Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken (original) (raw)
. Author manuscript; available in PMC: 2019 Nov 12.
Published in final edited form as: Clin Oncol (R Coll Radiol). 2019 Apr 19;31(7):407–415. doi: 10.1016/j.clon.2019.04.001
Abstract
FLASH radiotherapy (FLASH-RT) is a technology that could modify the way radiotherapy is delivered in the future. This technique involves the ultra-fast delivery of radiotherapy at dose rates several orders of magnitude higher than those currently used in routine clinical practice. This very short time of exposure leads to the striking observation of relative protection of normal tissues that are exposed to FLASH-RT as compared with conventional dose rate radiotherapy. Here we summarise the current knowledge about the FLASH effect and provide a synthesis of the observations that have been reported on various experimental animal models (mice, zebrafish, pig, cats), various organs (lung, gut, brain, skin) and by various groups across 40 years of research. We also propose possible mechanisms for the FLASH effect, as well as possible paths for clinical application.
Keywords: Differential effect, FLASH radiotherapy, normal tissue protection, oxygen
Introduction
Delivering high curative radiation doses to tumours depends on the ability to spare the involved normal tissues from the harmful effects of ionising radiation. During the last 100 years, both fractionation and precise volume optimisation have emerged as powerful tools to increase the differential radiation effect between tumours and normal tissues. In the last decade, important advances in image-guided radiotherapy, and in precision, increased significantly the rate of patients cured and free of recurrences. However, an important proportion of tumours remain radioresistant to conventional radiotherapy delivered at tolerance doses for involved normal tissues, defining a major unmet clinical need. The development of novel approaches to further optimise radiotherapy is needed, and promising options are coming from targeted therapies and biomodulatory agents [1,2]. Despite certain progress, other advancements in radiation delivery have been slow to materialise, and today most radiation therapy devices still use the same technology of waveguide acceleration as half a century ago. In fact, other opportunities to directly improve the biological efficacy of radiation therapy may have been under explored, including relatively straightforward options involving dose rate modulation. Brachytherapy using various dose rates has been very successful for treating certain types of tumour. By contrast, very high external beam dose rates have been less explored and underutilised, prompting the subject of this review.
FLASH-RT: when the Duration of Exposure Really Matters
One such advancement in beam delivery is termed FLASH radiotherapy (FLASH-RT), which involves the ultra-fast delivery of radiotherapeutic doses at dose rates several orders of magnitude higher than those currently used in routine clinical practice. This very short time of exposure leads to the striking observation of relative protection of various normal tissues when they are exposed to single doses of FLASH-RT, as compared with conventional dose rate radiotherapy; an effect that can be observed even when FLASH-RT was administered in a single fraction. Another clear clinical advantage of FLASH-RT derives from the very short time, which eliminates any effect of organ or tumour motion, provided targeting is well controlled. At the biological level, the reduced normal tissue toxicity induced has been named the FLASH effect. Notably, the FLASH effect was described as early as the 1970s for intestine and skin [3–5]. Interest in this development for the clinic was discussed concerning killing cells (normal and tumour) independent of oxygen tension and abrogating the problem of resistant hypoxic tumour cells at conventional dose rates. However, translation of these findings to the clinic did not materialise. It was considered that the total doses required to use up all local oxygen in fully oxic cells would be too high, the induced resistance was found only for cells already slightly hypoxic (implying a potential detrimental effect for tumour control) and the instantaneous dose rates were enormous and not widely available [6–8]. In addition, no tumour responses were studied. It took more than three decades for this phenomenon to be ‘rediscovered’ and further explored [9–14]. The robustness of the FLASH effect is validated by the fact that it has been reproduced in various animal models (mice, rat, zebrafish, pig, cats), various organs (lung, skin, gut, brain) and by various investigators across 40 years of research in radiobiology. Reproducibility is ensured by accurate dosimetric methodologies that were developed and calibrated for the precise determination of the delivered dose [15–17]. Three passive detectors, including thermoluminescent dosimeters, Gafchromic films and alanine pellets, composed this redundant dosimetric strategy and achieved reliable and consistent agreement (~3%). Today, and to ensure the proper development of FLASH-RT, it is of the utmost importance to precisely define the physical parameters able to trigger the FLASH effect in biological tissue. Although the field is in constant evolution and many parameters remain to be discovered, this review aims to provide a first operational definition of what precisely is FLASH-RT. We will start with a summary of the biological data collected over 40 years that constitute the FLASH effect, define the physical parameters required to obtain the FLASH effect and propose potential mechanisms associated with it.
Normal Tissue Response to FLASH-RT
An increased normal tissue tolerance to irradiation-delivered FLASH has been described in various experimental models (Table 1).
Table 1.
