Prophylactic use of ergot alkaloids in the third stage of labour (original) (raw)
Abstract
Background
Previous research has shown that the prophylactic use of uterotonic agents in the third stage of labour reduces postpartum blood loss and moderate to severe postpartum haemorrhage (PPH). PPH is defined as a blood loss of 500 mL or more within 24 hours after birth. This is one of a series of systematic reviews assessing the effects of prophylactic use of uterotonic drugs; in this review prophylactic ergot alkaloids as a whole, and different regimens of administration of ergot alkaloids, are compared with no uterotonic agents. This is an update of a Cochrane Review which was first published in 2007 and last updated in 2011.
Objectives
To determine the effectiveness and safety of prophylactic use of ergot alkaloids in the third stage of labour by any route (intravenous (IV), intramuscular (IM), or oral) compared with no uterotonic agents, for the prevention of PPH.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (19 September 2017); we also searched reference lists of retrieved studies.
Selection criteria
We included all randomised controlled trials or cluster‐randomised trials comparing prophylactic ergot alkaloids by any route (IV, IM, or oral) with no uterotonic agents in the third stage of labour among women giving birth vaginally.
Data collection and analysis
Two review authors independently assessed trials for inclusion, extracted data and checked them for accuracy; they also assessed the risk of bias in included studies. Two review authors assessed the quality of the evidence using the GRADE approach.
Main results
There were eight included studies: three studies had a low risk of bias and five studies had high risk of bias. The studies compared ergot alkaloids with no uterotonic agents, with a total of 2031 women in the ergot alkaloids group and 1978 women in the placebo or no treatment group. Seven studies used the IV/IM route of administration and one study used the oral route.
Ergot alkaloids (any route of administration) versus no uterotonic agents
Use of ergot alkaloids in the third stage of labour decreased mean blood loss (mean difference (MD) ‐80.52 mL, 95% confidence interval (CI) ‐96.39 to ‐64.65 mL; women = 2718; studies = 3; moderate‐quality evidence); decreased PPH of at least 500 mL (average risk ratio (RR) 0.52, 95% CI 0.28 to 0.94; women = 3708; studies = 5; I2 = 83%; low‐quality evidence); increased maternal haemoglobin concentration (g/dL) at 24 to 48 hours postpartum (MD 0.50 g/dL, 95% CI 0.38 to 0.62; women = 1429; studies = 1; moderate‐quality evidence); and decreased the use of therapeutic uterotonics (average RR 0.37, 95% CI 0.15 to 0.90; women = 2698; studies = 3; I2 = 89%; low‐quality evidence). There were no clear differences between groups in severe PPH of at least 1000 mL (average RR 0.32, 95% CI 0.04 to 2.59; women = 1718; studies = 2; I2 = 74%; very low‐quality evidence). The risk of retained placenta or manual removal of the placenta, or both, were inconsistent with high heterogeneity. Ergot alkaloids increased the risk of elevated blood pressure (average RR 2.60, 95% CI 1.03 to 6.57: women = 2559; studies = 3; low‐quality evidence) and pain after birth requiring analgesia (RR 2.53, 95% CI 1.34 to 4.78: women = 1429; studies = 1; moderate‐quality evidence) but there were no differences between groups in vomiting, nausea, headache or eclamptic fit.
Results for IV/IM ergot alkaloids versus no uterotonic agents were similar to those for the main comparison of ergot alkaloids administered by any route, since most of the studies (seven of eight) used the IV/IM route. Only one small study (289 women) compared oral ergometrine with placebo and it showed no benefit of ergometrine over placebo. No maternal adverse effects were reported.
None of the studies reported on any of our prespecified neonatal outcomes
Authors' conclusions
Prophylactic IM or IV injections of ergot alkaloids may be effective in reducing blood loss, reducing PPH (estimated blood loss of at least 500 mL), and increasing maternal haemoglobin. Ergot alkaloids may also decrease the use of therapeutic uterotonics, but adverse effects may include elevated blood pressure and pain after birth requiring analgesia. There were no differences between groups in terms of other adverse effects (vomiting, nausea, headache or eclamptic fit). There is a lack of evidence on the effects of ergot alkaloids on severe PPH, and retained or manual removal of placenta. There is also a lack of evidence on the oral route of administration of ergot alkaloids.
Plain language summary
Managing the end of childbirth (placenta delivery) with ergot alkaloid medications (e.g. ergometrine)
What is the issue?
The third stage of labour is the period from the birth of the baby to the expulsion of the placenta and membranes. As the placenta separates, there is inevitably some blood loss from the placental site until the muscles of the uterus clamp the blood vessels. Fit, healthy women cope with this normal blood loss without problems, but where poor nutrition, poor sanitation and limited or no access to clinical care are complications of pregnancy, severe morbidity and death can result from excessive blood loss at birth. This is very common in low‐ and middle‐income countries. Active intervention, called 'active management of third stage', is recommended for the third stage of labour to reduce excess blood loss. Active intervention incorporates: 1) the administration of a uterotonic medication (medicine that stimulates contractions), given just before or just after the baby is born to help the muscles of the uterus contract; 2) cord clamping, performed approximately one to three minutes after birth; and 3) the use of controlled cord traction to deliver the placenta in settings where skilled birth attendants are available. This review of studies looked at the use of one group of uterotonic medications called ergot alkaloids (e.g. ergometrine) as part of this active management.
Why is this important?
A previous systematic review showed that the combination of ergometrine and oxytocin was associated with a significantly lower postpartum haemorrhage (PPH) rate (defined as blood loss of at least 500 mL) but a greater incidence of side‐effects compared to the use of oxytocin alone. However, there was no review comparing ergometrine with no uterotonic medications and different routes or timings of administration for the prevention of PPH.
What evidence did we find?
We searched for evidence in September 2017 and included eight trials involving 4009 women receiving ergometrine by mouth (orally), into the muscle (intramuscularly (IM)) or into the vein (intravenously (IV)). Of eight trials, seven included studies were analysed in this updated review.
The evidence from the trials analysed suggests that ergot alkaloids may decrease mean blood loss, increase maternal haemoglobin levels in the blood, and may decrease both blood loss of at least 500 mL (PPH) and the use of therapeutic uterotonics. It is uncertain whether ergot alkaloids have any effect on numbers of women experiencing high blood loss of at least 1000 mL (severe PPH). The evidence also suggested that they may increase adverse effects such as increased blood pressure and pain after birth. They may make little or no difference between groups in terms of other adverse effects (vomiting, nausea, headache or eclamptic fit) and results were inconsistent on the risk of retained or manual removal of placenta. Most of the evidence came from trials that administered ergot alkaloids using the IM or IV route. There was only one small trial that looked at the use of oral ergot alkaloids and results were inconclusive. There were limited numbers of included studies and results between studies were not always consistent or precise. Overall quality of evidence across critical and important outcomes ranged from very low to moderate.
What does this mean?
The IV or IM route, although it may reduce blood loss and PPH, was associated with the adverse effects of raised blood pressure and pain due to contractions of the uterus. There was not enough evidence on the oral route of administering ergot alkaloids. There are other medications, namely oxytocin, syntometrine and prostaglandins (which are assessed in other Cochrane Reviews), that can be used and may be preferable.
Summary of findings
Background
Description of the condition
The third stage of labour is defined as the period of labour from birth of the baby to the expulsion or extraction of the placenta and membranes. Placental separation involves capillary haemorrhage and shearing of decidua spongiosa because of the mechanical action of uterine contraction. Blood loss during the third stage of labour depends on the time between placental separation and contraction of the placental bed by uterine activity. Most women experience mild to moderate blood loss. However, the third stage of labour can be a potentially hazardous period of childbirth resulting in postpartum haemorrhage (PPH). The World Health Organization (WHO) defines PPH as blood loss after delivery of 500 mL or more (WHO 2000).
In 2015, the estimate of global maternal mortality was approximately 303,000 (WHO 2015). Almost all maternal deaths occur in low‐ and middle‐income countries. Unfortunately, due to socio‐economic conditions, dwindling investment in health, and non‐ or poorly‐functioning health systems, many women are unable to access essential care during pregnancy, childbirth and the postpartum period (Acuin 2010). The most common preventable causes of maternal death are haemorrhage, pregnancy‐induced hypertension and sepsis. One of the most common causes of maternal death worldwide is PPH (Say 2014; McCormick 2002; WHO 2001).
Active management of the third stage of labour, before the occurrence of PPH, is better than treatment when blood loss is 500 mL or more. The third stage of labour is an important and critical period for interventions to reduce the incidence of PPH (De Groot 1995). Active management involves prophylactic use of oxytocic drugs; umbilical cord clamping; and controlled cord traction for delivery of the placenta (WHO 2003). Three recommendations for active management in the third stage of labour are administration of an uterotonic drug within one minute of the birth of the baby, clamping and cutting the umbilical cord after birth, and delivering the placenta by applying controlled cord traction during a strong uterine contraction (Den Hertog 2001; McCormick 2002). Combined controlled cord traction in active management of the third stage reduces the time of the third stage, the incidence of PPH and retained placenta and the need for additional oxytocic agents compared with using only uterotonic drugs (Brucker 2001). Recent WHO recommendation suggests late cord clamping, in which the umbilical cord is clamped approximately one to three minutes after birth of the baby, and controlled cord traction is recommended in settings where skilled birth attendants are available (WHO 2012). The recent updated Cochrane Review on active management of the third stage of labour showed a reduction of the incidence of severe blood loss and blood transfusion requirement but an increase in maternal blood pressure and pain, as well as reducing the baby's birthweight (Begley 2015). Likewise, the updated Cochrane Review on controlled cord traction for the third stage of labour found the reduction of blood loss and duration of the third stage of labour (Hofmeyr 2015).
Description of the intervention
Uterotonic agents are divided into three groups: ergot alkaloids, oxytocin and prostaglandins (De Groot 1998; Den Hertog 2001). Their mechanisms of preventing PPH are different. Methylergometrine is the most common type of ergot alkaloid; it increases the muscle tone of the uterus, with superimposed fast rhythmic contractions of the myometrium and tetanic contraction for several hours resulting in compressed myometrial blood vessels. Oxytocin acts through oxytocin receptors in myometrium and decidua, leading to fast and long‐lasting contractions upon basal tone of the myometrium. Syntometrine, consisting of five units of oxytocin and 0.5 mg of ergometrine, has been designed to take advantage of the rapid onset of action of oxytocin, combined with the longer action of ergometrine. Carbetocin is similar to oxytocin, but it has a rapid onset and prolonged duration of action relative to oxytocin. The effect of a room‐temperature stable formulation of carbetocin under the WHO CHAMPION Trial will be soon published (Widmer 2016). Finally, prostaglandins induce strong myometrial contractions by increasing uterine tone (De Groot 1995). There are several Cochrane systematic reviews already published about the use of various uterotonic drugs in the third stage of labour for preventing PPH (McDonald 2004; Su 2012; Tunçalp 2012; Westhoff 2013).
Recent studies have highlighted oxytocin as the first‐line drug used for prophylaxis based on the evidence of its benefit in terms of reducing PPH compared with using no uterotonic drugs, and its favourable side‐effect profile (Westhoff 2013). However, the use of the combination preparation of ergot alkaloid plus oxytocin, syntometrine, is associated with a statistically significant reduction of PPH when compared with oxytocin alone, attributable to the ergometrine effect (McDonald 2004). Because of the effect of strong and lasting uterine contractions, ergometrine has been used as one of the uterotonic drugs of choice for preventing PPH.
How the intervention might work
Ergometrine is ergot in origin and was recovered first as a product of a fungus, Claviceps purpurea, and used in obstetrics for the first time in 1582. This use ended in 1822 due to uterine rupture, stillbirth and maternal death from inaccurate doses and ergotism (gangrene and convulsive forms) (De Groot 1998; Van Dongen 1995). However, ergot alkaloids were found to be more useful and less harmful for obstetric practice in the form of ergometrine in 1932 by Moir and Dale (Dunn 2002). They have specific uterotonic action through adrenergic receptors with less vasoconstrictive ability, and they prevent excessive bleeding after childbirth. Two chemical ergot alkaloids are ergonovine/ergometrine maleate (ergotrate) and methylergonovine/methylergometrine maleate (methergine). They produce persistent uterine contractions in the inner zone of the myometrium through calcium channel mechanisms and actin‐myosin interaction, leading to the shearing effect on placental separation and less blood loss or PPH, but they may increase the risk of maternal side effects such as hypertension and other complications of vasoconstriction (Brucker 2001; De Groot 1998; Dua 1994; Gowri 2003; Sultatos 1997; Taylor 1985). In addition, the risks of partial retention or trapping of the placenta (or both), manual removal of placenta, or uterine inversion or cord avulsion are still concerns with ergometrine administration (Sorbe 1978).
Different types of ergot alkaloid, and different routes and timing of administration, have been used for both prophylactic and therapeutic purposes (Andersen 1998; Borri 1986; De Groot 1996b; Moir 1979; Van Selm 1995). The most common ergot alkaloids for obstetric treatment are ergometrine and methylergometrine. Both injectable, ergometrine and methylergometrine are very unstable when stored unrefrigerated, and deteriorate with higher storage temperatures and exposure to light; therefore, they need to be stored in a dark place at a temperature of 4 ºCelsius to 8 ºCelsius. Their oral forms also deteriorate within weeks or immediately after being taken from a sealed package or container or when stored in increased temperatures and high humidity. Intravenous methylergometrine administration induces both increased frequency of uterine contractions and basal tone, with a decrease of amplitude lasting at least 30 minutes and maintained for 60 to 90 minutes. The uterine effect following oral administration is detected in 20 to 30 minutes, peaks at 60 to 70 minutes and is maintained for 120 minutes, but its effect is unpredictable (De Groot 1996a; De Groot 1996b). Injectable oxytocin is much more stable than ergometrine and methylergometrine (De Groot 1996a; De Groot 1998; Hogerzeil 1996). Although the chemical instability and the side effects of ergot alkaloids are of concern, clinical trials on the use of ergot alkaloids in the third stage of labour for prevention of PPH have been conducted (Andersen 1998; De Groot 1996b; Sorbe 1978). Oral forms of ergot alkaloids might be useful for women in some areas where intravenous or intramuscular administration is not possible. A systematic review on the effectiveness and safety of ergot alkaloids in the third stage of labour is needed.
Why it is important to do this review
Systematic reviews on the comparison of ergot alkaloid versus oxytocin, prostaglandins, syntometrine and carbetocin are published in the_Cochrane Library_ (McDonald 2004; Su 2012; Tunçalp 2012; Westhoff 2013). Nevertheless, there still is a gap in knowledge on the effectiveness and safety of prophylactic use of ergot alkaloids in the third stage of labour compared with no uterotonic drugs, as well as of different routes or timings of administration for the prevention of PPH. This is an update of the review which was first published in 2007 (Liabsuetrakul 2007).
Objectives
To determine the effectiveness and safety of prophylactic use of ergot alkaloids in the third stage of labour by any route (intravenous, intramuscular, or oral) compared with no uterotonic agents, for the prevention of postpartum haemorrhage.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials or cluster‐randomised trials comparing prophylactic ergot alkaloids by any route (intravenous (IV), intramuscular (IM), or oral) with no uterotonic agents in the third stage of labour among women giving birth vaginally. We included trials that were presented only as abstracts if there was sufficient detail (published and unpublished) to confirm eligibility.
Types of participants
Pregnant women anticipating a vaginal delivery.
Types of interventions
Any ergot alkaloid given prophylactically, by whatever route or timing of administration, compared with no uterotonic agents. Due to pharmacokinetic differences between different routes and timings of administration, we planned to evaluate separately oral versus IV or IM ergot alkaloids and administration before versus after placental delivery.
We reviewed the following three comparisons:
- ergot alkaloids (any route of administration) versus no uterotonic agents;
- IV/IM ergot alkaloids versus no uterotonic agents;
- oral ergot alkaloids versus no uterotonic agents.
As comparisons of ergot alkaloids with other uterotonic agents have been assessed in other reviews, such studies were not eligible for inclusion in this review.
Types of outcome measures
We selected the outcome measures based on factors relating to the effectiveness and safety of ergot alkaloids in terms of clinical relevance for both maternal and neonatal outcomes.
Primary outcomes
- Mean blood loss (mL).
- Postpartum haemorrhage (PPH) (clinically estimated or measured blood loss of 500 mL or more).
- 'Severe' PPH (clinically estimated or measured blood loss of 1000 mL or more).
- Maternal haemoglobin concentration at 24 to 48 hours postpartum.
