Impact of disturbed areas on Theraphosidae spiders diversity (Araneae) and first population data of Grammostola rosea (Walckenaer) in Panul Park (original) (raw)
Abstract
Soil fauna constitutes one of the most abundant and richest environments on earth (Coleman et al. 2004, Fundamentals of solil ecology, 2nd ed. Elsevier Academic Press, London, UK). Different degrees of soil disturbance can affect arthropod diversity, which allows a correlation of biodiversity to quality of habitat. The present study aimed to evaluate the impact of habitat on Theraphosidae spiders, with special focus on Grammostola rosea. Slight differences in the diversity of Theraphosidae between the disturbed area of Cerro Huechuraba and the undisturbed Panul Park were found. However, a high dominance of G. rosea was observed in both study areas. G. rosea density 1,350 ind/ha in Panul Park, and 750 ind/ha in Cerro Huechuraba. UPGMA cluster analysis did not show significant differences between established environments. A standard methodology to develop inventories of Theraphosidae was proposed. The distribution of G. rosea and its natural history were reported.
Keywords: anthropic disturbance, anthropogenic impact, chilean tarantula, methodology environmental baseline
1. INTRODUCTION
Soil is one of the habitats with the greatest species richness on earth, but also one of the least studied (Decaëns, Jiménez, Gioia, Measey, & Lavelle, 2006; Giller, Beare, Lavelle, Izac, & Swift, 1997; Wolters, 2001). Arthropods represent 97% of soil fauna, and 12% of this corresponds to arachnids (Decaëns et al., 2006). Arthropod diversity can vary depending on degree of environmental disturbance, thereby indicating a correlation of biodiversity to quality of habitat (Duelli & Obrist, 1998). Spiders, considered as hyperdiverse groups, are very common in terrestrial ecosystems and have great ecological diversity (Jimenez‐Valverde & Hortal, 2003). Among the studies of spiders are those dealing with their potential to control pests by depredation (Greenstone & Sunderland, 1999; Harwood, Sunderland, & Symondson, 2004; Maloney, Drummond, & Alford, 2003; Nyffeler & Sunderland, 2003), with guild structure (Cardoso, Pekár, Jocqué, & Coddington, 2011; Uetz, Halaj, & Cady, 1999), with diversity, distribution, fragmentation, and competition effects (Torma, Gallé, & Bozsó, 2014), and with bioindicators of soil quality (Marc, Canard, & Ysnel, 1999; Willett, 2001). Given their prominence, several authors have studied the effect and relative importance of local environmental factors on spiders and other arachnids (Lo‐Man‐Hung et al., 2011).
However, studies of the biology, ecology, and taxonomy of spiders have been delayed compared to other groups (Bertani, 2001; Jimenez‐Valverde & Hortal, 2003; Marc et al., 1999). Even more scarce are those studies in tarantulas, mainly due to their way of life and the obtaining specimens (Ferretti, Schwerdt, Peralta, Farina, & Pompozzi, 2016).
Among the Theraphosidae present in Chile, Grammostola rosea (Walckenaer, 1837) is the most frequently mentioned in informal reports, but no data about its population distribution are given. Moreover, historical distributions may no longer be useful because humans in their imperative need for expansion have notoriously modified the environment. Consequently, land use has shifted to urban use along with alterations of vegetation and geography (Argañaraz & Gleiser, 2017), thereby producing local changes in arachnid diversity. In addition, some historical distributional data may be wrong, as is the case with G. rosea, which was cited for Mexico, a labeling error by Pickard‐Cambridge (1897) detected by Pocock (1903).
Another important factor is that wild specimens of this species are under great capture pressure to be sold abroad as pets, which could eventually cause a drastic decline in populations and possibly local extinctions (Aguilera, 2015; ODEPA, 2017).
The present study aim was to evaluate habitat impacts on communal and distributional parameters of Grammostola rosea, in two areas with different levels of anthropic disturbance and land use, and especially in an ecological preservation area. Furthermore, data related to habitat and natural history of the species, proposed methodologies to correctly evaluate Grammostola populations in environmental baseline are given.
2. MATERIALS AND METHODS
2.1. Study area
Forest of Panul Park, with an approximate sampling area of 13.5 ha (Figure 1a) in the eastern sector of La Florida (357,928 E, 6,288,800 S). Located in the foothills of one of the closest mountain ranges of Santiago, known as the Sierra de Ramón, where the highest elevations exceed 3,000 m above sea level. Recently, this sector has had an economic interest for real estate development, and therefore, some environmental studies have been performed in the area (Gesterra, 2011), but none about arachnids. Due to the importance of the area and the imminent urbanization, the Corporación Municipal de Fomento al Desarrollo Comunal y Productivo of La Florida (COFODEP, 2015) suggests Panul Park as an “environmental ecological preservation area of the foothills, municipality of La Florida.”