In vivo studies of FLASH response for various normal tissues
| Dose (Gy) at conventional dose rates | FLASH dose rate (Gy/s) | Dose modifying factor | System | Anaesthetic | Assay | Reference |
|---|---|---|---|---|---|---|
| Normal tissues | ||||||
| 11.9 | 17–83 | 1.13 | Mouse intestine | Nembutal | LD50/5 | [3] |
| 14.7 | 70–210 | 1.13–1.24 | Mouse intestine | ? | LD50/5 | [14] |
| 24 | 56–83 | 1.4 | Mouse foot skin | Sodium amytal | Early and late reactions | [4] |
| 50 | 17–170 | 1.36 | Mouse tail skin | None | Necrosis ND50 | [5] |
| 22–34 | 300 | ≥1.36 | Minipig and cat skin | General anaesthesia | Early and late reactions | [13] |
| 15–17 | 40 | 1.8 | Mouse lung | Ketamine/xylasine/acepromazine | Fibrosis | [9] |
| 10 | 100–106 | 1.4 | Mouse brain | Isoflurane | Memory | [10]Montay-Gruel et al. (in revision) |
Using the lung as a relevant model to investigate radiation-induced toxicity, C57BL6J mice were locally irradiated and the occurrence of pneumonitis and fibrosis was assessed after exposure to high single dose delivered FLASH (≥40 Gy/s) or conventional dose rates (≤0.03 Gy/s). Severe lung pneumonitis and fibrosis affected 100% of mice irradiated with 17 Gy at conventional dose rates; whereas mice were free of pneumonitis and fibrosis after similar doses of FLASH. Dose escalation up to 30 Gy FLASH was necessary to induce pneumonitis and fibrosis. In the normal lung, 17 Gy FLASH irradiation prevented transforming growth factor-β activation and acute apoptosis in bronchi and vessels [9].
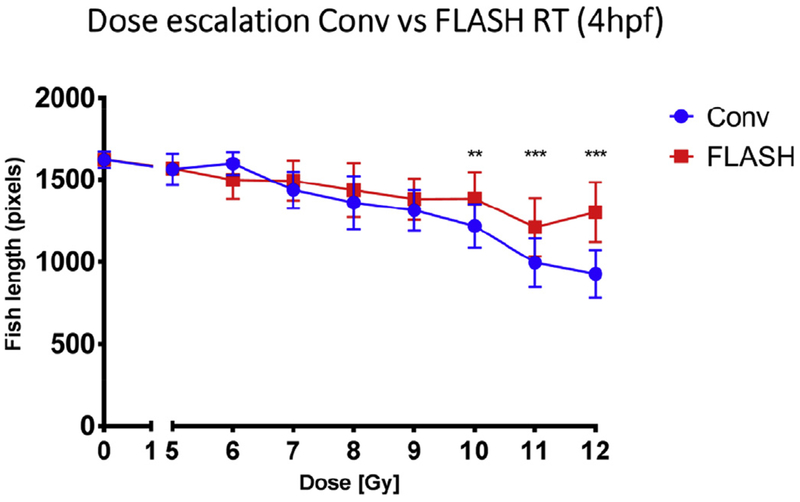
Next, using the brain as another clinically relevant model of radiation-induced toxicity in C57BL6 mice and neurocognition as a functional outcome, the physical parameters required to obtain the FLASH effect were investigated. A dose of 10 Gy FLASH-RT, delivered at a mean dose rate above 100 Gy/s, did not alter neurocognitive function of mice, whereas some cognitive impairment was observed at lower dose rates (60, 30 Gy/s) [10]. Moreover, the FLASH effect was reproduced after whole brain irradiation with ultra-high dose rates delivered with X-rays using synchrotron light [12] and was seen not only in late responding tissues, but also in the early responding gut after abdominal irradiation [3,14]. In addition, the FLASH effect was not restricted to rodent models, but was also observed in developing zebrafish embryos (Figure 1). These experiments showed that the benefits of FLASH over conventional dose rate exposure were especially prominent after exposure to high single doses of irradiation (> 10 Gy).
Fig 1.
Dose-escalation study. Irradiation was 4 hours post-fertilization (4hpf). Eggs were given 5–12 Gy delivered with FLASH or conventional dose rate irradiation. Radiation-induced alteration of zebrafish morphology was assessed 5 days post-fertilization (5dpf) by body length measurement. FLASH radiotherapy induced lower morphological alterations than conventional radiotherapy at doses above 10 Gy. Mean ± standard deviation and Mann–Whitney’s-test: *P < 0.05; **P < 0.01; ***P < 0.001 (n = 9–19 embryos/group).
To move closer to bona fide clinical applications, the advantages of FLASH-RT were confirmed in dose-escalation experiments comparing conventional dose rate and FLASH-RT on the skin of a mini-pig [18]. Single irradiation doses ranging from 22 to 34 Gy were delivered, with an applicator of 2.6 cm diameter to the same animal and at the same time. Using the absence of late skin necrosis at 9 months as a functional end point, 25 Gy delivered at a conventional dose rate yielded a similar outcome to 34 Gy delivered with FLASH-RT. This result suggests that the dose modifying factor for FLASH-RT is at least 1.36 compared with conventional dose rates. As discussed in the editorial by Harrington [19], the impact of FLASH-RT on a large irradiation field was then investigated, where a 31 Gy dose of FLASH-RT was administered to a 8 × 8 cm2 area on the skin of the mini-pig. This dose and volume led to the occurrence of vascular alterations (4 months after radiotherapy) and in-field ulceration (5–7 months after radiotherapy) that was transient and healed spontaneously 7.5 months after radiotherapy. Therefore, even at an extremely high single dose (31 Gy), this result suggests that the FLASH effect can still be observed after exposure of large volumes.