Secondary outcomes
Maternal outcomes
- Blood transfusion.
- Use of therapeutic uterotonics.
- Length of third stage of labour (minutes).
- Retained placenta or manual removal of the placenta, or both.
- Vomiting.
- Nausea.
- Elevation of blood pressure (mmHg).
- Pain after birth requiring analgesia (not prespecified).
- Headache (not prespecified).
- Eclamptic fit (not prespecified).
- Postnatal haemoglobin (Hb) less than 10 g/dL (not prespecified).
- Uterine subinvolution at routine follow‐up (not prespecified).
- Postpartum febrile morbidity (not prespecified).
Neonatal outcomes
- Apgar score equal to or less than six at five minutes.
- Jaundice.
- Not breastfeeding at discharge.
- Admission to neonatal intensive care unit.
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (19 September 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
- monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
- weekly searches of MEDLINE (Ovid);
- weekly searches of Embase (Ovid);
- monthly searches of CINAHL (EBSCO);
- handsearches of 30 journals and the proceedings of major conferences;
- weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (19 September 2017) (See Appendix 1 for search terms used).
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Liabsuetrakul 2007.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search. The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
In previous versions of the review and this updated version, the contact author (Tippawan Liabsuetrakul (TL)) assessed all potential studies identified as a result of the search strategy, using title and abstract, and searched for the full texts. Two review authors (TL and Krantarat Peeyananjarassri (KP)) independently reviewed the full texts regarding types of studies, participants, interventions and outcomes, based on the prespecified inclusion criteria and using a trial eligibility form. We resolved any disagreement through discussion or, if required, we consulted the third review author (Thanapan Choobun (TC)).
Data extraction and management
Two review authors (TL and TC)) independently assessed the validity of each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). There was no attempt to mask the authors' names, institutions, source of the publication or results when applying the inclusion criteria.
We designed a form to extract data. For eligible studies, two review authors (TL and KP) independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (TL and KP) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
- low risk of bias (any truly random process, e.g. random number table; computer random number generator);
- high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
- unclear risk of bias.
2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
- low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
- high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
- unclear risk of bias.
3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
- low, high or unclear risk of bias for participants;
- low, high or unclear risk of bias for personnel.
3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
- low, high or unclear risk of bias.
4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
- low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
- high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
- unclear risk of bias.
5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
- low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
- high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
- unclear risk of bias.
6) Other bias (checking for bias due to problems not covered by 1) to 5) above)
We described for each included study any important concerns we had about other possible sources of bias.
7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to 1) to 6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses; see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, TL assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook. These assessments were checked by a second person (a Researcher from Cochrane Pregnancy and Childbirth). We assessed the quality of the body of evidence relating to the following outcomes for the main comparison.
- Mean blood loss (mL).
- PPH (clinically estimated or measured blood loss of 500 mL or more).
- 'Severe' PPH (clinically estimated or measured blood loss of 1000 mL or more).
- Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL).
- Use of therapeutic uterotonics.
- Elevation of blood pressure.
- Pain after birth requiring analgesia.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014), in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates, if appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
None of the included studies were cluster‐randomised trials.
In future updates, if identified and eligible, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Handbook, using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion.
Other unit of analysis issues
Five of the included studies had more than two treatment groups (De Groot 1996b; Howard 1964; Ilancheran 1990; Kerekes 1979; McGinty 1956). For these studies we included only two of the relevant groups that met the inclusion criteria in order to include data into single pair‐wise comparisons.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include all participants randomised to each group in the analyses). The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses and to consider whether an overall summary was meaningful, and if so, to use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
- Use of ergot alkaloids with or without active management of third stage of labour.
- Use of ergot alkaloids before or after placental delivery.
- Use of ergot alkaloids in different doses.
There were too few studies included in any analyses with details on prespecified subgroups to make subgroup analysis possible.
If there are enough data in future updates of this review, we will use the following outcomes in subgroup analyses.
- Mean blood loss (mL).
- PPH (clinically estimated or measured blood loss of 500 mL or more).
- 'Severe' PPH (clinically estimated or measured blood loss of 1000 mL or more).
- Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL).
We will assess subgroup differences by interaction tests available within Review Manager (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of risk of bias assessed by concealment of allocation, high attrition rates, or both, with studies being at high risk of bias excluded from the analyses in order to assess whether this makes any difference to the overall result. We performed a sensitivity analysis for all the outcomes which showed substantial heterogeneity by excluding trials which we classified as at high risk of bias in the main comparison.
Results
Description of studies
Results of the search
See Figure 1.
1.
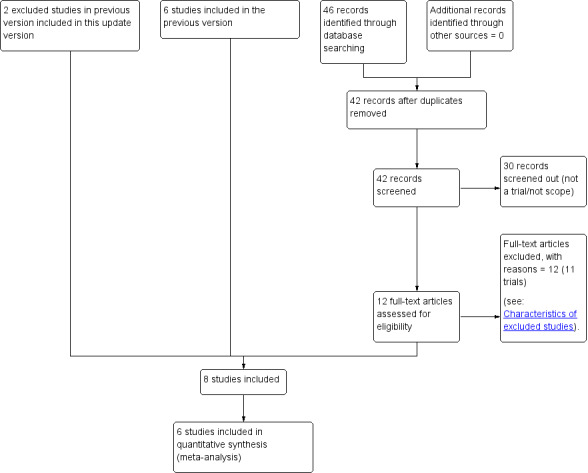
Study flow diagram.
An updated search on 19 September 2017 retrieved 42 trial reports. After screening, we excluded 30 reports and assessed 12 new reports of 11 studies. We excluded all 11 studies. For 10 of these studies, the intervention was not that of this review (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Ezeama 2014; Fawzy 2012; Gupta 2014; Is 2012; Patil 2013; Sharma 2014; Shrestha 2008). The remaining study, identified from the clinical trials registry search, did not provide details of a contact person and so it was not possible to obtain any further information relating to the results (EudraCT2010‐01980‐42).
We also reassessed and included two studies that were previously excluded due to not reporting outcomes of interest (Ilancheran 1990; Jolivet 1978). This updated review now includes eight randomised controlled trials.
Included studies
A total of 4009 women participated in the eight included studies comparing any ergot alkaloids with placebo or no treatment (Begley 1990; Daley 1951; De Groot 1996b; Howard 1964; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956).
1) Study location and settings
All studies were conducted in high‐income countries with low maternal mortality ratios, namely England, Hungary, Ireland, the Netherlands, the United States, France and Singapore.
2) Participants
All participants included in these studies were delivered vaginally. The criteria for inclusion and exclusion were clearly defined in three studies (Begley 1990; Daley 1951; De Groot 1996b). One study identified participants as women who had a spontaneous vaginal delivery without complications; definitions of complications were not given (Kerekes 1979). The remaining four studies included women who delivered vaginally; the exclusion criteria were not provided in the report (Howard 1964; McGinty 1956; Ilancheran 1990; Jolivet 1978). Women with hypertension or cardiovascular diseases were excluded from participating in two studies (Begley 1990; De Groot 1996b).
3) Interventions
The studies compared ergot alkaloids with no treatment (Begley 1990; Daley 1951; Ilancheran 1990; Jolivet 1978; Kerekes 1979), or a placebo (De Groot 1996b; Howard 1964; McGinty 1956). Three studies randomised participants into three comparison groups (De Groot 1996b; Howard 1964; Kerekes 1979), and two studies randomised into four comparison groups (Ilancheran 1990; McGinty 1956). Ergot alkaloids used were either ergometrine/ergonovine or methylergometrine/methylergonovine/methylergobasine. There were various routes of administration: intravenous in five studies (Begley 1990; Howard 1964; Ilancheran 1990; Kerekes 1979; McGinty 1956), intramuscular in two studies (Daley 1951; Jolivet 1978), and oral in one study (De Groot 1996b). Doses of intravenous or intramuscular ergometrine or methylergometrine varied from 0.2 mg (Howard 1964; Jolivet 1978; Kerekes 1979; McGinty 1956), to 0.5 mg (Begley 1990; Daley 1951), and no clearly defined dose (Ilancheran 1990); and the dose of oral ergometrine was 0.4 mg (De Groot 1996b). Most studies administered ergot alkaloids in the third stage of labour (Begley 1990; Daley 1951; De Groot 1996b; Howard 1964; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956); in one study administration occurred after placental delivery (Howard 1964). There were three studies which described the method of placental delivery: one by active management of third stage of labour (Begley 1990); two by physiological placental separation (Daley 1951; De Groot 1996b). The remaining studies gave no details of the method of placental delivery (Howard 1964; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956). There were five studies with three or more arms comparing ergot alkaloids with placebo or other uterotonic drugs (De Groot 1996b; Howard 1964; Ilancheran 1990; Kerekes 1979; McGinty 1956).
4) Outcomes
The largest study reported all prespecified outcome measures (Begley 1990). The following maternal outcomes not prespecified in the review are reported: postnatal haemoglobin (Hb) less than 10 g/dL (Begley 1990); headache (Begley 1990; McGinty 1956); pain after birth requiring analgesia (Begley 1990); eclamptic fit (Begley 1990; McGinty 1956); uterine subinvolution at routine follow‐up (Kerekes 1979); postpartum febrile morbidity (Kerekes 1979); prostaglandin levels (Ilancheran 1990); and newborn birthweight and quantity of milk in 24 hours (Jolivet 1978). We did not analyse the outcome of prostaglandins, newborn birthweight and quantity of milk in this review. Blood loss was observed in six studies: clinically estimated in three (Begley 1990; Daley 1951; Howard 1964), measured by gravimetric method (De Groot 1996b), collection of blood in a container (Kerekes 1979), and non‐specified method (Ilancheran 1990). Maternal Hb concentration was checked at 48 to 72 hours postpartum in two trials (Begley 1990; Kerekes 1979). However, mean blood loss and maternal Hb concentration data could not be extracted in one study because the authors did not report the number in the result and noted only a significant difference between the comparison groups (Kerekes 1979). Two studies reported manual removal of the placenta (Begley 1990; De Groot 1996b), and one study reported retained placenta for 60 minutes or more (Daley 1951). The incidence of blood transfusion was noted in three studies (Begley 1990; De Groot 1996b; McGinty 1956). The use of therapeutic uterotonics was described in three studies (Begley 1990; De Groot 1996b; Howard 1964). The duration of the third stage of labour was described as the mean length of the third stage (Begley 1990; Daley 1951; De Groot 1996b; Kerekes 1979), and not as third stage of labour lasting more than 30 minutes, which was a prespecified outcome of this review; one of the studies did not present the standard deviations (De Groot 1996b), so we only analysed three studies for this outcome. The elevation of blood pressure was measured in three studies (Begley 1990; Howard 1964; McGinty 1956), but the definitions varied: diastolic blood pressure greater than 95 mmHg (Begley 1990), the increase of systolic or diastolic blood pressure greater than 10 mmHg (Howard 1964), or the increase of systolic blood pressure 20 mmHg or more or systolic blood pressure greater than 170 mmHg (McGinty 1956). Vomiting and nausea were reported in two studies (Begley 1990; McGinty 1956). None of the neonatal outcomes were reported in the included studies.
5) Dates of study, funding sources and declarations of interest
Dates when the studies were conducted were reported as: 1 October 1987 to 31 October 1988 (Begley 1990); July 1993 to July 1994 (De Groot 1996b); and August 1962 to July 1963 (Howard 1964). In one study, it was reported that “The experiment was started in the spring of 1949”, but the completion date was not reported (Daley 1951). Study dates were not reported in four studies (Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956
Sources of funding included: the Research and Development Trust of the Coombe Hospital in one study (Begley 1990), and a Public Health Service Grant No. GM‐08943‐02 and No. AO‐00555‐03 in another one study (Howard 1964). Funding sources were not reported in the remaining studies (Daley 1951; De Groot 1996b; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956).
There was no information in any of the studies on declarations of interest among primary researchers.
Please see the table Characteristics of included studies for further details.
Excluded studies
The details of all excluded studies are in the table Characteristics of excluded studies.
In the previous version of the review (Liabsuetrakul 2007), we excluded 29 studies by screening using title, abstract or studies within the scope of other Cochrane Reviews. These include nine studies where the comparison was with prostaglandin (Amant 1999; Baumgarten 1983; Caliskan 2002; Chatterjee 2000; Diab 1999; Lam 2004; Penaranda 2002; Rajwani 2000; Vimala 2004); 11 studies where oxytocin was the comparator (Barbaro 1961; Bonham 1963; Docherty 1981; Francis 1965a; Francis 1965b; Fugo 1958; Huh 2004; Kikutani 2003; Soiva 1964; Stearn 1963; Symes 1984); seven studies where syntometrine was the comparator (Khan 1995; Lamont 2001; Mitchell 1993; Nieminen 1963; Vaughan 1974; Yardim 1967; Yuen 1995); one study of nipple stimulation (Badhwar 1991); and one study of syntometrine (OCM 505) (Carpén 1968). We then evaluated the full texts of the remaining 32 studies, and excluded 26 of these: 18 because the studied intervention was not that of this review (Chukudebelu 1963; Forster 1957; Groeber 1960; Jago 2007; Kemp 1963; Moir 1979; Moodie 1976; Moore 1956; Orji 2008; Paull 1977; Pei 1996; Ramesh 1983; Reddy 2001; Rooney 1985; Saito 2007; Singh 2009; Thilaganathan 1993; Thornton 1988); three because the studies were not randomised controlled trials (Friedman 1957; Hacker 1979; Sorbe 1978); one because it did not include women having a vaginal delivery (Dweck 2000); and four because there were no outcomes of interest (Ilancheran 1990; Jolivet 1978; Terry 1970; Weiss 1975). However, in this current update, we reassessed four studies to make sure the reasons for exclusion were still valid and could not introduce outcome reporting bias (Ilancheran 1990; Jolivet 1978; Terry 1970; Weiss 1975). Two studies were still excluded (Terry 1970; Weiss 1975): we excluded one because the comparison was with syntometrine (Terry 1970), and one because it did not measure any outcomes from this review (Weiss 1975). We included the remaining two studies (Ilancheran 1990; Jolivet 1978), but one did not contribute any data to the review (Jolivet 1978).
We excluded the 11 studies from the 2017 search. The intervention used in 10 studies was not that of this review (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Ezeama 2014; Fawzy 2012; Gupta 2014; Is 2012; Patil 2013; Sharma 2014; Shrestha 2008), and one study identified from the clinical trials registry search did not provide details of a contact person and so it was not possible to obtain any further information relating to the results (EudraCT2010‐01980‐42).
Risk of bias in included studies
According to the 'Risk of bias' tool, three studies had a low risk of bias (Begley 1990; De Groot 1996b; Howard 1964), and five studies showed a high risk of bias (Daley 1951; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956) (Figure 2; Figure 3).
2.
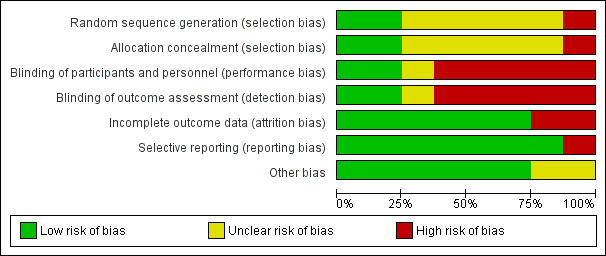
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
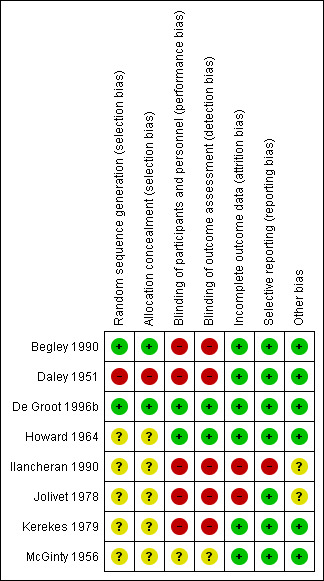
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered two studies to have adequate sequence generation and allocation concealment (Begley 1990; De Groot 1996b). Sequence generation and allocation concealment were inadequate in one study due to the use of alternation by weekends when teams of obstetricians and midwives changed (Daley 1951). We were unable to make a judgement about whether sequence generation and allocation concealment was adequate or inadequate in five studies due to there being no details of the methods used (Howard 1964; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956). We did not request additional information regarding allocation concealment from the trial authors of these studies because they were published before 1980.
