What are the most accurate categories for mammal tarsus arrangement? A review with attention to South American Notoungulata and Litopterna (original) (raw)
Abstract
The arrangement of the tarsus has been used to differentiate afrotherian and laurasiatherian ungulates for more than a century, and it is often present in morphological matrices that include appendicular features. Traditionally, it has two states: (i) an alternating tarsus, where proximal elements are interlocked with central and distal elements positioned like the bricks of a wall; and (ii) a serial tarsus, where elements are not interlocked. Over the years, these states became synonymous with the presence or absence of an astragalocuboid contact. Within the South American order Notoungulata, a third disposition was recognized: the reversed alternating tarsus, associated with a calcaneonavicular contact. This state was considered to be a synapomorphy of ‘advanced’ Toxodontia families (Notohippidae, Leontiniidae and Toxodontidae), but a further inspection of its distribution shows that it occurs throughout Mammalia. Additionally, it overlaps the serial tarsus condition as originally defined, and it probably has no functional or phylogenetic significance. Calcaneonavicular and astragalocuboid contacts are non‐exclusive, and their presence within a species, genus or family is not constant. Serial and alternating imply movements of the articulations of the mid‐tarsus in the transverse axis, while reverse alternating refers to a small calcaneonavicular contact that sometimes occurs in a serial condition or to a significant displacement of the tarsal articulations in a different (proximodistal) axis. The proximodistal arrangement of the joints could be functionally significant. Two new states are observed and defined: (i) ‘flipped serial’, present in Macropodidae, in which the calcaneocuboid articulation is medially displaced and significantly larger than the astragalonavicular contact, but the relationships between proximal and central elements are one to one; and (ii) ‘distal cuboid’, an extreme proximodistal displacement of the astragalonavicular joint. Serial and alternating, as originally defined (i.e. without any reference to which bone contacts which), seem to be the best states for classifying tarsal arrangement though as the disposition of distal or central bones in relationship to proximal bones.
Keywords: Mammalia, Meridiungulata, morphology, phylogeny, tarsus
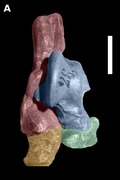
Dorsal view of right tarsus of Eutypotherium lehmannnistchei (MLP 12‐1701).
Introduction
The spatial arrangement of the ungulate basipodium was the subject of intense debate among zoological anatomists at the end of the XIX century and the beginning of the XX century (Cope, 1881, 1884a; Matthew, 1897, 1937; Osborn, 1898; Simpson, 1937). The serial (or taxeopod) arrangement and the alternating (or diplarthral) arrangement have been used to differentiate afrotherian and laurasiatherian ungulates, respectively, since then, although natural variation of this character is high and definitions of these conditions have varied (Cope, 1882; Thewissen, 1990). The serial arrangement refers to a condition in which bones are aligned proximodistally with one another with minimal interlocking between adjacent elements, while the alternating arrangement refers to the opposite condition, where the elements are positioned similar to the bricks of a wall (Cope, 1884a).
The difference between the serial and alternating states is not as clear as it may seem, especially in the carpus, where the alternating carpus was defined by Cope (1882) as one where the intermedium (middle proximal carpal; other names: lunar, semilunar, lunate) rests on the fourth distal carpal (unciform, hamate) and the scaphoid (radial plus central carpal in most eutherians) rests on the third distal carpal (magnum, capitate). However, the concept of ‘rest’ is vague; in the absence of a differentiated central carpal, the scaphoid usually articulates with the third distal carpal both in the serial and in the alternating conditions, with some exceptions, such as Elephas and Phenacodus. The absence of scaphoid/magnum articulation is rare, so an alternating or serial carpus cannot be determined by the articulations of isolated bones. This has led to confusion (Cope, 1884a; Matthew, 1897; Thewissen, 1990). Further contributing to this confusion is that, when the character state was defined, it was not known that the central carpal (os centrale) was incorporated into the scaphoid in several lineages; for example, Cope (1882: fig. 3) mistaken the central carpal with the second distal carpal (trapezoid) in Hyracoidea. For me, a clearer definition of an alternating carpus is one in which the second row of carpals has rotated toward the medial side relative to those of the first. This rotation was speculated by Cope (1882) to provide a double articulation that is mechanically more secure against dislocation or fracture. A serial carpus, on the contrary, favors movements among the joints. This definition is transferable to the tarsus.
In the tarsus, the original definitions of serial and alternating have not been used in several decades. Instead, they have been simplified: a serial tarsus is defined as one in which a proximal tarsal articulates distally with only one element (i.e. there are only astragalonavicular and calcaneocuboid contacts), and an alternating tarsus is defined as one with both astragalonavicular and astragalocuboid contact (Fig. 1; Bergqvist, 1996). In some cases, this can conflict with the original definitions of serial and alternating; for example, horses have a very small astragalocuboid contact, an alternating tarsus under this definition, but as there are minimal interlocking articulations, the tarsus is functionally serial (Hussain, 1975).
Figure 1.
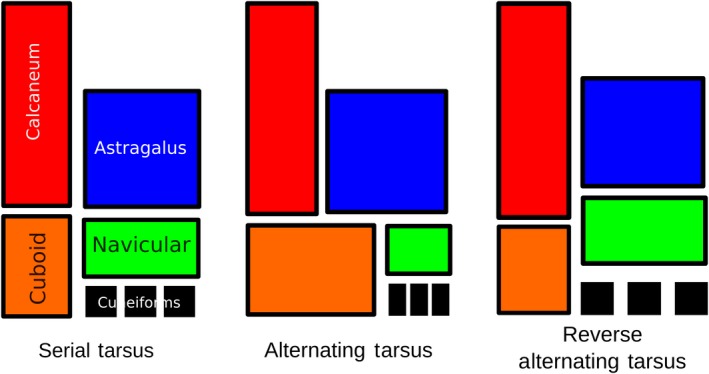
Outline of serial, alternating tarsal and previously proposed reverse alternating tarsal arrangements.