The biological data related to the FLASH effect produced at the CHUV (Lausanne University hospital) were obtained in very well-controlled dosimetric conditions with standardised physical parameters, summarised in Table 2. Further studies are ongoing to explore the impact of volume, number of pulses, pulse width, repetition frequency, but the data provided here can be considered as the minimal requirements to produce the FLASH effect.
Table 2.
Parameters with which the FLASH effect has been observed at the CHUV
| Model | Devices | Volume (ml) | Duration of radiotherapy (ms) | Dose delivered (Gy) | Mean dose rate (Gy/s) | Dose rate within the pulse (Gy/s) |
|---|---|---|---|---|---|---|
| Mice, zebrafish | Oriatron (eRT6) | <2 | <200 | >8 | >40 | >1.8 × 105 |
| Pig/cats | Oriatron (eRT6) | <12 | <200 | Up to 41 | 300–400 | >1.106 |
| Pig | Oriatron (eRT6) | 100 | <200 | 31 | 160 | 0.8 × 106 |
Tumour Response to FLASH-RT
The FLASH effect is defined by its non-toxic effect at the normal tissue level. However, if any clinical translation is foreseen, investigation of the tumour response to FLASH is required. The studies investigating FLASH on tumours are less numerous and this is an ongoing topic (Table 3).
Table 3.
In vivo studies of FLASH response in experimental tumour models
| Dose (Gy) at conventional dose rates | FLASH dose rate (Gy/s) | Dose modifying factor | System | Anaesthetic | Assay | Reference |
|---|---|---|---|---|---|---|
| 17–25 | 60 | ~1 | Human xenografts in mice | Ketamine/xylasine/acepromazine | Growth delay | [9] |
| 15–28 | 60 | ~1 | Orthotopic syngeneic lung model | Isoflurane | Growth delay | [9] |
Importantly, using subcutaneously xenografted tumours and orthotopic lung tumours, isoefficacy of FLASH-RT and conventional radiotherapy was shown [9]. The FLASH effect on the lung enabled safe dose escalation up to 28 Gy. Interestingly, 8 weeks after radiotherapy, 70% of the 28 Gy FLASH-irradiated mice were free of pulmonary tumours and lung complications, whereas only 20% of the 15 Gy mice irradiated at conventional dose rate were free of tumours, but exhibited severe pneumonitis and pre-fibrotic remodelling. These experiments were the first to show that FLASH-RT prevented acute and delayed complications, thereby enabling dose escalation that enhanced early anti-tumour efficacy. More experiments are currently underway to investigate the potential benefit of FLASH-RT on other tumour models using hypofractionated regimens delivered 24 h apart designed to approximate clinical scenarios.
Data to date indicate that in every instance the anti-tumour efficacy of FLASH-RT is equal to conventional dose rate radiotherapy when isodoses are used. These preclinical results are consistent with results obtained in the first veterinarian clinical trial conducted in feline patients with spontaneous squamous cell carcinomas of the nasal planum. Cats were treated with FLASH-RT in a dose-escalation study using single doses of irradiation ranging from 25 to 41 Gy and volumes ranging from 6 to 25 ml. Despite the high single doses administered, dose-limiting toxicity was not observed, and the maximal tolerated dose was not reached. Only minimal or mild mucosal and skin reactions were observed, without major disturbance of food intake and without subsequent late side-effects. The tumour control probability was high, compared with the literature, with a rate of 84% at 1 year. Further follow-up studies at the CHUV have begun in the form of a phase III trial conducted in cats with squamous cell carcinoma and treated with FLASH-RT. When comparing outcomes after a single dose of FLASH-RT with previous studies using fractionated radiotherapy, the tolerance/efficacy (therapeutic) ratio appeared markedly superior. The data obtained so far with normal tissue protection and tumour growth delay in animal systems suggest a potential role for FLASH in treating humans. Targeted studies investigating longer-term tumour control are still needed to capture late recurrence rates derived from surviving and slowly progressing tumour stem cells.
What Distinguishes FLASH-RT from Conventional Dose Rate Radiotherapy? A Primary Role for Oxygen
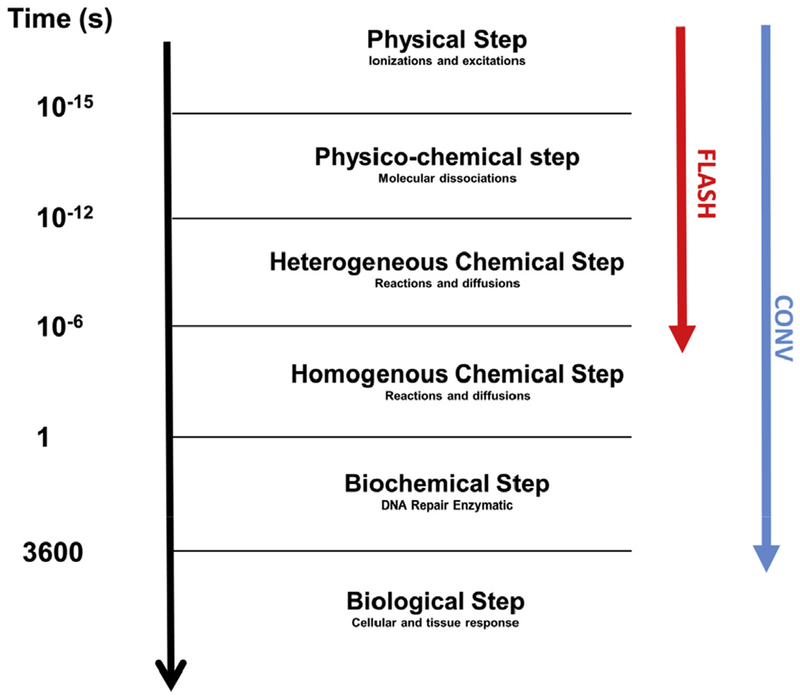
FLASH-RT uses ultra-rapid dose delivery (Table 2), which is the first and most obvious difference between FLASH-RT (microseconds) and conventional dose rate radiotherapy (minutes). These extremely high dose rates were postulated to be responsible for the very early divergence of radiochemical events that could distinguish FLASH-RT from more conventional dose rates used in the clinic (Figure 2).