Blinding
We assessed that the method of blinding (participants and caregiver) was of low risk of bias in two studies that compared ergometrine/methylergometrine with placebo (De Groot 1996b; Howard 1964). One study mentioned that normal saline was used as the control; however, the appearance of methergine or ergonovine was not described (i.e. whether it was identical with control) thus we judged its blinding as unclear (McGinty 1956). Due to the comparison being no treatment or different route of drug administration in the remaining studies (Begley 1990; Daley 1951; Ilancheran 1990; Jolivet 1978; Kerekes 1979), we considered that it was not possible to blind and so deemed these studies to be at high risk of performance bias.
In two studies we judged that it was possible for blinding of outcome assessment to be performed (De Groot 1996b; Howard 1964); we judged that it was unlikely that blinding of outcome assessment was possible in five studies (Begley 1990; Daley 1951; Ilancheran 1990; Jolivet 1978; Kerekes 1979); and we judged that it was not clear whether it was possible to perform blinding of outcome assessment in one study (McGinty 1956).
Incomplete outcome data
We assessed that all participants who entered the study were accounted for in the outcome measures and analyses in five trials (Begley 1990; Daley 1951; De Groot 1996b; Kerekes 1979; McGinty 1956). In Howard 1964, not all participants who entered the study were accounted for in the outcome measures and analyses, but the loss of participants at follow‐up was less than 10%. In two studies, a number of participants in the outcomes identified were not reported (Ilancheran 1990; Jolivet 1978), and so we deemed these studies to have high risk of bias for this domain.
Selective reporting
We assessed that in seven included trials published all expected prespecified outcomes in the reports. One study reported one additional outcome in the results which was not defined in the methods, thus we rated as high risk of reporting bias (Ilancheran 1990).
Other potential sources of bias
We did not identify other important potential source of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Ergot alkaloids (any route of administration) versus no uterotonic agents in the third stage of labour.
| Ergot alkaloids (any route of administration) versus no uterotonic agents in the third stage of labour | | | | | | | | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ------------------------------------------------------------------------------------------------------------ | ------------------------------------------------------------------------------------------------------------------------------------------------ | -------------------------------- | ----------------------------------- | ------------------ | | | Patient or population: women in the third stage of labour Settings: hospital settings in the USA, Ireland, and the Netherlands Intervention: ergot alkaloids (any route of administration)Comparison: no uterotonic agents | | | | | | | | Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | | | Assumed risk | Corresponding risk | | | | | | | Control | Ergot alkaloids (any routes) and no uterotonic agents | | | | | | | **Mean blood loss (mL)**visual estimation | The mean blood loss ranged across control groups from 234.8 to 520 mL | The mean blood loss in the intervention groups was 80.52 mL lower(96.39 to 64.65 lower) | | 2718 (3 studies) | ⊕⊕⊕⊝ moderate1 | | | **PPH (estimated or measured blood loss of at least 500 mL)**visual estimation | Study population | RR 0.52(0.28 to 0.94) | 3708 (5 studies) | ⊕⊕⊝⊝ low1, 2 | | | | 118 per 1000 | 61 per 1000(33 to 111) | | | | | | | Medium risk population | | | | | | | | 120 per 1000 | 62 per 1000(34 to 113) | | | | | | | **Severe PPH (estimated or measured blood loss of at least 1000 mL)**visual estimation | Study population | RR 0.32(0.04 to 2.59) | 1718 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | | | | 31 per 1000 | 10 per 1000(1 to 80) | | | | | | | Medium risk population | | | | | | | | 64 per 1000 | 20 per 1000(3 to 166) | | | | | | | Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL) | The mean maternal haemoglobin concentration at 24 to 48 hours postpartum in the control group was 12.09 g/dL | The mean maternal haemoglobin concentration at 24 to 48 hours postpartum in the intervention groups was 0.5 g/dL higher(0.38 to 0.62 higher) | | 1429 (1 study) | ⊕⊕⊕⊝1 moderate | | | Use of therapeutic uterotonics | Study population | RR 0.37(0.15 to 0.9) | 2698 (3 studies) | ⊕⊕⊝⊝ low1,2 | | | | 132 per 1000 | 49 per 1000(20 to 119) | | | | | | | Medium risk population | | | | | | | | 129 per 1000 | 48 per 1000(19 to 116) | | | | | | | Elevation of blood pressure | Study population | RR 2.6(1.03 to 6.57) | 2559 (3 studies) | ⊕⊕⊝⊝ low1,2 | | | | 133 per 1000 | 346 per 1000(137 to 874) | | | | | | | Medium risk population | | | | | | | | 120 per 1000 | 312 per 1000(124 to 788) | | | | | | | Pain after birth requiring analgesia (not prespecified) | Study population | RR 2.53(1.34 to 4.78) | 1429 (1 study) | ⊕⊕⊕⊝1 moderate | | | | 18 per 1000 | 46 per 1000(24 to 86) | | | | | | | Medium risk population | | | | | | | | 18 per 1000 | 46 per 1000(24 to 86) | | | | | | | *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PPH: postpartum haemorrhage; mL: millilitre; g/dL: grams per decilitre | | | | | | | | GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | | | | | | |
Summary of findings 2. Intravenous/intramuscular ergot alkaloids versus no uterotonic agents in the third stage of labour.
| Intravenous/intramuscular ergot alkaloids versus no uterotonic agents in the third stage of labour | | | | | | | | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ------------------------------------------------------------------------------------------------------------ | ------------------------------------------------------------------------------------------------------------------------------------------------ | -------------------------------- | ----------------------------------- | ------------------ | | | Patient or population: women in the third stage of labour Settings: hospital settings in USA and Ireland Intervention: intravenous/intramuscular ergot alkaloidsComparison: no uterotonic agents | | | | | | | | Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | | | Assumed risk | Corresponding risk | | | | | | | Control | Intravenous/intramuscular ergot alkaloids versus no uterotonic agents | | | | | | | **Mean blood loss (mL)**visual estimation | The mean blood loss ranged across control groups from 234.8 to 325.9 mL | The mean blood loss in the intervention groups was 81.75 mL lower(97.88 to 65.61 lower) | | 2429 (2 studies) | ⊕⊕⊕⊝ moderate1 | | | Estimated or measured blood loss of at least 500 mLvisual estimation | Study population | RR 0.41(0.22 to 0.75) | 3419 (4 studies) | ⊕⊕⊝⊝ low1,2 | | | | 96 per 1000 | 39 per 1000(21 to 72) | | | | | | | Medium risk population | | | | | | | | 83 per 1000 | 34 per 1000(18 to 62) | | | | | | | Estimated or measured blood loss of at least 1000 mLvisual estimation | Study population | RR 0.09(0.01 to 0.72) | 1429 (1 study) | ⊕⊕⊝⊝ low1,3 | | | | 15 per 1000 | 1 per 1000(0 to 11) | | | | | | | Medium risk population | | | | | | | | 15 per 1000 | 1 per 1000(0 to 11) | | | | | | | Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL) | The mean maternal haemoglobin concentration at 24 to 48 hours postpartum in the control group was 12.09 g/dL | The mean maternal haemoglobin concentration at 24 to 48 hours postpartum in the intervention groups was 0.5 g/dL higher(0.38 to 0.62 higher) | | 1429 (1 study) | ⊕⊕⊕⊝ moderate1 | | | Use of therapeutic uterotonics | Study population | RR 0.25(0.1 to 0.66) | 2409 (2 studies) | ⊕⊕⊝⊝ low1,2 | | | | 126 per 1000 | 32 per 1000(13 to 83) | | | | | | | Medium risk population | | | | | | | | 125 per 1000 | 31 per 1000(13 to 83) | | | | | | | Elevation of blood pressure | Study population | RR 2.6(1.03 to 6.57) | 2559 (3 studies) | ⊕⊕⊝⊝ low1,2 | | | | 133 per 1000 | 346 per 1000(137 to 874) | | | | | | | Medium risk population | | | | | | | | 120 per 1000 | 312 per 1000(124 to 788) | | | | | | | Pain after birth requiring analgesia (not prespecified) | Study population | RR 2.53(1.34 to 4.78) | 1429 (1 study) | ⊕⊕⊕⊝ moderate1 | | | | 18 per 1000 | 46 per 1000(24 to 86) | | | | | | | Medium risk population | | | | | | | | 18 per 1000 | 46 per 1000(24 to 86) | | | | | | | *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PPH: postpartum haemorrhage; mL: millilitre; g/dL: grams per decilitre | | | | | | | | GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | | | | | | |
Summary of findings 3. Oral ergot alkaloids versus no uterotonic agents in the third stage of labour.
| Oral ergot alkaloids versus no uterotonic agents in the third stage of labour | | | | | | | | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ---------------------------------------------------- | ------------------------------------------------------------------------------------------------ | -------------------------------- | ----------------------------------- | ------------------------- | | | Patient or population: women in the third stage of labour Settings: a single study, hospital setting in the Netherlands Intervention: oral ergot alkaloidsComparison: no uterotonic agents | | | | | | | | Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | | | Assumed risk | Corresponding risk | | | | | | | Control | Oral ergot alkaloids versus no uterotonic agents | | | | | | | **Mean blood loss (mL)**visual estimation | The mean blood loss in the control group was 520 mL | The mean blood loss in the intervention groups was 44 mL lower(132.08 lower to 44.08 higher) | | 289 (1 study) | ⊕⊕⊝⊝ low1 | | | Estimated or measured blood loss of at least 500 mLvisual estimation | Study population | RR 0.96(0.72 to 1.29) | 289 (1 study) | ⊕⊕⊝⊝ low1 | | | | 385 per 1000 | 370 per 1000(277 to 497) | | | | | | | Medium risk population | | | | | | | | 385 per 1000 | 370 per 1000(277 to 497) | | | | | | | Estimated or measured blood loss of at least 1000 mLvisual estimation | Study population | RR 0.73(0.36 to 1.5) | 289 (1 study) | ⊕⊕⊝⊝ low1 | | | | 112 per 1000 | 82 per 1000(40 to 168) | | | | | | | Medium risk population | | | | | | | | 112 per 1000 | 82 per 1000(40 to 168) | | | | | | | Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL) | Not estimable | Not estimable | | | Not reported in the trial | | | Use of therapeutic uterotonics | Study population | RR 0.79(0.47 to 1.34) | 289 (1 study) | ⊕⊕⊝⊝ low1 | | | | 182 per 1000 | 144 per 1000(86 to 244) | | | | | | | Medium risk population | | | | | | | | 182 per 1000 | 144 per 1000(86 to 244) | | | | | | | Elevation of blood pressure | Not estimable | Not estimable | | | Not reported in the trial | | | Pain after birth requiring analgesia (not prespecified) | Not estimable | Not estimable | | | Not reported in the trial | | | *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PPH: postpartum haemorrhage; mL: millilitre; g/dL: grams per decilitre | | | | | | | | GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | | | | | | |
We included eight studies with a total of 4009 women: 2031 women in the ergot alkaloids group and 1978 in the placebo or no treatment group. We analysed data from seven studies comparing ergot alkaloids with no uterotonic agents, with a total of 2001 women in the ergot alkaloids group and 1950 women in the placebo or no treatment group. Effects of interventions are presented in three comparisons: any route of administration, intravenous/intramuscular administration, or oral regimens, versus no uterotonic agents (seven, six and one included studies, respectively). We performed a sensitivity analysis for the outcomes which showed substantial heterogeneity by excluding trials which we classified as having high risk of bias. Where significant heterogeneity was observed, we analysed results using a random‐effects model.
1) Ergot alkaloids (any route of administration) versus no uterotonic agents
Primary outcomes
For four primary outcomes, five included studies compared any route of ergot alkaloids with no treatment, of which four studies used the intravenous/intramuscular route of administration (Begley 1990; Daley 1951; Howard 1964; Ilancheran 1990), and one study used the oral route (De Groot 1996b). The use of ergot alkaloids decreased mean blood loss (mean difference (MD) ‐80.52 mL, 95% confidence interval (CI) ‐96.39 to ‐64.65 mL; 3 studies, 2718 women; moderate‐quality evidence; Analysis 1.1). Postpartum haemorrhage (PPH), blood loss of at least 500 mL, was also reduced with ergot alkaloids (average risk ratio (RR) 0.52, 95% CI 0.28 to 0.94; random‐effects, Tau² = 0.32, I² = 83%; 123/1851 versus 220/1857 women; 5 studies, 3708 women, low‐quality evidence; Analysis 1.2). There were no clear differences in blood loss of at least 1000 mL between the ergot alkaloids and no treatment groups (average RR 0.32, 95% CI 0.04 to 2.59; random‐effects, Tau² = 1.74, I² = 74%; 13/851 versus 27/867 women; 1 study, 1429 women; very low‐quality evidence; Analysis 1.3). There was an increase in mean maternal haemoglobin concentration at 48 to 72 hours of postpartum in the ergot alkaloids group, but data were only reported in one study (MD 0.50 g/dL, 95% CI 0.38 to 0.62; 1 study, 1429 women; Analysis 1.4).
1.1. Analysis.
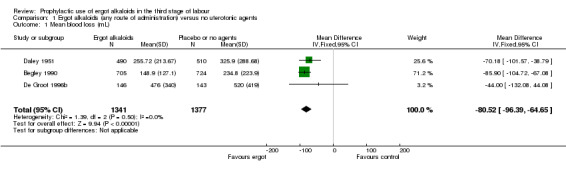
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 1 Mean blood loss (mL).
1.2. Analysis.
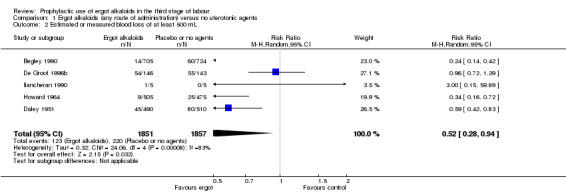
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 2 Estimated or measured blood loss of at least 500 mL.
1.3. Analysis.
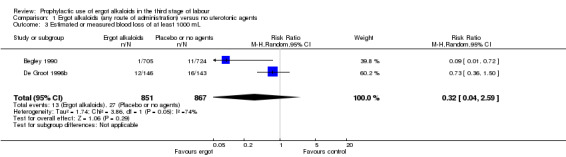
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 3 Estimated or measured blood loss of at least 1000 mL.
1.4. Analysis.
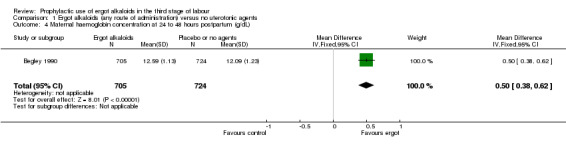
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 4 Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL).
Secondary outcomes
No difference was demonstrated in the incidence of blood transfusion when ergot alkaloid was compared with no uterotonic agents (RR 0.33, 95% CI 0.08 to 1.40; 2/951 versus 6/917 women; 3 studies, 1868 women; Analysis 1.5), but use of ergot alkaloid did reduce the use of therapeutic uterotonics (average RR 0.37, 95% CI 0.15 to 0.90; random‐effects, Tau² = 0.56, I² = 89%; 60/1356 versus 177/1342 women; 3 studies, 2698 women; low‐quality evidence, Analysis 1.6). Compared with no treatment, ergot alkaloids reduced the mean length of the third stage of labour by nearly two minutes (MD ‐1.70 minutes, 95% CI ‐3.33 to ‐0.06; random‐effects, Tau² = 3.68, I² = 94%; 3 studies, 2522 women, Analysis 1.7), but there were no differences between groups in the risk of retained placenta or manual removal of the placenta (average RR 3.86, 95% CI 0.36 to 41.78; random‐effects, Tau² = 3.43, I² = 81%; 38/1341 versus 20/1377 women; 3 studies, 2718 women; Analysis 1.8).
1.5. Analysis.
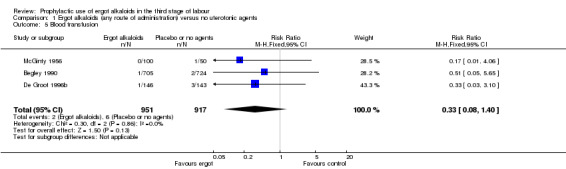
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 5 Blood transfusion.
1.6. Analysis.
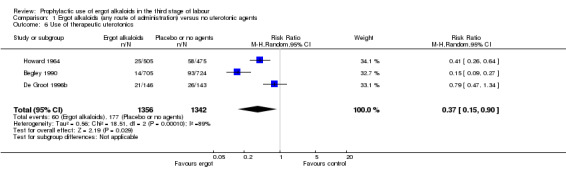
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 6 Use of therapeutic uterotonics.