In recent decades, a new disposition was recognized in Notoungulata: the reversed alternating tarsus. This state was defined by Cifelli (1993) as the loss of astragalocuboid contact (characteristic of an alternating tarsus) and establishment of calcaneonavicular contact. He proposed it as a synapomorphy for ‘advanced’ Toxodontia (Notohippidae, Leontiniidae and Toxodontidae). Later, because it also occurs in some interatheriid Typotheria, it was described as a non‐exclusive trait of advanced toxodonts (Shockey et al. 2012). However, no known notoungulate presents an alternating tarsus (i.e. one with astragalocuboid contact); only the reversed alternating and the serial arrangements have been identified. Thus, in 2016, I presented an alternative definition for the reversed condition: an astragalonavicular joint located more proximal than the calcaneocuboid joint, with the result that the navicular contacts the calcaneus to some degree (Fig. 1; Lorente, 2016). In that work, the reverse alternating tarsus (as newly defined) was mapped onto the phylogenetic trees of Billet (2011) for Notoungulata and of Welker et al. (2015) for Eutheria to see how the state was distributed among Notoungulata and Litopterna. The reversed alternating tarsus turned out to be the predominant condition within Notoungulata (no notoungulate was found with an alternating tarsus), and the reversed alternating tarsus seemed to be the only condition present in Litopterna. However, further investigation of other orders of mammals revealed that: (i) the reversed alternating condition is far more widespread than it was previously thought; (ii) tarsal arrangement is complex and may require more than one character to accurately describe it; (iii) a reverse alternating tarsus cannot be defined in a way that is compatible with the definitions of serial and alternating dispositions because some animals with an alternating tarsus have an astragalonavicular joint more proximal (e.g. Artiodactyla). Additionally, the reverse alternating tarsus (as traditionally defined) is present in mammals with very different tarsal conditions that are probably not functionally equivalent. For example, in many notoungulates, astragalonavicular and calcaneocuboid articulations are basically at the same level along the proximodistal axis (though the navicular is slightly more proximal because of its convex shape), while in Litopterna and some more cursorial notoungulates, these two articulations are much further apart. In the absence of phylogenetic or functional significance, the reversed alternating tarsus has little utility as a distinct character state.
In this study, I try to separate those two reverse conditions and I take them with a different approach by taking movements into account. Transverse displacement between the articulations evolves less frequently and is more conservative within groups, while sagittal displacements and the presence or absence of articulation facets are more variable. The presence of calcaneonavicular or astragalocuboid facets is especially variable within groups, and happens without any change in the dispositions of the bones taking place. Although almost any transverse shift of the cuboid bone to the medial side results in an astragalocuboid facet, proximodistal displacements of the astragalonavicular joint do not always produce a calcaneonavicular facet. This can be due to a very small calcaneonavicular contact that is only evident when examining soft tissues or no contact between navicular and calcaneus at all (as occurs, e.g. in primates).
Materials and methods
Abbreviations
AMNH, American Museum of Natural History, New York, USA; LIEB‐PV, Paleovertebrates Collection of Laboratorio de Investigaciones en Evolución y Biodiversidad, Universidad Nacional de la Patagonia ‘San Juan Bosco’, provincia del Chubut, Argentina; MACN, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina; MACN‐A, Ameghino Collection of MACN; MACN‐Ma, Collection of Mastozoology of MACN; MLP, Museo de la Plata, La Plata, Argentina, Mastozoology and Vertebrate Paleontology collections; MPEF‐PV, Museo Paleontológico Egidio Feruglio, Paleontología de Vertebrados, Trelew, Argentina; MCNAM‐PV, Museo de Ciencias Naturales y Antropológicas J. C. Moyano, Paleontología de Vertebrados, Mendoza, Argentina; PVL, Instituto Miguel Lillo, Paleontología de Vertebrados, San Miguel de Tucumán, Argentina; SALMA, South American Land Mammal Ages; SANU, South American Native Ungulates.
The collections of Museo de La Plata and Museo Argentino de Ciencias Naturales were consulted, as well as several authors (Matthew, 1897; Sinclair, 1908; Chaffee, 1952; Cifelli, 1993; Bergqvist, 1996; Elissamburu, 2007; Croft & Anderson, 2008; Shockey & Anaya, 2008; Shockey et al. 2012; Vera, 2012; O'Leary et al. 2013).
Results and discussion
Notoungulata, Litopterna and the reversed alternating tarsus
Notoungulata and Litopterna were small to large‐sized herbivorous South American native ungulates. Several recent studies have presented strong molecular evidence that they are most closely related to Perissodactyla among extant orders (Buckley, 2015; Welker et al. 2015; Westbury et al. 2017). Despite their close relationship, Notoungulata and Litopterna are morphologically very different from one another. Notoungulates were the more diverse group. They occupied a wide variety of niches in the middle Cenozoic but, by the Pleistocene, there were only a few lineages remaining (Patterson & Pascual, 1972; Simpson, 1980; Bond, 1986; Cifelli, 1993; Croft, 1999; Shockey et al. 2007; Elissamburu, 2012). Their lophodont dentition was specialized very early in the group's evolution (Paleocene, de Muizon, 1992) , while their appendicular morphology remained very similar to archaic ungulates and carnivores even in very late taxa (e.g. Pleistocene Paedotherium). Early notoungulates probably had a generalist or fossorial mode of locomotion, and were a lot more like a civet or a weasel than an extant ungulate such as an artiodactyl or perissodactyl (Croft & Anderson, 2008; Lorente, 2015; Muñoz, 2017; Muñoz et al. 2017; Lorente et al. 2018).
Litopterna was second in taxonomic diversity among South American native ungulates, but they had opposite traits: early species were specialized unguligrades with low morphological disparity (Protolipterna, Sao Jose de Itaborai, Brasil. Cifelli, 1983, 1993; Bergqvist, 1996), but their dentition was still brachydont and bunodont. Their general aspect was more similar to horses, deer or the mara, a cursorial rodent (Dolichotis patagonica).
The original definition of reversed alternating tarsus was introduced in a context where the alternating disposition was supposed to be the plesiomorphic condition within ‘archaic’ ungulates, the hypothesized ancestors of notoungulates (Matthew, 1897). Originally, Cope (1884b) described the condition in ‘Condylarthra’ as serial, but their classification changed as the recognition of an alternating tarsus became associated with astragalocuboid articulation. For example, Ectonus (Piveteau, 1958) and Hyopsodus (Penkrot et al. 2008) have a small cuboid facet in the astragalus; they are classified as serial following Cope's original definition (i.e. transverse alignment) but as alternating based on the presence of astragalocuboid articulation. Periptychus was classified as serial in Cope's description although its astragalus has a large cuboid contact. This cuboid articulation was probably disregarded by Cope, who was more interested in the medial incursion of the cuboid, because it is lateral to the astragalus in Periptychus. Protoungulatum and some ‘archaic’ ungulates also have been described as having a cuboid facet on the astragalus, but this facet has been inferred without knowledge of the navicular or cuboid. This inference is complicated by many factors (see below), and there is a high likelihood that most ‘archaic’ ungulates described as having an alternating tarsus have a strictly serial tarsus following Cope's original definition. The most common problems with inferring a serial or alternating tarsus (or an astragalocuboid articulation) from isolated elements include the following.