Figure 1.
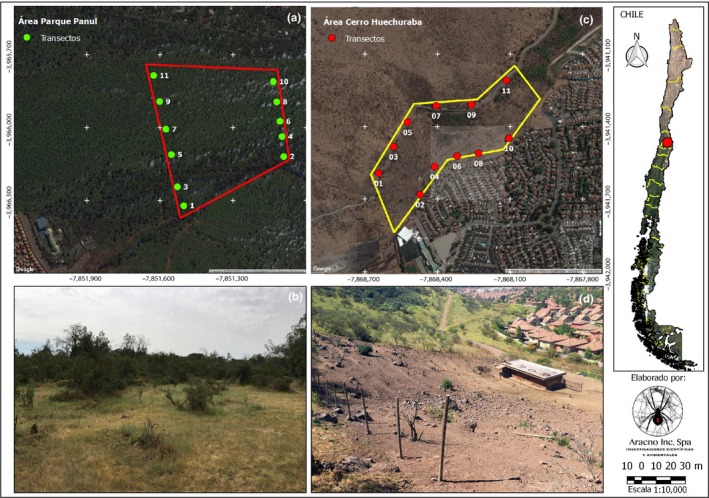
(a) Cartography of Panul Park study area. (b) Panul Park study area, annual Forest and Poaceae formation. (c) Cartography of Cerro Huechuraba study area. (d) Cerro Huechuraba study area, from top to bottom formation of wild allochthonous, Forest and devoid of vegetation with poaceas
Panul Park is known as the last native sclerophyllous forest in Santiago, alternating secondary sclerophyllous scrubs (Mann, 1964; Figure 1b). In this study area, two environments were characterized: a Forest composed of sclerophyllous forest patches and scrubs of Acacia caven (Molina) Molina and a second environment devoid of arboreal vegetation and covered mainly by annual poaceae and isolated specimens of A. caven.
The second study area was located in Cerro Huechuraba (343,367 E, 6,308,994 S) with an approximate inspection area of 13 ha (Figure 1c); at 3 km north of Route 70 (in the 1,700 block of Américo Vespucio Ave.) and 1.5 km east of Route 52 (in the 7,500 block of San Martin Ave.) toward the north of the Huechuraba urban zone, defined as an urban housing area according to the Communal Regulatory Plan. This last area has an important anthropic intervention, due to the presence of houses in the periphery and stables, grazing areas in the study area. Only this last one has flora and wild fauna. In this area, three environments were observed: Forest formed by small vegetational patches of A. caven, a second environment of wild allochthonous of dense patches of Rubus ulmifolius Schott 1818, Loasa tricolor Ker Gawl. 1822 and A. caven, and a third environment laking arboreal vegetation and covered mainly by annual poaceae (Figure 1d).
Geographical coordinates are expressed in UTM WGS84 19H.
2.2. Sampling
Collection was performed during the spring of November 2016, using stratified random sampling with eleven transects of 200 m length by 2 m width, with a pedestrian path at low speed for two specialists in each area for a minimum of 30 min by transect. To confirm inhabitation of burrows, a flexible endoscope with a video microcamera with a lighting source connected by fiber optic to a screen, which allowed insertion of the fiber optic cable inside the burrow for inspection, was used. A manual collection was performed (Sorensen, Coddington, & Schaft, 2002; Upton, 1991), and the individuals were stored in 95% ethanol prior to labeling, and later deposited in the arachnological collection of the Museum of Zoology of the University of Concepción (MZUC‐UCCC), Concepción, Chile and National Museum of Natural History (MNHN), Santiago, Chile. Collects were authorized by the Servicio Agrícola y Ganadero, Exempt Resolution No. 6382/2016 Metropolitan SAG.
In order to obtain data of the biology and current distribution of G. rosea, additional collects and observations were made throughout the potential distribution of the species. (29°S and 40°S) in Chile, between 2016 and 2018, and the collections of MNHN and UCCC‐UDEC were revised (see Data accessibility).
2.3. Breeding
30 specimens were raised in glass boxes of 30 × 30 × 30 cm. Artificial burrows attached to glass were built for observation. All containers had a substrate of soil, Sphagnum sp. and water provision. They were fed once every 2 weeks mainly with cockroaches (Blatta sp.), crickets (Gryllidae), Tenebrio sp., and Zophobas sp. Temperature in laboratory varied between 16°C and 34°C. Behaviors were studied by direct observations and registered by notes and photographs.