1.7. Analysis.
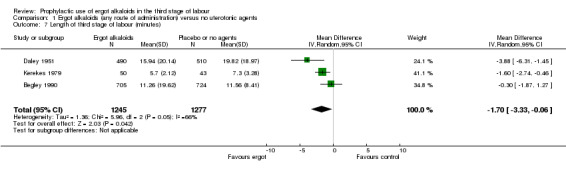
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 7 Length of third stage of labour (minutes).
1.8. Analysis.
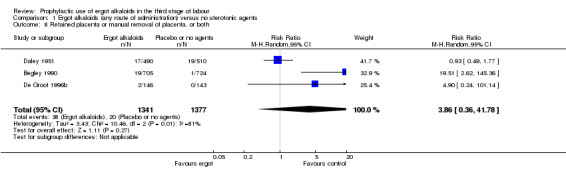
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 8 Retained placenta or manual removal of placenta, or both.
For the remaining maternal secondary outcomes, only the intravenous or intramuscular route of administration was used (three studies: Begley 1990; Howard 1964; McGinty 1956); therefore, the results were similar to the findings of comparisons of intramuscular or intramuscular ergot alkaloids compared with no uterotonic agents, reported below in comparison two. We could not find the studies reporting neonatal secondary outcomes.
The number of included studies was too few to conduct subgroup analyses based on the use of ergot alkaloids with or without active management of third stage of labour, before or after placental delivery, and in different doses.
None of the studies reported on any of our prespecified neonatal outcomes: Apgar score equal to or less than six at five minutes; jaundice; not breastfeeding at discharge; and admission to neonatal intensive care.
Sensitivity analyses
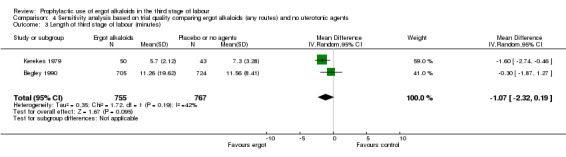
There were three studies at high risk of bias for either allocation concealment or attrition bias (Daley 1951; Ilancheran 1990; Jolivet 1978). We excluded these studies for the following analyses: mean blood loss (mL) (Analysis 1.1); estimated or measured blood loss of at least 500 mL (Analysis 1.2); length of third stage of labour (minutes) (Analysis 1.7); retained placenta or manual removal of placenta, or both (Analysis 1.8). This made little difference to the overall results for mean blood loss (mL) (Analysis 4.1). After excluding two trials (Daley 1951; Ilancheran 1990), for estimated or measured blood loss of at least 500 mL the confidence intervals increased slightly to cross the line of no effect and heterogeneity increased (average RR 0.44, 95% CI 0.16 to 1.24; 3 studies, 2698 women; I2 = 92%; Analysis 4.2). For length of third stage of labour (minutes), excluding one study, Daley 1951, again resulted in the confidence interval increasing slightly to cross the line of no effect, but heterogeneity decreased from 66% to 42% (MD ‐1.07 minutes, 95% CI ‐2.32 to 0.19; 2 studies, 1522 women; I2 = 42%; Analysis 4.3). For the outcome retained placenta or manual removal of placenta, removing one study, Daley 1951, from the analysis changed the direction of effect from no difference to a reduction in retained placenta or manual removal of placenta in favour of the control group and heterogeneity completely disappeared (RR 12.79, 95% CI 2.40 to 68.19; 2 studies, 1718 women; I2 = 0%; Analysis 4.4).
4.1. Analysis.
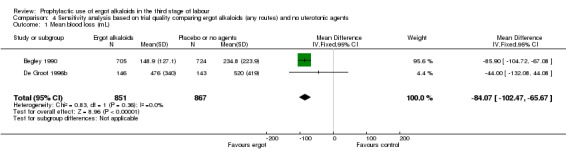
Comparison 4 Sensitivity analysis based on trial quality comparing ergot alkaloids (any routes) and no uterotonic agents, Outcome 1 Mean blood loss (mL).
4.2. Analysis.
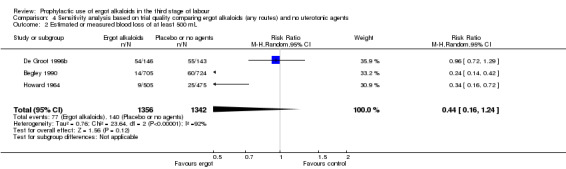
Comparison 4 Sensitivity analysis based on trial quality comparing ergot alkaloids (any routes) and no uterotonic agents, Outcome 2 Estimated or measured blood loss of at least 500 mL.
4.3. Analysis.
Comparison 4 Sensitivity analysis based on trial quality comparing ergot alkaloids (any routes) and no uterotonic agents, Outcome 3 Length of third stage of labour (minutes).
4.4. Analysis.
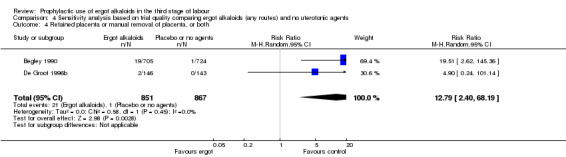
Comparison 4 Sensitivity analysis based on trial quality comparing ergot alkaloids (any routes) and no uterotonic agents, Outcome 4 Retained placenta or manual removal of placenta, or both.
2) Intravenous or intramuscular ergot alkaloids compared with no uterotonic agents
Primary outcomes
Four included studies compared the intravenous/intramuscular route of administration of ergot alkaloids with no treatment (Begley 1990; Daley 1951; Howard 1964; Ilancheran 1990). Two studies comparing intravenous/intramuscular ergot alkaloids with no treatment, found that the use of ergot alkaloids decreased mean blood loss (mean difference (MD) ‐81.75 mL, 95% confidence interval (CI) ‐97.88 to ‐65.61 mL; 2 studies, 2429 women; moderate‐quality evidence; Analysis 2.1). When blood loss of at least 500 mL (moderate PPH) was considered, there was significant heterogeneity, thus we analysed this outcome using a random‐effects model and ergot alkaloids were associated with a lower moderate PPH rate (average RR 0.41, 95% CI 0.22 to 0.75; random‐effects, Tau² = 0.22, I² = 67%; 69/1705 versus 165/1714 women; 4 studies, 3419 women; low‐quality evidence; Analysis 2.2). One study comparing ergot alkaloids with no treatment, reported a reduction in blood loss of at least 1000 mL (RR 0.09, 95% CI 0.01 to 0.72; 1 study, 1429 women; low‐quality evidence; Analysis 2.3) and increased mean maternal haemoglobin concentration at 48 to 72 hours postpartum (MD 0.50 g/dL, 95% CI 0.38 to 0.62; 1 study, 1429 women; moderate‐quality evidence; Analysis 2.4) with ergot alkaloids.
2.1. Analysis.
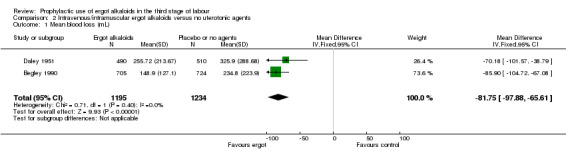
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 1 Mean blood loss (mL).
2.2. Analysis.
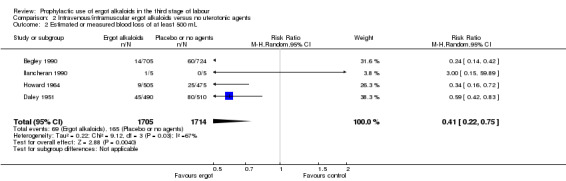
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 2 Estimated or measured blood loss of at least 500 mL.
2.3. Analysis.
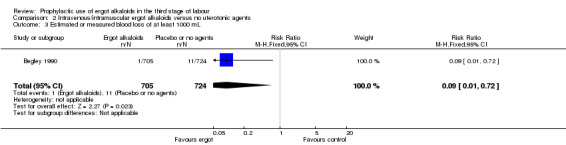
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 3 Estimated or measured blood loss of at least 1000 mL.
2.4. Analysis.
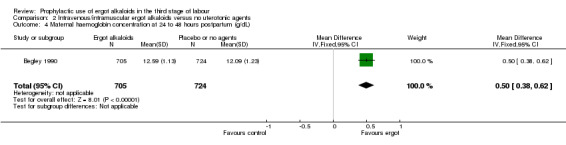
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 4 Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL).
Secondary outcomes
No difference was demonstrated in the incidence of blood transfusion when ergot alkaloid was compared with no uterotonic agents (RR 0.34, 95% CI 0.05 to 2.16; 1/805 versus 3/774 women; 2 studies, 1579 women; Analysis 2.5), but use of an ergot alkaloid did reduce the use of therapeutic uterotonics (average RR 0.25, 95% CI 0.10 to 0.66; random‐effects, Tau² = 0.41, I² = 86%; 39/1210 versus 151/1199 women; 2 studies, 2409 women; low‐quality evidence; Analysis 2.6). Mean length of third stage of labour was significantly less in the ergot alkaloid group (MD ‐1.70 minutes, 95% CI ‐3.33 to ‐0.06; Tau² = 1.36, I² = 66%; 3 studies, 2522 women; Analysis 2.7) using random‐effects analysis.
2.5. Analysis.
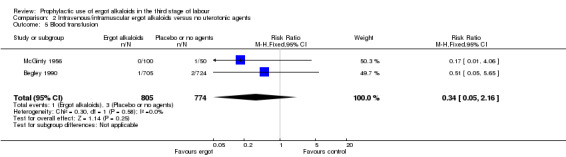
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 5 Blood transfusion.
2.6. Analysis.
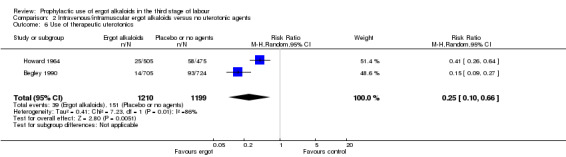
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 6 Use of therapeutic uterotonics.
2.7. Analysis.
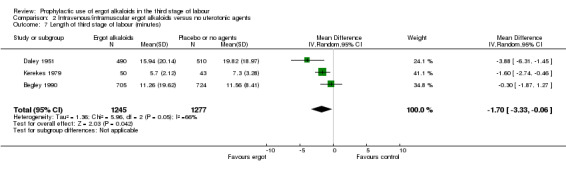
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 7 Length of third stage of labour (minutes).
The risk of retained placenta or manual removal of the placenta, or both, was not demonstrated (average RR 3.75, 95% CI 0.14 to 99.71; random‐effects, Tau² = 5.06, I² = 90%; 36/1195 versus 20/1234 women; 2 studies, 2429 women; Analysis 2.8). It should be noted that the outcomes of these two studies had opposite directions of effect. In one study (Begley 1990), the risk of manual removal of placenta was increased in the ergot alkaloid group (RR 19.51, 95% CI 2.62 to 145.36), but in the other study (Daley 1951), the risk of retained placenta at 60 minutes or more was not increased.
2.8. Analysis.
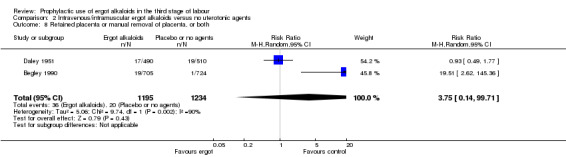
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 8 Retained placenta or manual removal of placenta, or both.
In two studies, vomiting and nausea were increased with ergot alkaloids (vomiting: average RR 6.82, 95% CI 0.37 to 126.26; random‐effects, Tau² = 2.10, I² = 47%; 13/805 versus 0/774 women; 2 studies, 1579 women; Analysis 2.9; nausea: average RR 8.63, 95% CI 0.26 to 284.55; random‐effects, Tau² = 4.04, I² = 63%; 21/805 versus 0/774 women; 2 studies, 1579 women; Analysis 2.10). The following maternal adverse effects were increased with intravenous or intramuscular ergot alkaloids compared with no treatment: elevation of blood pressure (average RR 2.60, 95% CI 1.03 to 6.57; random‐effects; Tau² = 0.55, I² = 84%; 299/1310 versus 166/1249 women; 3 studies, 2559 women; low‐quality evidence; Analysis 2.11); and pain after birth requiring analgesia (RR 2.53, 95% CI 1.34 to 4.78; 1 study, 1429 women; moderate‐quality evidence; Analysis 2.12). Significant heterogeneity for the outcome elevation of blood pressure might be because different definitions of elevated blood pressure were used.
2.9. Analysis.
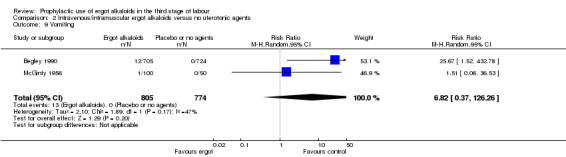
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 9 Vomiting.
2.10. Analysis.
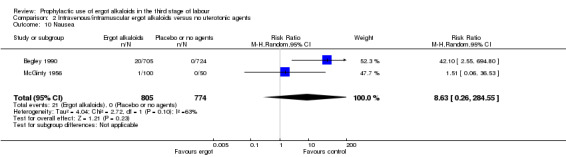
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 10 Nausea.
2.11. Analysis.
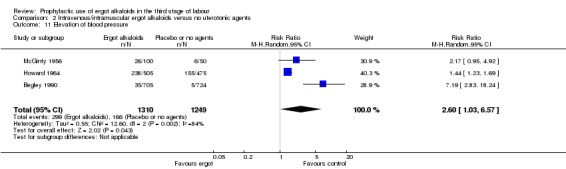
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 11 Elevation of blood pressure.
2.12. Analysis.
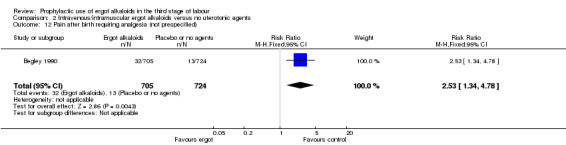
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 12 Pain after birth requiring analgesia (not prespecified).
None of the studies reported on any of our prespecified neonatal outcomes: Apgar score equal to or less than six at five minutes; jaundice; not breastfeeding at discharge; and admission to neonatal intensive care.
Outcomes not prespecified
There was no evidence of a difference in the incidence of headache (Analysis 2.13) and eclamptic fits (Analysis 2.14). One study reported no difference of uterine subinvolution at routine follow‐up (Analysis 2.15), and postpartum febrile morbidity (Analysis 2.16). One study reported a significant reduction of postnatal haemoglobin less than 10 g/dL (RR 0.30, 95% CI 0.14 to 0.67; 1 study, 1429 women; Analysis 2.17).
2.13. Analysis.
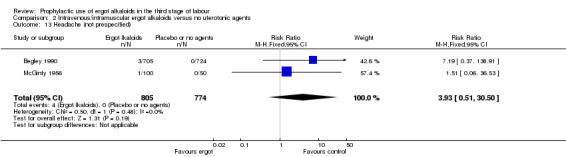
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 13 Headache (not prespecified).
2.14. Analysis.
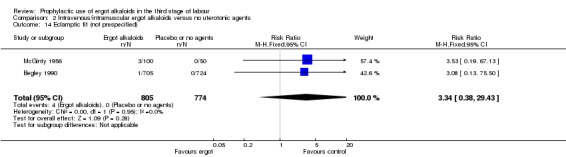
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 14 Eclamptic fit (not prespecified).
2.15. Analysis.
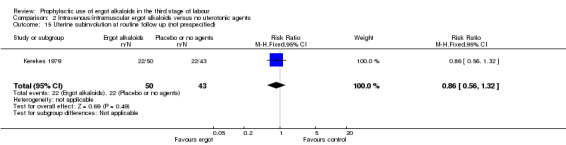
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 15 Uterine subinvolution at routine follow up (not prespecified).
2.16. Analysis.
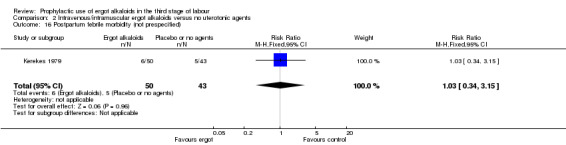
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 16 Postpartum febrile morbidity (not prespecified).
2.17. Analysis.
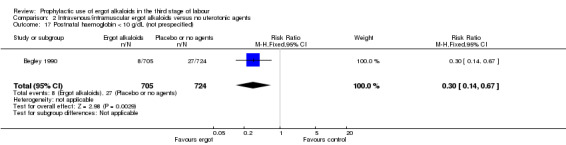
Comparison 2 Intravenous/intramuscular ergot alkaloids versus no uterotonic agents, Outcome 17 Postnatal haemoglobin < 10 g/dL (not prespecified).