- The possible presence of additional calcaneoastragalar facets in a position similar to that of the astragalocuboid facet in other taxa (e.g. Perissodactyla, Fig. 2C, pink shading). Tarsus may appear to be alternating, but it could be serial.
Figure 2.
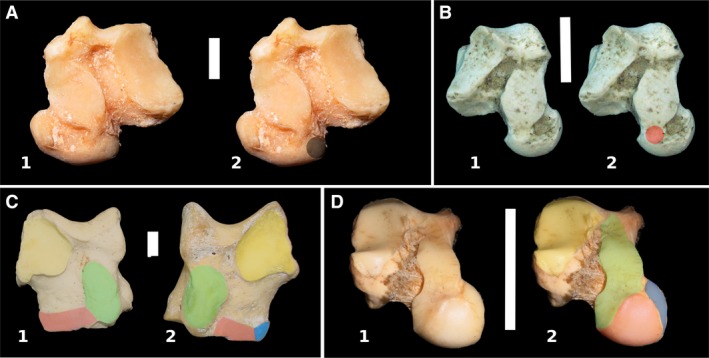
(A) Plantar view of right astragalus of Tremarctos ornatus (MLP 1.I.03.62); shading in the surface of contact of cuboid bone. (B) Plantar view of left astragalus of Notostylops sp. (LIEB‐PV 4016); shading in the space between navicular and sustentacular facets. (C) Plantar view of left astragalus of: (1) a juvenile of perissodactyl Tapirus terrestris (MLP 1); (2) an adult Tapirus terretris (MLP 1070). In yellow shading, ectal facet; in green shading, sustentacular facet; in pink shading, anterior astragalocalcaneal facet. Observe the disconnection between sustentacular and anterior astragalocalcaneal facet in the adult. (D) Left astragalus of Lagostomus maximus (MLP 1683). Plantar view, slightly oblique to better observe the facets. Facets as previous image; in soft dark blue shading, the facet for the sesamoid. Scale bar: 10 mm.
2. The absence of a discernible astragalocuboid facet in the astragalus even though there is an astragalocuboid articulation (e.g. Tremarctos, Periptychus. Fig. 2A, shading). Tarsus may appear to be serial, but its condition could be alternating.
3. The presence of a connection between navicular and sustentacular facets of the astragalus that may be interpreted as a different facet (either for the calcaneus or the cuboid). In more complete tarsi, this intermediate articulation does not appear to articulate with any bone (Fig. 2B, shading). It is usually present in highly mobile astragali and can be a surface of extension of the sustentacular facet. Tarsus could be described as alternating but is more likely serial. Also, the joint capsules of different articulation are confluent in early ontogenetic stages and separate as the bone develops, so such conditions can also vary with age (Scheuer & Black, 2004; Fig. 2C). This is an important factor in determining the final configuration of the tarsus and the carpus, and it has yet to be studied; the number of joints in living animals can be less than that expected by the number of bones involved.
4. The presence of a facet for a sesamoid bone between the astragalus and navicular that resembles an astragalar cuboid or navicular facet (e.g. Caviomorpha, Fig. 2D). Tarsus may appear to be alternating, but it is more likely serial.
5. In several mammals, especially those with a generalist mode of locomotion, astragalonavicular and calcaneocuboid joints are at the same level along the proximodistal axis, allowing great variation of contacts between the bones. This appears to be normal intraspecific variation rather than a pathological condition, and paleontological studies usually lack samples large enough to be able to see such polymorphic character states (e.g. Equus, Canis). Hussain (1975) found that six out of 11 Mesohippus specimens he studied had a calcaneonavicular articulation. Domestic horses, for example, most commonly have a small astragalocuboid articulation, but in a sample of fewer than 20 individuals, I observed all possible facet configurations: no astragalocuboid nor calcaneonavicular facets, both facets, and only one facet (either astragalocuboid or calcaneonavicular). In young horses with calcaneocuboid and calcaneonavicular articulations or astragalocuboid and astragalonavicular articulations, the facets are undifferentiated in the calcaneus or the astragalus, respectively.
The positional relationships among the calcaneus, astragalus, cuboid and navicular are very difficult to assess and, surprisingly, the navicular (rather than the astragalus) is the best element to make such inferences. The navicular presents distinct facets for the astragalus and the cuboid, the distal tarsals and, in some cases, also for the calcaneus (Fig. 3). The shape of those facets also gives a general idea of the distribution of the other tarsal elements. By contrast, several facets can be superposed or found in similar places on the astragalus and calcaneus, and as the cuboid and navicular share the same joint capsule, the astragalus does not always bear distinct articular facets for them.
Figure 3.
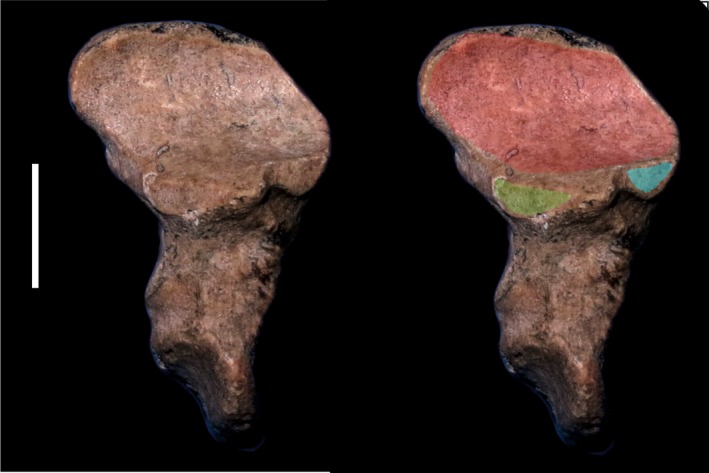
Proximal view of the left navicular of proterotheriid Eoauchenia (MLP 48‐XII‐16‐1). In light blue shading, sesamoid facet; in green shading, calcaneal facet; in red shading, astragalar facet. Scale bar: 10 mm.
In most animals with a calcaneonavicular contact, the calcaneocuboid and the astragalonavicular joints are at a similar distance between each other, and this contact is very small and probably incidental. In animals where the navicular is medial to the calcaneus, as in Litopterna, the presence of a calcaneonavicular articulation is variable; for example, in Macrauchenia, the calcaneus and navicular contact each other, but there is no clear facet on either bone. Perhaps some tissue separated them in life, but it is unknown. If the articulation was small enough, it could have been present in the articular cartilages of the synovial joint without leaving a trace in the bone. Calcaneonavicular contact is present in notoungulates and some perissodactyls and litopterns, but also in several Carnivora (e.g. Chrysocyon), Chiroptera, Rodentia (Fig. 4), Loxodonta (Smuts & Bezuidenhout, 1994), some primates (e.g. Lemur catta; Fig. 4), and ancient ‘insectivores’ and Dermoptera (Szalay & Lucas, 1996). The presence of a calcaneonavicular articulation facet seems to be independent of the displacement of the astragalonavicular joint to a more proximal position (compare Figs 4 and 5).