2.4. Taxonomy
For identification of theraphosids, the morphology of the tibial processes by Montes de Oca, D'Elía, and Pérez‐Miles (2016) and Montenegro, Aguilera, and Casanueva (2018), the palpal bulb structure by Bertani (2000), the spermatheca morphology by Schiapelli and Gerschman (1961), and the urticating setae by Cooke, Roth, and Miller (1972) were used. Identifying images were obtained with a stereoscopic magnifier Motic SMZ‐140 and ZeissStemi SR with its supplement for a Nikon Coolpix P600 camera.
2.5. Statistics
In order to quantify Grammostola rosea and the other Theraphosidae in the study sites, a diversity analysis was performed using the computer program PAST 3.16 (Hammer, Harper, & Ryan, 2001) to determine S richness, _H_′ diversity according to Shannon–Weaver (Shannon & Weaver, 1949), and _J_′ uniformity according to Simpson (1949). The Shannon–Weaver and Simpson indices were used because they allow comparisons of Araneofauna in terms of richness proportional to abundance, and evenness of abundance among the species in the two study areas. Moreover, these indices are frequently used in the literature (Jaksic, 2001) and therefore facilitate comparisons with similar studies (Aguilera, Casanueva, & Hernández, 2006). In order to compare alpha biodiversity estimators between both study sites, a comparison test was performed with 1,000 iterations in the PAST program, based on the bootstrapping technique (Manly, 1997). To evaluate the degree of similarity of tarantulas between the two study areas, the Jaccard similarity coefficient was used based on presence/absence taxa records. In order to evaluate the existence of groupings according to localities and/or vegetation formation, a cluster analysis was performed using Jaccard similarity values. For this conglomerate, the abundance data of each transect were analyzed and the unweighted pair‐group method using arithmetic averages (UPGMA) was used (Sokal & Rohlf, 1995). Species accumulation curve was calculated (Collwel & Coddington, 1994; Jimenez‐Valverde & Hortal, 2003) to evaluate the information collected in the field and also to determine the efficiency of the characterization of the influence area. Additionally, density of species per m2 was also estimated for each study area.
3. RESULTS
A total of 86 individuals were recorded from the transect sampling, 30 in the Huechuraba area and 56 in Panul Park, belonging to Grammostola rosea, Homoeomma chilensis Montenegro & Aguilera, 2018 and one Euathlus sp. (Table 1). In the Huechuraba area, only G. rosea and Euathlus sp. were recorded with a relative abundance of 96.7% and 3.3%, respectively, while in Panul Park, 96.4% abundance of G. rosea was observed, and Euathlus sp. and Homoeomma chilensis. represented 1.8% each with an estimated G. rosea density of 1,350 ind/ha for Panul Park, and 750 ind/ha for Cerro Huechuraba.
Table 1.
Number of individuals per study area and environments
| Environment | Transect | Grammostola rosea | Euathlus sp. | Homoeomma sp. |
|---|---|---|---|---|
| Forest | P1 | 10 | 0 | 0 |
| Forest | P2 | 0 | 0 | 0 |
| Forest | P3 | 2 | 0 | 0 |
| Forest | P4 | 18 | 0 | 0 |
| Scrub/Poaceas | P5 | 4 | 0 | 0 |
| Scrub/Poaceas | P6 | 0 | 0 | 0 |
| Scrub/Poaceas | P7 | 0 | 0 | 0 |
| Scrub/Poaceas | P8 | 9 | 0 | 1 |
| Scrub/Poaceas | P9 | 4 | 1 | 0 |
| Scrub/Poaceas | P10 | 0 | 0 | 0 |
| Scrub/Poaceas | P11 | 7 | 0 | 0 |
| Forest | H1 | 4 | 0 | 0 |
| Forest | H2 | 4 | 0 | 0 |
| Scrub/Poaceas | H3 | 3 | 0 | 0 |
| Scrub/Poaceas | H4 | 3 | 0 | 0 |
| Allochthonous | H5 | 0 | 0 | 0 |
| Allochthonous | H6 | 1 | 0 | 0 |
| Scrub/Poaceas | H7 | 3 | 0 | 0 |
| Scrub/Poaceas | H8 | 6 | 0 | 0 |
| Scrub/Poaceas | H9 | 2 | 0 | 0 |
| Forest | H10 | 3 | 1 | 0 |
| Allochthonous | H11 | 0 | 0 | 0 |
In relation to the communal parameters (Table 2), it can be observed that Panul Park had a greater _H_′ diversity (H = 0.178) compared to Huechuraba (H = 0.146), but without significant statistical differences, while the J’ index indicates that the abundances are similarly distributed in both study areas, but since the _J_′ values are considerably low indicate a dominance given by G. rosea. On the other hand, G. rosea density values are greater in Panul Park than in Huechuraba. The cluster analysis (UPGMA + Jaccard) evaluated Theraphosidae similarity between the two study sites and did not show significant groupings according to environments, or they would be expected to occur by chance (Figure 2a). In the species accumulation analysis, it was observed that the asymptotic number of species was not reached in any of the two study areas, so objective criteria cannot be applied to determine whether the species inventory was sufficiently complete (Figure 2b).