Fig 2.
Chronology of physical, physicochemical, chemical, biochemical and biological events occuring after irradiation in the biological tissue. The difference between FLASH radiotherapy and radiotherapy delivered at the conventional dose rate is the duration of the exposure to the ionising radiation during the chemical step of the cascade. The chemical steps are highly dependent on dioxygen concentration within tissues.
Given the known role of oxygen in modulating radiosensitivity, we rationalised that FLASH-RT could cause a rapid consumption of local oxygen that would occur much faster than any tissue reoxygenation kinetics. Rapid depletion of oxygen would therefore elicit a transient radiation-induced hypoxia, as described in several past publications (Table 4). The FLASH effect has been investigated using bacteria, various mammalian cell lines and human lymphocytes (Table 4).
Table 4.
In vitro studies of FLASH response for cells at different oxygen levels
| Dose (Gy) at conventional dose rates* | FLASH dose rate (Gy/s)† | FLASH breakpoint dose (Gy)‡ | Dose modifying factor versus air§ | System | Conditions | Assay end point | Reference‡‡ |
|---|---|---|---|---|---|---|---|
| 250 | 1010 | 370 | 1.1 | Bacteria‖ | 11% oxygen | Colony, SF = 0.001** | [20] |
| 200 | 1.8 | 5.4% oxygen | |||||
| 80 | 2.7 | 2.4% oxygen | |||||
| 30 | 2.9 | 1% oxygen | |||||
| 60 | 3 × 108 | 40 | 2.0 | Bacteria¶ | 5% oxygen | Colony, SF = 0.01 | [21] |
| 30 | 2.3 | 2.4% oxygen | |||||
| 15 | 3.0 | 1% oxygen | |||||
| 9.5 | 107 | 7 | 1.8 | HeLa cells | 0.35% oxygen | Colony, SF = 0.01 | [22] |
| 12 | 1010 | 7.7 | 1.7 | P388 cells | 0.35% oxygen | TD50, SF = 0.001†† | [6] |
| 0–19 | 102–1010 | None | 1 | Mammalian cell lines | Air (21% oxygen) | Colonies | [22,23] |
| Colonies and SLDR | [24] | ||||||
| Colonies | [25]x | ||||||
| Colonies | [26] | ||||||
| Colonies | [27] | ||||||
| Colonies, G2 arrest, apoptosis | [28]p | ||||||
| [29,30] | |||||||
| Colonies, residual DNA dsb | [31] | ||||||
| Colonies, residual DNA dsb | |||||||
| 1–2 | 4.5 × 108 | None | 1 | Human lymphocytes | Air | Chromosome aberration repair time | [32]x |
| 1–5 | 106–2.5 × 108 | None | 1 | Human lymphocytes | Air | Unstable chromosome aberrations | [33] |
| 0.5–8 | 3.6 × 109 | None | 1 | Human–hamster cells | Air | Chromosome aberrations | [34] |
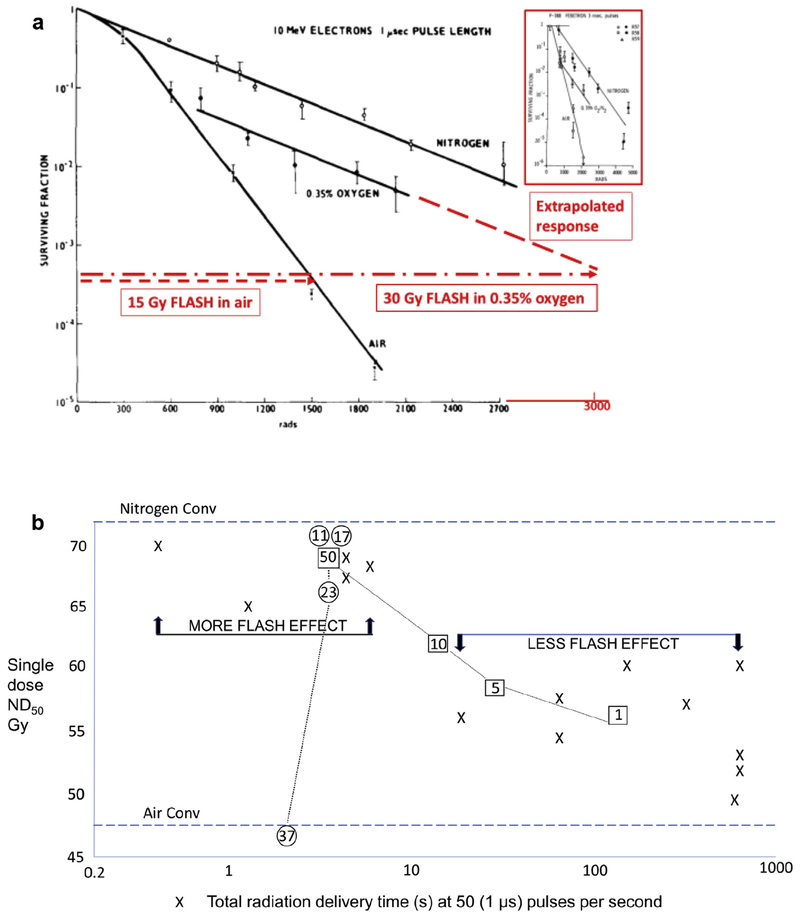
For aerobic cells (21% oxygen) irradiated in vitro, at least 13 publications of studies using single FLASH doses of electrons, protons, X-rays, ranging up to 19 Gy, have shown no FLASH effect. This is expected because of the high, non-physiological oxygen tension where oxygen depletion was insufficient (0.44 μM/Gy, [35]), requiring a dose as high as 90 Gy FLASH to deplete aerobic cells to anoxic cells. A diagrammatic representation of isoeffective cell survival to FLASH irradiation in air versus in 0.35% oxygen (~2 mm Hg), or in nitrogen, is shown in Figure 3a, based on primary published data. It underscores the importance of oxygen tension and provides an explanation of why the FLASH effect is observed only under physiological oxygen tension.