Figure 4.
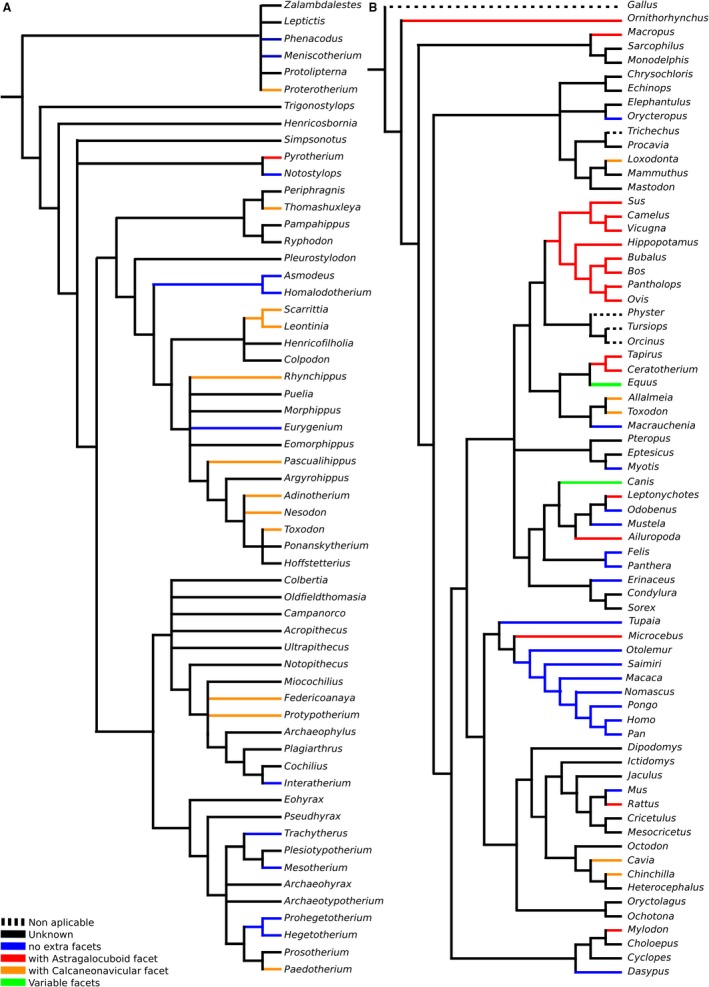
Presence of facets mapped over: (A) strict consensus cladogram from the analysis of Billet (2011, fig. 9); and (B) Bayesian consensus tree of COL1 protein sequence data, with chicken (Gallus) as outgroup from the analysis of Welker et al. (2015).
Figure 5.
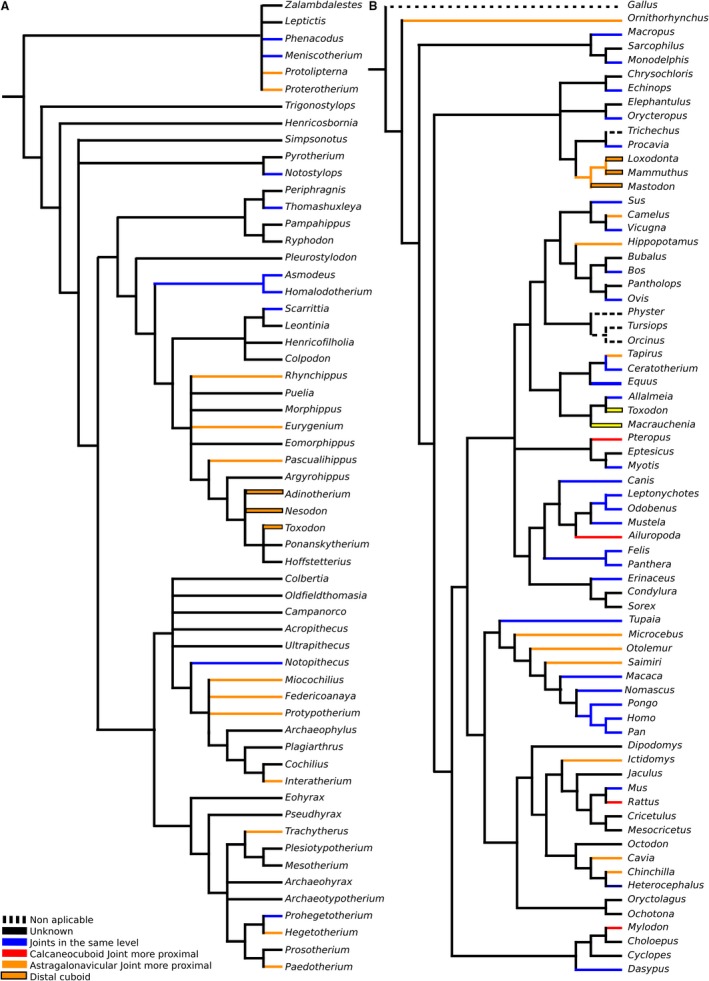
Displacement of joints in the proximodistal axis mapped over: (A) strict consensus cladogram from the analysis of Billet (2011, fig. 9); and (B) Bayesian consensus tree of COL1 protein sequence data, with chicken (Gallus) as outgroup from the analysis of Welker et al. (2015).
Although notoungulates can have a serial or reversed alternating tarsus, in most cases it is functionally serial, with minimal interlocking articulations (Fig. 6). Most notoungulates were plantigrade or digitigrade (Loomis, 1914; Shockey & Flynn, 2007; Elissamburu, 2010; Vera, 2012; Lorente et al. 2014; Lorente, 2015) and had a considerable amount of movement in the articulations (Lorente, 2015; Muñoz, 2017). The astragalar head is almost spherical in notoungulates, particularly in basal groups (Fig. 7A), allowing movements in the three axes. Notoungulates were very different from extant specialized ungulates.
Figure 6.