Table 2.
Alpha biodiversity estimators in the two study areas
| Alfa biodiversity | Panul Park | Cerro Huechuraba |
|---|---|---|
| Richness S | 3 (2–3) | 2 (2–2) |
| Shannon H | 0.18 (0.08–0.36) | 0.14 (0.14–0.39) |
| Simpson J | 0.06 (0.03–0.16) | 0.06 (0.06–0.23) |
| Density (m2) G. rosea | 0.135 | 0.073 |
| Density (m2) Euathlus sp. | 0.003 | 0.003 |
| Density (m2) Homoeomma cf chilensis | 0.003 | 0 |
Figure 2.
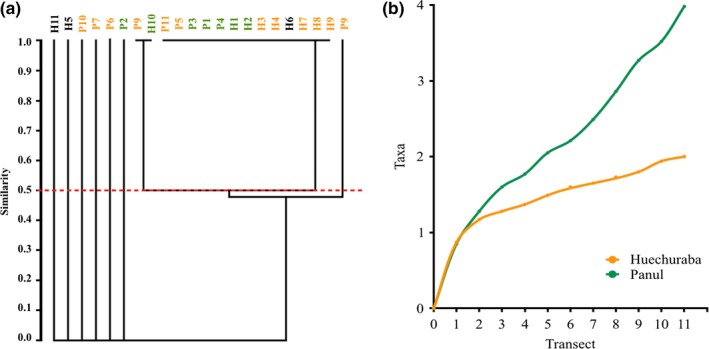
(a) Similarity phenogram obtained from UPGMA analysis based on presence or absence of theraphosid spider within the two study areas. H: Cerro Huechuraba; P: Panul Park; Green, Forest; Orange, Scrub and poaceas; Black, allochthonous (b) Species accumulation curve for both sampling areas
Figure 3.

Grammostola rosea. (a) rose variety. (b) brown variety. (c) Tibiae I apophysis, ventral view. (d) spermathecae. (e) right palp, retrolateral view. (f) right palp, prolateral view. (g) embolus apex, detail. LP: Lower prolateral keel; PB: prolateral branch; RB: retrolateral branch; UP: Upper prolateral keel
3.1. Natural history
Grammostola rosea (Figure 1a–g). This species lives at low altitudes, ranging between 0 and 1,500 m above sea level, with fragmented populations distributed between 28.5°S (Vallenar, Atacama Region) and 37.8°S (Angol, Biobio Region) (see data accessibility). In the field, it has been observed that males of G. rosea are living in burrows built directly into the ground with little stony substrates, between undergrowth and under large stones. Females build burrows of different depths, reaching a maximum of 40 cm. Generally, the burrows are a straight tunnel with one chamber at the bottom and sometimes a second chamber at two thirds of depth. The entrance frequently covered with silk. Sexual activity periods were estimated through the presence of living mature males in the field during the study period and occur mainly between September and March (spring and summer seasons in the Southern Hemisphere). A second activity period occurs between May and July. Egg‐sacs of G. rosea were observed throughout December to February.
In Laboratory, two females copulated in June. Six months after copulation (December), the females perform egg‐sac oviposition and spiderlings emerged 60 days after (February). As average, a total of 253 spiderlings were counted.
Regarding diet, this species consumes crickets of various species from the Gryllidae family as well as beetles, mainly Scarabidae. For predators, it was possible to observe wasps from the family Pompilidae (Pepsis sp. and Pompilocalus sp.), hunting spiders, and small lizards such as Liolaemus lemiscatus Gravenhorst (1838).
It has been observed that G. rosea has various color patterns, frequently with reddish to dark brown tones in the setae, cephalothorax, and abdomen (Figure 1a,b). Additionally, the characters of the spermatheca of the female, bulb, and tibial apophysis of the male for G. rosea are given in Figure 1c–g.
In particular in the areas of study, high burrow density was observed for some of the sectors sampled, with distances of no more than 1 m between burrows. In Panul Park, burrows were found among different vegetational formations, both in sclerophyllous forests and in environments devoid of vegetation or with annual isolated individual poaceae and A. caven, while in Cerro Huechuraba burrows were mainly found in vegetational formations of forest and scrub.