3) Oral ergot alkaloids compared with no uterotonic agents
One study, De Groot 1996b, compared oral ergometrine with placebo and showed no significant benefit of ergometrine over placebo in terms of mean blood loss (Analysis 3.1); blood loss of at least 500 mL (Analysis 3.2) and 1000 mL (Analysis 3.3); need for blood transfusion (Analysis 3.4); use of further oxytocics (Analysis 3.5); and manual removal of the placenta (Analysis 3.6). Data presented for length of third stage of labour could not be extracted. No maternal adverse effects were reported. None of our prespecified neonatal outcomes were reported: Apgar score equal to or less than six at five minutes; jaundice; not breastfeeding at discharge; and admission to neonatal intensive care.
3.1. Analysis.
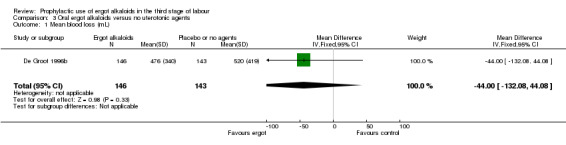
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 1 Mean blood loss (mL).
3.2. Analysis.
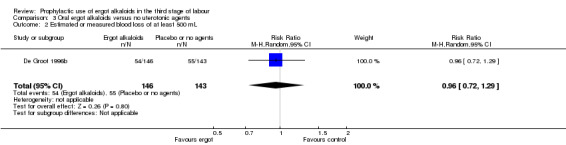
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 2 Estimated or measured blood loss of at least 500 mL.
3.3. Analysis.
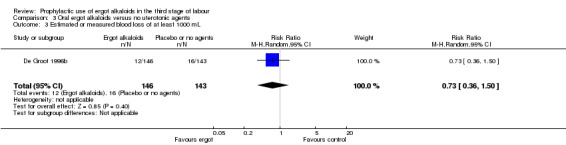
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 3 Estimated or measured blood loss of at least 1000 mL.
3.4. Analysis.
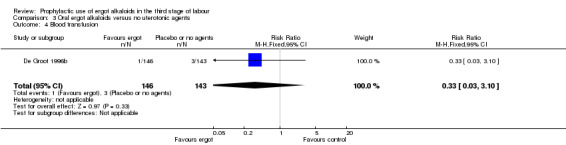
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 4 Blood transfusion.
3.5. Analysis.
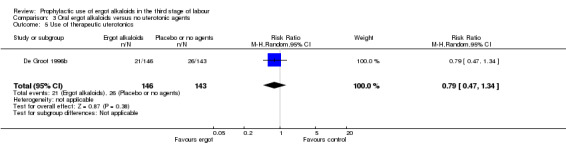
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 5 Use of therapeutic uterotonics.
3.6. Analysis.
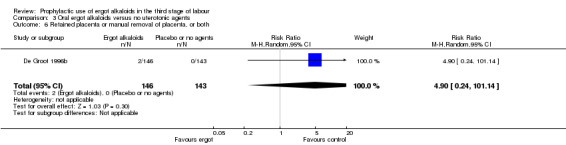
Comparison 3 Oral ergot alkaloids versus no uterotonic agents, Outcome 6 Retained placenta or manual removal of placenta, or both.
Discussion
Summary of main results
There was moderate‐quality evidence that the use of ergot alkaloids probably reduces mean blood loss, probably slightly reduces moderate postpartum haemorrhage (PPH) (low‐quality evidence) and the use of therapeutic uterotonics (low‐quality evidence), and probably increases postnatal haemoglobin (Hb) level. Nevertheless, there was very low‐quality evidence that the intervention probably makes little difference to severe PPH. Furthermore, there was moderate‐quality evidence that the use of ergot alkaloids probably leads to an increase in the frequency of women requiring analgesia after birth due to pain. There was also low‐quality evidence to suggest that ergot alkaloids may slightly increase elevation of blood pressure. The use of intravenous/intramuscular ergot alkaloids may make little or no difference in terms of other adverse effects (vomiting, nausea, headache or eclamptic fit) and results were inconsistent on the risks of retained or manual removal of placenta.
Overall completeness and applicability of evidence
Intravenous/intramuscular ergot alkaloids decreased mean blood loss and reduced the incidence of postpartum blood loss of at least 500 mL, which is in accordance with the results of a Cochrane Review assessing the effectiveness of active versus expectant management in the third stage of labour (Begley 2015). Postpartum blood loss of 1000 mL or more and postnatal Hb concentration less than 10 g/dL were decreased, and there was an increase in mean postnatal Hb in the intravenous ergot alkaloids group, but data were from one study only (Begley 1990). These effects can result from strong uterine contractions after giving ergot alkaloids (De Groot 1995; De Groot 1996a; De Groot 1998), leading to a reduction in the need for therapeutic uterotonics, but no change in the incidence of blood transfusion. The reduction in the need for therapeutic uterotonics was also found in the Cochrane Reviews of oxytocin versus no uterotonic agents (Westhoff 2013) and active versus expectant management in the third stage of labour (Begley 2015). Mean length of the third stage of labour in the ergot alkaloid group in this review was minimally decreased (two minutes); however, previous Cochrane Reviews on prophylactic use of other uterotonics in the third stage of labour compared with no uterotonic agents have not shown this benefit (Tunçalp 2012; Westhoff 2013). Two‐minute reduction in the third stage of labour seems unlikely to be clinically significant; however, it is a very critical period in case of bleeding.
No difference was demonstrated for the risk of retained placenta or manual removal of the placenta, or both; heterogeneity was high (81%) and confidence intervals wide (three trials: Begley 1990; Daley 1951; De Groot 1996b). This finding was in accordance with the results of the Cochrane Reviews of uterotonic versus no uterotonic agents (Tunçalp 2012; Westhoff 2013) and active versus expectant management in the third stage of labour (Begley 2015). Likewise, this lack of a difference was also found in a Cochrane review comparing a combination of ergot alkaloid and oxytocin versus oxytocin (McDonald 2004).
Significant adverse events for elevation of blood pressure and pain after birth requiring analgesia result from the effects of ergot alkaloids caused by persistent uterine contraction and vasoconstriction (De Groot 1998; Den Hertog 2001; Van Dongen 1995), in accordance with the Cochrane Reviews (Begley 2015;McDonald 2004). Data from case reports reported severe adverse effects (cerebral ischaemia, vasospasm and hypertensive encephalopathy) (Dua 1994; Taylor 1985), indicating that the drug should be used with caution. There was only one study, De Groot 1996b, comparing oral ergometrine versus placebo, which showed no significant risk or benefit of ergometrine. This may be explained by there being only a small number of women in the study, and therefore insufficient power to detect any difference. Easy deterioration of oral ergot alkaloids immediately after being taken from a sealed package or container, and from being exposed to increased temperature and high humidity (De Groot 1998), or longer latency time and less effect on uterine motility when compared with the intravenous route, can lead to unpredictable bioavailability (De Groot 1996a).
According to the magnitude of ergot alkaloid effect, 19 pregnant women would need to be given ergot alkaloids to prevent one additional pregnant woman from experiencing moderate PPH (number needed to treat for an additional beneficial outcome (NNTB) 19, 95% confidence interval (CI) 14 to 30), with simultaneous harm of pain after birth (number needed to treat for an additional harmful outcome (NNTH) 36, 95% CI 22 to 108) and elevation of blood pressure (NNTH 10, 95% CI 8 to 15). Thus, with the possible elevation in blood pressure, ergot alkaloids should be used with caution in those women with high blood pressure.
Quality of the evidence
We included eight randomised controlled trials in this review. According to the 'Risk of bias' tool, three studies had a low risk of bias (Begley 1990; De Groot 1996b; Howard 1964), and five studies showed a high risk of bias (Daley 1951; Ilancheran 1990; Jolivet 1978; Kerekes 1979; McGinty 1956) (Figure 2; Figure 3).
For the main comparison, ergot alkaloids versus no uterotonic agents, the quality of the evidence for the outcomes ranged from very low to moderate (see Table 1). The quality of the evidence for mean blood loss was moderate; there were limitations in the study design, which gave us reason to downgrade our assessment of the evidence. The quality of evidence for moderate PPH and severe PPH was low and very low. We downgraded our quality assessments for PPH due to there being limitations in study design, inconsistency in results, and imprecision. Only one study contributed to the evidence for maternal haemoglobin level 24 to 48 hours postpartum, and pain after birth requiring the need for analgesia. We assessed the quality of evidence for these outcomes as moderate, due to limitations in study design. We also noted limitations in study design and inconsistency among the studies contributing to the evidence for the use of therapeutic uterotonics and elevation of blood pressure, so we judged the quality of evidence as low. The quality of the evidence for the comparison of intravenous/intramuscular ergot alkaloids versus no uterotonic agents was similar to the main comparison of administration by any route, but most of the studies (seven of eight) used the intravenous/intramuscular route (see Table 2). Only one small study (289 women) compared oral ergometrine with placebo and it showed no benefit of ergometrine over placebo. We judged the quality of this evidence to be low for all outcomes; we downgraded our assessment because of imprecision due to the evidence coming from a single study with a small sample size and wide confidence intervals (see Table 3).
Potential biases in the review process
The possibility of introducing bias was present at every stage of the review process. In order to minimise bias, two review authors independently assessed studies for eligibility, undertook data extraction and carried out GRADE quality assessments. We undertook a comprehensive search strategy in order to minimise the potential for publication bias. We also reassessed previously excluded studies to ensure they had not been excluded due to not reporting outcomes of interest in order to avoid outcome reporting bias.
Agreements and disagreements with other studies or reviews
To date, there are no other published systematic reviews comparing ergot alkaloids to no uterotonics. Two Cochrane Reviews compared oxytocin versus no uterotonics or any uterotonics including ergot alkaloids (Westhoff 2013), or ergot alkaloids plus oxytocin versus oxytocin (McDonald 2004).
The effects found in this review, of ergot alkaloids administered in the third stage of labour on reduction of moderate PPH and use of therapeutic uterotonics (when compared with no uterotonics), were similar to the findings of the oxytocin review by Westhoff and colleagues (Westhoff 2013). A lower mean blood loss was noted in our review, but it was not identified in any study included in Westhoff 2013. No difference in the rate of manual removal of the placenta was noted in our review which is in accordance with the Cochrane Reviews on oxytocin, misoprostol, or syntometrine versus no uterotonic agents or other uterotonics (McDonald 2004; Tunçalp 2012; Westhoff 2013). The combination of 5 IU of oxytocin plus 0.5 mg of ergot alkaloids (syntometrine) showed a greater reduction of the risk of moderate PPH and therapeutic uterotonics compared with 10 IU of oxytocin, but the risks of elevation of diastolic blood pressure, vomiting, nausea and combined vomiting and nausea were reported (McDonald 2004). The adverse effects associated with syntometrine may be due to higher doses of ergot alkaloids in the combination.
One recently published network meta‐analysis (NMA), Gallos 2018, suggests that ergot alkaloids are also beneficial in terms of preventing PPH, though they appeared to be most effective when combined with oxytocin. The authors of this NMA concluded that 'ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination were most effective for preventing PPH ≥ 500 mL than the current standard oxytocin. Ergometrine plus oxytocin combination was more effective for preventing PPH ≥ 1000 mL than oxytocin.'
Authors' conclusions
Implications for practice.
Prophylactic intramuscular (IM) or intravenous (IV) injections of ergot alkaloids may be effective in reducing blood loss and postpartum haemorrhage (PPH) (estimated blood loss of at least 500 mL), and increasing maternal haemoglobin. They may also decrease the use of therapeutic uterotonics, but adverse effects may include elevated blood pressure and pain after birth requiring analgesia. There were no differences between use of ergot alkaloids and no uterotonics in terms of other adverse effects (vomiting, nausea, headache or eclamptic fit). There is a lack of evidence on the effects on severe PPH, retained or manual removal of the placenta and on the oral route of administering ergot alkaloids.
Implications for research.
When ergot alkaloids were compared with no uterotonic agents, there was some evidence to suggest beneficial effects in terms of reduced postpartum blood loss and reduced use of additional therapeutic uterotonics, but higher risk of elevation of blood pressure. However, the participants in these trials were not at increased risk of PPH and so the possible benefits to this group of women have not really been assessed. The optimal dosing of, and route of administration for, ergot alkaloids is inconclusive and future trials could address this. The adverse effects of ergot alkaloids also need to be seriously considered. None of the studies on prophylactic use of uterotonics included in this review addressed neonatal outcomes or serious morbidity from PPH, thus there is the need in future trials to measure neonatal outcomes, serious morbidity in the mother and possible adverse effects of ergometrine on lactation.
What's new
| Date | Event | Description |
|---|---|---|
| 19 September 2017 | New search has been performed | Search updated. We assessed 12 new reports (11 studies) and all were excluded. We reassessed two previously excluded studies and included them in this update (Ilancheran 1990; Jolivet 1978). Therefore, this review now includes eight randomised controlled trials. The comparisons have been restructured so that they are not subgrouped by route, but different routes are analysed in separate comparisons. We have produced three 'Summary of findings' tables for this update. |
| 19 September 2016 | New citation required but conclusions have not changed | The conclusions remain unchanged. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 30 April 2011 | New search has been performed | Search updated. Three new reports identified and excluded (Kemp 1963; Orji 2008; Singh 2009). Conclusions not changed. |
| 7 September 2008 | New search has been performed | Search updated. Three new studies identified and excluded (Jago 2007; Moodie 1976; Saito 2007) and another report of the Thornton 1988 excluded study added. |
| 4 April 2008 | Amended | Converted to new review format. |
| 12 February 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health. Anna Cuthbert, Research Associate at Cochrane Pregnancy and Childbirth, University of Liverpool, helped with assessing risk of bias and extracting data.
Appendices
Appendix 1. Search terms used for ClinicalTrials.gov and ICTRP
ergot AND labo(u)r
ergometrin(e) AND labo(u)r
ergonovine
methylergonovine
methergine
Data and analyses
Comparison 1. Ergot alkaloids (any route of administration) versus no uterotonic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean blood loss (mL) | 3 | 2718 | Mean Difference (IV, Fixed, 95% CI) | ‐80.52 [‐96.39, ‐64.65] |
| 2 Estimated or measured blood loss of at least 500 mL | 5 | 3708 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.94] |
| 3 Estimated or measured blood loss of at least 1000 mL | 2 | 1718 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.59] |
| 4 Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL) | 1 | 1429 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.38, 0.62] |
| 5 Blood transfusion | 3 | 1868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.40] |
| 6 Use of therapeutic uterotonics | 3 | 2698 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.90] |
| 7 Length of third stage of labour (minutes) | 3 | 2522 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.33, ‐0.06] |
| 8 Retained placenta or manual removal of placenta, or both | 3 | 2718 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.36, 41.78] |
| 9 Vomiting | 2 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 6.82 [0.37, 126.26] |
| 10 Nausea | 2 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 8.63 [0.26, 284.55] |
| 11 Elevation of blood pressure | 3 | 2559 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.03, 6.57] |
| 12 Pain after birth requiring analgesia (not prespecified) | 1 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.34, 4.78] |
| 13 Headache (not prespecified) | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [0.51, 30.50] |
| 14 Eclamptic fit (not prespecified) | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.38, 29.43] |
| 15 Uterine subinvolution at routine follow up (not prespecified) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.32] |
| 16 Postpartum febrile morbidity (not prespecified) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.15] |
| 17 Postnatal haemoglobin < 10 g/dL (not prespecified) | 1 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
1.9. Analysis.
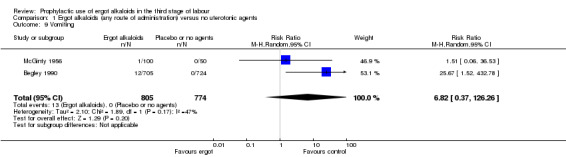
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 9 Vomiting.
1.10. Analysis.
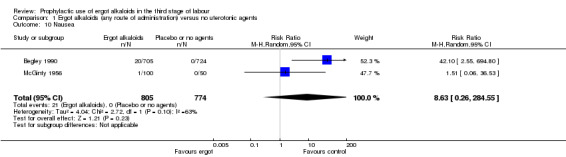
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 10 Nausea.