Fig 3.
(a) Effect of FLASH in vitro. Representation of isoeffective cell survival to FLASH irradiation in air versus in 0.35% oxygen (~2 mm Hg). The slope in the latter condition becomes parallel to that in nitrogen, i.e. the cells in 0.35% oxygen are depleted to zero oxygen by FLASH irradiation to result in a radioresistant anoxic response. The ratio of the slopes (0.35% oxygen versus air) in this example is ~2.6 and the ratio of isoeffective doses is ~ 30/15 = 2. The difference in these ratios is because of the presence of the breakpoint dose of ~7 Gy needed to deplete the oxygen from 0.35% to zero. The breakpoint dose is higher for higher initial levels of oxygen. Figure based on the results of Nias et al. [22] using Hela cells and 10 MeV linac electrons delivered in a single 1 μs pulse. Very similar results were reported by Berry and Stedeford [6], using P-388 murine leukaemia cell survival assayed in vivo after a single 3 ns pulse from a Febetron-706 400 keV electron generator (see inset graph). 3000 rads = 30 Gy. (b) Effect of FLASH delivery times at 50 (1 μs) pulses per second in vivo. Data taken from [5]. ND50 = dose to produce necrosis in 50% of murine tails by 6 weeks after irradiation. Each data point was derived from two to four experiments; standard errors are ~ ±3% of the mean values plotted. Numbers in circles: tail temperature at the time of irradiation, showing loss of the FLASH effect, presumably due to improved blood flow and higher oxygen tension in the tissue. Numbers in squares: number of (1 μs) pulses per second using the same intrapulse dose rate of 0.4 × 106 Gy/s, showing loss of the FLASH effect with protraction of overall dose delivery.
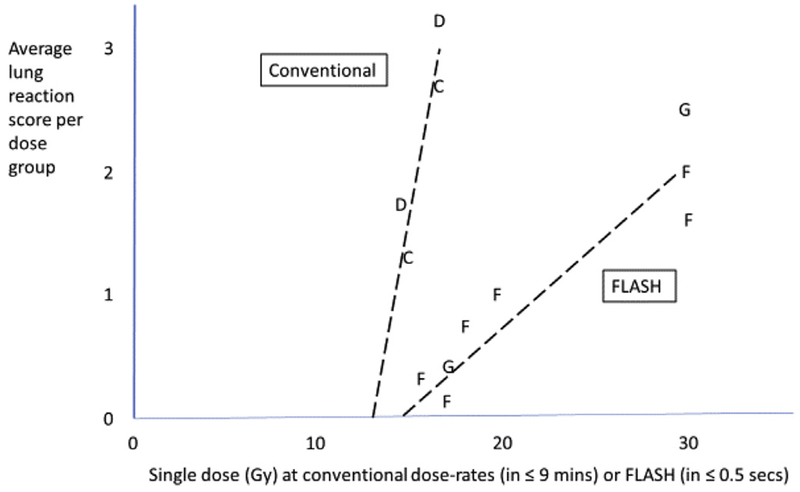
Interestingly, Hendry et al. [5] rescaled the graphical data of reaction scores presented in Favaudon et al. [9] and then plotted average values per group against the dose of conventional dose rate radiotherapy or FLASH-RT (Figure 4). The isoeffective dose ratio at an average score of 2 is ~30/15 = ~2.0. Note that if FLASH resistance starts at ~15 Gy, the target cells would initially be at 15 × [0.44 μM oxygen depleted per Gy] = ~6.6 μM oxygen. Also, the FLASH-RT slope is lower than for conventional dose rate radiotherapy by a factor ~2, as expected for anoxic versus aerobic slopes (Figure 3a), hence implicating oxygen depletion as a mechanism for the induced resistance. Thus, the role of oxygen seems to be a primary variable regulating the FLASH effect, and reinforces the need to specifically evaluate the critical physical parameters necessary for the FLASH effect, where depending on the end point, total doses must be sufficiently high and exposure durations must be sufficiently short to adequately consume local oxygen from a given volume of irradiated tissue.