4. DISCUSSION
Theraphosidae spiders inhabiting areas with various degrees of anthropic disturbance respond in different ways to habitat changes, especially because they depend on the landscape for food and shelter (Goncalves‐Souza, Matallana, & Brescovit, 2008). This study showed theraphosid communities vary slightly according to conservation status of the area, which mainly affected richness and abundance of Theraphosidae, in alignment with Goncalves‐Souza et al. (2008). Comparison of diversity indices pointed to a slightly higher diversity in the Panul forest area, along with a marked dominance of G. rosea, accounting for 96% of the samples obtained. G. rosea, although sympatric with the found other two Theraphosidae species were dominant in the study areas, perhaps due to its way of life and the micro environmental requirements needed for its development. Notably, this species can build its burrow directly on the ground, unlike Euathlus and Homoeomma chilensis, which require a stony substrate for their burrows. It is that _G. rose_a has larger number of offspring than Euathlus and H. chilensis (Aguilera, 2015) and therefore explains the higher population density. Goncalves‐Souza et al. (2008) indicated that specific environmental factors such as microhabitats, closed vegetation as protection against predators, temperatures, humidity, and light intensity can explain variations of spider diversity among different habitats.
Diversity differed slightly between the two study areas (see _H_′ and _J_′ indices), with a notable abundance of G. rosea, although 46% lower in Cerro Huechuraba (anthropically intervened) than in Panul Forest. In both study areas, no significant cluster was observed when considering the present environments (see UPGMA analysis). This showed high similarity between the environments; therefore, these would behave as habitat units structurally homogeneous for the spiders, and G. rosea would not be restricted by the types of environments present (forest/scrub and cover of poaceas).
Despite this, in Cerro Huechuraba, the relative abundances were considerably lower in wild allochthonous environment than in the forest/scrub and cover of poaceas environments. Furthermore, density followed the same pattern indicated for relative abundance and was estimated at 1,350 ind/ha for Panul Park, and 750 ind/ha for Cerro Huechuraba.
As for the obtained species accumulation curve, it was not possible to reach asymptotic numbers of species. In this context, there are no objective criteria that determine when the Theraphosidae inventory in both study areas is sufficiently complete. As reported by Jimenez‐Valverde and Hortal (2003), the fact that it was not possible to reach asymptotic numbers could be due to temporal or spatial biases in the sampling effort distribution that could affect the curve shape. In the present study, collection was performed during spring; therefore, temporal bias was considered as spring and to a lesser extent summer is the ideal sampling times for Theraphosidae. This is on account of its seasonal life cycles, and these times correspond with a higher activity cycle, including reproductive and growing seasons (Aguilera, 2015). During these seasons, adult males and females are more easily found, along with younger populations (personal observations), therefore facilitating proper estimation of Theraphosidae population densities. Additionally, in order to avoid generating spatial bias of the sampling effort distribution a stratified random technique was employed, with a greater sampling effort in those strata that presented conditions of specific environments or microhabitats suitable for the presence of Theraphosidae and also to maximize the probability to inventory all species. It is important to mention that it has been observed that local distribution of Theraphosidae population can be fragmented in other areas of the country and that even though the sampling sites are adequated, these spiders may not be registered. For this reason, it is recommended to perform samplings in more extensive areas and thus increase the possibility of registering these fragment distributions.
Although possible errors associated with the species accumulation curve were considered, an initial exponential growth was not possible, neither an asymptotic number of species. This fact has been observed in other studies of spider diversity, in which the quality of spider inventory is evaluated and subsequently it is observed that it is not possible to register all species, causing accumulation curves to vary greatly from the asymptote (Brennan, Majer, & Reygaert, 1999; Coddington, Young, & Coyle, 1996; Edwards, 1993; Jimenez‐Valverde & Hortal, 2003; Samu & Lövei, 1995; Sorensen et al., 2002; Toti, Coyle, & Miller, 2000).