1.11. Analysis.
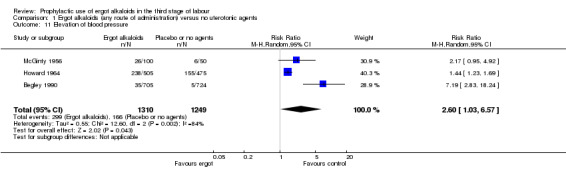
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 11 Elevation of blood pressure.
1.12. Analysis.
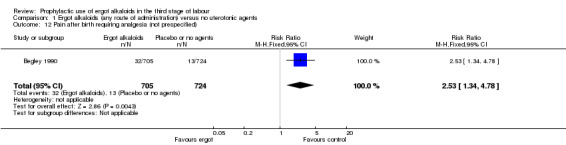
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 12 Pain after birth requiring analgesia (not prespecified).
1.13. Analysis.
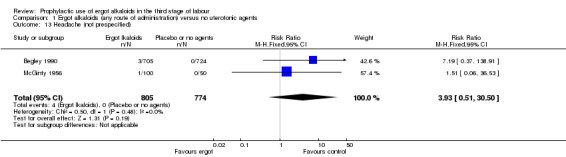
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 13 Headache (not prespecified).
1.14. Analysis.
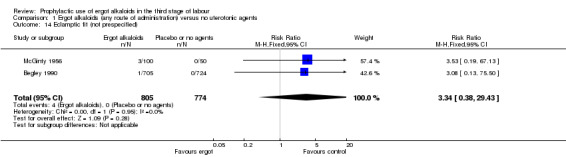
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 14 Eclamptic fit (not prespecified).
1.15. Analysis.
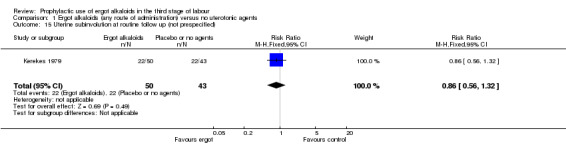
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 15 Uterine subinvolution at routine follow up (not prespecified).
1.16. Analysis.
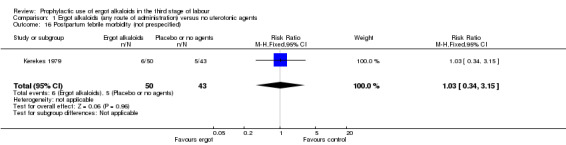
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 16 Postpartum febrile morbidity (not prespecified).
1.17. Analysis.
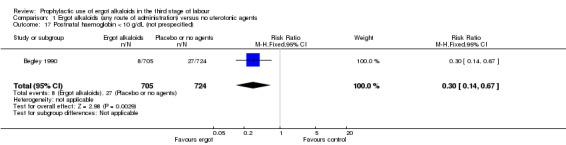
Comparison 1 Ergot alkaloids (any route of administration) versus no uterotonic agents, Outcome 17 Postnatal haemoglobin < 10 g/dL (not prespecified).
Comparison 2. Intravenous/intramuscular ergot alkaloids versus no uterotonic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean blood loss (mL) | 2 | 2429 | Mean Difference (IV, Fixed, 95% CI) | ‐81.75 [‐97.88, ‐65.61] |
| 2 Estimated or measured blood loss of at least 500 mL | 4 | 3419 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.22, 0.75] |
| 3 Estimated or measured blood loss of at least 1000 mL | 1 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.72] |
| 4 Maternal haemoglobin concentration at 24 to 48 hours postpartum (g/dL) | 1 | 1429 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.38, 0.62] |
| 5 Blood transfusion | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.05, 2.16] |
| 6 Use of therapeutic uterotonics | 2 | 2409 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.10, 0.66] |
| 7 Length of third stage of labour (minutes) | 3 | 2522 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.33, ‐0.06] |
| 8 Retained placenta or manual removal of placenta, or both | 2 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [0.14, 99.71] |
| 9 Vomiting | 2 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 6.82 [0.37, 126.26] |
| 10 Nausea | 2 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 8.63 [0.26, 284.55] |
| 11 Elevation of blood pressure | 3 | 2559 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.03, 6.57] |
| 12 Pain after birth requiring analgesia (not prespecified) | 1 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.34, 4.78] |
| 13 Headache (not prespecified) | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [0.51, 30.50] |
| 14 Eclamptic fit (not prespecified) | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.38, 29.43] |
| 15 Uterine subinvolution at routine follow up (not prespecified) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.32] |
| 16 Postpartum febrile morbidity (not prespecified) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.15] |
| 17 Postnatal haemoglobin < 10 g/dL (not prespecified) | 1 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
Comparison 3. Oral ergot alkaloids versus no uterotonic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean blood loss (mL) | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐44.0 [‐132.08, 44.08] |
| 2 Estimated or measured blood loss of at least 500 mL | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.72, 1.29] |
| 3 Estimated or measured blood loss of at least 1000 mL | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.50] |
| 4 Blood transfusion | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.10] |
| 5 Use of therapeutic uterotonics | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.34] |
| 6 Retained placenta or manual removal of placenta, or both | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 4.90 [0.24, 101.14] |
| 7 Elevation of blood pressure | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Pain after birth requiring analgesia (not prespecified) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Sensitivity analysis based on trial quality comparing ergot alkaloids (any routes) and no uterotonic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean blood loss (mL) | 2 | 1718 | Mean Difference (IV, Fixed, 95% CI) | ‐84.07 [‐102.47, ‐65.67] |
| 2 Estimated or measured blood loss of at least 500 mL | 3 | 2698 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 3 Length of third stage of labour (minutes) | 2 | 1522 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐2.32, 0.19] |
| 4 Retained placenta or manual removal of placenta, or both | 2 | 1718 | Risk Ratio (M‐H, Random, 95% CI) | 12.79 [2.40, 68.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Begley 1990.
| Methods | Randomisation in batches of 100 and allocation by sealed envelopes. No blinding. | |
|---|---|---|
| Participants | Eligibility: women attending the antenatal clinic at Coombe hospital who were public and semi‐private clients, singleton cephalic presentation, gestational age of 35‐36 weeks, no medical complications which would contraindicate the use of ergometrine or increase risk of bleeding (such as cardiovascular disease, use of heparin and hypertension), low risk to haemorrhage such as age 35 years or less, parity 5 or less, no previous history of primary PPH, Hb 11 g/dL or more (IV sample) or 10.6 or more (capillary sample).Exclusions: women who had hypertension (140/95 or greater), epidural anaesthesia, antenatal haemorrhage, first stage of labour in excess of 15 hours and operative delivery were excluded. | |
| Interventions | IV ergometrine 0.5 mg following the birth of the baby (n = 705) versus no ergometrine (n = 724) | |
| Outcomes | Mean blood loss; blood loss of at least 500 mL and 1000 mL; mean postnatal Hb (48‐72 hours); Hb less than 10 g/dL; manual removal of placenta; blood transfusion; elevation of diastolic blood pressure (> 95 mmHg); eclamptic fit; vomiting; nausea; headache; atonic haemorrhage requiring IV or IM ergot; length of third stage of labour; after‐birth pain needing IM or oral analgesia | |
| Notes | If eligible pregnant women were excluded, the envelope was returned unopened and reallocated to next batch.Dates of when the studies were conducted: 1 October 1987 and 31 October 1988Any details of funding sources: Research and Development Trust of the Coombe HospitalAny declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "random number tables were used. The first number was selected from the table by a disinterested observer and the numbers were allocated in blocks of 100 following the sequence."Comment: randomised |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "the envelopes remained sealed until the woman was in the second stage of labour and the midwife was certain a normal delivery would ensure."Comment: adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (from correspondence): "it was not possible to blind either women or the clinicians as ergometrine given intravenously causes a strong contraction that is felt and seen by both parties."Comment: intervention or control was not blinded which is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (from correspondence): "The technicians conducting the haemoglobin estimations were blind to trial allocation."Comment: incomplete blinding of outcome assessment such as mean blood loss which is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "same number of participants allocated and outcomes identified were reported."Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Daley 1951.
| Methods | Randomisation by alternative weekends changing with each team of obstetricians and midwives No blinding | |
|---|---|---|
| Participants | Eligibility: women having delivered spontaneously of a single fetus after less than 48 hours of labour, parity less than 5 and no antepartum haemorrhage or hydramnios at St Helier Hospital | |
| Interventions | IM ergometrine 0.5 mg as soon as the head was crowned (n = 490) versus no ergometrine (n = 510) | |
| Outcomes | Mean blood loss; blood loss of at least 500 mL; retained placenta for 60 min or more; mean length of third stage of labour | |
| Notes | 2 women excluded because of traumatic haemorrhage as clinicians felt convinced of the value of ergometrine. All outcomes were stratified by gravida.Dates of when the studies were conducted: written that “The experiment was stated in the spring of 1949” but ended period was not written.Any details of funding sources: no details given.Any declarations of interest among primary researchers: no information of declarations of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "treated cases were divided from controls by the simple method of the techniques changing with the team of obstetricians on duty. Two teams changed at 9 am each weekday and worked alternate weekends."Comment: sequence generated by some rule based on day of admission. |
| Allocation concealment (selection bias) | High risk | Participants were assigned based on alternation or rotation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded or incomplete blinding and the outcome or outcome measurement is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded or incomplete blinding and the outcome or outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "same number of participants allocated and outcomes identified were reported."Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
De Groot 1996b.
| Methods | A double‐blind multicentre trial with randomisation by computer‐generated randomisation list. Ergometrine and placebo were identical. The boxes of ergometrine and placebo were numbered by hospital pharmacy. | |
|---|---|---|
| Participants | Eligibility: all delivered women in 2 university hospitals (Leiden, Nijmegen), a midwifery school (Kerkrade) and by independent midwives in the area of the university hospital of Nijmegen in the study periodExclusions: refusal to participate, cardiovascular diseases, multiple pregnancies, non‐cephalic presentation, polyhydramnios, tocolysis given 2 hours prior to delivery, anticoagulant therapy, stillbirth, antepartum haemorrhage, induction or augmentation, operative vaginal deliveries, anaemia less than 6.8 g, former complications in the third stage of labour or women who wish natural births | |
| Interventions | Total of 367 women were assigned to 2:2:1 of oral ergometrine 0.4 mg (n = 146), oral placebo tablets (n = 143) and standard IM oxytocin (n = 78) immediately after birth. | |
| Outcomes | Mean blood loss measured by gravimetric method; blood loss of at least 500 mL and 1000 mL; removal of placenta; requiring blood transfusion; use of further oxytocics; length of third stage | |
| Notes | Data for length of third stage were presented as mean only, no standard deviation given.Dates of when the studies were conducted: July 1993 to July 1994Any details of funding sources: no details givenAny declarations of interest among primary researchers: no information of declarations of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the hospital pharmacy of the Univeristy of Nijmegen supplied numbered boxes containing 0.4 mg ergometrine tablets, placebo tablets or 5 IU oxytocin according a computer‐generated randomisation list."Comment: randomised |
| Allocation concealment (selection bias) | Low risk | Quote: "the hospital pharmacy of the Univeristy of Nijmegen supplied numbered boxes.... randomisation list. Care was taken that no difference could be seen or heard between the packages of the ergometrine/placebo tablets and the oxytocin ampoules."Comment: adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the hospital pharmacy of the Univeristy of Nijmegen supplied numbered boxes containing 0.4 mg ergometrine tablets, placebo tablets or 5 IU oxytocin according a computer‐generated randomisation list."Comment: a placebo tablet of ergometrine tablet was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the hospital pharmacy of the Univeristy of Nijmegen supplied numbered boxes containing 0.4 mg ergometrine tablets, placebo tablets or 5 IU oxytocin according a computer‐generated randomisation list."Comment: a placebo tablet of ergometrine tablet was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "same number of participants allocated and outcomes identified were reported."Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Howard 1964.
| Methods | A double‐blind trial with simple randomisation. The vials were coded and identical in appearance. | |
|---|---|---|
| Participants | Eligibility: all vaginally delivered women at the University of Iowa Hospital Exclusion: women delivered by caesarean section | |
| Interventions | 3 groups of comparisons: IV methylergonovine maleate 0.2 mg (n = 505), IV 0.9% sodium chloride (n = 475) and oxytocin (n = 479) following placental delivery | |
| Outcomes | Blood loss of at least 500 mL; elevation of systolic or diastolic blood pressure greater than 10 mmHg; need further treatment (vigorous uterine massage, IV or IM methylergonovine and/or oxytocin) | |
| Notes | Elevation of blood pressure was stratified by normotensive or hypertensive and pre‐eclamptic women.Dates of when the studies were conducted: August 1962 to July 1963Any details of funding sources: Public Health Service Grant No. GM‐08943‐02 and No. AO‐00555‐03Any declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly selected for 1 of 3 study groups."Comment: insufficient information about the sequence generation process to permit judgement of "Yes" or "No" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No" as the method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blinded technique was used..... The vials were identical in appearance and the contents were not known until completion of the study."Comment: blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a double‐blinded technique was used..... The vials were identical in appearance and the contents were not known until completion of the study."Comment: blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2% to 8% of recruited subjects were missing at measuring outcomes."Comment: for dichotomous outcome data, the proportion of missing outcomes compared with observed events risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ilancheran 1990.
| Methods | Women were equally divided into 4 groups on a random basis. No blinding. | |
|---|---|---|
| Participants | Eligibility: 20 consecutive women with spontaneous labour, between 38‐42 weeks who had normal vertex deliveryExclusion: no details given | |
| Interventions | 4 groups of comparison: no oxytocics in the third stage (n = 5), IV syntocinon (n = 5), IV syntometrine (n = 5) and IV ergometrine (n = 5) when anterior shoulder of baby was delivered. All the drugs were given intravenously and in standard doses. | |
| Outcomes | Serum levels of prostaglandins in the third stage of labour; postpartum haemorrhage at least 500 mL. | |
| Notes | In the results, we could not identify the actual figures of mean and standard deviation of prostaglandin levels. Postpartum haemorrhage was not defined for outcome in the methods but postpartum haemorrhage (600 mL) was found in 1 woman to whom ergometrine was given. No haemorrhage found in other groups. The correspondence author was contacted and no available data existed as the study was conducted in past 30 years.Dates of when the studies were conducted: no details givenAny details of funding sources: no details givenAny declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were equally divided on a random basis".Comment: insufficient information about the sequence generation process to permit judgement of "Yes" or "No" |
| Allocation concealment (selection bias) | Unclear risk | Methods not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind this type of intervention when not placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind this type of intervention when not placebo‐controlledOutcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants in outcome identified were not reported |
| Selective reporting (reporting bias) | High risk | Postpartum haemorrhage not prespecified in methods section but reported |
| Other bias | Unclear risk | Baseline characteristics not listed, difficult to identify other sources of bias |
Jolivet 1978.
| Methods | Women were randomised into 2 groups. No blinding. | |
|---|---|---|
| Participants | Eligibility: full‐term healthy women at the first 6 days of postpartumExclusion: no details given | |
| Interventions | 2 groups of comparison: no ergot at delivery or in the following 6 days (n = 28) and IM 0.2 mg methylergobasine maleate (Methergine) immediately after delivery followed by 3 x 1 mg tablets of ergotamine tartrate (Gynergen) by mouth daily (n = 30) | |
| Outcomes | Newborn birthweight and quantity of milk in 24 hours were measured. | |
| Notes | A paper was published in French and Anna Cuthbert, Research Associate at Cochrane Pregnancy and Childbirth, University of Liverpool, helped with assessing risk of bias and data extraction.Dates of when the studies were conducted: no details givenAny details of funding sources: no details givenAny declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2 groups were formed at random – no further detail given |
| Allocation concealment (selection bias) | Unclear risk | Methods not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind this type of intervention when not placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind this type of intervention when not placebo‐controlled Outcome assessors not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Neonatal denominators not included in tables |
| Selective reporting (reporting bias) | Low risk | 2 outcomes prespecified in methods section, and reported |
| Other bias | Unclear risk | Baseline characteristics not listed, difficult to identify other sources of bias |
Kerekes 1979.