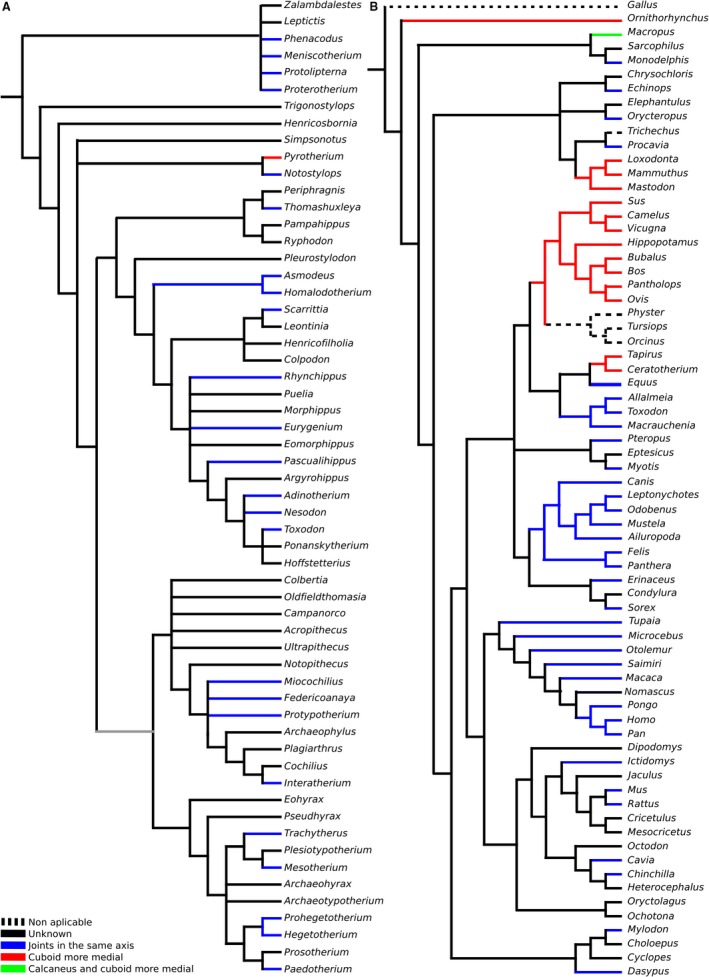
Displacement of joints in the transversal axis mapped over: (A) strict consensus cladogram from the analysis of Billet (2011, fig. 9); and (B) Bayesian consensus tree of COL1 protein sequence data, with chicken (Gallus) as outgroup from the analysis of Welker et al. (2015).
Figure 7.
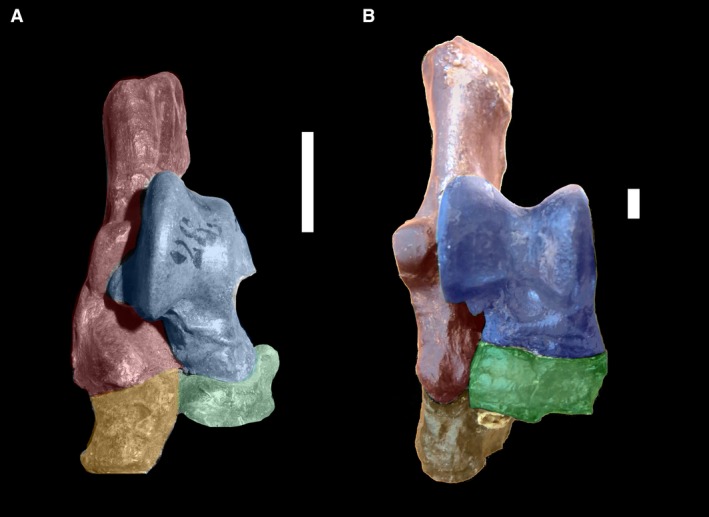
(A) Dorsal view of right tarsus of Eutypotherium lehmannnistchei (MLP 12‐1701). (B) Dorsal view of right tarsus of Theosodon sp. (MLP 700). In blue shading, astragalus; in green shading, navicular; in red shading, calcaneus; in yellow shading, cuboid. Scale bar: 10 mm.
The opposite was true of Litopterna, which were specialized unguligrades since very early in their evolution (early Eocene; Cifelli, 1983; Bergqvist, 1996). Litopterna had a tarsus in which the cuboid and calcaneus are clearly lateral to the navicular (half or more of the navicular is medial to the calcaneus; Fig. 7B), although a clear calcaneonavicular facet is not always present, making this condition distinct from the one in basal notoungulates. In Litopterna, the astragalonavicular joint does not have the enarthrosis condition present in notoungulates; instead, it is a hinge joint, a synovial joint that allows movement in only one plane, as can be inferred by the shape of the astragalar navicular facet. As navicular movements would be constrained to the sagittal plane, it would not have affected the calcaneus.
This condition was not unique to Litopterna; some ancient horses present a similar case. A more proximal astragalonavicular joint, in which the navicular and the calcaneus are side by side, is present, with or without calcaneonavicular facets, in Heptodon (Radinsky, 1965), Eomoropus (Osborn, 1913; Radinsky, 1964), Helaletes, Colodon and Hyracotherium (Holbrook, 2001). Holbrook (2001) noted that the presence of this state in Hyracotherium and Heptodon suggests that it is ancestral for Perissodactyla, but its absence in Homogalax, Cardiolophus, brontotheres and phenacodonts indicates it more likely is a derived condition within Perissodactyla. Phenacodontids, which are archaic ungulates, generally have a serial tarsus (e.g. Phenacodus, Tetraclaenodon). Hyracotherium shows a similar arrangement as Litopterna, with the astragalonavicular joint more proximal than the calcaneocuboid joint, but no facet (Wood et al. 2011). Cambaytherium, considered primitive perissodactyl, have been described as having an alternating tarsus, but cuboid and navicular bones of these taxa are unknown (Rose et al. 2014), and the condition is difficult to infer with the proximal elements only (see above).
A condition similar to that of Litopterna, in which the astragalonavicular joint is more proximally placed than the calcaneocuboid joint, is present in artiodactyls, some caviomorphs rodents, such as Lagostomus, specialized notoungulates (such as Miocene interatheriids and notohippids).
As all these animals share mobility restricted to the sagittal plane, I propose that the proximodistal distance between the calcaneocuboid and the astragalonavicular joints is a much more relevant functional character than the presence or absence of calcaneonavicular facets of the tarsus. (Litopterna, Caviomorpha, Notoungulata and Perissodactyla have a serial tarsus following the definition of Cope, while Artiodactyla has an alternating tarsus.) A more proximally located astragalonavicular joint in a mammal with a serial tarsus could help to provide integrity to the tarsus and to prevent dislocations in a different plane, as in an alternating tarsus. Most mammals with such an anteroposteriorly displaced tarsus are cursorial or graviportal, in several cases both. Some artiodactyls have both an alternating tarsus and a more proximally located astragalonavicular joint. Why do modern horses, which are heavy animals specialized for speed, lack both of these traits? Is the hypothesis about the preventive nature of displacement of the joints in any axis wrong or they have resolved the mechanical stress in the joints in another way? A major difference between the three‐first orders noted and horses is that the astragalonavicular joint is very mobile in the former whereas its mobility is restricted in horses (and in perissodactyls in general; Wood et al. 2011).