The present work confirms the presence of G. rosea in the Metropolitan Region, Chile, specifically in the eastern zone in the Andean foothills and associated with a type of sclerophyllous forest vegetation with Acacia scrub. The largest number of individuals was found on the slopes or plains at low altitude, less than 700 meters above sea level, with population numbers decreasing as altitude increases (see altitude in data accessibility). G. rosea has been cited for several cities in Chile, in the northern zone of the IV Region of Coquimbo (Coquimbo), central zone for the V Region of Valparaíso (Valparaíso) and Metropolitan Region (Santiago), and in the southern zone from the VI Region to the VIII Region of Bio Bio(Talcahuano) (Aguilera, 2015; Legendre & Calderón, 1984; Pocock, 1903). This work establishes the northern limit of the 28.5°S Atacama Region (Vallenar) and the southern limit of 37.8°S of the Biobio Region (Angol) for the studied species. This wide distribution of G. rosea facilitates evaluating the state of the different populations throughout the country, with studies of the genetic structure of each population, to analyze the distribution of inter‐ and intranatural population variability. Additionally, future studies could examine the processes involved, to later infer regarding relationships among different entities, as well as deterministic evolutionary mechanisms (natural selection, migration and mutation) and stochastic (genetic drift), as well as demographic processes (changes in population size) involved in the maintenance of variability in a certain group of organisms. This context could facilitate interpretation of the adaptive strategies of these spiders or be used to implement management units in biodiversity conservation plans (Remis, 2011), and also analyze phylogenetic aspects to better understand the presence of this genus in Chile.
The methodology proposed here could be used in future records of Theraphosidae and especially for environmental impact studies and environmental baselines.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MAA and RM conceived the ideas and did the field trip and collect data; MAA and MEC designed methodology and analyzed the data. All authors wrote the manuscript and contributed critically to the drafts and gave final approval for publication.
ACKNOWLEDGMENTS
Publication funded by VRID No. 214.113.088‐1.0 University of Concepción and Aracno Inc. SpA.
Aguilera MA, Montenegro R V ., Casanueva ME. Impact of disturbed areas on Theraphosidae spiders diversity (Araneae) and first population data of Grammostola rosea (Walckenaer) in Panul Park. Ecol Evol. 2019;9:5802–5809. 10.1002/ece3.5163
DATA ACCESSIBILITY
Data available from Figshare Knowledge Repository: https://doi.org/10.6084/m9.figshare.6493454.v1.
REFERENCES
- Aguilera, M. A. (2015). Recopilación, sistematización de datos e información sobre especies de la Clase Arachnida regulados por el Reglamento (D.S. 05/1998 y sus modificaciones) de la Ley de Caza N °4.601, sustituida por la Ley Nº 19.473. Mediante licitación pública ID 612‐47‐L115, aprobáda por Resolución Exenta No4897 02‐07‐2015. Santiago, Chile: Servicio Agricola y Ganadero. [Google Scholar]
- Aguilera, M. A. , Casanueva, M. E. , & Hernández, C. E. (2006). Composición de la araneofauna en dos especies de árboles nativos_Peumus boldus_Mol. y_Luma apiculata_(D.C.) Burret en el parque botánico Pedro del río Zañartu (Hualpen), Concepción, VIII Región, Chile. Gayana (Concepción), 70, 176–185. 10.4067/S0717-65382006000200004 [DOI] [Google Scholar]
- Argañaraz, C. , & Gleiser, R. M. (2017). Does urbanization have positive or negative effects on Crab spider (Araneae: Thomisidae) diversity? Zoologia, 34, 1–8. [Google Scholar]
- Bertani, R. (2000). Male palpal bulbs and homologous features in Theraphosinae (Araneae, Theraphosidae). Journal of Arachnology, 28(1), 29–42. 10.1636/0161-8202(2000)028[0029:MPBAHF]2.0.CO;2 [DOI] [Google Scholar]
- Bertani, R. (2001). Revision, cladistic analysis, and zoogeography of Vitalius, Nhandu, and Proshapalopus; with notes on other Theraphosine Genera (Araneae, Theraphosidae). Arquivos De Zoologia (São Paulo), 36(3), 92 10.11606/issn.2176-7793.v36i3p265-356 [DOI] [Google Scholar]
- Brennan, K. E. C. , Majer, J. D. , & Reygaert, N. (1999). Determination of an optimal pitfall trap size for sampling spiders in a Western Australian Jarrah Forest. Journal of Insect Conservation, 3(4), 297–307. 10.1023/A:1009682527012 [DOI] [Google Scholar]
- Cardoso, P. , Pekár, S. , Jocqué, R. , & Coddington, J. A. (2011). Global patterns of guild composition and functional diversity of spiders. PLoS ONE, 6(6), e21710 10.1371/journal.pone.0021710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington, J. , Young, L. H. , & Coyle, F. A. (1996). Estimating spider species richness in a southern Appalachian cove hardwood forest. Journal of Arachnology, 24, 111–128. [Google Scholar]
- COFODEP (2015). Áreas de preservación ecológica ambiental de la pre cordillera, comuna de La Florida: Anexo 3 Fauna. La Florida, Santiago, Chile: S.G.A. [Google Scholar]