| Methods | Simple randomisation into 3 comparison groups without concealment or blinding | |
|---|---|---|
| Participants | Eligibility: women with spontaneous uncomplicated vaginal deliveries at Korvin HospitalExclusions: no details given | |
| Interventions | 3 groups of comparisons were IV ergometrine 0.2 mg (n = 50), no treatment (n = 43) and intramyometrial prostaglandins (PGF2alpha) 1 mg (n = 47) after clamping of umbilical cord. | |
| Outcomes | Mean blood loss measured by cylinder after the collection of blood from container; maternal Hb concentration at 48 hours postpartum; duration of third stage of labour; uterine subinvolution at routine follow‐up; postpartum febrile morbidity. | |
| Notes | No data shown for mean blood loss or maternal HbDates of when the studies were conducted: no details givenAny details of funding sources: no details givenAny declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "three groups were formed by assigning the patients randomly to no treatment and to treatment with PGF2α and ergometrin."Comment: insufficient information about the sequence generation process to permit judgement of "Yes" or "No" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No" as the method of concealment is not described in sufficient detail to allow a definite judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded or incomplete blinding and the outcome or outcome measurement is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded or incomplete blinding and the outcome or outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "same number of participants allocated and outcomes identified were reported."Comment: no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
McGinty 1956.
| Methods | Simple randomisation into 4 comparison groups. | |
|---|---|---|
| Participants | Eligibility: women delivering vaginally at the Creighton Memorial St Joseph's Hospital and Bramwell Booth Memorial Hospital. Exclusions: no details provided. | |
| Interventions | 4 groups of comparisons were IV methergine 0.2 mg (n = 50), ergonovine 0.2 mg (n = 50), pitocin 5 units (n = 50) and normal saline 1 mL (n = 50) after birth of anterior shoulder. | |
| Outcomes | Blood transfusion, elevation of blood pressure (increase of systolic blood pressure 20 or more) and severe elevation (systolic blood pressure more than 170); vomiting; nausea; headache; eclamptic fit. | |
| Notes | Elevation of blood pressure were stratified by normotensive or hypertensive women. 5 severe PPH (no criteria shown) were found in the placebo group. Dates of when the studies were conducted: no details given Any details of funding sources: no details given Any declarations of interest among primary researchers: no information of declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "these cases were picked at random manner."Comment: insufficient information about the sequence generation process to permit judgement of "Yes" or "No" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No" as the method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the series has been broken down into four groups:........... of methergine intravenously;...... of ergonovine intravenously;..... of pitocin intravenously and five units intramuscularly.... the control group ... one cubic centimeter of normal saline intravenously."Comment: insufficient information to permit judgement of "Yes" or "No" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the series has been broken down into four groups:........... of methergine intravenously;...... of ergonovine intravenously;..... of pitocin intravenously and five units intramuscularly.... the control group ... one cubic centimeter of normal saline intravenously."Comment: insufficient information to permit judgement of "Yes" or "No" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "same number of participants allocated and outcomes identified were reported."Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports include all expected outcomes specified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adhikari 2007 | Comparisons of 0.2 mg IM methylergometrine and 10 IU IM oxytocin |
| Amant 1999 | Comparisons of oral misoprostol and IV methylergometrine included in previous Cochrane Review |
| Badhwar 1991 | Management with nipple stimulation |
| Barbaro 1961 | Comparisons of IM syntometrine and ergometrine included in previous Cochrane Review |
| Baumgarten 1983 | Comparisons of ergometrine, oxytocin and sulprostone on uterine contractility by intra‐catheter pressure excluded in previous Cochrane Review due to no possible outcomes |
| Bonham 1963 | Comparisons of syntometrine, ergometrine and ergometrine plus hyaluronidase included in previous Cochrane Review |
| Boopathi 2014 | Comparisons of 0.2 mg IV methylergometrine and 10 IU IM oxytocin |
| Caliskan 2002 | Comparisons of misoprostol plus oxytocin, misoprostol, oxytocin and oxytocin plus ergometrine included in previous Cochrane Review |
| Carpén 1968 | Comparisons of IM OCM 505 and IV methergine |
| Chatterjee 2000 | No data can be extracted, excluded in previous Cochrane Review |
| Chukudebelu 1963 | Not randomised controlled trial comparing 0.5 U of syntocinon plus 0.5 mg of ergometrine, 0.5 mg of ergometrine and 1 mg of ergometrine |
| Dhananjaya 2014 | Comparison of 0.2 mg IM methylergometrine and 10 IU IM oxytocin |
| Diab 1999 | Comparisons of misoprostol and ergometrine excluded in previous Cochrane Review |
| Docherty 1981 | Data were not suitable for extraction and failed contact with author excluded in previous 2 Cochrane Reviews |
| Dweck 2000 | Participants were women who underwent caesarean section and received 0.2 mg of methergine orally every 6 hours until hospital discharge with the first dose being within the first 6 hours after caesarean section. The outcome was diagnosed endometritis. |
| EudraCT2010‐01980‐42 | Only a protocol registered in the EU clinical trial register on 13 July 2010 was found but no results and publication were available. It was not possible to identify a contact person and so we were unable to contact anyone for any further details about the results of the study. |
| Ezeama 2014 | Comparison of 0.5 mg IM ergometrine and 10 IU IM oxytocin |
| Fawzy 2012 | Comparison of 10 mL IV ergotmetrine and 200 mcg rectal or sublingual tablet of misoprostol |
| Forster 1957 | Not randomised controlled trial comparing 0.2 mg of methergine versus ergometrine given IV immediately after the delivery of the baby for 24 weeks and for next 8 weeks, 0.2 mg of methergine or ergometrine given additionally by IM injection after the expression of the placenta |
| Francis 1965a | Comparisons of syntometrine before placental delivery and ergometrine after placental delivery excluded in previous Cochrane Review |
| Francis 1965b | Comparisons of IM syntometrine and ergometrine included in previous Cochrane Review |
| Friedman 1957 | Not randomised controlled trial comparing no medication as a control, 10 units of oxytocin IM, 0.2 mg of ergonovine maleate IM or IV, 0.2 mg of methylergonovine tartrate IM or IV and 1 mg of dihydroergotamine methanesulfonate IM after delivery of placenta |
| Fugo 1958 | Comparisons of IV oxytocin, syntometrine, U3772 and ergometrine included in previous Cochrane Review |
| Groeber 1960 | Comparisons of 0.2 mg of methergine and 0.2 mg of ergonovine IV after delivery of placenta |
| Gupta 2014 | Comparisons of 0.2 mg IM methoergin and 125 mcg IM carboprost |
| Hacker 1979 | Not randomised controlled trial comparing no drug as a control, syntometrine (combining 5 U of oxytocin and 0.5 mg of ergometrine maleate) IM and 0.5 mg of ergometrine maleate IV |
| Huh 2004 | Comparisons of oxytocin administered before and after placental delivery excluded in previous Cochrane Review due to comparison of oxytocin with time difference |
| Is 2012 | Comparisons of IM ergometrine and 400 mcg rectal misoprostol |
| Jago 2007 | Comparisons of IM 0.5 mg of ergometrine and IV 10 IU of oxytocin |
| Kemp 1963 | Not randomised controlled trial comparing syntometrine (combining 5 IU of syntocinon and 0.5 mg of ergometrine) IM with 0.5 mg of ergometrine IM |
| Khan 1995 | Comparisons of IM syntometrine and oxytocin included in previous Cochrane Review |
| Kikutani 2003 | Comparisons of oxytocin and ergometrine on epidural pressure |
| Lam 2004 | Comparisons of sublingual misoprostol and IV syntometrine |
| Lamont 2001 | Comparisons of syntometrine and prostaglandin |
| Mitchell 1993 | Comparisons of syntometrine and oxytocin included in previous Cochrane Review |
| Moir 1979 | Comparisons of 0.5 mg of ergometrine and 10 IU of oxytocin given at the time of delivery of anterior shoulder |
| Moodie 1976 | Comparisons of IV 0.5 mg of ergometrine and IV 5 IU of oxytocin |
| Moore 1956 | Not randomised controlled trial comparing 0.2 mg of ergonovine maleate and 0.2 mg of methylergonovine tartrate IV after expulsion of placenta |
| Nieminen 1963 | Comparisons of ergometrine, syntometrine and oxytocin included in previous Cochrane Review |
| Orji 2008 | Comparisons of 0.25 mg of ergometrine and 10 IU oxytocin IV at the delivery of anterior shoulder of the baby |
| Patil 2013 | Comparisons of 0.2 mg IV methylergometrine and 600 mcg oral tablet misoprostol |
| Paull 1977 | Comparisons of 0.25 mg and 0.5 mg of ergometrine maleate IV after completion of second stage of labour |
| Pei 1996 | Not randomised controlled trial and no outcomes of interest comparing oxytocin and ergotocin on postpartum lactation |
| Penaranda 2002 | Comparisons of sublingual misoprostol, oxytocin and methylergometrine included in previous Cochrane Review |
| Rajwani 2000 | No data can be extracted, excluded in previous Cochrane Review |
| Ramesh 1983 | Not randomised controlled trial comparing 0.5 mg of PGE2 intramyometrial at the fundus of the uterus at the time of crowning of fetal head and 0.25 mg of methylergotamine maleate |
| Reddy 2001 | Comparisons of 0.2 mg of methylergometrine IV at the time of anterior shoulder delivery, 10 IU of oxytocin diluted with 10 mL of normal saline via umbilical cord immediately after clamping the cord and IM 250 mg of carboprost with the delivery of anterior shoulder of the baby |
| Rooney 1985 | Quasi‐randomised controlled trial using odd‐even cases comparing syntometrine with the delivery of anterior shoulder IM and no syntometrine |
| Saito 2007 | Quasi‐randomised controlled trial (samples were allocated using weekly or monthly as determined by each hospital in alternate shifts) comparing 5 IU of oxytocin and 0.2 mg of methylergometrine IM administered shortly after delivery of the baby |
| Sharma 2014 | Not randomised controlled trial comparing 0.2 mg IV methylergometrine and 10 IU IV oxytocin and 600 mcg sublingual misoprostol |
| Shrestha 2008 | Comparisons of 0.2 mg IM methergine Intramyometrial 250 mcg 15‐methyl PGF2a and IM 250 mcg 15‐methyl PGF2a |
| Singh 2009 | Comparisons of sublingual 400 microgram and 600 microgram of misoprostol, 5 IU of oxytocin IV and 200 microgram of methylergometrine IV at the delivery of anterior shoulder of the baby |
| Soiva 1964 | Comparisons of IV methylergometrine and IM oxytocin included in previous Cochrane Review |
| Sorbe 1978 | Not randomised controlled trial comparing 0.2 mg of ergometrine, 10 IU of oxytocin IV after delivery of anterior shoulder and control group which was not described how to select |
| Stearn 1963 | Comparisons of syntometrine and ergometrine excluded in previous Cochrane Review due to allocation |
| Symes 1984 | Comparisons of oxytocin and oxytocin plus ergometrine excluded in previous Cochrane Review due to no clinical outcomes |
| Terry 1970 | Quasi‐randomised controlled trial comparing IM syntometrine (0.5 mg of ergometrine and 5 IU of oxytocin) at the delivery of anterior shoulder with IM syntometrine with free bleeding. Outcome of interest was fetal cells in maternal blood. The comparison group contained another uterotonic and so this is not eligible for inclusion. |
| Thilaganathan 1993 | Comparison of 1 mL of syntometrine after the delivery of baby and no drug |
| Thornton 1988 | Quasi‐randomised controlled trial comparing IM oxytocin on the delivery of anterior shoulder and no treatment |
| Vaughan 1974 | Comparisons of syntometrine and oxytocin excluded in previous Cochrane Review due to 1 outcome on central venous pressure |
| Vimala 2004 | Comparisons of sublingual misoprostol and IM methylergometrine |
| Weiss 1975 | Quasi‐randomised controlled trial comparing 0.2 mg of methylergonovine maleate after delivery of placenta and 1 mL of normal saline IM. Only serum prolactin before and after 80‐90 min after injection was measured. Excluded because none of the outcomes of the review were measured. |
| Yardim 1967 | Comparisons of oxytocin plus ergometrine and no drug |
| Yuen 1995 | Comparisons of syntometrine and oxytocin included in previous Cochrane Review |
Differences between protocol and review
We listed 11 maternal outcomes related to both efficacy and safety, and four neonatal outcomes, in the original protocol. However, six additional outcomes were identified in the review published in 2007 and 2008. In this updated version, we divided the outcomes into primary and secondary outcomes. We chose the outcomes which directly represented "haemorrhage" as the primary outcomes and considered all other maternal and neonatal outcomes as secondary outcomes. We changed the outcome "third stage of labour lasting more than 30 minutes" to "length of third stage of labour (minutes)".
For this 2017 update, we added an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). Two excluded studies in the previous review were included (Ilancheran 1990; Jolivet 1978). They had previously been excluded due to not reporting outcomes of the review.
In previous version of this review, there was one comparison which was subgrouped by route of administration (intravenous/intramuscular and oral). These have now been separated out into three separate comparisons in this update:
- ergot alkaloids (any route of administration) versus no uterotonic agents
- intravenous/intramuscular ergot alkaloids versus no uterotonic agents
- oral ergot alkaloids versus no uterotonic agents
The original prespecified subgroup analyses were:
- risk of having a postpartum haemorrhage: high risk versus low risk;
- route of administration of ergot alkaloids: intramuscular or intravenous compared with oral route.
In this update (2017), they have been amended slightly to:
- use of ergot alkaloids with or without active management of third stage of labour;
- use of ergot alkaloids before or after placental delivery;
- use of ergot alkaloids in different doses.
Contributions of authors
Tippawan Liabsuetrakul (TL) TL was involved in all parts of preparing the review, and wrote the first and final draft of the review. For the 2011 update, TL assessed the full texts of the additional three references, identified from the updated search, against the criteria for inclusion; appraised methodological quality of all included studies; and prepared the updated review. For the 2017 update, TL assessed all 12 new reports from the updated search, updated the review in accordance with the new format and prepared the final updated review.
Krantarat Peeyananjarassri (KP) KP was involved in assessing the included or excluded studies, and commented on the first draft of the review. For the 2011 update, KP assessed the full texts of the additional three references, identified from the updated search, against the criteria for inclusion; appraised methodological quality of all included studies; and approved the final version of the updated review. For the 2017 update, KP assessed all 12 new reports from the updated search and approved the final version of the updated review.
Thanapan Choobun (TC) TC was involved in assessing the quality of included studies and extracting the data. TC commented on the first draft of the review. For the 2011 and 2017 update, TC approved the final version of the updated review.
Monir Islam (MI) MI commented on the draft review. For the 2011 and 2017 update, MI approved the final version of the updated review.
Sources of support
Internal sources
- Faculty of Medicine, Prince of Songkla University, Thailand.
External sources
- Thailand Research Fund (Distinguished Research Professor Award), Thailand.
Declarations of interest
Tippawan Liabsuetrakul: none known.
Thanapan Choobun: none known.
Krantarat Peeyananjarassri: none known.
Q Monir Islam: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Begley 1990 {published data only}
- Begley CM. Comparative studies in the third stage of labour [MSc thesis]. Dublin: Trinity College, University of Dublin, 1990. [Google Scholar]
- Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3‐17. [DOI] [PubMed] [Google Scholar]
- Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60‐72. [DOI] [PubMed] [Google Scholar]
Daley 1951 {published data only}
- Daley D. The use of intramuscular ergometrine at the end of the second stage of normal labour. Journal of Obstetrics and Gynaecology of the British Empire 1951;58(3):388‐97. [DOI] [PubMed] [Google Scholar]
De Groot 1996b {published data only}
- Groot ANJA, Roosmalen J, Dongen PWJ, Borm GF. A placebo‐controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75(5):464‐8. [DOI] [PubMed] [Google Scholar]
Howard 1964 {published data only}
- Howard WF, McFadden PR, Keettel WC. Oxytocic drugs in fourth stage of labor. JAMA 1964;189:411‐3. [DOI] [PubMed] [Google Scholar]
Ilancheran 1990 {published data only}
- Ilancheran A, Ratnam SS. Effect of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29(3):177‐80. [DOI] [PubMed] [Google Scholar]
Jolivet 1978 {published data only}
- Jolivet A, Robyn C, Huraux‐Rendu C, Gautray JP. Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1978;7(1):129‐34. [PubMed] [Google Scholar]
Kerekes 1979 {published data only}
- Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage labour. Prostaglandins 1979;18:161‐6. [DOI] [PubMed] [Google Scholar]
McGinty 1956 {published data only}
- McGinty LB. A study of the vasopressor effects of oxytocics when used intravenously in the third stage of labour. Western Journal of Surgery 1956;64:22‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Adhikari 2007 {published data only}
- Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal Journal of Obstetrics and Gynaecology 2007;2(2):24‐8. [Google Scholar]
Amant 1999 {published data only}
- Amant F, Spitz B, Timmerman D, Corresmans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double‐blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066‐70. [DOI] [PubMed] [Google Scholar]
Badhwar 1991 {unpublished data only}
- Badhwar L, Singh K, Sethi N, Gupta I, Aggarwal N. The value of nipple stimulation in the management of third stage of labour. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 September; Singapore. 1991:16.