Fig 4.
Average lung reaction scores per dose group = sum of percentage of animals × 1, 2, 3, 4 unitised scores of ±, +, ++, +++, read from [9, figure 1]. C,D, conventional dose rate, assay at 24 and 36 weeks; F,G, FLASH dose rate, assay at 24 and 36 weeks. Dashed lines are drawn for visual trend, no strict linear model is implied.
Physical parameters are essential for the FLASH effect and other influential aspects include the pulse size and pulse repetition frequency of the FLASH dose, when delivered as a series rather than a single pulse. In the mouse tail skin studies using 10 MeV electrons, the FLASH dose was delivered at 50 pulses/s, varying the pulse size and duration (Figure 3b) [5], whereas in the lung and brain studies [9,10] the FLASH dose was delivered in one to 10 pulses of 1.8–2 μs, suggesting that the dominant variable for response was the overall duration of the dose. In the older studies, the FLASH effect was observed for irradiation times <4.5 s, with a small influence of intrapulse dose rate, whereas in recent studies irradiation time less than 200 ms was required and a high intra-pulse dose rate.
If the oxygen depletion mechanism is considered speculatively for the neuroprotection effect reported after 10 Gy FLASH irradiation of the brain [10], it would follow that the initial oxygen tension of the target tissue may be lower than that for skin. Also, 100 Gy/s was found to be the lower limit for full preservation of memory functions after 10 Gy, and the neuroprotective effect was lost using ≤20 Gy/s, i.e. ≥0.5 s exposure duration. This is compatible in principle with the time scales discussed for skin. For interest, measured values of oxygen tension in brain vary widely with location, technique and anaesthetic use, but the maintenance of a good oxygenation is an highly controlled process in the brain, although some low values are reported, in particular for peri-tumoural human brain tissues [36,37].
The role of oxygen also provides a testable hypothesis that allows one to make certain predictions. Modulation of oxygen conditions by supplementation experiments might abolish the FLASH effect, whereas depletion may have little or no additional impact. Similar arguments can be brought forth to predict that the impact of scavengers would be marginal during FLASH-RT compared with conventional dose rate radiotherapy, as these compounds minimise the impact of oxygen and free radicals derived from the indirect radiolysis of water. Data obtained in zebrafish embryos incubated with millimolar levels of either n-acetyl-cysteine or amifostine support this hypothesis and showed little impact of the scavengers on FLASH-irradiated embryos, whereas each compound afforded significant improvement in embryo morphology after conventional dose rate radiotherapy, as compared with the absence of the scavengers (Bourhis et al., in revision [38]).
Another important feature is the oxygen tensions between normal tissue and tumours. Whereas normal tissues are thought to be homogeneous, tumours contain oxic, hypoxic and anoxic cell populations.
What Distinguishes FLASH-RT from Conventional Dose Rate Radiotherapy? A Role for Redox Biology
The physicochemical basis of the FLASH effect is also related to the instantaneous production of free radicals and to the inherent differences in redox and free radical chemistry that distinguish normal tissue from tumour tissue. For a given isodose, a given pulse of FLASH-RT deposits significantly more energy (i.e. eV/kg) and liberates significantly more electrons, which result in tens of thousands more ionisation events than from conventional dose rate radiotherapy. The resultant redox reactions of the reactive species following this ‘instantaneous’ FLASH pulse would propagate in the biological tissues and eventually decay in a series of biochemical and biophysical reactions that would be expected to take kinetically a different path than similar reactions following a conventional pulse of radiation. The end result is that normal tissues have lower pro-oxidant burdens during steady-state metabolism than tumours and eliminate FLASH-mediated free radicals much more effectively. The differences in redox metabolism between cancerous and normal tissues can thus be maximised. Fenton chemistry and peroxidation chain reactions persist longer in tumours, extending the half-life of many reactive species and reactions that promote the accumulation of oxidative injury and death. Importantly, FLASH-RT inundates the cellular systems that remove copious organic hydroperoxides generated during irradiation, which then provides a key explanation for why the FLASH effect is most readily demonstrated in vivo as robust normal tissue sparing. The differential rate of removal and decay of radiation-induced free radicals between normal tissue and tumours defines the beneficial therapeutic index we call the FLASH effect. The foregoing considerations and quantitative calculations in support of these ideas are the topic of a recent review (Spitz et al., in revision [39]).
Conclusions: FLASH-RT, a Potential Paradigm-changing Technology
- FLASH-RT enables higher doses to be tolerated by normal tissues (optimised when the mean dose rate ≥100 Gy/s).
- FLASH-RT enhances the differential effect between normal tissue reactions and tumour growth restraint.
- Experimental studies of tumour control after FLASH irradiation need to be carried out.
- Oxygen consumption probably mediates the FLASH effect.
- The biological mechanisms of FLASH also include redox biology, which provides further modification between normal tissue and tumour responses.