- Coleman, D. C. , Crossley, D. A. , & Hendrix, P. F. (2004). Fundamentals of solil ecology, 2nd ed London, UK: Elsevier Academic Press. [Google Scholar]
- Collwel, R. , & Coddington, J. (1994). Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London B, 345, 101–118. [DOI] [PubMed] [Google Scholar]
- Cooke, J. A. L. , Roth, V. D. , & Miller, F. H. (1972). The urticating hairs of Theraphosid spider. American Museum Novitates, 2498, 1–43. [Google Scholar]
- Decaëns, T. , Jiménez, J. J. , Gioia, C. , Measey, G. J. , & Lavelle, P. (2006). The values of soil animals for conservation biology. European Journal of Soil Biology, 42(Supplement 1), S23–S38. 10.1016/j.ejsobi.2006.07.001 [DOI] [Google Scholar]
- Duelli, P. , & Obrist, M. K. (1998). In search of the best correlates for local organismal biodiversity in cultivated areas. Biodiversity & Conservation, 7(3), 297–309. 10.1023/A:1008873510817 [DOI] [Google Scholar]
- Edwards, R. L. (1993). Can the species richness of spiders be determined? Psyche, 100(3–4), 185–208. 10.1155/1993/20674 [DOI] [Google Scholar]
- Ferretti, N. , Schwerdt, L. , Peralta, L. , Farina, J. , & Pompozzi, G. (2016). Nuevos datos de distribución de Grammostola burzaquensis Ibarra ‐Grasso, 1946 (Araneae, Theraphosidae) en el Sistema serrano de Tandilia. Historia Natural, 6(1), 75–82. [Google Scholar]
- Gesterra . (2011). Estudio de Impacto Ambiental “Proyecto Inmobiliario El Panul”: Fauna. Santiago, Chile: Sustentable S.A. [Google Scholar]
- Giller, K. E. , Beare, M. H. , Lavelle, P. , Izac, A. M. N. , & Swift, M. J. (1997). Agricultural intensification, soil biodiversity and agroecosystem function. Applied Soil Ecology, 6(1), 3–16. 10.1016/S0929-1393(96)00149-7 [DOI] [Google Scholar]
- Goncalves‐Souza, T. , Matallana, G. , & Brescovit, A. D. (2008). Effects of habitat fragmentation on the spider community (Arachnida: Araneae) in three Atlantic forest remnants in southeastern Brazil. Revista Ibérica De Aracnología, 16, 35–42. [Google Scholar]
- Greenstone, M. H. , & Sunderland, K. D. (1999). Why a symposium on spider agroecosystem now? Journal of Arachnology, 27, 267–269. [Google Scholar]
- Hammer, Ø. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 1–9. [Google Scholar]
- Harwood, J. D. , Sunderland, K. D. , & Symondson, W. O. C. (2004). Prey selection by linyphiid spiders: Molecular tracking of the effects of alternative prey on rates of aphid consumption in the field. Molecular Ecology, 13(11), 3549–3560. 10.1111/j.1365-294X.2004.02331.x [DOI] [PubMed] [Google Scholar]
- Jaksic, F. (2001). Ecología de Comunidades. Santiago, Chile: Ediciones Universidad Católica de Chile. [Google Scholar]
- Jimenez‐Valverde, A. , & Hortal, J. (2003). Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Revista Ibérica De Aracnología, 8, 151–161. [Google Scholar]
- Legendre, R. , & Calderón, G. R. (1984). Liste systématique des araignées mygalomorphes du Chili. Bulletin Du Muséum National D'histoire Naturelle De Paris, 4(6A), 1021–1065. [Google Scholar]
- Lo‐Man‐Hung, N. F. , Marichal, R. , Candiani, D. F. , Carvalho, L. S. , Indicatti, R. P. , Bonaldo, A. B. , … Lavelle, P. (2011). Impact of different land management on soil spiders (Arachnida: Araneae) in two Amazonian areas of Brazil and Colombia. Journal of Arachnology, 39(2), 296–302. 10.1636/CP10-89.1 [DOI] [Google Scholar]
- Maloney, D. , Drummond, F. A. , & Alford, R. (2003). Spider predation in agroecosystems: Can spiders effectively control pest populations? Technical Bulletin: Maine Agricultural and Forest Experiments Station, 190, 1–32. [Google Scholar]
- Manly, B. F. J. (1997). Randomization, bootstrap and Monte Carlo methods in Biology. London, UK: Chapman and Hall. [Google Scholar]
- Mann, G. (1964). Compendio de Zoología I. Ecología y Biogeografía. Santiago, Chile: Centro de Investigación Zoologica. Universidad de Chile. [Google Scholar]
- Marc, P. , Canard, A. , & Ysnel, F. (1999). Spiders (Araneae) useful for pest limitation and bioindication. Agriculture, Ecosystems & Environment, 74(1), 229–273. 10.1016/S0167-8809(99)00038-9 [DOI] [Google Scholar]
- Montenegro, R. , Aguilera, A. M. , & Casanueva, C. M. E. (2018). First record of _Homoeomma_Ausserer, 1871 in Chile and description of two new species. Spixiana, 41(1), 13–25. [Google Scholar]
- Montes de Oca, L. , D'Elía, G. , & Pérez‐Miles, F. (2016). An integrative approach for species delimitation in the spider genus Grammostola (Theraphosidae, Mygalomorphae). Zoologica Scripta, 45(3), 322–333. 10.1111/zsc.12152 [DOI] [Google Scholar]
- Nyffeler, M. , & Sunderland, K. D. (2003). Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and US studies. Agriculture, Ecosystems & Environment, 95(2), 579–612. 10.1016/S0167-8809(02)00181-0 [DOI] [Google Scholar]
- ODEPA . (2017). Oficina de estudios y políticas agrarias: Estadisticas de exportación Araña pollito (Migalomorfas, suborden Araneae) Código SACH 01069020. Período anual desde 1998 hasta 2017. Retrieved from http://www.odepa.cl.