Barbaro 1961 {published data only}
- Barbaro CA, Smith GO. Clinical trial of SE505 ‐ a new oxytocic mixture. Australian and New Zealand Journal of Obstetrics and Gynaecology 1961;1:147‐50. [Google Scholar]
Baumgarten 1983 {published data only}
- Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post‐partum application of sulprostone and other oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16(3):181‐92. [DOI] [PubMed] [Google Scholar]
Bonham 1963 {published data only}
- Bonham DG. Intramuscular oxytocics and cord traction in third stage of labour. British Medical Journal 1963;2:1620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boopathi 2014 {published data only}
- Boopathi A, Nayak SR, Rao A, Rao B. Oxytocin versus methylergometrine in the active management of third stage of labour. Open Journal of Obstetrics and Gynecology 2014;4:666‐71. [Google Scholar]
Caliskan 2002 {published data only}
- Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187(4):1038‐45. [DOI] [PubMed] [Google Scholar]
Carpén 1968 {published data only}
- Carpén E, Koistinen O, Virkkunen A, Laakso L. Clinical trial of intramuscular OCM 505 and intravenous methergin in the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1968;57(4):473‐5. [PubMed] [Google Scholar]
Chatterjee 2000 {unpublished data only}
- Chatterjee A. Misoprostol and the 3rd stage. XVI FIGO World Congress of Obstetrics & Gynecology (Book 4); 2000 Sept 3‐8; Washington DC, USA. 2000:29.
Chukudebelu 1963 {published data only}
- Chukudebelu WO, Marshall AT, Chalmers JA. Use of "syntometrine" in the third stage of labour. British Medical Journal 1963;1(5342):1390‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhananjaya 2014 {published data only}
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734‐9. [Google Scholar]
Diab 1999 {published data only}
- Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. Journal of Obstetrics & Gynaecology Research 1999;25(5):327‐32. [DOI] [PubMed] [Google Scholar]
Docherty 1981 {published data only}
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
Dweck 2000 {published data only}
- Dweck MF, Lynch CM, Spellacy WN. Use of methergine for the prevention of postoperative endometritis in non‐elective cesarean section patients. Infectious Diseases in Obstetrics & Gynecology 2000;8(3‐4):151‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
EudraCT2010‐01980‐42 {published data only}
- EudraCT2010‐01980‐42. Efficacy of methylergometrine daily administration in puerperium. clinicaltrialsregister.eu/ctr‐search/trial/2010‐01980‐42/GB (first received 22 July 2010).
Ezeama 2014 {published data only}
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynecology and Obstetrics 2014;124(1):67‐71. [DOI] [PubMed] [Google Scholar]
- PACTR201105000292708. Prophylactic ergometrine versus oxytocin for women in the third stage of labour: a double‐blind, randomized trial. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201105000292708 (first received: 2 May 2011).
Fawzy 2012 {published data only}
- Fawzy AEMA, Swelem M, Abdelrehim AI, Titeli S, Elghazal ZS, El‐Gahwagi MM, et al. Active management of third stage of labor by intravenous ergometrine and rectal versus sublingual misoprostol (a double‐center study). Alexandria Journal of Medicine 2012;48(4):381‐5. [Google Scholar]
Forster 1957 {published data only}
- Forster FMC. A comparative study of ergometrine and 'methergin' used in the management of the third stage of labour. Medical Journal of Australia 1957;2:155‐6. [Google Scholar]
Francis 1965a {published data only}
- Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin‐ergometrine mixture (first of two trials). Australian and New Zealand Journal of Obstetrics and Gynaecology 1965;5:47‐51. [DOI] [PubMed] [Google Scholar]
Francis 1965b {published data only}
- Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin‐ergometrine mixture (second of two trials). Australian and New Zealand Journal of Obstetrics and Gynaecology 1965;5:47‐51. [DOI] [PubMed] [Google Scholar]
Friedman 1957 {published data only}
- Friedman EA. Comparative clinical evaluation of postpartum oxytocics. American Journal of Obstetrics and Gynecology 1957;73(6):1306‐13. [DOI] [PubMed] [Google Scholar]
Fugo 1958 {published data only}
- Fugo NW, Dieckmann WJ. A comparison of oxytocic drugs in the management of the placental stage. American Journal of Obstetrics and Gynecology 1958;76(1):141‐6. [DOI] [PubMed] [Google Scholar]
Groeber 1960 {published data only}
- Groeber WR, Bishop EH. Methergine and ergonovine in the third stage of labor. Obstetrics & Gynecology 1960;15:85‐8. [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta M, Bhosale U. Comparative study of methylergometrine and low dose carboprost (PGF2‐x) in active management of 3rd stage labor. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):139. [Google Scholar]
Hacker 1979 {published data only}
- Hacker NF, Biggs JSG. Blood pressure changes when uterine stimulants are used after normal delivery. British Journal of Obstetrics and Gynaecology 1979;86(8):633‐6. [DOI] [PubMed] [Google Scholar]
Huh 2004 {published data only}
- Huh W, Chelmow D, Malone FD. A randomized, double‐blinded, placebo controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S130. [DOI] [PubMed] [Google Scholar]
Is 2012 {published data only}
- Is S, Gr V, Keranahalli S. Comparison of intramuscular ergometrine and per rectal misoprostol for prophylaxis against atonic post partum haemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S797‐8. [Google Scholar]
Jago 2007 {published data only}
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal‐Fetal and Neonatal Medicine 2007;20(9):703‐5. [DOI] [PubMed] [Google Scholar]
Kemp 1963 {published data only}
- Kemp J. Clinical trial of "syntometrine" in the third stage of labour. British Medical Journal 1963;1(5342):1391‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1995 {published data only}
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin vs syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147‐51. [DOI] [PubMed] [Google Scholar]
Kikutani 2003 {published data only}
- Kikutani T, Shimada Y. Effects of methylergometrine and oxytocin on thoracic epidural pressure during cesarean section. Journal of Obstetrics and Gynaecology Research 2003;29(3):180‐5. [DOI] [PubMed] [Google Scholar]
Lam 2004 {published data only}
- Lam H, Tang OS, Lee CP, Ho PC. A pilot‐randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83(7):647‐50. [DOI] [PubMed] [Google Scholar]
Lamont 2001 {published data only}
- Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203‐10. [DOI] [PubMed] [Google Scholar]
Mitchell 1993 {published data only}
- Mitchell GG, Elbourne DR. The Salford third stage trial: oxytocin plus ergometrine vs oxytocin alone in the active management of the third stage of labor. Online Journal of Current Clinical Trials 1993;2:Doc 83. [PubMed] [Google Scholar]
Moir 1979 {published data only}
- Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side‐effects at spontaneous vertex delivery. British Journal of Anaesthesia 1979;51:113‐7. [DOI] [PubMed] [Google Scholar]
Moodie 1976 {published data only}
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571‐4. [DOI] [PubMed] [Google Scholar]
Moore 1956 {published data only}
- Moore JH. Is methylergonovine tartrate superior to ergonovine maleate. American Journal of Obstetrics and Gynecology 1956;71(4):908‐11. [DOI] [PubMed] [Google Scholar]
Nieminen 1963 {published data only}
- Nieminen U, Jaervinen PA. A comparative study of different medical treatments of the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:424‐9. [PubMed] [Google Scholar]
Orji 2008 {published data only}
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynecology & Obstetrics 2008;101(2):129‐32. [DOI] [PubMed] [Google Scholar]
Patil 2013 {published data only}
- Patil NB, Patted SS. A randomised controlled trial of oral misoprostol vs injection methylergometrine for prevention of post partum hemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(3):296‐303. [Google Scholar]
Paull 1977 {published data only}
- Paull JD, Ratten GJ. Ergometrine and third stage blood loss. Medical Journal of Australia 1977;1:178‐9. [Google Scholar]
Pei 1996 {published data only}
- Pei JL, Zhao DF. Study of the effects of using uterine stimulants on milk secretion during delivery. Zhonghua Hu Li Za Zhi [Chinese Journal of Nursing] 1996;31(7):384‐5. [PubMed] [Google Scholar]
Penaranda 2002 {published data only}
- Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92. [Google Scholar]
Rajwani 2000 {unpublished data only}
- Rajwani J, Survana K. Active management of third stage of labor ‐ a comparative study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 3); 2000 Sept 3‐8; Washington DC, USA. 2000:54.
Ramesh 1983 {published data only}
- Ramesh S, Bhatnagar B, Karna S, Ranjana. Role of intramyometrial prostaglandin E2 in management of third stage of labour. Indian Journal of Medical Research 1983;77:642‐7. [PubMed] [Google Scholar]
Reddy 2001 {published data only}
- Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. Journal of Obstetrics and Gynecology of India 2001;51(2):44‐7. [Google Scholar]
Rooney 1985 {published data only}
- Rooney I, Hughes P, Calder AA. Is routine administration of syntometrine still justified in the management of the third stage of labour?. Health Bulletin 1985;43(3):99‐101. [PubMed] [Google Scholar]
Saito 2007 {published data only}
- Saito K, Haruki A, Ishikawa H, Takahashi T, Nagase H, Koyama M, et al. Prospective study of intramuscular ergometrine compared with intramuscular oxytocin for prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2007;33(3):254‐8. [DOI] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma M, Kaur P, Kaur K, Kaur A, Kaur PK, Kaur MM. A comparative study of oxytocin/misoprostol/methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynecology of India 2014;64(3):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrestha 2008 {published data only}
- Shrestha A, Urala MS, Upreti D, Niraula S. Comparison of intramyometrial and intramuscular 15 methyl PGF2x against traditional prophylactic intramuscular methergin for the active management of third stage of labor. Nepal Journal of Obstetrics and Gynaecology 2008;3(2):35‐9. [Google Scholar]
Singh 2009 {published data only}
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. [DOI] [PubMed] [Google Scholar]
Soiva 1964 {published data only}
- Soiva K, Koistinen O. Clinical experience with simultaneous intramuscular injection of oxytocin and methylergometrine. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:173‐8. [PubMed] [Google Scholar]
Sorbe 1978 {published data only}
- Sorbe B. Active pharmacologic management of the third stage of labor. A comparison of oxytocin and ergometrine. Obstetrics & Gynecology 1978;52(6):694‐7. [PubMed] [Google Scholar]
Stearn 1963 {published data only}
- Stearn RH. Syntometrine in the management of the third stage of labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1963;70:593‐6. [DOI] [PubMed] [Google Scholar]
Symes 1984 {published data only}
- Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. Journal of Obstetrics and Gynaecology 1984;5:36‐8. [Google Scholar]
Terry 1970 {published data only}
- Terry MF. A management of the third stage to reduce feto‐maternal transfusion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77(2):129‐32. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1993 {published data only}
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48(1):19‐22. [DOI] [PubMed] [Google Scholar]
Thornton 1988 {published data only}
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 1988;297(6642):167‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin in the third stage of human labour with and without syntometrine [abstract]. Clinical Science 1987;73 Suppl(17):2P. [Google Scholar]
Vaughan 1974 {published data only}
- Vaughan Williams CA, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. Journal of Obstetrics and Gynaecology of the British Commonwealth 1974;81(8):596‐9. [DOI] [PubMed] [Google Scholar]
Vimala 2004 {published data only}
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87(1):1‐5. [DOI] [PubMed] [Google Scholar]
Weiss 1975 {published data only}
- Weiss G, Klein S, Shenkman L, Kataoka K, Hollander CS. Effect of methylergonovine on puerperal prolactin secretion. Obstetrics & Gynecology 1975;46(2):209‐10. [PubMed] [Google Scholar]
Yardim 1967 {published data only}
- Yardim T. Active direction of the postpartum period with Syntometrin. Der Landarzt 1967;43(25):1223‐5. [PubMed] [Google Scholar]
Yuen 1995 {published data only}
- Yuen PM, Chan NST, Yim SF, Chang AMZ. A randomised double blind comparison of syntometrine and syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102(5):377‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Acuin 2010
- Acuin CS, Khor GL, Liabsuetrakul T, Achadi EL, Htay TT, Firestone R, et al. Maternal, neonatal, and child health in southeast Asia: towards greater regional collaboration. Lancet 2011;377(9764):516‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 1998
- Andersen B, Andersen LL, Sorensen T. Methylergometrine during the early puerperium; a prospective randomized double blind study. Acta Obstetricia et Gynecologica Scandinavica 1998;77(1):54‐7. [DOI] [PubMed] [Google Scholar]
Begley 2015
- Begley CM, Gyte GML, Murphy DJ, Devane D, McDonald SJ, McGuire W. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Borri 1986
- Borri P, Gerli P, Antignani FL, Bindi L, Cozzi C, Moscarella G, et al. Methylergonovine maleate: a proposal for its more specific use. Biological Research in Pregnancy and Perinatology 1986;7(3):128‐30. [PubMed] [Google Scholar]
Brucker 2001
- Brucker MC. Management of the third stage of labor: an evidence‐based approach. Journal of Midwifery and Women's Health 2001;46(6):381‐92. [DOI] [PubMed] [Google Scholar]
De Groot 1995
- Groot AN. Prevention of postpartum haemorrhage. Bailliere’s Clinical Obstetrics and Gynaecology 1995;9(3):619‐31. [DOI] [PubMed] [Google Scholar]
De Groot 1996a
- Groot AN. The role of oral (methyl)ergometrine in the prevention of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69(1):31‐6. [DOI] [PubMed] [Google Scholar]
De Groot 1998
- Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56(4):523‐35. [DOI] [PubMed] [Google Scholar]
Den Hertog 2001
- Hertog CE, Groot AN, Dongen PW. History and use of oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 2001;94(1):8‐12. [DOI] [PubMed] [Google Scholar]
Dua 1994
- Dua JA. Postpartum eclampsia associated with ergometrine maleate administration. British Journal of Obstetrics and Gynaecology 1994;101(1):72‐3. [DOI] [PubMed] [Google Scholar]
Dunn 2002
- Dunn PM. John Chassar Moir (1900‐1977) and the discovery of ergometrine. Archives of Diseases in Childhood. Fetal & Neonatal Edition 2002;87:F152‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallos 2018
- Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD011689.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gowri 2003
- Gowri V, Al Hinai A. Postpartum second degree heart block induced by methergine. International Journal of Gynecology & Obstetrics 2003;81(2):227‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2015
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogerzeil 1996
- Hogerzeil HV, Walker GJ. Instability of (methyl)ergometrine in tropical climates: an overview. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69(1):25‐9. [DOI] [PubMed] [Google Scholar]
McCormick 2002
- McCormick ML, Sanghvi HC, Kinzie B, McIntosh N. Preventing postpartum hemorrhage in low‐resource settings. International Journal of Gynecology & Obstetrics 2002;77(3):267‐75. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald S, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2014
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
Su 2012
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultatos 1997
- Sultatos LG. Mechanisms of drugs that affect uterine motility. Journal of Nurse‐Midwifery 1997;42(4):367‐70. [DOI] [PubMed] [Google Scholar]
Taylor 1985
- Taylor GJ, Cohen B. Ergonovine‐induced coronary artery spasm and myocardial infarction after normal delivery. Obstetrics & Gynecology 1985;66(6):821‐2. [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dongen 1995
- Dongen PW, Groot AN. History of ergot alkaloids from ergotism to ergometrine. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;60(2):109‐16. [DOI] [PubMed] [Google Scholar]
Van Selm 1995
- Selm M, Kanhai HH, Keirse MJ. Preventing the recurrence of atonic postpartum hemorrhage: a double‐blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74(4):270‐4. [DOI] [PubMed] [Google Scholar]
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Managing Complications in Pregnancy and Childbirth: a Guide for Midwives and Doctors. Geneva: World Health Organization, 2000. [Google Scholar]
WHO 2001
- World Health Organization. Maternal Mortality in 1995: Estimates Developed by WHO, UNICEF, UNFPA. Geneva: World Health Organization, 2001. [Google Scholar]
WHO 2003
- World Health Organization. Pregnancy, Childbirth, Postpartum and Newborn Care: a Guide for Essential Practice. Geneva: World Health Organization, 2003. [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. Trends in Maternal Mortality:1990 to 2015 estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization, 2015. [Google Scholar]
Widmer 2016
- Widmer M, Piaggio G, Abdel‐Aleem H, Carroli G, Chong YS, Coomarasamy A, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trial 2016;17(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Liabsuetrakul 2007
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PubMed] [Google Scholar]