Does tarsal configuration have any useful phylogenetic signal?
The serial/alternating dichotomy is much more complex than which bone contacts which or how they are organized. There are plenty of different variations of the distribution of the tarsals and their contacts. Also, calcaneonavicular and astragalocuboid contact are not mutually exclusive; modern horses can have both. Tarsal arrangement is affected by several other factors, such as:
- proximodistal distance of calcaneocuboid and astragalonavicular joints;
- transverse width of the cuboid relative to the navicular;
- dorsoplantar extent of navicular;
- transverse width of the astragalus relative to the calcaneus;
- mobility of the hindfoot (astragalus and calcaneus) relative to the midfoot (navicular, cuboid and cuneiforms; transverse tarsal joint of Szalay, 1994).
The positions of contacts among tarsal bones can vary depending on how the tarsals are positioned relative to one another. In different mammals, the astragalocuboid facet can be positioned laterally (e.g. Periptychus, Ailuropoda), plantarly (e.g. Astrapotheria), distally (e.g. Pyrotheria), or a combination (Artiodactyla). Something similar also happens with the contact between the navicular and calcaneus. The variation is so great, both intraspecifically and interspecifically, that it cannot reasonably have any functional significance. For most mammals, the facets are so small that they may be incidental, non‐pathological contacts that form during development due to mobility between bones. The talocalcaneonavicular joint, the joint that relates these three bones, is the most mobile of the foot. Only in animals with an interlocking tarsus, an alternating tarsus beyond any doubt, does there seem to be some functional or phylogenetic correspondence. This is mainly the case in cetartiodactyls, but also in fossil and extant rhinoceros and tapirs. In these taxa, the cuboid is more developed than in horses and occupies half of the lateral width of the tarsus, whereas the navicular is small. The navicular is usually wider than the cuboid in the serial condition. This is particularly so for artiodactyls and rhinoceros. Tapirs present an intermediate condition where the cuboid is less developed, and the astragalocuboid contact is smaller than in rhinoceroses. Horses, traditionally considered as having an alternating tarsus, have a small plantar cuboid facet in the astragalus that usually is not visible when the tarsus is articulated. Therefore, horses have a serial tarsus as defined by Hussain (1975). In other mammals with the serial tarsal disposition, the relationships among the bones are generally as follows: the calcaneus mainly articulates with the cuboid, the astragalus mainly articulates with the navicular, the navicular and cuboid are mainly next to each other, and the astragalus is wider in dorsal view than the calcaneus.
‘Serial’ and ‘alternating’ can be considered states of displacement of the articulations in the transverse axis. Serial refers to the state where there is almost no displacement of the cuboid–navicular pair with respect to the calcaneus–astragalus pair. In the serial state, astragalocuboid and calcaneonavicular facets can be present, but they are small and do not significantly modify the movements permitted by the joints. The alternating state is that in which the navicular–cuboid articulation has moved considerably to the internal side relative to the calcaneus–astragalus articulation. An extreme displacement is present in Macropodidae, where the cuboid is significantly larger than the navicular but, in this case, the astragalar head is small and there is no astragalocuboid contact. This is here a termed a ‘flipped serial’ tarsus (Fig. 8A).
Figure 8.
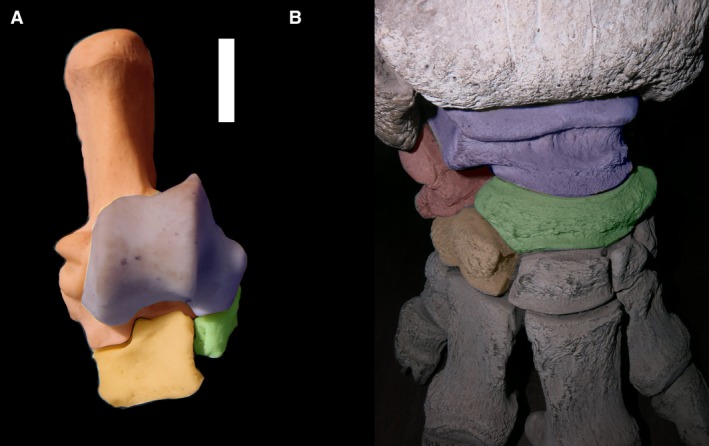
(A) Dorsal view of right tarsus of Macropodidae indet. (MLP 951). (B) Dorsal view of right tarsus of Loxodonta africana (MLP 1123; specimen in exposition). In blue shading, astragalus; in green shading, navicular; in red shading, calcaneus; in orange shading, cuboid.
The reverse alternating tarsus appears to encompass (and therefore obscure) two different conditions: a serial tarsus with a small calcaneonavicular contact and a displacement of the articulations in a different axis, the proximodistal axis. The astragalonavicular pair now changes with respect to the calcaneocuboid pair. Basal notoungulates and perissodactyls usually show the former condition, while some later cursorial notoungulates, litopterns and other specialized animals show the latter. The most extreme displacement of the astragalonavicular joint is present in elephants and toxodontids, where the navicular is positioned above the cuboid instead of being strictly medial to it. The navicular is always more medial than the cuboid but, in these animals, the calcaneocuboid joint is as distal as the naviculocuneiform joints, and the navicular facet in the cuboid is almost at the same position as the calcaneal facet. This condition is defined here as a new state, the ‘distal cuboid’. The state generally appears in graviportal mammals, and may have more morphofunctional than phylogenetic significance (Fig. 8B). Both extant and extinct elephants have a medial incursion of the cuboid (an alternating tarsus), although they are typically classified as having a serial tarsus. This incursion is hidden by the extreme displacement in the proximodistal axis.
Conclusion
Calcaneonavicular contact is not an uncommon condition restricted to Notoungulata, nor is it independent of other tarsal conditions. How tarsal bones contact each other is highly variable and not easy to determine without a complete tarsus, something that is exceptional in the paleontological record.