- Pickard‐Cambridge, F. O. (1897). Arachnida. Araneida. Biologia CentraliAmericana Zoology London, 1, 225–232. [Google Scholar]
- Pocock, R. I. (1903). On some genera and species of South‐American Aviculariidae. Annals and Magazine of Natural History, 7(11), 81–115. [Google Scholar]
- Remis, M. I. (2011). Análisis de la estructura poblacional. BAG. Journal of Basic and Applied Genetics, 22(1), 1–6. [Google Scholar]
- Samu, F. , & Lövei, G. L. (1995). Species richness of a spider community (Araneae): Extrapolation from simulated increasing sampling effort. EJE, 92(4), 633–638. [Google Scholar]
- Schiapelli, R. D. , & Gerschman, B. (1961). Las especies del género Grammostola Simon 1892, en la República Argentina (Araneae, Theraphosidae). Actas Y Trabajos Del Congreso Sudamericano De Zoologia, La Plata I (La Plata, 1959) 3, 199–208. [Google Scholar]
- Shannon, C. E. , & Weaver, W. (1949). The mathematical theory of communication. Champaign, IL: University of Illinois Press, Urbana. [Google Scholar]
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688 10.1038/163688a0 [DOI] [Google Scholar]
- Sokal, R. , & Rohlf, J. (1995). Biometría: The principles and practice of statistics in biological research. San Francisco, CA: W. H. Freeman. [Google Scholar]
- Sorensen, L. , Coddington, J. , & Schaft, N. (2002). Inventorying and estimating subcanopy spiders diversity using semiquantitative sampling methods in an Afromontane forest. Environmental Entomology, 31(2), 319–330. [Google Scholar]
- Torma, A. , Gallé, R. , & Bozsó, M. (2014). Effects of habitat and landscape characteristics on the arthropod assemblages (Araneae, Orthoptera, Heteroptera) of sand grassland remnants in Southern Hungary. Agriculture, Ecosystems & Environment, 196, 42–50. 10.1016/j.agee.2014.06.021. [DOI] [Google Scholar]
- Toti, D. S. , Coyle, F. A. , & Miller, J. A. (2000). A structured inventory of Appalachian grass bald and heath bald spider assemblages and a test of species richness estimator performance. Journal of Arachnology, 28(3), 329–345. 10.1636/0161-8202(2000)028[0329:ASIOAG]2.0.CO;2 [DOI] [Google Scholar]
- Uetz, G. W. , Halaj, J. , & Cady, A. B. (1999). Guild Structure of Spiders in Major Crops. The Journal of Arachnology, 27(1), 270–280. [Google Scholar]
- Upton, M. S. (1991). Methods for collecting, preserving, and studying insects and allied forms. Brisbane, QLD: Australian Entomological Society. [Google Scholar]
- Willett, T. R. (2001). Spiders and other arthropods as indicators in old‐growth versus logged redwood stands. Restoration Ecology, 9(4), 410–420. 10.1046/j.1526-100X.2001.94010.x [DOI] [Google Scholar]
- Wolters, V. (2001). Biodiversity of soil animals and its function. European Journal of Soil Biology, 37(4), 221–227. 10.1016/S1164-5563(01)01088-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from Figshare Knowledge Repository: https://doi.org/10.6084/m9.figshare.6493454.v1.