Currently, few devices are able to deliver ultra-high dose rate irradiation on large volumes of tissue. One of them is the experimental electron linac (6 MeV, FLASH-RT, eRT6) located at the CHUV [15]. In parallel, other groups are beginning to be equipped with preclinical devices in Europe and the USA [40,41]. These experimental devices will enable the studies required to enhance our understanding of the FLASH effect, but not without the significant technological shift necessary to transfer this novel radiotherapy technology to patients within the next few years. Proton therapy facilities might be optimised to deliver FLASH-RT. In addition, a new generation of linacs will be able to readily deliver FLASH radiation with photons and very-high energy electrons. Advancements include magnetic focusing of electron beams on the target providing highly improved conformality and the construction of more efficient and cheaper linacs for FLASH. The brief radiation exposure also largely obviates the need for techniques compensating for organ and tumour movement during irradiation. Prevailing limitations with many of these upcoming modalities persist, however, and are largely related to volume effects, where larger treatment volumes covered by defocused, dispersed or scanned beam approaches invariably compromise exposure durations and intrapulse dose rates; this reality reduces oxygen consumption rates to levels suboptimal with respect to second-order rate constants of free radical reactions involving oxygen that define prerequisite radiochemical conditions for the FLASH effect. Resolving these current limitations will require advanced technologies, which may expedite the advent of a new era of radiotherapy largely improved by less normal tissue complications.
Acknowledgments
The authors would like to thank the FLASH team of the CHUV: J. Bourhis, P. Montay-Gruel, B. Petit and J. Ollivier who were involved in the biology studies; C. Bailat, F. Bochud, J-F Germond, R. Moeckli, P. Gonçalves Jorge who were involved in the physics studies. We would like to thank the animal facilities of Epalinges and zebrafish platform of UNIL. The FLASH studies are supported by grants from the Swiss National fund FNS no. 31003A_156892, Swiss lead agency grant FNS/ANR CR32I3L_156924 and ISREC Foundation in Switzerland thanks to a Biltema donation (MCV) and by NINDS, United States grant NS089575 (CLL).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67(1):65–85. 10.3322/caac.21358. [DOI] [PubMed] [Google Scholar]
- [2].Bristow RG, Alexander B, Baumann M, Bratman SV, Brown JM, Camphausen K, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol 2018;19(5):e240–e251. 10.1016/S1470-2045(18)30096-2. [DOI] [PubMed] [Google Scholar]
- [3].Hornsey S, Bewley DK. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med 1971;19(5):479–483. [DOI] [PubMed] [Google Scholar]
- [4].Field SB, Bewley DK. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med 1974;26(3):259–267. [DOI] [PubMed] [Google Scholar]
- [5].Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res 1982;92(1):172–181. [PubMed] [Google Scholar]
- [6].Berry RJ, Stedeford JB. Reproductive survival of mammalian cells after irradiation at ultra-high dose-rates: further obser-vations and their importance for radiotherapy. Br J Radiol 1972;45(531):171–177. 10.1259/0007-1285-45-531-171. [DOI] [PubMed] [Google Scholar]
- [7].Berry RJ. Effects of radiation dose-rate from protracted, continuous irradiation to ultra-high dose-rates from pulsed accelerators. Br Med Bull 1973;29(1):44–47. [DOI] [PubMed] [Google Scholar]
- [8].Hall EJ, Brenner DJ. The dose-rate effect revisited: radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys 1991;21(6):1403–1414. [DOI] [PubMed] [Google Scholar]
- [9].Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6(245):245ra93 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- [10].Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017;124:365–369. 10.1016/j.radonc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- [11].Montay-Gruel P, Meziani L, Yakkala C, Vozenin MC. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br J Radiol 2019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-rays can trigger the FLASH effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 2018;129(3): 582–588. 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- [13].Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2018. 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- [14].Loo BW Jr, Schuler E, Lartey F, Rafat M, King GJ, Trovatin S, et al. Delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys 2017;98(2 sup):E16. [Google Scholar]
- [15].Jaccard M, Duran MT, Petersson K, Germond JF, Liger P, Vozenin MC, et al. High dose-per-pulse electron beam dosimetry: commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys 2018;45(2): 863–874. 10.1002/mp.12713. [DOI] [PubMed] [Google Scholar]
- [16].Jaccard M, Petersson K, Buchillier T, Germond JF, Duran MT, Vozenin MC, et al. High dose-per-pulse electron beam dosimetry: usability and dose-rate independence of EBT3 Gafchromic films. Med Phys 2017;44(2):725–735. 10.1002/mp.12066. [DOI] [PubMed] [Google Scholar]
- [17].Petersson K, Jaccard M, Germond JF, Buchillier T, Bochud F, Bourhis J, et al. High dose-per-pulse electron beam dosimetry - a model to correct for the ion recombination in the advanced Markus ionization chamber. Med Phys 2017;44(3): 1157–1167. 10.1002/mp.12111. [DOI] [PubMed] [Google Scholar]