Serial and alternating arrangements of the tarsals, as originally defined (i.e. without reference to articulations between specific tarsals) seem to be the best character states for describing the positions of tarsal elements in mammals. To further clarify these states, I define a serial tarsus as one in which the calcaneus is positioned mostly on the cuboid and the astragalus is positioned mostly on the navicular, and an alternating tarsus as one in which the navicular and cuboid have a contact with the astragalus of similar size. Serial and alternating states can be also defined based on relative displacement of articulations in the transverse axis, and alternating tarsus also can be defined as a medial incursion of the cuboid bone, as a navicular incursion to the lateral side has not been observed. Serial refers to the state where there is almost no displacement of the cuboid–navicular pair with respect to the calcaneus–astragalus pair. In the serial state, astragalocuboid and calcaneonavicular facets can be present, but they are usually small and do not significantly modify the movements permitted by the joints. The alternating state is that in which the navicular–cuboid articulation has moved considerably to the medial side relative to the calcaneus–astragalus articulation. An extreme displacement is present in Macropodidae, where the cuboid is significantly larger than the navicular but, in this case, the astragalar head is small and there is no astragalocuboid contact. This is here called a ‘flipped serial tarsus’. The ‘reversed alternating’ state is simply a subset of the ‘serial’ state.
What joint (calcaneocuboid or astragalonavicular) is more proximal, and which bones contact which can be taken as different and independent characters. These conditions are much more variable than the movements permitted in the transverse axis. Along the proximodistal axis, the most common states are as follows: (i) calcaneocuboid and astragalonavicular joints at the same level; or (ii) an astragalonavicular joint more proximal than the calcaneocuboid. However, a calcaneocuboid joint more proximal than the astragalonavicular also has been observed. Bone contacts are not necessarily congruent with the displacements of these joints in the different axis. Notoungulata and Litopterna have a serial tarsus. Basal notoungulates have a serial tarsus with astragalonavicular and calcaneocuboid articulations at the same proximodistal level, similar to ‘archaic’ ungulates. Litopterns and more advanced notoungulates have a serial tarsus with an astragalonavicular joint that is considerably more proximal, convergently evolved with artiodactyls and caviomorphs, and in concordance with their alleged specialization in a cursorial mode of locomotion.
Acknowledgements
The author is grateful to M. Reguero, A. Scarano. I. Olivares (MLP), A. Kramarz, P. Teta and S. Lucero (MACN) for allowing access to specimens under their care. A special thanks to Emilia Rafasquino, from the Museo de Anatomía Veterinaria, Facultad de Ciencias Veterinarias (UNLP), who allowed the author to check a great number of horses’ tarsi. The author wants to thank Ken Rose for all the suggestions, and specially thanks Darin Croft for all his advices and patience.
References
- Bergqvist LP (1996) Reassociação do pós‐cránio às espécies de ungulados da Bacia de S. J. de Itaboraí (Paleoceno), estado do Rio de Janeiro, e filogenia dos ‘condylarthra’ e ungulados sul‐americanos com base no póscrânio. Doctoral dissertation, Universidade Federal do Rio Grande do Sul. [Google Scholar]
- Billet G (2011) Phylogeny of the Notoungulata (Mammalia) based on cranial and dental characters. J Syst Palaeontol 9, 481–497. [Google Scholar]
- Bond M (1986) Los ungulados fósiles de Argentina: evolución y paleoambientes. Actas, IV Congreso Argentino de Paleontología y Bioestratigrafía, Mendoza 2, 173–185.
- Buckley M (2015) Ancient collagen reveals evolutionary history of the endemic South American ‘ungulates’. Proc Biol Sci 282, 20142671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee R (1952) The Deseadan vertebrate fauna of the Scarritt Pocket, Patagonia. Bull Am Mus Nat Hist 98, 503–562. [Google Scholar]
- Cifelli RL (1983) Eutherian tarsals from the late Paleocene of Brazil. Am Mus Novit 2761, 1–31. [Google Scholar]
- Cifelli RL (1993) The phylogeny of the native South American ungulates In: Mammal Phylogeny: Placentals (eds Szalay FS, Novacek MJ, McKenna MC.), pp. 195–216. New York: Springer; [Google Scholar]
- Cope ED (1881) On the origin of the foot structures of the ungulates. Am Nat 15, 269–273. [Google Scholar]
- Cope ED (1882) The classification of the ungulate Mammalia. Proc Am Philos Soc 20, 438–447. [Google Scholar]
- Cope ED (1884a) The Vertebrata of the Tertiary Formations of the West, Report of the United States Geological Survey of the Territories. Vol. 3, pp. 1–1003. [Google Scholar]
- Cope ED (1884b) The condylarthra. Am Nat 18, 790–805. [Google Scholar]
- Croft DA (1999) Placentals: South American ungulates In: Encyclopedia of Paleontology (ed. Singer R.). pp. 890–806, Chicago, IL: Fitzroy‐Dearborn. [Google Scholar]
- Croft DA, Anderson LC (2008) Locomotion in the extinct notoungulate Protypotherium. Palaeontol Electronica 11, 1–20. [Google Scholar]
- Elissamburu A (2007) Estudio Biomecánico del Aparato Locomotor de Ungulados Nativos Sudamericanos (Notoungulata). Doctoral dissertation, Universidad Nacional de la Plata. [Google Scholar]
- Elissamburu A (2010) Estudio biomecánico y morfofuncional del esqueleto apendicular de Homalodotherium Flower 1873 (Mammalia, Notoungulata). Ameghiniana 47, 25–43. [Google Scholar]
- Elissamburu A (2012) Estimación de la masa corporal en géneros del Orden Notoungulata. Estud Geol 68, 91–111. [Google Scholar]
- Holbrook LT (2001) Comparative osteology of early Tertiary tapiromorphs (Mammalia, Perissodactyla). Zool J Linn Soc 131, 1–55. [Google Scholar]
- Hussain TS (1975) Evolutionary and functional anatomy of the pelvic limb in fossil and Recent Equidae (Perissodactyla, Mammalia). Anat Histol Embryol 4, 193–222. [DOI] [PubMed] [Google Scholar]
- Loomis FB (1914) The Deseado Formation of Patagonia. Amherst, Massachusetts: The Rumford Press. [Google Scholar]
- Lorente M (2015) Desarrollo de modelos de asociación y clasificaciones de restos postcraneanos aislados de ungulados nativos del Paleoceno‐Eoceno de América del Sur. Doctoral dissertation. Universidad Nacional de La Plata. [Google Scholar]
- Lorente M (2016) Reverse alternating tarsus, a derived or a basal trait?. XI Congreso de la Asociación Paleontológica Argentina, General Roca, Argentina.
