PubMed] [Google Scholar]
Sparrow 1986
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. American Guidance Service, Circle Pines. Journal of Educational Measurement 1986;23(4):389‐91. [Google Scholar]
Sparrow 1993
- Sparrow SS, Carter AS, Cicchetti DV. Vineland Screener: Overview, Reliability, Validity, Administration and Scoring. Interview Edition, Survey Form Manual. New Haven (CT): Yale University Child Study Centre, 1993. [Google Scholar]
Stangel 1997
- Stangel M, Schumacher HC, Ruprecht K, Boegner F, Marx P. Immunoglobulins for intravenous use inhibit TNF alpha cytotoxicity in vitro. Immunological Investigations 1997;26:569‐78. [DOI] [PubMed] [Google Scholar]
Tha‐In 2008
- Tha‐In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends in Immunology 2008;29(12):608‐15. [PUBMED: PMID: 18926775] [DOI] [PubMed] [Google Scholar]
Thompson 2000
- Thompson KA, Blessing W, Wesselingh SL. Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis. Journal of Neurovirology 2000;6:25‐32. [DOI] [PubMed] [Google Scholar]
Thompson 2012
- Thompson C, Kneen R, Riordan A, Kelly D, Pollard A J. Encephalitis in children. Archives of Disease in Childhood 2012;97:150‐61. [PUBMED: PMID: 21715390] [DOI] [PubMed] [Google Scholar]
Titulaer 2013
- Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurology 2013;12(2):157‐65. [PUBMED: PMCID: PMC3563251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trinath 2013
- Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase‐2‐dependent prostaglandin E2 in human dendritic cells. Blood 2013;122(8):1419‐27. [PUBMED: PMID: 23847198] [DOI] [PubMed] [Google Scholar]
UK Trialists
- UK Trialists. Individual patient data (as supplied 30 April 2013). Data on File.
van Swieten 1988
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; Vol. 19, issue 5:604‐7. [DOI] [PubMed]
Varni 1999
- Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Medical Care 1999; Vol. 37, issue 2:126‐39. [DOI] [PubMed]
Venkatesan 2013
- Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical Infectious Diseases 2013;57(8):1114‐28. [PUBMED: PMID 23861361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vora 2014
- Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis‐associated hospitalizations in the United States, 1998‐2010. Neurology 2014;82(5):443‐51. [PUBMED: PMID: 24384647] [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang SM, Lei HY, Huang MC, Su LY, Lin HC, Yu CK, et al. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71‐associated brainstem encephalitis. Journal of Clinical Virology 2006;37(1):47‐52. [PUBMED: PMID: 16861032] [DOI] [PubMed] [Google Scholar]
Wechsler 2002
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence (WPPSI ‐ III). 3rd Edition. Ontario (Canada): Pearson Canada Assessment Inc, 2002. [Google Scholar]
Wechsler 2003
- Wechsler D. Wechsler Intelligence Scale for Children (WISC‐IV). 4th Edition. Ontario (Canada): Pearson Canada Assessment Inc, 2003. [Google Scholar]
Zuliani 2012
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83:638‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]