- [18].Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of Flash radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2019;25(1): 35–42. 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- [19].Harrington KJ. Ultrahigh dose-rate radiotherapy: next steps for FLASH-RT. Clin Cancer Res 2018. 10.1158/1078-0432.CCR-18-1796. [DOI] [PubMed] [Google Scholar]
- [20].Epp ER, Weiss H, Santomasso A. The oxygen effect in bacterial cells irradiated with high-intensity pulsed electrons. Radiat Res 1968;34(2):320–325. [PubMed] [Google Scholar]
- [21].Phillips TL, Worsnop BR. Ultra-high dose-rate effects in radiosensitive bacteria. Int J Radiat Biol Relat Stud Phys Chem Med 1969;14(6):573–575. [DOI] [PubMed] [Google Scholar]
- [22].Nias AH, Swallow AJ, Keene JP, Hodgson BW. Effects of pulses of radiation on the survival of mammalian cells. Br J Radiol 1969;42(499):553 10.1259/0007-1285-42-499-553-b. [DOI] [PubMed] [Google Scholar]
- [23].Nias AH, Swallow AJ, Keene JP, Hodgson BW. Survival of HeLa cells from 10 nanosecond pulses of electrons. Int J Radiat Biol Relat Stud Phys Chem Med 1970;17(6):595–598. [DOI] [PubMed] [Google Scholar]
- [24].Schulz RJ, Nath R, Testa JR. The effects of ultra-high dose rates on survival and sublethal repair in Chinese-hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med 1978;33(1):81–88. [DOI] [PubMed] [Google Scholar]
- [25].Tillman C, Grafstrom G, Jonsson AC, Jonsson BA, Mercer I, Mattsson S, et al. Survival of mammalian cells exposed to ultrahigh dose rates from a laser-produced plasma x-ray source. Radiology 1999;213(3):860–865. 10.1148/radiology.213.3.r99dc13860. [DOI] [PubMed] [Google Scholar]
- [26].Shinohara K, Nakano H, Miyazaki N, Tago M, Kodama R. Effects of single-pulse (< or = 1 ps) X-rays from laser-produced plasmas on mammalian cells. J Radiat Res 2004;45(4): 509–514. [DOI] [PubMed] [Google Scholar]
- [27].Sorensen BS, Vestergaard A, Overgaard J, Praestegaard LH. Dependence of cell survival on instantaneous dose rate of a linear accelerator. Radiother Oncol 2011;101(1):223–225. 10.1016/j.radonc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- [28].Auer S, Hable V, Greubel C, Drexler GA, Schmid TE, Belka C, et al. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol 2011;6:139 10.1186/1748-717X-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laschinsky L, Baumann M, Beyreuther E, Enghardt W, Kaluza M, Karsch L, et al. Radiobiological effectiveness of laser accelerated electrons in comparison to electron beams from a conventional linear accelerator. J Radiat Res 2012;53(3): 395–403. [DOI] [PubMed] [Google Scholar]
- [30].Laschinsky L, Karsch L, Lessmann E, Oppelt M, Pawelke J, Richter C, et al. Radiobiological influence of megavoltage electron pulses of ultra-high pulse dose rate on normal tissue cells. Radiat Environ Biophys 2016;55(3):381–391. 10.1007/s00411-016-0652-7. [DOI] [PubMed] [Google Scholar]
- [31].Beyreuther E, Karsch L, Laschinsky L, Lessmann E, Naumburger D, Oppelt M, et al. Radiobiological response to ultra-short pulsed megavoltage electron beams of ultra-high pulse dose rate. Int J Radiat Biol 2015;91(8):643–652. 10.3109/09553002.2015.1043755. [DOI] [PubMed] [Google Scholar]
- [32].Prempree T, Michelsen A, Merz T. The repair time of chromosome breaks induced by pulsed x-rays on ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med 1969;15(6): 571–574. [DOI] [PubMed] [Google Scholar]
- [33].Purrott RJ, Reeder EJ. Chromosome aberration yields induced in human lymphocytes by 15 MeV electrons given at a conventional dose-rate and in microsecond pulses. Int J Radiat Biol Relat Stud Phys Chem Med 1977;31(3):251–256. [DOI] [PubMed] [Google Scholar]
- [34].Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, et al. The effectiveness of 20 mev protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiat Res 2011; 175(6):719–727. 10.1667/RR2465.1. [DOI] [PubMed] [Google Scholar]
- [35].Michaels HB. Oxygen depletion in irradiated aqueous-solutions containing electron affinic hypoxic cell radiosensitizers. Int J Radiat Oncol 1986;12(7):1055–1058. 10.1016/0360-3016(86)90224-5. [DOI] [PubMed] [Google Scholar]
- [36].Cruickshank GS, Rampling R. Peri-tumoural hypoxia in human brain: peroperative measurement of the tissue oxygen tension around malignant brain tumours. Acta Neurochir Suppl (Wien) 1994;60:375–377. [DOI] [PubMed] [Google Scholar]
- [37].Collingridge DR, Piepmeier JM, Rockwell S, Knisely JP. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol 1999;53(2):127–131. [DOI] [PubMed] [Google Scholar]
- [38].Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, Ollivier J, et al. Clinical translation of FLASH radiotherapy: why and how? Radiother Oncol 2019. [in press]. [DOI] [PubMed] [Google Scholar]
- [39].Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, et al. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol 2019. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schuler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys 2017;97(1):195–203. 10.1016/j.ijrobp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- [41].Lempart M, Blad B, Adrian G, Back S, Knoos T, Ceberg C, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother Oncol 2019. 10.1016/j.radonc.2019.01.031. [DOI] [PubMed] [Google Scholar]