- Lorente M, Gelfo JN, López GM (2014) Postcranial anatomy of the early notoungulate Allalmeia atalaensis from the Eocene of Argentina. Alcheringa 38, 398–411. [Google Scholar]
- Lorente M, Gelfo JN, López GM (2018) First skeleton of the notoungulate mammal Notostylops murinus and palaeobiology of Eocene Notostylopidae. Lethaia 52, 244–259. [Google Scholar]
- Matthew W (1897) A revision of the Puerco fauna. Bull Am Mus Nat Hist 9, 259–323. [Google Scholar]
- Matthew WD (1937) Paleocene Faunas of the San Juan Basin, New Mexico. Trans Am Philos Soc 30, 1–510. [Google Scholar]
- de Muizon C (1992) La fauna de mamfferos de Tiupampa (Paleoceno inferior, Formación Santa Lucia), Bolivia. Revista Técnica de Yacimientos Petroliferos Fiscales de Bolivia 12, 575–624. [Google Scholar]
- Muñoz NA (2017) Esqueleto apendicular de tipoterios (Notoungulata) y caviomorfos (Rodentia) de la Formación Santa Cruz (Mioceno Inferior alto). Doctoral dissertation, Facultad de Ciencias Naturales y Museo. [Google Scholar]
- Muñoz NA, Cassini GH, Candela AM, et al. (2017) Ulnar articular surface 3‐D landmarks and ecomorphology of small mammals: a case study of two early Miocene typotheres (Notoungulata) from Patagonia. Earth Environ Sci Trans R Soc Edinb 106, 315–323. [Google Scholar]
- O'Leary MA, Bloch JI, Flynn JJ, et al. (2013) The placental mammal ancestor and the post‐K‐Pg radiation of placentals. Science 339, 662–7. [DOI] [PubMed] [Google Scholar]
- Osborn HF (1898) Evolution of the Amblypoda. Part I. Taligrada and Pantodonta. Bull Am Mus Nat Hist 10, 169–218. [Google Scholar]
- Osborn HF (1913) Eomoropus, an American Eocene chalicothere. Bull Am Mus Nat Hist 32, 261–274. [Google Scholar]
- Patterson B, Pascual R (1972) The fossil mammal fauna of South America In: Evolution, Mammals, and Southern Continents. (eds Keast A, Erk FC, Glass B.), pp. 247–309. Albany, NY: State University of New York Press. [Google Scholar]
- Penkrot TA, Zack SP, Rose KD, et al. (2008) Postcranial morphology of Apheliscus and Haplomylus (Condylarthra, Apheliscidae): evidence for a Paleocene Holarctic origin of Macroscelidea In: Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. (eds Sargis EJ, Dagosto M.), pp. 73–106. Dordrecht: Springer. [Google Scholar]
- Piveteau J (1958) L'origine des mammifères et les aspects fondamentaux de leur evolution. Traité de Paléontologie 2, 1138. [Google Scholar]
- Radinsky LB (1964) Paleomoropus, a new early Eocene chalicothere (Mammalia, Perissodactyla), and a revision of Eocene chalicotheres. Am Mus Novit 2179, 1–28. [Google Scholar]
- Radinsky LB (1965) Evolution of the tapiroid skeleton from Heptodon to Tapirus. Bull Mus Comp Zool 134, 69–106. [Google Scholar]
- Rose KD, Holbrook LT, Rana RS, et al. (2014) Early Eocene fossils suggest that the mammalian order Perissodactyla originated in India. Nat Commun 5, 5570. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black S (2004) The Juvenile Skeleton. Elsevier. [Google Scholar]
- Shockey BJ, Anaya F (2008) Postcranial osteology of mammals from Salla, Bolivia (Late Oligocene): form, function, and phylogenetic implications In: Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. (eds Sargis EJ, Dagosto M.), pp. 135–157. Netherlands: Springer. [Google Scholar]
- Shockey BJ, Flynn JJ (2007) Postcranium of Casamayoran notoungulates from Patagonia. Am Mus Novit 3601, 1–26. [Google Scholar]
- Shockey BJ, Croft DA, Anaya Daza F (2007) Analysis of function in the absence of extant functional homologues: a case study using mesotheriid notoungulates (Mammalia). Paleobiology 33, 227–247. [Google Scholar]
- Shockey BJ, Flynn J, Croft D, et al. (2012) New Leontiniid Notoungulata (Mammalia) from Chile and Argentina: comparative anatomy, character analysis, and phylogenetic hypotheses. Am Mus Novit 3737, 1–64. [Google Scholar]
- Simpson GG (1937) The Fort Union of the Crazy Mountain Field, Montana, and Its Mammalian Faunas. Bulletin of the United States National Museum, 1–10, 1–287. [Google Scholar]
- Simpson GG (1980) Splendid Isolation: The Curious History of South American Mammals. New Haven: Yale University Press. [Google Scholar]
- Sinclair W (1908) The Santa Cruz Typotheria. Proc Am Philos Soc 47, 64–78. [Google Scholar]
- Smuts MMS, Bezuidenhout AJ (1994) Osteology of the pelvic limb of the African elephant (Loxodonta africana). Onderstepoort J Vet Res 61, 51–66. [PubMed] [Google Scholar]
- Szalay, FS (1994). Evolutionary history of the marsupials and an analysis of osteological characters. New York, NY: Cambridge University Press; 496 pp. [Google Scholar]
- Szalay FS, Lucas SG (1996) The Postcranial Morphology of Paleocene Chiacus and Mixodectes and the Phylogenetic Relationships of Archontan Mammals: Bulletin 7. New Mexico Museum of Natural History and Science. [Google Scholar]
- Thewissen J (1990) Evolution of Paleocene and Eocene Phenacodontidae (Mammalia, Condylarthra). Papers on Paleontology 29, 1–120. [Google Scholar]
- Vera B (2012) Postcranial morphology of Notopithecus Ameghino, 1897 (Notoungulata, Interatheriidae) from the middle Eocene of Patagonia, Argentina. J Vertebr Paleontol 32, 37–41. [Google Scholar]
- Welker F, Collins MJ, Thomas JA, et al. (2015) Ancient proteins resolve the evolutionary history of Darwin's South American ungulates. Nature 522, 81–84. [DOI] [PubMed] [Google Scholar]
- Westbury M, Baleka S, Barlow A, et al. (2017) A mitogenomic timetree for Darwin's enigmatic South American mammal Macrauchenia patachonica. Nat Commun 8, 15 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AR, Bebej RM, Manz CL, et al. (2011) Postcranial functional morphology of Hyracotherium (Equidae, Perissodactyla) and locomotion in the earliest horses. J Mamm Evol 18, 1–32. [Google Scholar]